Clinical Correlation of Transcription Factor SOX3 in Cancer: Unveiling Its Role in Tumorigenesis
Abstract
1. Introduction
2. Functional Implications of OX3 Modifications
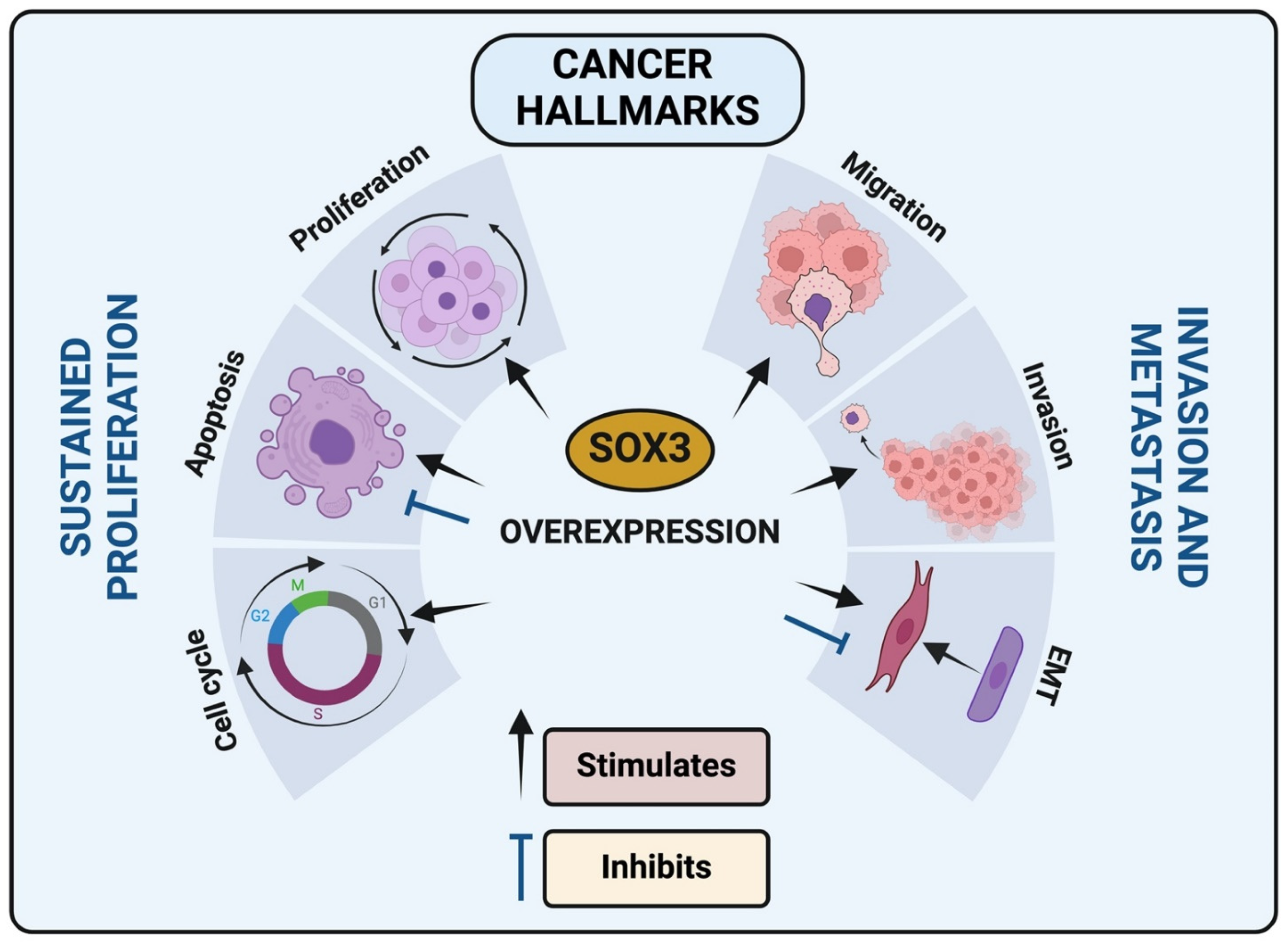
3. SOX3 Involvement and Regulation of Cancer Hallmarks
3.1. SOX3 and Cell Death by Apoptosis
3.2. SOX3 and Epithelial-Mesenchymal Transition (EMT)
3.3. SOX3 and Cell Invasion and Migration
3.4. SOX3 Interaction with Cell Cycle Regulators
4. SOX3 Investigation and Clinical Correlation in Different Types of Cancer
4.1. SOX3 in Osteosarcoma
4.2. SOX3 in Ovarian Cancer
4.3. SOX3 in Breast Cancer
4.4. SOX3 in Esophageal Cancer
4.5. SOX3 in Gastric Cancer (GC)
4.6. SOX3 in Glioma and Glioblastoma (GBM)
4.7. SOX3 in Hepatocellular Carcinoma (HCC)
4.8. SOX3 in Endometrial Carcinoma (EC)
4.9. Acute Myeloid Leucemia (AML)
5. Conclusions and Future Perspective
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BAX | BCL2 associated X—Pro-apoptotic |
| BCL-2 | B-cell lymphoma 2—Regulates apoptosis |
| BH3 | Pro-apoptotic proteins from BCL-2 family |
| Cdc25A | Cell division cycle 25 A |
| CDKN2A Gene | Cyclin Dependent Kinase Inhibitor 2A |
| CELF2 | CUGBP Elav-like family member 2—RNA-binding protein |
| HER2 (ERBB2) | Receptor tyrosine-protein kinase erbB-2 |
| Ki67 | nuclear protein associated with cellular proliferation. |
| Nanog | Transcription factor protein |
| Notch | notch receptor 1—Type I transmembrane protein |
| Oct4 | Transcription factor protein |
| p53 (TP53) | Tumor suppressor protein 53 |
| PCNA | Proliferating cell nuclear antigen |
| SLUG | snail family transcriptional repressor 2 |
| SNAIL | snail family transcriptional repressor 1 |
| TWIST | twist family bHLH transcription factor 1 |
| Wnt | Wnt family member 1 |
| ZEB1 | zinc finger E-box binding homeobox 1 |
| ZEB2 | zinc finger E-box binding homeobox 2 |
References
- Lambert, S.A.; Jolma, A.; Campitelli, L.F.; Das, P.K.; Yin, Y.; Albu, M.; Chen, X.; Taipale, J.; Hughes, T.R.; Weirauch, M.T. The Human Transcription Factors. Cell 2018, 172, 650–665. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Hochedlinger, K. The Sox Family of Transcription Factors: Versatile Regulators of Stem and Progenitor Cell Fate. Cell Stem Cell 2013, 12, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Kashimada, K.; Koopman, P. Sry: The master switch in mammalian sex determination. Development 2010, 137, 3921–3930. [Google Scholar] [CrossRef] [PubMed]
- Berta, P.; Hawkins, J.B.; Sinclair, A.H.; Taylor, A.; Griffiths, B.L.; Goodfellow, P.N.; Fellous, M. Genetic evidence equating SRY and the testis-determining factor. Nature 1990, 348, 448–450. [Google Scholar] [CrossRef] [PubMed]
- Harley, V.R.; Lovell-badge, R.; Goodfellow, P.N. Definition of a consensus DNA binding site for SRY. Nucleic Acids Res. 1994, 22, 1500–1501. [Google Scholar] [CrossRef] [PubMed]
- Harley, V.R.; Jackson, D.I.; Hextall, P.J.; Hawkins, J.R.; Berkovitz, G.D.; Sockanathan, S.; Lovell-Badge, R.; Goodfellow, P.N. DNA Binding Activity of Recombinant SRY from Normal Males and XY Females. Science 1992, 255, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.E.; Ely, D.; Prokop, J.; Milsted, A. Sry, more than testis determination? Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2011, 301, R561–R571. [Google Scholar]
- Hou, L.; Srivastava, Y.; Jauch, R. Molecular basis for the genome engagement by Sox proteins. Semin. Cell Dev. Biol. 2017, 63, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Hur, W.; Rhim, H.; Jung, C.K.; Kim, J.D.; Bae, S.H.; Jang, J.W.; Yang, J.M.; Oh, S.T.; Kim, D.G.; Wang, H.J.; et al. SOX4 overexpression regulates the p53-mediated apoptosis in hepatocellular carcinoma: Clinical implication and functional analysis in vitro. Carcinogenesis 2010, 31, 1298–1307. [Google Scholar] [CrossRef]
- Bernard, P.; Harley, V.R. Acquisition of SOX transcription factor specificity through protein-protein interaction, modulation of Wnt signalling and post-translational modification. Int. J. Biochem. Cell Biol. 2010, 42, 400–410. [Google Scholar] [CrossRef]
- Swain, N.; Thakur, M.; Pathak, J.; Swain, B. SOX2, OCT4 and NANOG: The core embryonic stem cell pluripotency regulators in oral carcinogenesis. J. Oral Maxillofac. Pathol. 2020, 24, 368. [Google Scholar] [CrossRef]
- Xu, Y.R.; Yang, W.X. SOX-mediated molecular crosstalk during the progression of tumorigenesis. Semin. Cell Dev. Biol. 2017, 63, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Bowles, J.; Schepers, G.; Koopman, P. Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev. Biol. 2000, 227, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Wegner, M. From head to toes: The multiple facets of Sox proteins. Nucleic Acids Res. 1999, 27, 1409–1420. [Google Scholar] [CrossRef] [PubMed]
- Chew, L.J.; Gallo, V. The Yin and Yang of Sox proteins: Activation and repression in development and disease. J. Neurosci. Res. 2009, 87, 3277–3287. [Google Scholar] [CrossRef] [PubMed]
- Abadi, A.J.; Zarrabi, A.; Hashemi, F.; Zabolian, A.; Najafi, M.; Entezari, M.; Hushmandi, K.; Aref, A.R.; Khan, H.; Makvandi, P.; et al. The role of SOX family transcription factors in gastric cancer. Int. J. Biol. Macromol. 2021, 180, 608–624. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, Y.; Sun, M.; Luo, X.; Zhang, Z.; Wang, Y.; Li, S.; Hu, D.; Zhang, J.; Wu, Z.; et al. SOX on tumors, a comfort or a constraint? Cell Death Discov. 2024, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Castillo, S.D.; Sanchez-Cespedes, M. The SOX family of genes in cancer development: Biological relevance and opportunities for therapy. Expert Opin. Ther. Targets 2012, 16, 903–919. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Taeb, S.; Hushmandi, K.; Orouei, S.; Shahinozzaman, M.; Zabolian, A.; Moghadam, E.R.; Raei, M.; Zarrabi, A.; Khan, H.; et al. Cancer and SOX proteins: New insight into their role in ovarian cancer progression/inhibition. Pharmacol. Res. 2020, 161, 105159. [Google Scholar] [CrossRef] [PubMed]
- Thu, K.L.; Becker-Santos, D.D.; Radulovich, N.; Pikor, L.A.; Lam, W.L.; Tsao, M.S. SOX15 and other SOX family members are important mediators of tumorigenesis in multiple cancer types. Oncoscience 2014, 1, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.S.; Holzner, M.; Weng, M.; Srivastava, Y.; Jauch, R. SOX17 in cellular reprogramming and cancer. Semin. Cancer Biol. 2020, 67, 65–73. [Google Scholar] [CrossRef]
- Underwood, A.; Rasicci, D.T.; Hinds, D.; Mitchell, J.T.; Zieba, J.K.; Mills, J.; Arnold, N.E.; Cook, T.W.; Moustaqil, M.; Gambin, Y.; et al. Evolutionary Landscape of SOX Genes to Inform Genotype-to-Phenotype Relationships. Genes 2023, 14, 222. [Google Scholar] [CrossRef]
- Qian, M.; Wang, D.C.; Chen, H.; Cheng, Y. Detection of single cell heterogeneity in cancer. Semin. Cell Dev. Biol. 2017, 64, 143–149. [Google Scholar] [CrossRef]
- Wu, F.; Fan, J.; He, Y.; Xiong, A.; Yu, J.; Li, Y.; Zhang, Y.; Zhao, W.; Zhou, F.; Li, W.; et al. Single-cell profiling of tumor heterogeneity and the microenvironment in advanced non-small cell lung cancer. Nat. Commun. 2021, 12, 2540. [Google Scholar] [CrossRef]
- Nguyen, A.; Yoshida, M.; Goodarzi, H.; Tavazoie, S.F. Highly variable cancer subpopulations that exhibit enhanced transcriptome variability and metastatic fitness. Nat. Commun. 2016, 7, 11246. [Google Scholar] [CrossRef]
- Ilan, Y.; Spigelman, Z. Establishing patient-tailored variability-based paradigms for anti-cancer therapy: Using the inherent trajectories which underlie cancer for overcoming drug resistance. Cancer Treat. Res. Commun. 2020, 25, 100240. [Google Scholar] [CrossRef]
- Yang, J.; Xu, J.; Wang, W.; Zhang, B.; Yu, X.; Shi, S. Epigenetic regulation in the tumor microenvironment: Molecular mechanisms and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 210. [Google Scholar] [CrossRef]
- Wuebben, E.L.; Rizzino, A. The dark side of SOX2: Cancer—A comprehensive overview. Oncotarget 2017, 8, 44917–44943. [Google Scholar] [CrossRef]
- Mirzaei, S.; Paskeh, M.D.A.; Entezari, M.; Mirmazloomi, S.R.; Hassanpoor, A.; Aboutalebi, M.; Rezaei, S.; Hejazi, E.S.; Kakavand, A.; Heidari, H.; et al. SOX2 function in cancers: Association with growth, invasion, stemness and therapy response. Biomed. Pharmacother. 2022, 156, 113860. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Almeida, C.P.; Oliveira, M.C.M.; Ferreira, E.; Ribeiro, T.S.; Borges, I.T.; Gomes, H.W.; Oliveira, C.A.; Puerto, H.L.D.; Martins, A.S. Overexpression of SOX2 is associated with poor prognosis in human breast cancer. J. Clin. Images Med. Case Rep. 2021, 1–4. Available online: https://jcimcr.org/articles/JCIMCR-v2-1182.html (accessed on 23 May 2024). [CrossRef]
- Lengerke, C.; Fehm, T.; Kurth, R.; Neubauer, H.; Scheble, V.; Müller, F.; Schneider, F.; Petersen, K.; Wallwiener, D.; Kanz, L.; et al. Expression of the embryonic stem cell marker SOX2 in early-stage breast carcinoma. BMC Cancer 2011, 11, 42. [Google Scholar] [CrossRef]
- Feng, X.; Lu, M. Expression of sex-determining region Y-box protein 2 in breast cancer and its clinical significance. Saudi Med. J. 2017, 38, 685–690. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, L.; Zhang, L.; Li, R.; Liang, J.; Yu, W.; Sun, L.; Yang, X.; Wang, Y.; Zhang, Y.; et al. The molecular mechanism governing the oncogenic potential of SOX2 in breast cancer. J. Biol. Chem. 2008, 283, 17969–17978. [Google Scholar] [CrossRef]
- Savare, J.; Bonneaud, N.; Girard, F. SUMO Represses Transcriptional Activity of the Drosophila SoxNeuro and Human Sox3 Central Nervous System–specific Transcription Factors. Mol. Biol. Cell 2005, 16, 2660–2669. [Google Scholar] [CrossRef]
- Lamoliatte, F.; McManus, F.P.; Maarifi, G.; Chelbi-Alix, M.K.; Thibault, P. Uncovering the SUMOylation and ubiquitylation crosstalk in human cells using sequential peptide immunopurification. Nat. Commun. 2017, 8, 14109. [Google Scholar] [CrossRef]
- Queiroz, L.Y.; Kageyama, R.; Cimarosti, H.I. SUMOylation effects on neural stem cells self-renewal, differentiation, and survival. Neurosci. Res. 2024, 199, 1–11. [Google Scholar] [CrossRef]
- Alfassam, H. SUMOylation as a Regulatory Mechanism for the Sox3 Transcription Factor; University of Nottingham: Nottingham, UK, 2019. [Google Scholar]
- Serrano-Gomez, S.J.; Maziveyi, M.; Alahari, S.K. Regulation of epithelial-mesenchymal transition through epigenetic and post-translational modifications. Mol. Cancer 2016, 15, 18. [Google Scholar] [CrossRef]
- Ullmann, R.; Chien, C.D.; Avantaggiati, M.L.; Muller, S. An Acetylation Switch Regulates SUMO-Dependent Protein Interaction Networks. Mol. Cell 2012, 46, 759–770. [Google Scholar] [CrossRef]
- Zheng, G.; Yang, Y.C. Sumoylation and acetylation play opposite roles in the transactivation of PLAG1 and PLAGL2. J. Biol. Chem. 2005, 280, 40773–40781. [Google Scholar] [CrossRef]
- Salas-Lloret, D.; González-Prieto, R. Insights in Post-Translational Modifications: Ubiquitin and SUMO. Int. J. Mol. Sci. 2022, 23, 3281. [Google Scholar] [CrossRef]
- Seo, Y.; Kim, D.K.; Park, J.; Park, S.J.; Park, J.J.; Cheon, J.H.; Kim, T.I. A Comprehensive Understanding of Post-Translational Modification of Sox2 via Acetylation and O-GlcNAcylation in Colorectal Cancer. Cancers 2024, 16, 1035. [Google Scholar] [CrossRef]
- Han, S.; Ren, Y.; He, W.; Liu, H.; Zhi, Z.; Zhu, X.; Yang, T.; Rong, Y.; Ma, B.; Purwin, T.J.; et al. ERK-mediated phosphorylation regulates SOX10 sumoylation and targets expression in mutant BRAF melanoma. Nat. Commun. 2018, 9, 28. [Google Scholar] [CrossRef]
- Lee, P.C.; Taylor-Jaffe, K.M.; Nordin, K.M.; Prasad, M.S.; Lander, R.M.; LaBonne, C. SUMOylated SoxE factors recruit Grg4 and function as transcriptional repressors in the neural crest. J. Cell Biol. 2012, 198, 799–813. [Google Scholar] [CrossRef]
- Girard, M.; Goossens, M. Sumoylation of the SOX10 transcription factor regulates its transcriptional activity. FEBS Lett. 2006, 580, 1635–1641. [Google Scholar] [CrossRef]
- Dutta, H.; Jain, N. Post-translational modifications and their implications in cancer. Front. Oncol. 2023, 13, 1240115. [Google Scholar] [CrossRef]
- Li, K.; Xia, Y.; He, J.; Wang, J.; Li, J.; Ye, M.; Jin, X. The SUMOylation and ubiquitination crosstalk in cancer. J. Cancer Res. Clin. Oncol. 2023, 149, 16123–16146. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, R.; Hei, H. Advances in post-translational modifications of proteins and cancer immunotherapy. Front. Immunol. 2023, 14, 1229397. [Google Scholar] [CrossRef]
- Dunphy, K.; Dowling, P.; Bazou, D.; O’Gorman, P. Current methods of post-translational modification analysis and their applications in blood cancers. Cancers 2021, 13, 1930. [Google Scholar] [CrossRef]
- Guo, Y.; Yin, J.; Tang, M.; Yu, X. Downregulation of SOX3 leads to the inhibition of the proliferation, migration and invasion of osteosarcoma cells. Int. J. Oncol. 2018, 52, 1277–1284. [Google Scholar] [CrossRef]
- Vicentic, J.M.; Drakulic, D.; Garcia, I.; Vukovic, V.; Aldaz, P. SOX3 can promote the malignant behavior of glioblastoma cells. Cell. Oncol. 2018, 42, 41–54. [Google Scholar] [CrossRef]
- Yan, Q.; Wang, F.; Miao, Y.; Wu, X. Sex-determining region Y-box3 (SOX3) functions as an oncogene in promoting epithelial ovarian cancer by targeting Src kinase. Tumor Biol. 2016, 37, 12263–12271. [Google Scholar] [CrossRef]
- Cui, K.; Zhang, H.; Wang, G. MiR-483 suppresses cell proliferation and promotes cell apoptosis by targeting SOX3 in breast cancer. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2069–2074. [Google Scholar]
- Almeida, C.P.; Ferreira, M.C.F.; Silveira, C.O.; Campos, J.R.; Borges, I.T.; Baeta, P.G.; Silva, F.H.S.; Reis, F.M.; Del Puerto, H.L. Clinical correlation of apoptosis in human granulosa cells—A review. Cell Biol. Int. 2018, 42, 1276–1281. [Google Scholar] [CrossRef]
- Lu, S.; Yu, Z.; Zhang, X.; Sui, L. MiR-483 targeted SOX3 to suppress glioma cell migration, invasion and promote cell apoptosis. Onco. Targets. Ther. 2020, 13, 2153–2161. [Google Scholar] [CrossRef]
- Silva, F.H.D.S.; Underwood, A.; Almeida, C.P.; Ribeiro, T.S.; Souza-Fagundes, E.M.; Martins, A.S.; Eliezeck, M.; Guatimosim, S.; Andrade, L.O.; Rezende, L.; et al. Transcription factor SOX3 upregulated pro-apoptotic genes expression in human breast cancer. Med. Oncol. 2022, 39, 212. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Hugo, H.; Ackland, M.L.; Blick, T.; Lawrence, M.G.; Clements, J.A.; Williams, E.D.; Thompson, E.W. Epithelial—Mesenchymal and mesenchymal—Epithelial transitions in carcinoma progression. J. Cell. Physiol. 2007, 213, 374–383. [Google Scholar] [CrossRef]
- Lee, T.K.; Poon, R.T.P.; Yuen, A.P.; Ling, M.T.; Kwok, W.K.; Wang, X.H.; Wong, Y.C.; Guan, X.; Man, K.; Chau, K.L.; et al. Twist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin. Cancer Res. 2006, 12, 5369–5376. [Google Scholar] [CrossRef]
- Kase, S.; Sugio, K.; Yamazaki, K.; Okamoto, T.; Yano, T.; Sugimachi, K. Expression of E-cadherin and β-catenin in human non-small cell lung cancer and the clinical significance. Clin. Cancer Res. 2000, 6, 4789–4796. [Google Scholar] [CrossRef]
- Pirinen, R.T.; Hirvikoski, P.; Johansson, R.T.; Hollmén, S.; Kosma, V.M. Reduced expression of α-catenin, β-catenin, and γ-catenin is associated with high cell proliferative activity and poor differentiation in non-small cell lung cancer. J. Clin. Pathol. 2001, 54, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Chen, D.; Shen, C.; Shen, J.; Zhao, H.; He, Y. Sex-determining region Y-box protein 3 induces epithelial-mesenchymal transition in osteosarcoma cells via transcriptional activation of Snail1. J. Exp. Clin. Cancer Res. 2017, 36, 46. [Google Scholar] [CrossRef]
- Gong, B.; Yue, Y.; Wang, R.; Zhang, Y.; Jin, Q.; Zhou, X. Overexpression of microRNA-194 suppresses the epithelial-mesenchymal transition in targeting stem cell transcription factor Sox3 in endometrial carcinoma stem cells. Tumor Biol. 2017, 39, 1010428317706217. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.H.S.; Underwood, A.; Almeida, C.P.; Lima, B.M.; Veloso, E.S.; Carvalho, B.A.; Ribeiro, T.S.; Cassali, G.D.; Ferreira, E.; Del Puerto, H.L. Transcription Factor SOX3 Regulates Epithelial—Mesenchymal Transition in Human Breast Cancer Cell Line MDA-MB-231. Ann. Breast Cancer 2024, 7, 1026. [Google Scholar]
- Acloque, H.; Ocaña, O.H.; Matheu, A.; Rizzoti, K.; Wise, C.; Lovell-Badge, R.; Nieto, M.A. Reciprocal repression between Sox3 and Snail transcription factors defines embryonic territories at gastrulation. Dev. Cell 2011, 21, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhai, J.; Wu, X.; Xie, G.; Shen, L. Serum proteome profiling reveals SOX3 as a candidate prognostic marker for gastric cancer. J. Cell. Mol. Med. 2020, 24, 6750–6761. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Liang, L.; Wang, Z.; Zhang, B.; Li, Q.; Tian, Y.; Yu, Y.; Chen, Z.; Wang, X.; Liu, H. Expression and significance of SOX B1 genes in glioblastoma multiforme patients. J. Cell. Mol. Med. 2021, 26, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, R.; Jiang, Y.; Zou, Y.; Guo, W. Overexpression of Sox3 is Associated with Diminished Prognosis in Esophageal Squamous Cell Carcinoma. Ann. Surg. Onco. 2013, 20, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xiao, F.; Yang, N.; Zhu, N.; Fu, Y.; Zhang, H.; Yang, G. Overexpression of Sox3 is associated with promoted tumor progression and poor prognosis in hepatocellular carcinoma. Int. J. Exp. Pathol. 2017, 10, 7873–7881. [Google Scholar]
- Matthews, H.K.; Bertoli, C.; de Bruin, R.A.M. Cell cycle control in cancer. Nat. Rev. Mol. Cell Biol. 2022, 23, 74–88. [Google Scholar] [CrossRef]
- Bylund, M.; Andersson, E.; Novitch, B.G.; Muhr, J. Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat. Neurosci. 2003, 6, 1162–1168. [Google Scholar] [CrossRef]
- Holmberg, J.; He, X.; Peredo, I.; Orrego, A.; Hesselager, G.; Ericsson, C.; Hovatta, O.; Oba-Shinjo, S.M.; Marie, S.K.N.; Nistér, M.; et al. Activation of neural and pluripotent stem cell signatures correlates with increased malignancy in human glioma. PLoS ONE 2011, 6, e18454. [Google Scholar] [CrossRef] [PubMed]
- Muhr, J.; Hagey, D.W. The cell cycle and differentiation as integrated processes: Cyclins and CDKs reciprocally regulate Sox and Notch to balance stem cell maintenance. BioEssays 2021, 43, 2000285. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Tang, H.; Song, C.; Wang, J.; Chen, B.; Huang, X.; Pei, X.; Liu, L. SOX2 promotes cell proliferation and metastasis in triple negative breast cancer. Front. Pharmacol. 2018, 9, 942. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.Y.; Liang, G.Y.; Zheng, Y.F.; Tan, Q.Y.; Wang, R.W.; Li, K. Sox3 silencing inhibits metastasis and growth of esophageal squamous cell carcinoma cell via down-regulating GSK-3β. Int. J. Clin. Exp. Pathol. 2016, 9, 2939–2949. [Google Scholar]
- Zheng, Y.F.; Li, K.; Cai, Q.Y.; Yang, L.; Tan, Q.Y.; Guo, W.; Wang, R.W. The effect of high Sox3 expression on lymphangiogenesis and lymph node metastasis in esophageal squamous cell carcinoma. Am. J. Transl. Res. 2017, 9, 2684–2693. [Google Scholar]
- Sa, J.K.; Kim, S.H.; Lee, J.K.; Cho, H.J.; Shin, Y.J.; Shin, H.; Koo, H.; Kim, D.; Lee, M.; Kang, W.; et al. Identification of genomic and molecular traits that present therapeutic vulnerability to HGF-targeted therapy in glioblastoma. Neuro. Oncol. 2019, 21, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Scuderi, S.A.; Lanza, M.; Casili, G.; Esposito, F.; Colarossi, C.; Giuffrida, D.; Irene, P.; Cuzzocrea, S.; Esposito, E.; Campolo, M. TBK1 Inhibitor exerts antiproliferative effect on glioblastoma multiforme cells. Oncol. Res. 2021, 28, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Turchi, L.; Sakakini, N.; Saviane, G.; Polo, B.; Saurty-Seerunghen, M.S.; Gabut, M.; Gouillou, C.A.; Guerlais, V.; Pasquier, C.; Vignais, M.L.; et al. CELF2 Sustains a Proliferating/OLIG2+ Glioblastoma Cell Phenotype via the Epigenetic Repression of SOX3. Cancers 2023, 15, 5038. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, P.; Lu, Y.; Zhang, A.; Chen, X. LINC00662 Promotes Proliferation and Invasion and Inhibits Apoptosis of Glioma Cells Through miR-483-3p/SOX3 Axis. Appl. Biochem. Biotechnol. 2022, 194, 2857–2871. [Google Scholar] [CrossRef]
- Güre, A.O.; Stockert, E.; Scanlan, M.J.; Keresztes, R.S.; Jäger, D.; Altorki, N.K.; Old, L.J.; Chen, Y.T. Serological identification of embryonic neural proteins as highly immunogenic tumor antigens in small cell lung cancer. Proc. Natl. Acad. Sci. USA 2000, 97, 4198–4203. [Google Scholar] [CrossRef]
- Matsumoto, T.; Oda, Y.; Hasegawa, Y.; Hashimura, M.; Oguri, Y.; Inoue, H.; Yokoi, A.; Tochimoto, M.; Nakagawa, M.; Jiang, Z.; et al. Anaplastic Lymphoma Kinase Overexpression Is Associated with Aggressive Phenotypic Characteristics of Ovarian High-Grade Serous Carcinoma. Am. J. Pathol. 2021, 191, 1837–1850. [Google Scholar] [CrossRef]
- Tosic, N.; Petrovic, I.; Grujicic, N.K.; Davidovic, S.; Virijevic, M.; Vukovic, N.S.; Pavlovic, S.; Stevanovic, M. Prognostic significance of SOX2, SOX3, SOX11, SOX14 and SOX18 gene expression in adult de novo acute myeloid leukemia. Leuk. Res. 2018, 67, 32–38. [Google Scholar] [CrossRef]
- Misaghi, A.; Goldin, A.; Awad, M.; Kulidjian, A.A. Osteosarcoma: A comprehensive review. Sicot-J. 2018, 4, 12. [Google Scholar] [CrossRef]
- Sambasivan, S. Epithelial ovarian cancer: Review article. Cancer Treat. Res. Commun. 2022, 33, 100629. [Google Scholar] [CrossRef]
- Swaminathan, H.; Saravanamurali, K.; Yadav, S.A. Extensive review on breast cancer its etiology, progression, prognostic markers, and treatment. Med. Oncol. 2023, 40, 238. [Google Scholar] [CrossRef]
- Dai, X.; Xiang, L.; Li, T.; Bai, Z. Cancer hallmarks, biomarkers and breast cancer molecular subtypes. J. Cancer 2016, 7, 1281–1294. [Google Scholar] [CrossRef]
- Rodriguez-Pinilla, S.M.; Sarrio, D.; Moreno-Bueno, G.; Rodriguez-Gil, Y.; Martinez, M.A.; Hernandez, L.; Hardisson, D.; Reis-Filho, J.S.; Palacios, J. Sox2: A possible driver of the basal-like phenotype in sporadic breast cancer. Mod. Pathol. 2007, 20, 474–481. [Google Scholar] [CrossRef]
- Mehta, G.A.; Khanna, P.; Gatza, M.L. Emerging Role of SOX Proteins in Breast Cancer Development and Maintenance. J. Mammary Gland Biol. Neoplasia 2019, 24, 213–230. [Google Scholar] [CrossRef]
- Xu, J.; Cao, W.; Shao, A.; Yang, M.; Andoh, V.; Ge, Q.; Pan, H.W.; Chen, K.P. Metabolomics of Esophageal Squamous Cell Carcinoma Tissues: Potential Biomarkers for Diagnosis and Promising Targets for Therapy. Biomed Res. Int. 2022, 2022, 7819235. [Google Scholar] [CrossRef]
- Sexton, R.E.; Al Hallak, M.N.; Diab, M.; Azmi, A.S. Gastric cancer: A comprehensive review of current and future treatment strategies. Cancer Metastasis Rev. 2020, 39, 1179–1203. [Google Scholar] [CrossRef] [PubMed]
- Nørøxe, D.S.; Poulsen, H.S.; Lassen, U. Hallmarks of glioblastoma: A systematic review. ESMO Open 2016, 1, e000144. [Google Scholar] [CrossRef] [PubMed]
- Balogh, J.; Victor, D.; Asham, E.H.; Burroughs, S.G.; Boktour, M.; Saharia, A.; Li, X.; Ghobrial, M.; Monsour, H. Hepatocellular carcinoma: A review. J. Hepatocell. Carcinoma 2016, 3, 41–53. [Google Scholar] [CrossRef] [PubMed]



| Author | Tumor Type | Specimens/Samples | Methodology/Technique | Main Results/Clinical Correlation |
|---|---|---|---|---|
| Cui et al., 2019 [53] | Breast | Clinical specimens: 62 patients. Cell lines: MCF-7, SKBR3, LCC2, MDA-MB-453, T-47D, LCC9, and normal human breast cell line MCF-10A | Cell transfection; qRT-PCR; WB; luciferase; cell viability and proliferation (MTT); colony formation and apoptosis detection assay. | The miR-483 inhibitor upregulated the protein level of SOX3. SOX3 expression was negatively correlated with miR-483 expression in breast cancer tissues. The miR-483 could suppress breast cancer cell proliferation and promote cell apoptosis via targeting SOX3. |
| Silva et al., 2022 [56] | Breast | Cell line: MDA-MB-231 | Cell transfection with SOX3 expression vector; immunofluorescence; cell viability and proliferation (MTT); flow cytometry (apoptosis); RT-qPCR. | The apoptotic rate was higher in cells transfected with pEF1-SOX3+ than in controls. MDA-MB-231 transfected with pEF1-SOX3+ showed upregulation of pro-apoptotic CASP3, CASP8, CASP9, and BAX mRNA, contrasting with downregulation of BCL2 anti-apoptotic mRNA, compared to controls. |
| Silva et al., 2022 [56] | Breast | Clinical specimens: 27 patients with breast invasive ductal carcinoma | Immunohistochemistry | The nuclear expression of the SOX3 protein was detected in 14% of the cases of ductal carcinoma, and the expression of pro-Caspase-3 was positive in 50%. The IHC negative nuclear expression of SOX3 in ductal carcinoma can be related to cells resistant to apoptosis. |
| Silva et al., 2024 [66] | Breast | Cell lines: MDA-MB-231 | Cell transfection with SOX3 expression vector; viability test (MTT); RT-qPCR. | A downregulation in NCAD and an upregulation of ECAD expression, followed by SOX3 protein expression in the triple-negative breast cancer MDA-MB-231 cell line. |
| Gong et al., 2017 [65] | Endometrial carcinoma | Clinical specimens: 19 endometrial carcinoma patients. Samples (stage IB, n = 11; stage IC, n = 5; stage IIa, n = 3; age = 37–72 years). Primary cell culture with the 19 EC, forming tumorspheres of in vitro experiments. Implantation of tumorsphere cells into mice nude for in vivo experiments. | Constructs for overexpression and silencing SOX3; cell transfection; flow cytometry, RT-qPCR, WB, immunohistochemistry. | SOX3 contributes to endometrial cancer stem cell invasion and suggests that repression of SOX3 by microRNA-194 may have therapeutic potential to suppress endometrial carcinoma metastasis. |
| Li et al., 2013 [70] | Esophageal squamous | Clinical specimens: 30 patients | RT-qPCR; WB; tissue microarrays; immunohistochemistry. | The expression of SOX3 in esophageal squamous carcinoma (ESCC) was significantly higher than in non-neoplastic samples. SOX3 expression in ESCC significantly correlated with regional lymph node metastasis (RLNM) and advanced TNM. SOX3 may be a valuable biomarker for predicting prognosis and a potential therapeutic target for ESCC. |
| Cai et al., 2016 [77] | Esophageal squamous | Cell lines: ECA109, SKGT-5, SKTG-4, TE-1, TE-3, TE-8; AND SV40-immortalized non-tumorigenic | Proliferation and cytotoxicity assays (LDH); oncomine; migration and invasion; WB; MMPs activity; RT-qPCR; xenograft model. | SOX3 protein is involved in esophageal squamous carcinoma cell (ESCC) metastasis. SOX3 disruption impaired ESCC cell migration and invasion. Metastasis was significantly inhibited when the SOX3 gene was disrupted by insertional mutagenesis. |
| Zheng et al., 2017 [78] | Esophageal squamous | Cell lines: TE-1, TE-10, TE-11, EC109, EC9706. Animal model: tumorigenesis and axillary lymph node metastasis in nude mice. | WB; RT-qPCR; invasion; scratch; MTT; tube formation test; ELISA; animal experiments; immunohistochemistry. | SOX3 promotes tumor cell proliferation, migration, and invasion in vitro. SOX3 promotes lymph node metastasis of the tumor in vivo. SOX3 could increase the VEGF-C/D expression in ESCC cells both in vivo and in vitro. The high expression of SOX3 upregulated the expression of VEGF-C and VEGF-D in ESCC and promoted lymph node metastasis. |
| Shen et al., 2020 [68] | Gastric | Clinical specimens: 60 patients—5 cases of early gastric carcinoma and 55 locally advanced gastric carcinoma cases. | Protein extraction; TMT/iTRAQ labeling; HPLC fractionation; liquid chromatography; WB; ELISA; immunohistochemistry, immunofluorescence. | Serum proteome profiling reveals differential expression of SOX3 protein, between pre- and post-operation for locally advanced gastric cancer. SOX3 is overexpressed in gastric cancer tissues and is associated with poor outcomes for gastric cancer. This study highlights the potentiality of the paired pre- and post-operation serum proteome signature for detecting putative biomarkers for gastric carcinoma and reveals that SOX3 may serve as a candidate molecular marker for the prognosis and outcomes of gastric cancer patients. |
| Shen et al., 2020 [68] | Gastric | Cell lines: AGS and MKN45 human gastric adenocarcinoma | SOX3 mRNA silencing; invasion assay; xenograft model in zebrafish (zPDX); chromatin immunoprecipitation. | SOX3 promotes gastric cancer cell invasion and migration through MMP9. |
| Jason et al., 2019 [79] | Glioblastoma | Clinical specimens: glioblastoma (GMB) obtained from patients undergoing surgery was used to obtain primary glioblastoma stem cells (GSCs). | DNA and RNA sequencing; invasion; proliferation; immunoblot assay; flow cytometry; immunohistochemistry. | Identification of several differentially expressed genes, including SOX3, associated with tumor invasiveness, malignancy, and unfavorable prognosis in glioblastoma patients. |
| Vicentic et al., 2019 [51] | Glioblastoma | Clinical specimens: 13 samples for immunohistochemistry, 27 samples for RT-qPCR, control non-tumoral brain RNAs, and 23 samples for Ambion. | Immunohistochemistry and RT-qPCR | SOX3 expression is higher in glioblastoma samples than in non-tumoral brain tissues. SOX3 protein expression in cell nuclei was observed in all analyzed tumor samples. |
| Vicentic et al., 2019 [51] | Glioblastoma | Cell lines: U87, U373, U251, A172 and T98; GNS166 and GNS179 (stem cell); GB1 and GB2 (oncopheres). | Transfection and luciferase assay; WB; MTT; immunocytochemistry; Transwell migration and invasion. | Exogenous overexpression of SOX3 enhances proliferation, viability, migration, and invasion of glioblastoma cells. The upregulation of SOX3 was accompanied by improved Hedgehog signaling pathway activity and autophagy suppression in glioblastoma cells. SOX3 expression was elevated in patient-derived glioblastoma stem cells as well as oncospheres derived from glioblastoma cell lines compared to their differentiated counterparts. |
| Pan et al., 2021 [69] | Glioblastoma | Cell line: U251 | Bioinformatic; cell transfection; migration; Transwell invasion assays; RT-qPCR. | Oncomine indicated in the CCLE database shows that SOX3 overexpressed in glioblastoma with a fold change (FC) of 1.184 compared to normal tissue. The LinkedOmics and GEPIA databases showed that increased SOX3 improved overall survival (Logrank p = 0.0432). The survival rate of high SOX3 patients is much higher than low SOX3 patients (HR = 0.825), and SOX3 may serve as a prognostic biomarker set for GBM patients. Downregulation of SOX3 increased the wound-healing rate in U251 cells at 48 h, suggesting SOX3 as an antioncogenic. Downregulation of SOX3 has no significant effect on U251 cell invasion. |
| Scuderi et al., 2021 [80] | Glioblastoma Multiforme | Cell lines: U-138MG, U-87 MG, U-138 and U-87. | Cell viability; RT-qPCR; WB and ELISA. | Treatment of GBM with BX795 (inhibitor of TBK1- TANK-binding kinase) showed a significant reduction in SOX3 gene and protein expression in GBM cells. |
| Turchi et al., 2023 [81] | Glioblastoma | Cell lines: plasmacytoid dendritic cells (PDC) from primary glioblastoma tumor (GB1, GB5, GB11) and TG6 cells (T lymphoblast). | Bioinformatics; cell transfection with SOX3 specific siRNAs and CELF2-specific shRNA; orthotopic xenografts animal model; RNA sequencing; ChIP sequencing (ChIP-Seq); spheroid formation assays; immunofluorescence; immunohistochemistry; immunohistofluorescence; RNA immunoprecipitation and PCR. | The protein CELF2 acts as an epigenetic regulator in glioma stem cells and can repress the SOX3 gene, promoting a proliferating tumor cell phenotype. CELF2 was found to be a significant point of tumor vulnerability as its repression is sufficient to convert aggressive tumor cells into cells without the ability to form tumors in vivo. |
| Holmberg et al., 2011 [74] | Glioma (Glioblastoma, Astrocytoma, Oligoastrocytoma) | Clinical specimens: 24 human glioma samples. Cell line: primary cell culture. Animal model: nude mice. | RT-qPCR; WB; immunohistochemistry; immunofluorescence and in situ hybridization. | SOX3 maintains neural cells as self-renewing progenitors, keeping cells on the cell cycle and in a proliferative state. |
| Shujing et al., 2020 [55] | Glioma | Clinical specimens: 40 patients’ glioma samples and corresponding adjacent tissue samples. Cell lines: gliocyte HEB and glioma cells LN18 and LN229 | Bioinformatic; cells transfection with SOX3 expression vector and miR-483; luciferase assay; RT-qPCR; WB and Transwell invasion assay. | The expression level of SOX3 in glioma was significantly higher compared with the normal tissues. SOX3 upregulation was associated with patients predicted poor outcomes. SOX3 mRNA expression was higher in glioma cell lines (LN18 and LN229) than in normal cell lines (HEB). SOX3 is downregulated by miR-483, inhibiting invasion, migration and promoting apoptosis of glioma cells, suggesting that miR- 483 can be a potential target for glioma treatment. |
| Yuan et al., 2022 [82] | Glioma | Clinical specimens: 50 glioma tumor tissues and adjacent normal brain tissues Cell line: HEB (human normal glial cell) and human glioma cell lines: U87, U251, LN229, and A172. | Bioinformatic; RT-qPCR; WB; cell proliferation assay; 5-ethynyl-2′-deoxyuridine (EdU) assay; flow cytometry; Transwell assay; RNA immunoprecipitation (RIP) assay. | SOX3 gene expression in glioma tissue clinical specimens is upregulated compared to that in adjacent normal tissues. The bioinformatic tool TargetScan predicted SOX3 as the downstream target of miR-483-3p. Functional experiments of dual-luciferase reporter assay confirmed that miR- 483-3p inhibited the activity of the SOX3-WT reporter. In vitro upregulation of SOX3 expression or the inhibition of miR-483-3p expression promotes the proliferation of U87 cells, which was blocked by LINC00662 silencing. Anti-apoptotic protein Bcl-2 expression was inhibited and reversed by co-transfection with SOX3 overexpression plasmids or miR-483-3p inhibitors. LINC00662 triggered tumor-promoting effects in gliomas via modulating the miR-483-3p/SOX3 axis. |
| Feng et al., 2017 [71] | Hepatocellular carcinoma | Clinical specimens: 50 patients | RT-qPCR; WB; immunohistochemistry. | The mRNA expression of SOX3 is upregulated in hepatocellular carcinoma (HCC) tissues. The recurrence-free survival (RFS) rate of patients with high SOX3 expression was considerably lower than that of patients with basal SOX3 expression. SOX3 overexpression was statistically correlated with less tumor capsule formation, worse degrees of tumor differentiation, and worse TNM classification. Results suggested SOX3 plays an oncogenic role in HCC. |
| Gure et al., 2000 [83] | Lung | Clinical specimens: 17 patients’ serum with lung cancer and 23 control patients. Cell line SK-LC-13; NCI-H69, 128, 146, 187, 209, 378, 889, 740; | RT-qPCR; Northern blot (NB). | SOX3 mRNA was not detected in serum from normal adult tissues. SOX3 mRNA was detected in 2 out of 10 cell lines. SOX3 is not detectable in normal lung adult tissues. SOX3 expression was detected in 10% of adult lung cancer tissue. All patients with antibodies against SOX3 or SOX21 had higher reactivity against SOX1 and SOX2. The seroreactivity to SOX3 and SOX21 might be secondary to the shared antigenic epitopes located within the highly conserved HMG box of SOX proteins. |
| Qiu et al., 2017 [64] | Osteosarcoma | Clinical specimens: 42 osteosarcoma tissues; non-tumor samples 42; and bone cysts 28. | RT-qPCR; WB and immunohistochemistry. | SOX3 was overexpressed in most osteosarcoma tissues compared with that in bone cysts. SOX3 expression correlates with Snail1 and E-cadherin in human OS tissues. The mechanistic link among SOX3, Snail1, and EMT indicates SOX3 as a potential therapeutic target for osteosarcoma metastasis. |
| Qiu et al., 2017 [64] | Osteosarcoma | Cell lines: U2OS, SoSP-M, and MG-63 | RT-qPCR; WB; luciferase assay; chromatin immunoprecipitation; cell migration and matrigel invasion; in vivo lung metastasis model; immunohistochemistry. | SOX3 promotes osteosarcoma cell migration invasion and induces EMT upregulating Snail1 expression in osteosarcoma cells. |
| Guo et al., 2018 [50] | Osteosarcoma | Clinical specimens: 70 patients with primary osteosarcoma and 20 patients with bone cysts | RT-qPCR and WB | Upregulation of SOX3 mRNA and protein expression level in human osteosarcoma tissues. SOX3 acts as an oncogene in osteosarcoma, and SOX3 inhibitors or downstream effectors may be attractive targets for osteosarcoma therapy. |
| Guo et al., 2018 [50] | Osteosarcoma | Cell lines: MG63 and U2OS human osteosarcoma cells | SOX3 mRNA silencing; WB; cell proliferation; cell cycle; cell migration and invasion assays and cell apoptosis analysis. | SOX3 knockdown in osteosarcoma cells inhibits the proliferation, induces G1 phase arrest, induces apoptosis, suppresses the migration and invasion, suppresses tumor growth in a xenograft mouse model, decreases the EMT-promoting proteins (Twist, Snail, and MMP-9) and increases E-cadherin. SOX3 acts as an oncogene in osteosarcoma, and SOX3 inhibitors or downstream effectors may be interesting targets for osteosarcoma therapy. |
| Yan et al., 2016 [52] | Ovarian | Clinical specimens: 142 patients with ovarian carcinoma, 28 patients with borderline ovarian cystadenoma, 33 patients with ovarian cystadenoma, and 25 as normal controls. | Immunohistochemistry. | SOX3 immunoreactivity in human ovarian tumor cells was mainly localized to the nuclei. None of the normal ovarian tissue samples were positive for SOX3 expression, whereas SOX3-positive epithelial cells were detected in ovarian cystadenoma, borderline ovarian tumors, and ovarian cancer epithelial tissues. SOX3 expression gradually increased from benign and borderline to malignant ovarian tumors. SOX3 may be involved in the malignant transformation of ovarian tumors and may be used as a supplementary indication in the diagnosis of epithelial ovarian cancer. |
| Yan et al., 2016 [52] | Ovarian | Cell lines: HO8910; HO8910-pm; SKOV3; SKOV3-ip; ES2; MCV-152; and Moody. | Cell transfection; RT-PCR; WB; cell immunofluorescence; cell proliferation; colony formation; cell migration and invasion; ECM and apoptosis analysis. | SOX3 expression was different in each cell line. SOX3 promotes proliferation, migration, invasionand inhibits the adhesion of ovarian cancer cells. SOX3 inhibits apoptosis of ovarian cancer cells. Overexpression of SOX3 leads to high phosphorylation of pro-metastatic proteins. SOX3 expression was relatively higher in highly metastatic cell lines SKOV3-ip compared to SKOV3 cell line, suggesting that SOX3 may play a key role in cell migration and tumor metastasis. |
| Matsumoto et al., 2021 [84] | Ovarian Serous Carcinoma | Clinical specimens: 135 cases of ovarian carcinomas. Cell lines: high-grade serous carcinoma (HGSC) cell lines OVSAHO, OVKATE, and OVCAR-3, and ovarian clear cell carcinoma (CCC) cell lines, OVISE, ES-2, OVTOKO, KOC7C, and TOV-21 G. | Immunohistochemistry; in situ hybridization fluorescence; mutation analysis of the ALK and TP53 genes. Cells transfection; RT-qPCR; WB; flow cytometry; spheroid assay; cell counting assay; wound-healing assay and RNA sequencing. | Overexpression of SOX2 or SOX3 enhanced both ALK and ELAVL3 promoter activities, suggesting the existence of ALK/Sox/HuC signaling loops. ALK overexpression was attributed to increased expression of neuroendocrine markers, including synaptophysin, CD56, and B-cell lymphoma 2, in HGSC tissues. These findings suggest that overexpression of full-length ALK may influence the biological behavior of HGSC through cooperation with ELAVL3 and Sox factors, leading to the establishment and maintenance of the aggressive phenotypic characteristics of HGSC. SOX3 expression increased in transfected cells with ALK-overexpressing vector but not in ALK-knockdown cells. Expression of SOX proteins was increased following ALK overexpression, suggesting the existence of a positive feedback loop between ALK and Sox factors. SOX3 induces ALK (anaplastic lymphoma kinase) overexpression in ovarian serous carcinoma. |
| Tosic et al., 2018 [85] | Acute myeloid leukemia (AML) | Clinical specimens: 50 AML patients with bone marrow and 12 healthy controls (bone marrow donors). | RT-qPCR | SOX3 gene expression was not different from healthy individuals. After the implementation of the “cut-off” value (3.60), 11 (22%) patients with high SOX3 expression were detected. The complete remission rate of patients with high expression of SOX3 was 55%. In the survival analyses, patients with increased expression of SOX3 showed lower disease-free survival (DFS) compared to patients with low expression of SOX3 (4 vs. 14 months). Patients with SOX3 high expression had an overall survival (OS) of only 3 months, but it was not significantly shorter compared to the 7 months found in the patients with low SOX3 expression (Log- Rank = 3.434; p = 0.064). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Puerto, H.L.; Miranda, A.P.G.S.; Qutob, D.; Ferreira, E.; Silva, F.H.S.; Lima, B.M.; Carvalho, B.A.; Roque-Souza, B.; Gutseit, E.; Castro, D.C.; et al. Clinical Correlation of Transcription Factor SOX3 in Cancer: Unveiling Its Role in Tumorigenesis. Genes 2024, 15, 777. https://doi.org/10.3390/genes15060777
Del Puerto HL, Miranda APGS, Qutob D, Ferreira E, Silva FHS, Lima BM, Carvalho BA, Roque-Souza B, Gutseit E, Castro DC, et al. Clinical Correlation of Transcription Factor SOX3 in Cancer: Unveiling Its Role in Tumorigenesis. Genes. 2024; 15(6):777. https://doi.org/10.3390/genes15060777
Chicago/Turabian StyleDel Puerto, Helen Lima, Ana Paula G. S. Miranda, Dinah Qutob, Enio Ferreira, Felipe H. S. Silva, Bruna M. Lima, Barbara A. Carvalho, Bruna Roque-Souza, Eduardo Gutseit, Diego C. Castro, and et al. 2024. "Clinical Correlation of Transcription Factor SOX3 in Cancer: Unveiling Its Role in Tumorigenesis" Genes 15, no. 6: 777. https://doi.org/10.3390/genes15060777
APA StyleDel Puerto, H. L., Miranda, A. P. G. S., Qutob, D., Ferreira, E., Silva, F. H. S., Lima, B. M., Carvalho, B. A., Roque-Souza, B., Gutseit, E., Castro, D. C., Pozzolini, E. T., Duarte, N. O., Lopes, T. B. G., Taborda, D. Y. O., Quirino, S. M., Elgerbi, A., Choy, J. S., & Underwood, A. (2024). Clinical Correlation of Transcription Factor SOX3 in Cancer: Unveiling Its Role in Tumorigenesis. Genes, 15(6), 777. https://doi.org/10.3390/genes15060777





