Genome-Wide Identification and Expression Profiling of the α-Amylase (AMY) Gene Family in Potato
Abstract
:1. Introduction
2. Materials and Methods
2.1. Identification of StAMY Genes
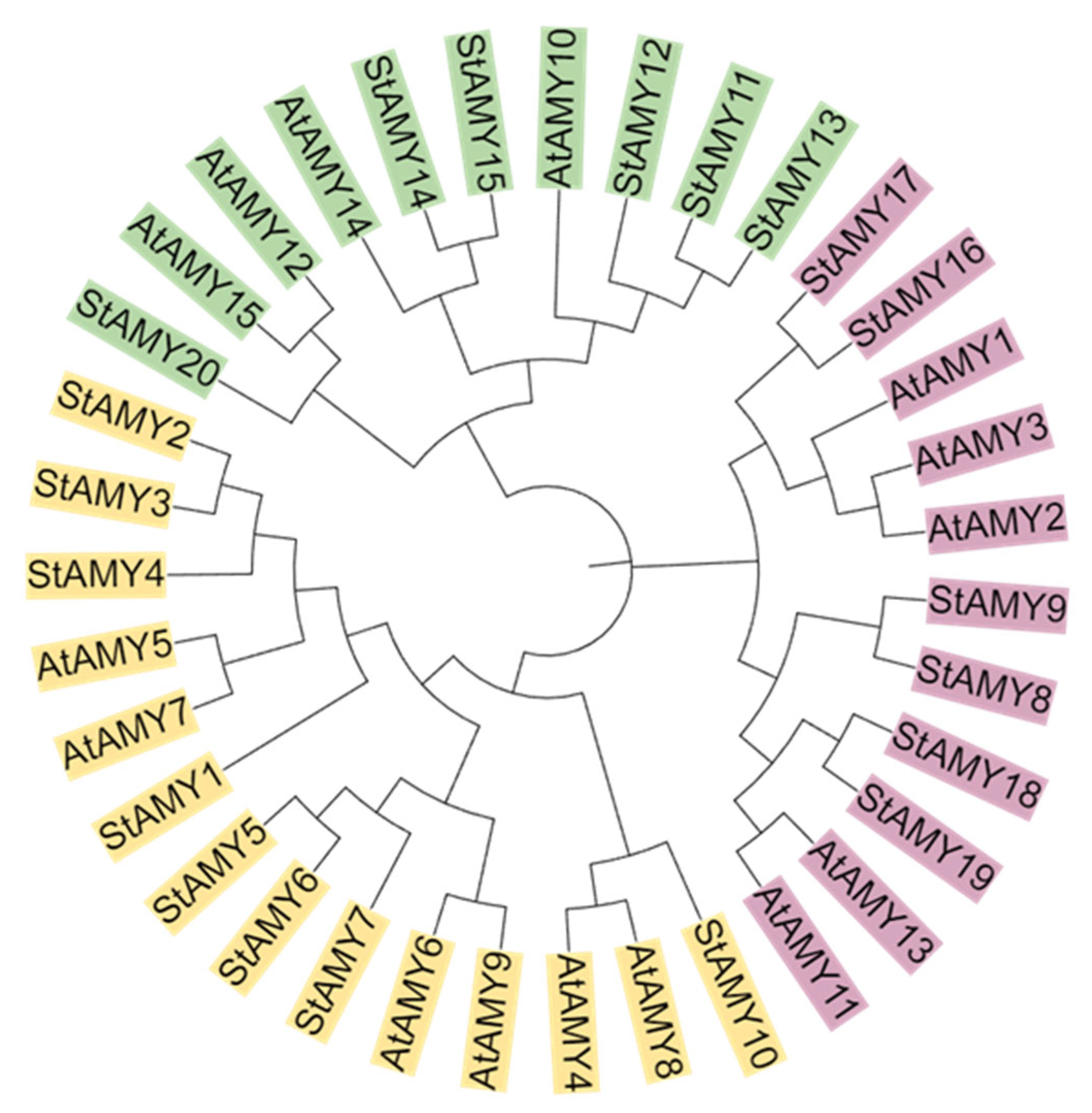
2.2. Multiple Sequence Alignment and Phylogenetic Analysis of StAMY Genes
2.3. Gene Structure and Conserved Motif Analysis of StAMY Genes
2.4. Promoter cis-Element Analysis of StAMY Genes
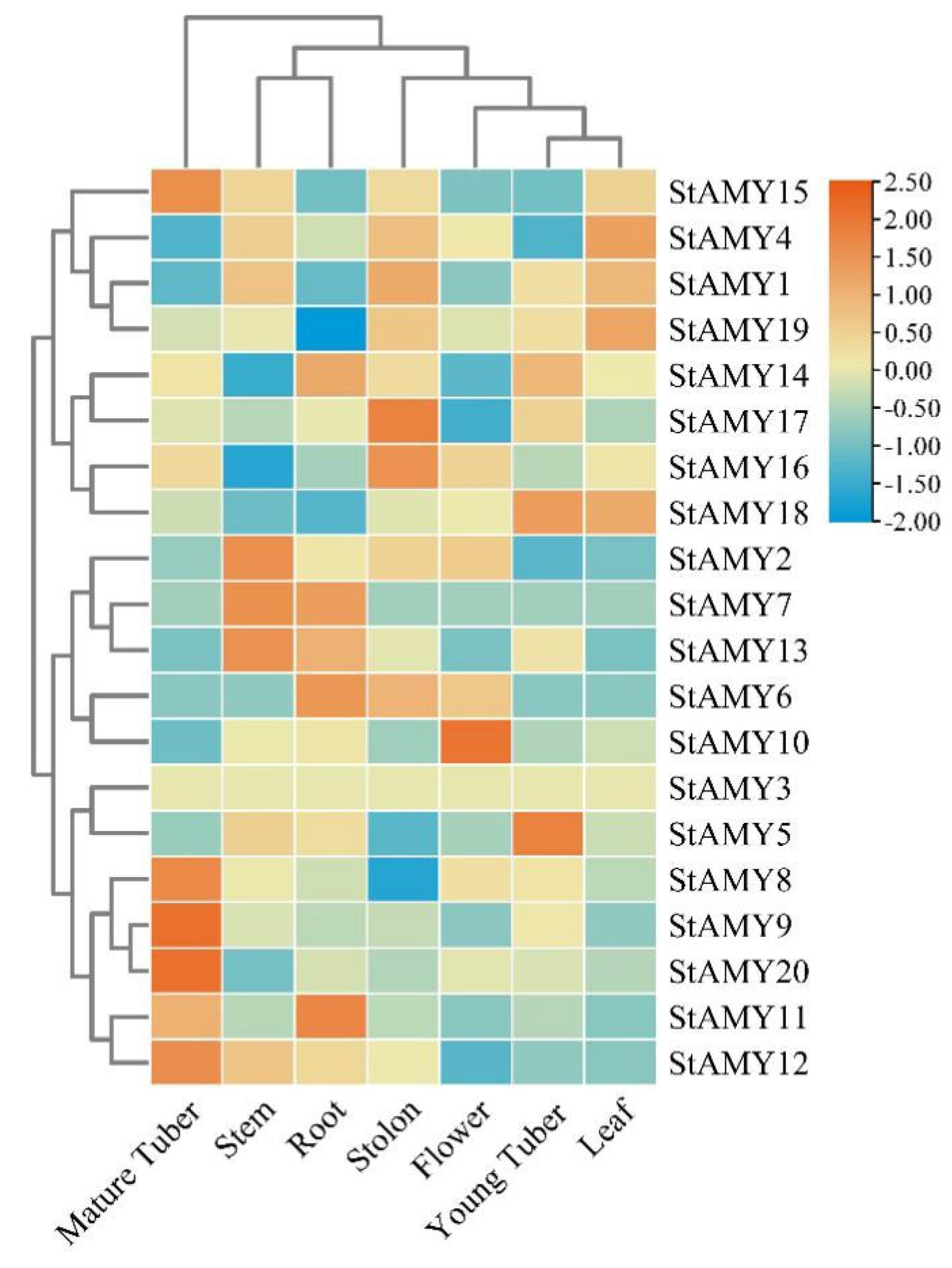
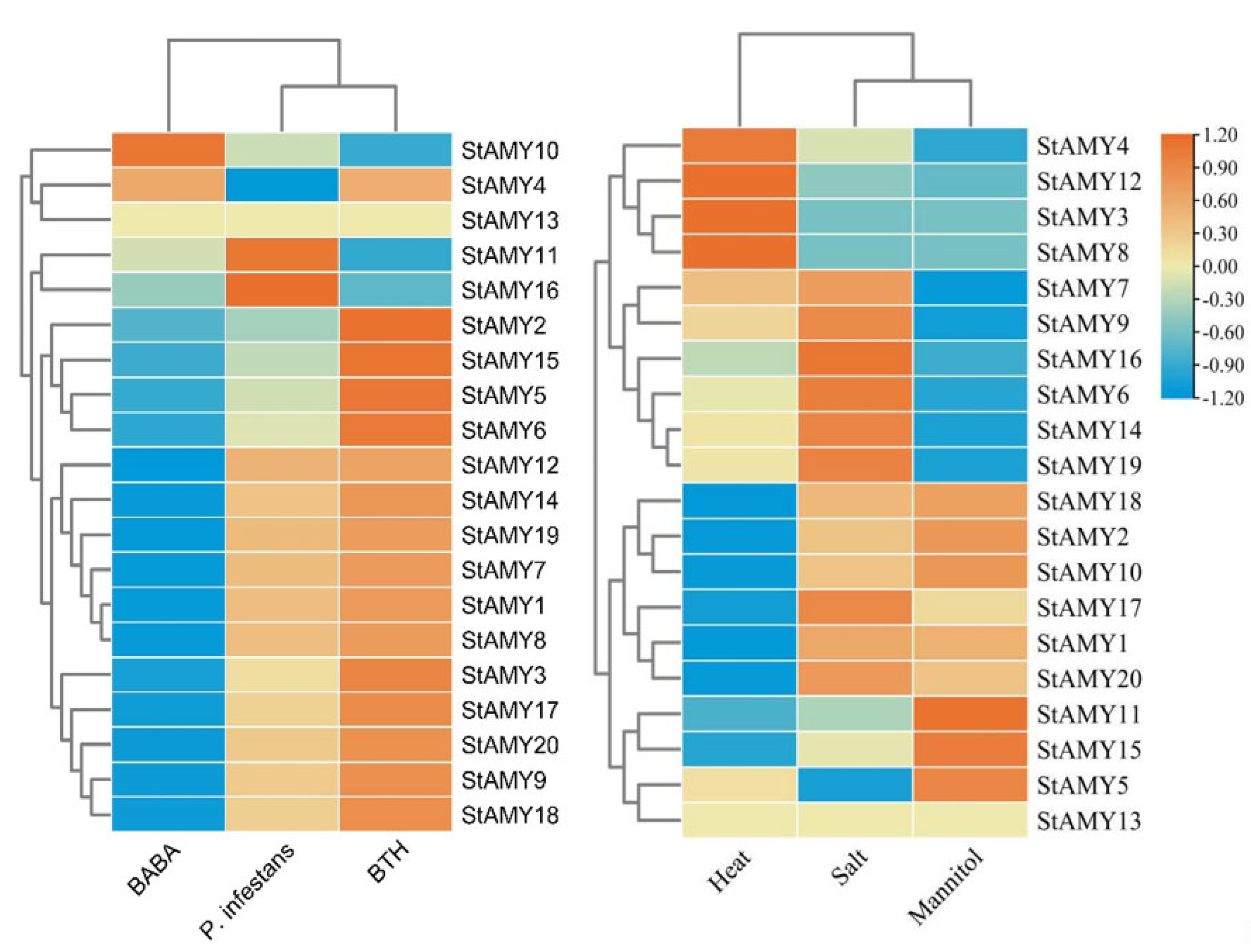
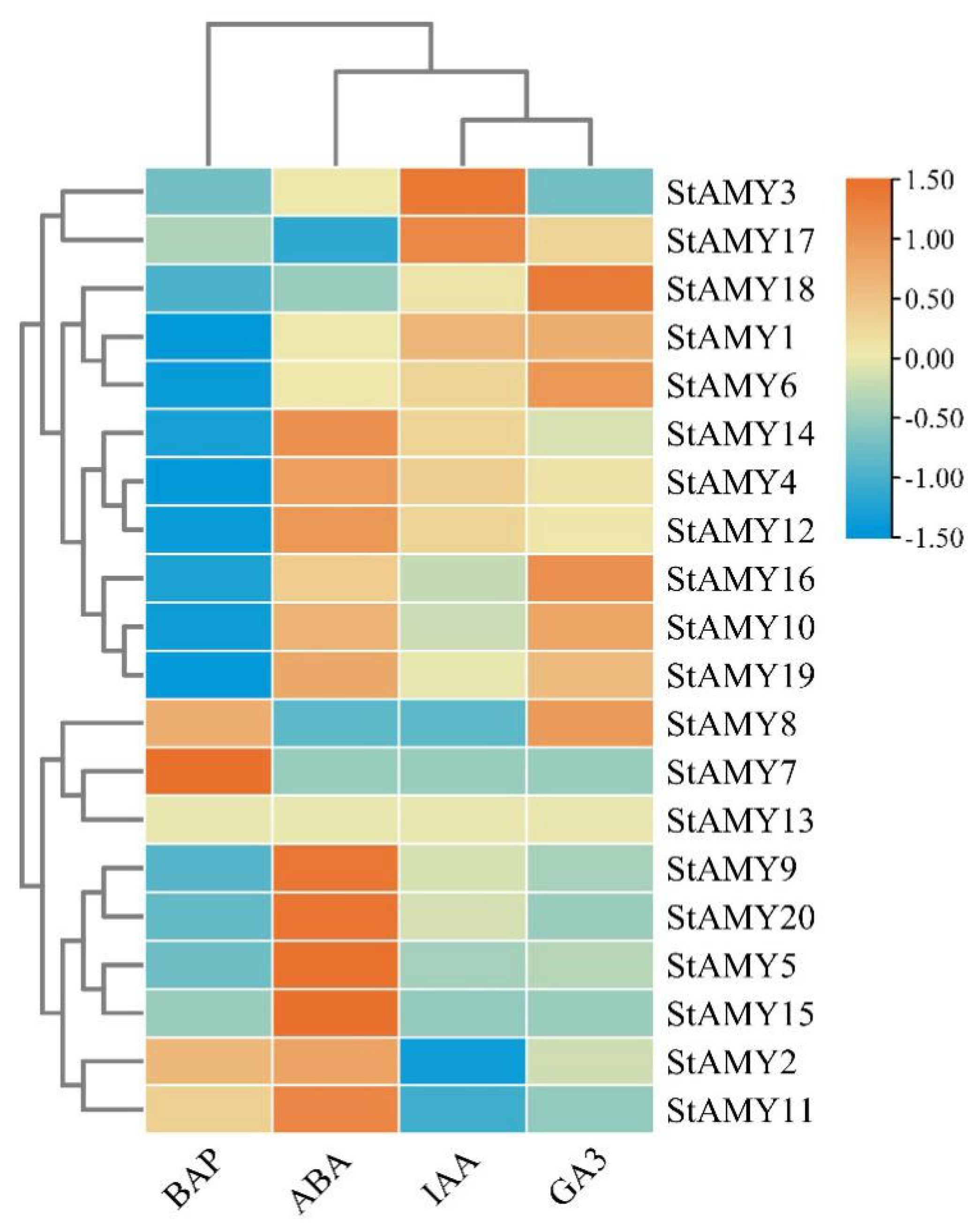
2.5. Expression Analysis of StAMY Genes
3. Results
3.1. Genome-Wide Identification of StAMY Genes
3.2. Chromosomal Locations of StAMY Genes
3.3. Gene Structures and Conserved Motifs of StAMY Genes
3.4. Promoter cis-Acting Elements of StAMY Genes
3.5. Tissue-Specific Expression Profiles of StAMY Genes
3.6. Changes in StAMY Gene Expression in Response to Biotic and Abiotic Stress
3.7. Changes in StAMY Gene Expression in Response to Phytohormones
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Beumer, K.; Stemerding, D. A breeding consortium to realize the potential of hybrid diploid potato for food security. Nat. Plants 2021, 7, 1530–1532. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Jia, Y.X.; Zhang, J.Z.; Li, H.B.; Cheng, L.; Wang, P.; Bao, Z.G.; Liu, Z.H.; Feng, S.S.; Zhu, X.J.; et al. Genome evolution and diversity of wild and cultivated potatoes. Nature 2022, 606, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Camire, M.E.; Kubow, S.; Donnelly, D.J. Potatoes and human health. Crit. Rev. Food Sci. Nutr. 2009, 49, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Isherwood, F.A. Starch-sugar interconversion in Solanum Tuberosum. Phytochemistry 1973, 12, 2579–2591. [Google Scholar] [CrossRef]
- Sołtys-Kalina, D.; Szajko, K.; Stefańczyk, E.; Smyda-Dajmund, P.; Śliwka, J.; Marczewski, W. eQTL mapping of the 12S globulin cruciferin gene PGCRURSE5 as a novel candidate associated with starch content in potato tubers. Sci. Rep. 2020, 10, 17168. [Google Scholar] [CrossRef] [PubMed]
- Kawochar, M.A.; Cheng, Y.X.; Begum, S.; Wang, E.S.; Zhou, T.T.; Liu, T.T.; Liu, T.F.; Song, B.T. Suppression of the tonoplast sugar transporter StTST3.2 improves quality of potato chips. J. Plant Physiol. 2022, 269, 153603. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.D. Problems of the Potato Chip Industry, Processing and Technology. Adv. Food Res. 1961, 10, 181–232. [Google Scholar]
- Sowokinos, J.R. Biochemical and molecular control of cold-induced sweetening in potatoes. Am. J. Potato Res. 2001, 78, 221–236. [Google Scholar] [CrossRef]
- Jaiswal, S.; Paul, K.; Raman, K.V.; Tyagi, S.; Saakre, M.; Tilgam, J.; Bhattacharjee, S.; Vijayan, J.; Mondal, K.K.; Sreevathsa, R.; et al. Amelioration of cold-induced sweetening in potato by RNAi mediated silencing of StUGPase encoding UDP-glucose pyrophosphorylase. Front. Plant Sci. 2023, 14, 1133029. [Google Scholar] [CrossRef] [PubMed]
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Acrylamide is formed in the Maillard reaction. Nature 2002, 419, 448–449. [Google Scholar] [CrossRef] [PubMed]
- Hogervorst, J.G.; Baars, B.J.; Schouten, L.J.; Konings, E.J.; Goldbohm, R.A.; Van, D.B.P.A. The carcinogenicity of dietary acrylamide intake: A comparative discussion of epidemiological and experimental animal research. Crit. Rev. Toxicol. 2010, 40, 485–512. [Google Scholar] [CrossRef] [PubMed]
- Muttucumaru, N.; Elmore, J.S.; Curtis, T.; Mottram, D.S.; Parry, M.A.; Halford, N.G. Reducing acrylamide precursors in raw materials derived from wheat and potato. J. Agric. Food Chem. 2008, 56, 6167–6172. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Zeeman, S.C.; Smith, S.M. Starch degradation. Annu. Rev. Plant Biol. 2005, 56, 73–98. [Google Scholar] [CrossRef] [PubMed]
- Zeeman, S.C.; Kossmann, J.; Smith, A.M. Starch: Its Metabolism, Evolution, and Biotechnological Modification in Plants. Annu. Rev. Plant Biol. 2010, 61, 209–234. [Google Scholar] [CrossRef] [PubMed]
- Mahlow, S.; Orzechowski, S.; Fettke, J. Starch phosphorylation: Insights and perspectives. Cell. Mol. Life Sci. 2016, 73, 2753–2764. [Google Scholar] [CrossRef] [PubMed]
- Thalmann, M.; Santelia, D. Starch as a determinant of plant fitness under abiotic stress. New. Phytol. 2017, 214, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Sun, F.L.; Wang, P.L.; Yusuyin, M.; Kuerban, W.; Lai, C.X.; Li, C.P.; Ma, J.; Xiao, F. Genome-Wide Identification and Preliminary Functional Analysis of BAM (β-Amylase) Gene Family in Upland Cotton. Genes 2023, 14, 2077. [Google Scholar] [CrossRef] [PubMed]
- Czyzewska, D.; Marczewski, W. Starch metabolism in potato tubers. Postep. Biochem. 2009, 55, 441–446. [Google Scholar]
- Van Der Maarel, M.J.; Van der Veen, B.; Uitdehaag, J.C.M.; Leemhuis, H.; Dijkhuizen, L. Properties and applications of starch-converting enzymes of the α-amylase family. J. Biotechnol. 2002, 94, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Ali, S.; Hassan, A.; Tahir, H.M.; Mumtaz, S.; Mumtaz, S. Biosynthesis and industrial applications of α-amylase: A review. Arch. Microbiol. 2021, 203, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.Y.; Yang, G.Y. α-amylase detection methods and applications. Sheng Wu Gong Cheng Xue Bao 2023, 39, 898–911. [Google Scholar] [PubMed]
- Janecek, S.; Svensson, B.; Henrissat, B. Domain evolution in the alpha-amylase family. J. Mol. Evol. 1997, 45, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Prakash, O.; Jaiswal, N. alpha-Amylase: An ideal representative of thermostable enzymes. Appl. Biochem. Biotechnol. 2010, 160, 2401–2414. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Cao, H.L.; Lin, H.Z.; Hu, J.; Ye, Y.J.; Li, J.M.; Hao, Z.; Hao, X.; Sun, Y.; Yang, Y.; et al. Expression patterns of alpha-amylase and beta-amylase genes provide insights into the molecular mechanisms underlying the responses of tea plants (Camellia sinensis) to stress and postharvest processing treatments. Planta 2019, 250, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Janeček, Š.; Svensson, B.; MacGregor, E.A. α-Amylase: An enzyme specificity found in various families of glycoside hydrolases. Cell Mol. Life Sci. 2014, 71, 1149–1170. [Google Scholar] [CrossRef] [PubMed]
- Apriyanto, A.; Compart, J.; Fettke, J. A review of starch, a unique biopolymer-Structure, metabolism and in planta modifications. Plant Sci. 2022, 318, 111223. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.Q.; Zhang, H.L.; Li, Z.C.; Bennett, A.J. Characterization of Evolution and Tissue-Expression of Rice (Oryza sativa L.) α-Amylase Genes. Acta Agron. Sin. 2024, 36, 17–27. [Google Scholar] [CrossRef]
- Kaplan, F.; Sung, D.Y.; Guy, C.L. Roles of β-amylase and starch breakdown during temperatures stress. Physiol. Plant. 2006, 126, 120–128. [Google Scholar] [CrossRef]
- Doyle, E.A.; Lane, A.M.; Sides, J.M.; Mudgett, M.B.; Monroe, J.D. An alpha-amylase (At4g25000) in Arabidopsis leaves is secreted and induced by biotic and abiotic stress. Plant Cell. Environ. 2007, 30, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.W.; Chiang, C.M.; Tseng, T.H.; Yu, S.M. Interaction between rice MYBGA and the gibberellin response element controls tissue-specific sugar sensitivity of alpha-amylase genes. Plant Cell 2006, 18, 2326–2340. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Kim, S.J.; Han, S.K.; An, G.; Kim, S.R. A gibberellin-stimulated transcript, OsGASR1, controls seedling growth and alpha-amylase expression in rice. J. Plant Physiol. 2017, 214, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Thalmann, M.; Pazmino, D.; Seung, D.; Horrer, D.; Nigro, A.; Meier, T.; Kölling, K.; Pfeifhofer, H.W.; Zeeman, S.C.; Santelia, D. Regulation of Leaf Starch Degradation by Abscisic Acid Is Important for Osmotic Stress Tolerance in Plants. Plant Cell 2016, 28, 1860–1878. [Google Scholar] [CrossRef] [PubMed]
- Geigenberger, P. Regulation of sucrose to starch conversion in growing potato tubers. J. Exp. Bot. 2003, 54, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Hajirezaei, M.R.; Börnke, F.; Peisker, M.; Takahata, Y.; Lerchl, J.; Kirakosyan, A.; Sonnewald, U. Decreased sucrose content triggers starch breakdown and respiration in stored potato tubers (Solanum tuberosum). J. Exp. Bot. 2003, 54, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.F.; Kawochar, M.A.; Liu, S.X.; Cheng, Y.X.; Begum, S.; Wang, E.S.; Zhou, T.T.; Liu, T.T.; Cai, X.K.; Song, B.T. Suppression of the tonoplast sugar transporter, StTST3.1, affects transitory starch turnover and plant growth in potato. Plant J. 2023, 113, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Prada, M.D.M.; Curtin, S.J.; Gutiérrez-González, J.J. Potato improvement through genetic engineering. GM. Crops Food. 2021, 12, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.M. Improving the Processing Quality of Existing Cultivars by Suppressing the Vacuolar Acid Invertase Gene. Plant Physiol. 2024, 154, 939–948. [Google Scholar]
- Van Harsselaar, J.K.; Lorenz, J.; Senning, M.; Sonnewald, U.; Sonnewald, S. Genome-wide analysis of starch metabolism genes in potato (Solanum tuberosum L.). BMC Genom. 2017, 18, 37. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, J.E.; Duffus, C.M.; Paterson, L.; Mackay, G.R.; Allison, M.J.; Bain, H. The effect of storage temperature on reducing sugar concentration and the activities of three amylolytic enzymes in tubers of the cultivated potato, Solanum tuberosum L. Potato Res. 1993, 36, 107–117. [Google Scholar] [CrossRef]
- Zhang, H.L.; Hou, J.; Liu, J.; Xie, C.H.; Song, B.T. Amylase Analysis in Potato Starch Degradation during Cold Storage and Sprouting. Potato Res. 2014, 57, 47–58. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, H.L.; Liu, J.; Reid, S.; Liu, T.F.; Xu, S.J.; Tian, Z.D.; Sonnewald, U.; Song, B.T.; Xie, C.H. Amylases StAmy23, StBAM1 and StBAM9 regulate cold-induced sweetening of potato tubers in distinct ways. J. Exp. Bot. 2017, 68, 2317–2331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Liu, J.; Hou, J.; Yao, Y.; Lin, Y.; Ou, Y.B.; Song, B.T.; Xie, C.H. The potato amylase inhibitor gene SbAI regulates cold-induced sweetening in potato tubers by modulating amylase activity. Plant Biotechnol. 2015, 12, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Sverrisdóttir, E.; Byrne, S.; Sundmark, H.E.R.; Johnsen, H.L.; Nielsen, K.L. Genomic prediction of starch content and chipping quality in tetraploid potato using genotyping-by-sequencing. Theor. Appl. Genet. 2017, 130, 2091–2108. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.; Fitzgerald, A.M.; Farnden, K.J.F.; Macrae, E.A.; Macrae, K.J.F. Characterisation of putative α-amylases from apple (Malus domestica) and arabidopsis thaliana. Biol.-Sect. Cell. Mol. Biol. 2002, 11, 137–148. [Google Scholar]
- Gong, X.; Westcott, S.; Zhang, X.Q.; Yan, G.J.; Lance, R.; Zhang, G.P.; Sun, D.F.; Li, C.D.; Turgay, U. Discovery of novel bmy1 alleles increasing β-amylase activity in chinese landraces and tibetan wild barley for improvement of malting quality via mas. PLoS ONE 2013, 8, e72875. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.L.; Wang, Y.; Jia, B.C.; Tian, X.Q.; Chu, J.; Yin, H.B.; Jameson, P.E.; Chen, S.H.; Guo, S.L. Genome-wide identification and expression analysis of the beta-amylase gene family in chenopodium quinoa. DNA Cell Biol. 2021, 7, 40. [Google Scholar]
- Yang, T.Y.; Li, H.R.; Li, L.W.; Wei, W.L.; Huang, Y.H.; Xiong, F.Q.; Wei, M.G. Genome-wide characterization and expression analysis of α-amylase and β-amylase genes underlying drought tolerance in cassava. BMC Genom. 2023, 24, 190. [Google Scholar] [CrossRef] [PubMed]
- Kouzai, Y.; Noutoshi, Y.; Inoue, K.; Shimizu, M.; Onda, Y.; Mochida, K. Benzothiadiazole, a plant defense inducer, negatively regulates sheath blight resistance in Brachypodium distachyon. Sci. Rep. 2018, 8, 17358. [Google Scholar] [CrossRef] [PubMed]
- Baccelli, I.; Mauch-Mani, B. Beta-aminobutyric acid priming of plant defense: The role of ABA and other hormones. Plant. Mol. Biol. 2016, 91, 703–711. [Google Scholar] [CrossRef]
- Birch, P.R.; Whisson, S.C. Phytophthora infestans enters the genomics era. Mol. Plant Pathol. 2001, 2, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Mugao, L. Morphological and Molecular Variability of Alternaria solani and Phytophthora infestans Causing Tomato Blights. Int. J. Microbiol. 2023, 2023, 8951351. [Google Scholar] [CrossRef] [PubMed]
- Benková, E. Plant hormones in interactions with the environment. Plant. Mol. Biol. 2016, 91, 597. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Van Lammeren, A.A.; Vermeer, E.; Vreugdenhil, D. The role of gibberellin, abscisic acid, and sucrose in the regulation of potato tuber formation in vitro. Plant Physiol. 1998, 117, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Zeng, L.Z.; Yang, J.L.; Zheng, X.D.; Yu, T. 6-Benzylaminopurine inhibits growth of Monilinia fructicola and induces defense-related mechanism in peach fruit. Food Chem. 2015, 187, 210–217. [Google Scholar] [CrossRef] [PubMed]



| Gene ID | CDS Length (bp) | Start–End Positions | Protein Length (aa) | Molecular Weight (Da) | Isoelectric Point (PI) | Subcellular Localization |
|---|---|---|---|---|---|---|
| StAMY1 | 2964 | 24230843-24247840 | 987 | 112,118.25 | 5.77 | cytosol |
| StAMY2 | 1326 | 35837209-35840386 | 441 | 49,246.7 | 5.43 | vacuole |
| StAMY3 | 1122 | 35837209-35840386 | 373 | 41,687.07 | 5.17 | cytosol |
| StAMY4 | 1224 | 65328724-65330894 | 407 | 45,723.43 | 5.83 | chloroplast |
| StAMY5 | 1224 | 68257647-68262571 | 407 | 46,346.11 | 6.74 | cytosol |
| StAMY6 | 1224 | 68257647-68262571 | 407 | 46,346.11 | 6.74 | cytosol |
| StAMY7 | 1188 | 68257647-68262571 | 395 | 44,870.42 | 6.67 | cytosol |
| StAMY8 | 2547 | 68516172-68508497 | 848 | 96,766.73 | 4.9 | nucleus |
| StAMY9 | 2745 | 68516457-68508497 | 914 | 104,165.09 | 5.09 | chloroplast |
| StAMY10 | 3999 | 6053915-6037246 | 1332 | 150,181.53 | 6.05 | mitochondrion |
| StAMY11 | 1185 | 715327-700273 | 394 | 45,248.83 | 6.27 | peroxisome |
| StAMY12 | 1350 | 715327-700273 | 449 | 51,028.05 | 6.1 | peroxisome |
| StAMY13 | 1143 | 715327-700273 | 380 | 43,513.8 | 6.27 | peroxisome |
| StAMY14 | 2382 | 7370232-7354469 | 793 | 89,389.69 | 5.54 | nucleus |
| StAMY15 | 1866 | 7370232-7354469 | 621 | 70,484.13 | 5.19 | nucleus |
| StAMY16 | 1947 | 55405743-55386292 | 648 | 73,772.35 | 6.89 | chloroplast |
| StAMY17 | 2709 | 55936361-55953226 | 902 | 103,776.35 | 6.4 | cytosol |
| StAMY18 | 2631 | 3555467-3535898 | 876 | 100,067.77 | 5.02 | chloroplast |
| StAMY19 | 2316 | 3555467-3535898 | 771 | 88,867.57 | 4.94 | cytosol |
| StAMY20 | 2637 | 53271759-53267937 | 878 | 97,998.1 | 6.8 | chloroplast |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Y.; Jin, L. Genome-Wide Identification and Expression Profiling of the α-Amylase (AMY) Gene Family in Potato. Genes 2024, 15, 793. https://doi.org/10.3390/genes15060793
Duan Y, Jin L. Genome-Wide Identification and Expression Profiling of the α-Amylase (AMY) Gene Family in Potato. Genes. 2024; 15(6):793. https://doi.org/10.3390/genes15060793
Chicago/Turabian StyleDuan, Yudan, and Liping Jin. 2024. "Genome-Wide Identification and Expression Profiling of the α-Amylase (AMY) Gene Family in Potato" Genes 15, no. 6: 793. https://doi.org/10.3390/genes15060793
APA StyleDuan, Y., & Jin, L. (2024). Genome-Wide Identification and Expression Profiling of the α-Amylase (AMY) Gene Family in Potato. Genes, 15(6), 793. https://doi.org/10.3390/genes15060793





