Identification and Characterization of the miRNA Transcriptome Controlling Green Pigmentation of Chicken Eggshells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chickens and Sample Collection
2.2. RNA Preparation
2.3. High-Throughput Sequencing
2.4. miRNA Target Gene Prediction and Functional Analysis
2.5. Quantitative Real-Time PCR
2.6. Statistical Analysis
3. Results
3.1. Eggshell Quality and Phenotypic Results
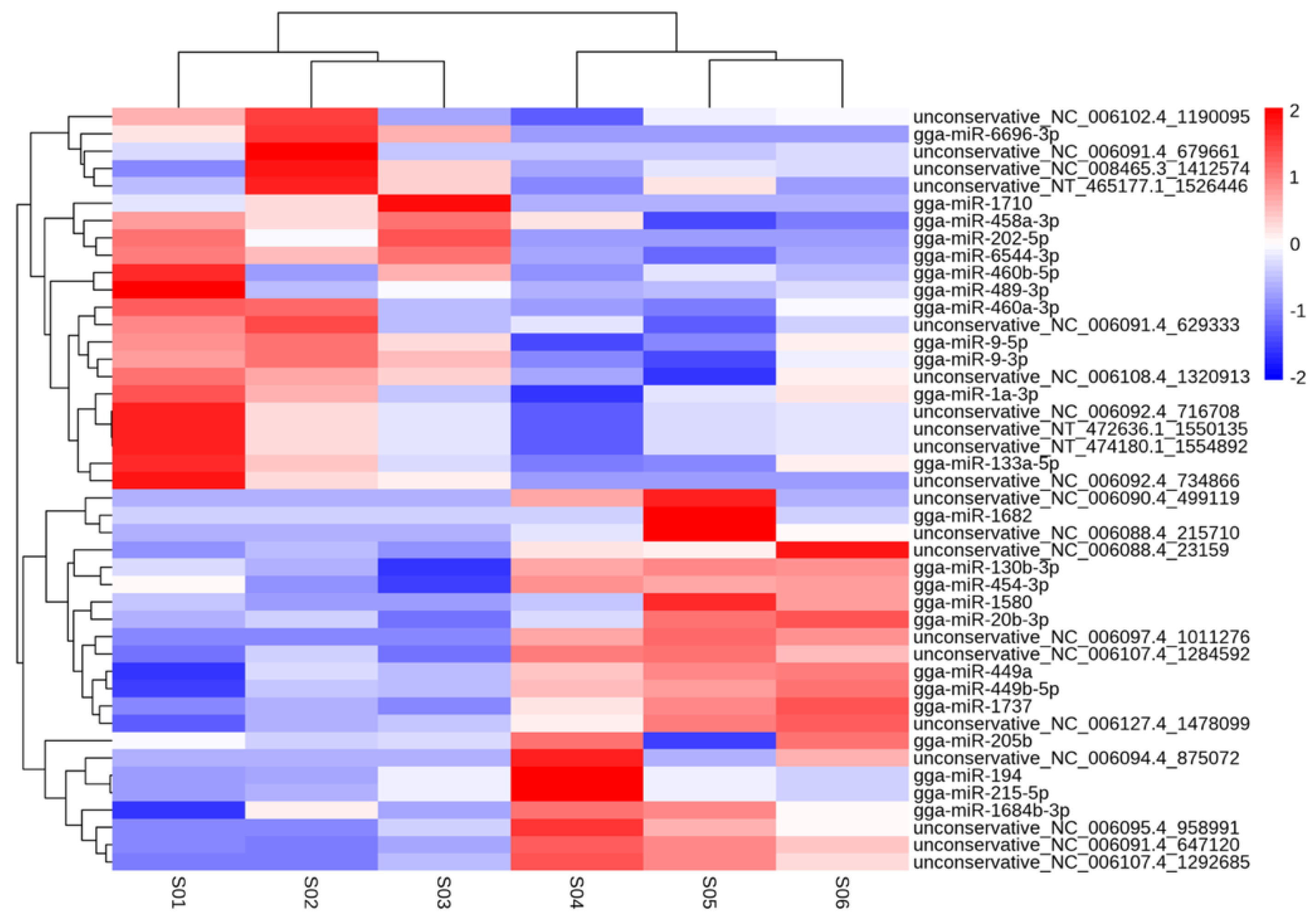
3.2. Overview of miRNA Sequencing Results
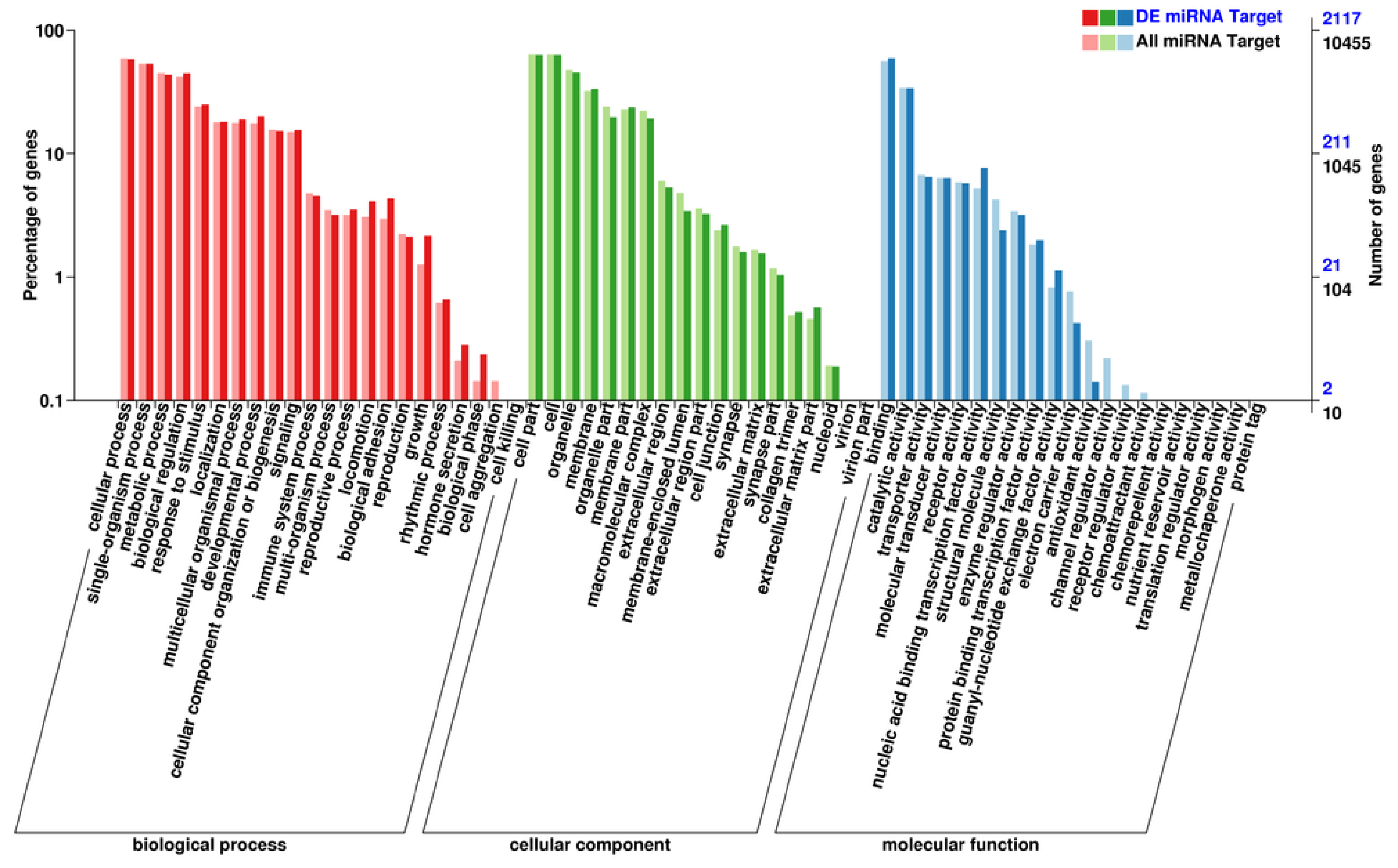
3.3. Prediction of Target Genes and Functional Bioinformatics Analysis
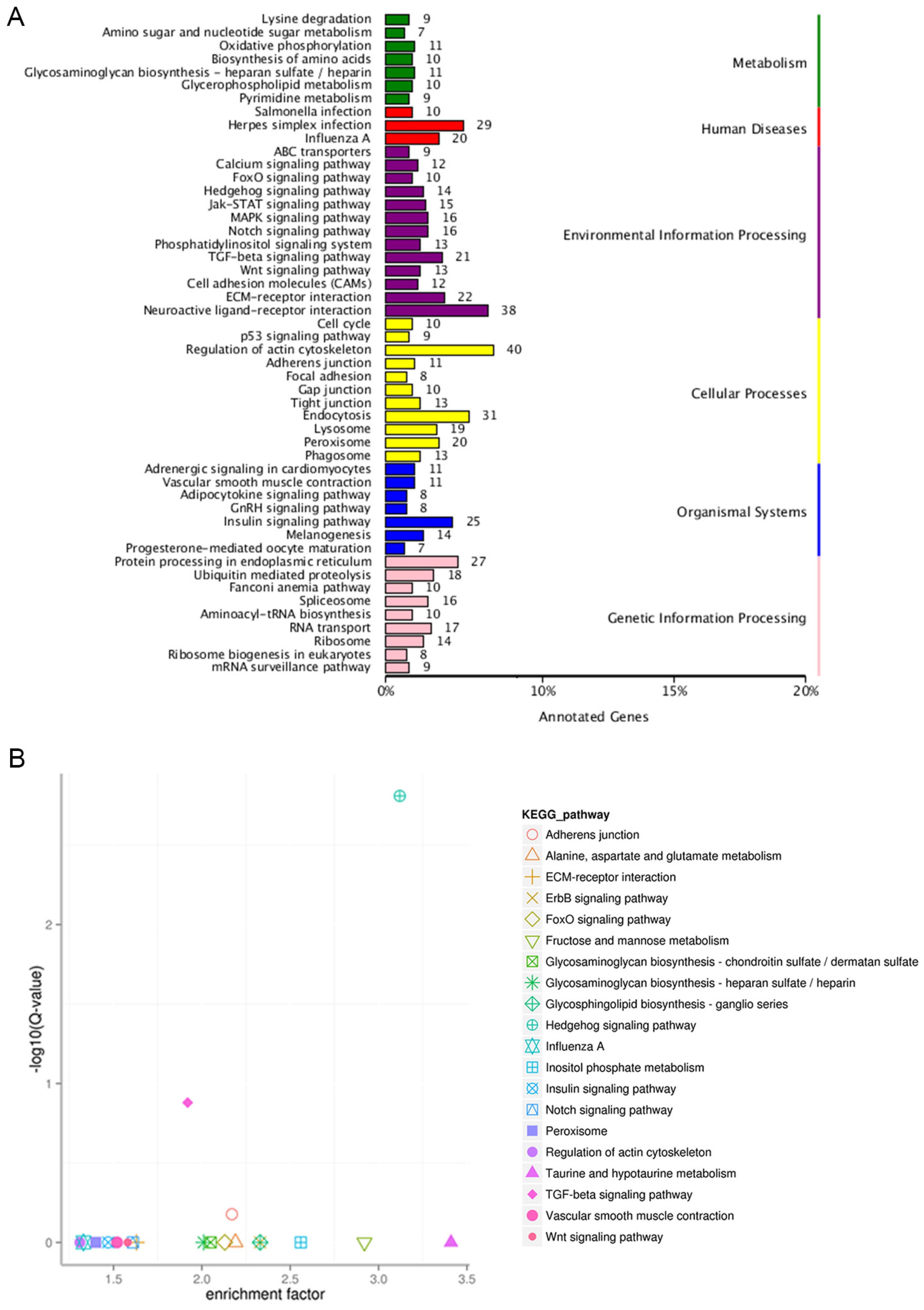
3.4. Analysis of KEGG Pathways
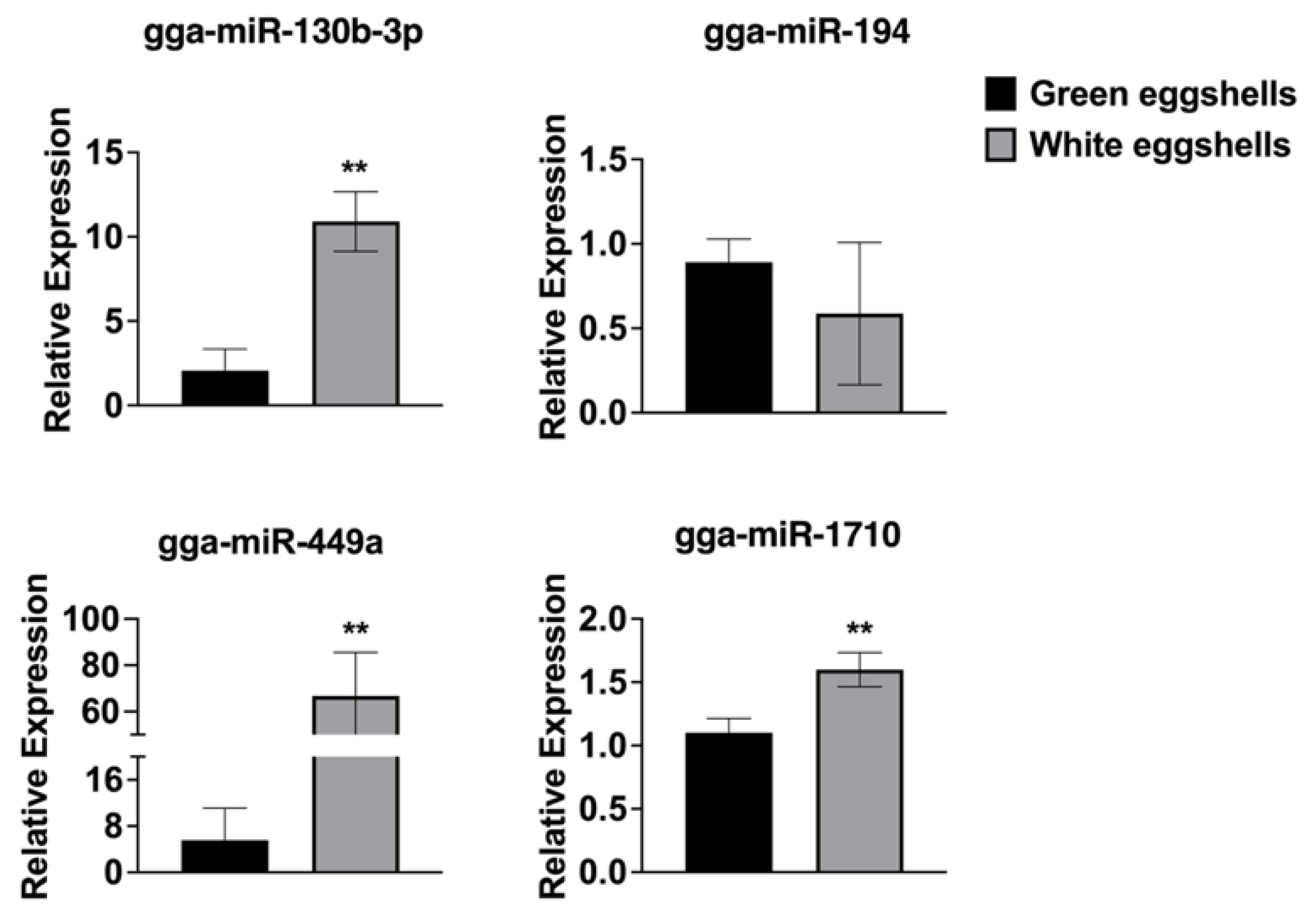
3.5. Validation of Sequencing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, G.; Sun, C.; Wu, G.; Shi, F.; Liu, A.; Yang, N. iTRAQ-Based Quantitative Proteomics Identifies Potential Regulatory Proteins Involved in Chicken Eggshell Brownness. PLoS ONE 2016, 11, e0168750. [Google Scholar] [CrossRef]
- Widjastuti, T.; Haetami, K.; Abun, T.W.; Rusmana, D.; Austin, P.R.; Tan, E. Bacillus Subtilis Spores Improve Brown Egg Colour. Int. J. Poult. Sci. 2017, 18, 55–60. [Google Scholar]
- Somes, R.G.; Francis, P.V.; Tlustohowicz, J.J. Protein and Cholesterol Content of Araucana Chicken Eggs. Poult. Sci. 1977, 56, 1636–1640. [Google Scholar] [CrossRef]
- Wang, X.L.; Zheng, J.X.; Ning, Z.H.; Qu, L.J.; Xu, G.Y.; Yang, N. Laying Performance and Egg Quality of Blue-Shelled Layers as Affected by Different Housing Systems. Poult. Sci. 2009, 88, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.W.; Lilyblade, A.; Clifford, C.K.; Ernst, R.; Clifford, A.J.; Dunn, P. Composition of and Cholesterol in Araucana and Commercial Eggs. J. Am. Diet. Assoc. 1978, 72, 45–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qu, L.; Yao, J.; Yang, X.; Li, G.; Zhang, Y.; Li, J.; Wang, X.; Bai, J.; Xu, G.; et al. An EAV-HP Insertion in 5′ Flanking Region of SLCO1B3 Causes Blue Eggshell in the Chicken. PLoS Genet. 2013, 9, e1003183. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Gu, X.; Huang, X.; Liu, R.; Li, J.; Hu, Y.; Li, G.; Zeng, T.; Tian, Y.; Hu, X.; et al. Two Cis-Regulatory SNPs Upstream of ABCG2 Synergistically Cause the Blue Eggshell Phenotype in the Duck. PLoS Genet. 2020, 16, e1009119. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hu, J.; Guo, Z.; Fan, W.; Xu, Y.; Liang, S.; Liu, D.; Zhang, Y.; Xie, M.; Tang, J.; et al. A Single Nucleotide Polymorphism Variant Located in the Cis-Regulatory Region of the ABCG2 Gene Is Associated with Mallard Egg Colour. Mol. Ecol. 2021, 30, 1477–1491. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, K.; Xu, J.; Li, F.; Ding, J.; Ma, Z.; Li, G.; Li, H. Insights Into mRNA and Long Non-Coding RNA Profiling RNA Sequencing in Uterus of Chickens with Pink and Blue Eggshell Colors. Front. Vet. Sci. 2021, 8, 736387. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Llave, C.; Kasschau, K.D.; Rector, M.A.; Carrington, J.C. Endogenous and Silencing-Associated Small RNAs in Plants. Plant Cell 2002, 14, 1605–1619. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Z.; Li, L.; Lodish, H.F.; Bartel, D.P. MicroRNAs Modulate Hematopoietic Lineage Differentiation. Science 2004, 303, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, I.; David, M. MicroRNAs in the Immune Response. Cytokine 2008, 43, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Bobe, M.S.R.; Al Kobaisi, M.; Bhosale, S.V.; Bhosale, S.V. Solvent-Tuned Self-Assembled Nanostructures of Chiral l/d-Phenylalanine Derivatives of Protoporphyrin IX. ChemistryOpen 2015, 4, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.Q.; Li, A.; Lan, J.J.; Wang, Y.M.; Yan, M.J.; Lian, S.Y.; Wu, X. Study of Formation of Green Eggshell Color in Ducks through Global Gene Expression. PLoS ONE 2018, 13, e0191564. [Google Scholar] [CrossRef] [PubMed]
- Papapetrou, E.P.; Korkola, J.E.; Sadelain, M. A Genetic Strategy for Single and Combinatorial Analysis of miRNA Function in Mammalian Hematopoietic Stem Cells. Stem Cells 2010, 28, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.; Sekeroglu, A.; Koç, H.; Eleroglu, H.; Duman, M.; Tahtali, Y.; Elmastas, M.; Sen Mutlu, M.I. Egg Production and Quality Characteristics of Laying Hens Fed Diets Supplemented with Dry Caper (Capparis spinosa) Leaf Powder. Indian J. Anim. Res. 2018, 52, 72–78. [Google Scholar] [CrossRef]
- Yıldırım, A.; Şekeroğlu, A.; Koç, H.; Eleroğlu, H.; Tahtali, Y.; Sen, M.I.; Duman, M.; Genc, N. THE EFFECT OF DRY CAPER (Capparis spinosa) fruit on egg production and quality characteristics of laying hens. Pak. J. Agric. Sci. 2014, 51, 217–224. [Google Scholar]
- Vaidya, K.; Ghosh, A.; Kumar, V.; Chaudhary, S.; Srivastava, N.; Katudia, K.; Tiwari, T.; Chikara, S.K. De Novo Transcriptome Sequencing in Trigonella Foenum-Graecum L. to Identify Genes Involved in the Biosynthesis of Diosgenin. Plant Genome 2013, 6, plantgenome2012.08.0021. [Google Scholar] [CrossRef]
- Xiong, J.; Lu, X.; Zhou, Z.; Chang, Y.; Yuan, D.; Tian, M.; Zhou, Z.; Wang, L.; Fu, C.; Orias, E.; et al. Transcriptome Analysis of the Model Protozoan, Tetrahymena Thermophila, Using Deep RNA Sequencing. PLoS ONE 2012, 7, e30630. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.P.; Shih, I.; Jones-Rhoades, M.W.; Bartel, D.P.; Burge, C.B. Prediction of Mammalian microRNA Targets. Cell 2003, 115, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, W.; Rasko, J.E.J.; Flamant, S. MicroRNA Target Prediction and Validation. Adv. Exp. Med. Biol. 2013, 774, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Shenker, S.; Miura, P.; Sanfilippo, P.; Lai, E.C. IsoSCM: Improved and Alternative 3′ UTR Annotation Using Multiple Change-Point Inference. RNA 2015, 21, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Dennis, G.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003, 4, P3. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Samiullah, S.; Roberts, J.R.; Chousalkar, K. Eggshell Color in Brown-Egg Laying Hens—A Review. Poult. Sci. 2015, 94, 2566–2575. [Google Scholar] [CrossRef]
- Li, G.; Chen, S.; Duan, Z.; Qu, L.; Xu, G.; Yang, N. Comparison of Protoporphyrin IX Content and Related Gene Expression in the Tissues of Chickens Laying Brown-Shelled Eggs. Poult. Sci. 2013, 92, 3120–3124. [Google Scholar] [CrossRef]
- Mateo, R.; Castells, G.; Green, A.J.; Godoy, C.; Cristòfol, C. Determination of Porphyrins and Biliverdin in Bile and Excreta of Birds by a Single Liquid Chromatography-Ultraviolet Detection Analysis. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 810, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Wragg, D.; Mwacharo, J.M.; Alcalde, J.A.; Wang, C.; Han, J.-L.; Gongora, J.; Gourichon, D.; Tixier-Boichard, M.; Hanotte, O. Endogenous Retrovirus EAV-HP Linked to Blue Egg Phenotype in Mapuche Fowl. PLoS ONE 2013, 8, e71393. [Google Scholar] [CrossRef] [PubMed]
- Letschert, K.; Keppler, D.; König, J. Mutations in the SLCO1B3 Gene Affecting the Substrate Specificity of the Hepatocellular Uptake Transporter OATP1B3 (OATP8). Pharmacogenetics 2004, 14, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Boivin, A.-A.; Cardinal, H.; Barama, A.; Naud, J.; Pichette, V.; Hébert, M.-J.; Roger, M. Influence of SLCO1B3 Genetic Variations on Tacrolimus Pharmacokinetics in Renal Transplant Recipients. Drug Metab. Pharmacokinet. 2013, 28, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Samiullah, S.; Roberts, J.; Wu, S.-B. Downregulation of ALAS1 by Nicarbazin Treatment Underlies the Reduced Synthesis of Protoporphyrin IX in Shell Gland of Laying Hens. Sci. Rep. 2017, 7, 6253. [Google Scholar] [CrossRef]
- Kolaja, G.J.; Hinton, D.E. Effects of DDT on Eggshell Quality and Calcium Adenosine Triphosphatase. J. Toxicol. Environ. Health 1977, 3, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Şekeroğlu, A.; Duman, M.; Tahtalı, Y.; Yıldırım, A.; Eleroğlu, H. Effect of Cage Tier and Age on Performance, Egg Quality and Stress Parameters of Laying Hens. S. Afr. J. Anim. Sci. 2014, 44, 288–297. [Google Scholar] [CrossRef]
- Treiber, T.; Treiber, N.; Meister, G. Regulation of microRNA Biogenesis and Its Crosstalk with Other Cellular Pathways. Nat. Rev. Mol. Cell Biol. 2019, 20, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Nematbakhsh, S.; Pei Pei, C.; Selamat, J.; Nordin, N.; Idris, L.H.; Abdull Razis, A.F. Molecular Regulation of Lipogenesis, Adipogenesis and Fat Deposition in Chicken. Genes 2021, 12, 414. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Jia, X.; Li, H.; Tan, S.; Nie, Q.; Yu, H.; Yang, Y. Characterization of microRNA and mRNA Expression Profiles in Skin Tissue between Early-Feathering and Late-Feathering Chickens. BMC Genom. 2018, 19, 399. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Zhang, Z.; Amevor, F.K.; Du, X.; Li, L.; Tian, Y.; Kang, X.; Shu, G.; Zhu, Q.; Wang, Y.; et al. Circadian miR-449c-5p Regulates Uterine Ca2+ Transport during Eggshell Calcification in Chickens. BMC Genom. 2021, 22, 764. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Lan, J.; Xu, M.; Qin, K.; Liu, H.; Sun, G.; Liu, X.; Chen, Y.; He, Z. Impact of KIT Editing on Coat Pigmentation and Fresh Meat Color in Yorkshire Pigs. CRISPR J. 2022, 5, 825–842. [Google Scholar] [CrossRef]
- Kutty, R.K.; Kutty, G.; Rodriguez, I.R.; Chader, G.J.; Wiggert, B. Chromosomal Localization of the Human Heme Oxygenase Genes: Heme Oxygenase-1 (HMOX1) Maps to Chromosome 22q12 and Heme Oxygenase-2 (HMOX2) Maps to Chromosome 16p13.3. Genomics 1994, 20, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Bourguinat, C.; Che, H.; Mani, T.; Keller, K.; Prichard, R.K. ABC-B Transporter Genes in Dirofilaria Immitis. Int. J. Parasitol. Drugs Drug Resist. 2016, 6, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Riou, M.; Koch, C.; Delaleu, B.; Berthon, P.; Kerboeuf, D. Immunolocalisation of an ABC Transporter, P-Glycoprotein, in the Eggshells and Cuticles of Free-Living and Parasitic Stages of Haemonchus Contortus. Parasitol. Res. 2005, 96, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, T.; Sassa, S.; Kappas, A. The Occurrence of Molecular Interactions among NADPH-Cytochrome c Reductase, Heme Oxygenase, and Biliverdin Reductase in Heme Degradation. J. Biol. Chem. 1982, 257, 7786–7793. [Google Scholar] [CrossRef]
- Panhéleux, M.; Kälin, O.; Gautron, J.; Nys, Y. Features of Eggshell Formation in Guinea Fowl: Kinetics of Shell Deposition, Uterine Protein Secretion and Uterine Histology. Br. Poult. Sci. 1999, 40, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.R.; Huber, W.J.; Backes, W.L. Human Heme Oxygenase-1 Efficiently Catabolizes Heme in the Absence of Biliverdin Reductase. Drug Metab. Dispos. 2010, 38, 2060–2066. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.P.; Liu, R.F.; Wang, A.R.; Li, J.Y.; Deng, X.M. Expression and Activity Analysis Reveal That Heme Oxygenase (Decycling) 1 Is Associated with Blue Egg Formation. Poult. Sci. 2011, 90, 836–841. [Google Scholar] [CrossRef]
- Balogun, E.; Hoque, M.; Gong, P.; Killeen, E.; Green, C.J.; Foresti, R.; Alam, J.; Motterlini, R. Curcumin Activates the Haem Oxygenase-1 Gene via Regulation of Nrf2 and the Antioxidant-Responsive Element. Biochem. J. 2003, 371, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-T.; Chi, K.-H.; Ho, F.-M.; Tsao, W.-C.; Lin, W.-W. Proteasome Inhibitors Up-Regulate Haem Oxygenase-1 Gene Expression: Requirement of P38 MAPK (Mitogen-Activated Protein Kinase) Activation but Not of NF-kappaB (Nuclear Factor kappaB) Inhibition. Biochem. J. 2004, 379, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Perron, M.; Boy, S.; Amato, M.A.; Viczian, A.; Koebernick, K.; Pieler, T.; Harris, W.A. A Novel Function for Hedgehog Signalling in Retinal Pigment Epithelium Differentiation. Development 2003, 130, 1565–1577. [Google Scholar] [CrossRef] [PubMed]
- Dakubo, G.D.; Mazerolle, C.; Furimsky, M.; Yu, C.; St-Jacques, B.; McMahon, A.P.; Wallace, V.A. Indian Hedgehog Signaling from Endothelial Cells Is Required for Sclera and Retinal Pigment Epithelium Development in the Mouse Eye. Dev. Biol. 2008, 320, 242–255. [Google Scholar] [CrossRef]
| Eggshell Quality | Light Green Eggshell Group (N = 3) | Dark Green Eggshell Group (N = 3) | Population (N = 856) |
|---|---|---|---|
| Egg weight (g) | 40.13 ± 1.91 | 39.03 ± 3.09 | 39.18 ± 4.21 |
| ESW (g) | 5.76 ± 0.26 | 5.63 ± 0.41 | 5.46 ± 0.56 |
| ESI | 1.31 ± 0.04 | 1.32 ± 0.04 | 1.31 ± 0.04 |
| ESC (L value) | 86.33 ± 1.29 ** | 79.79 ± 1.69 ** | 82.93 ± 1.79 ** |
| ESC (a value) | −2.5 ± 0.87 * | −6.3 ± 0.85 * | −4.07 ± 1.19 * |
| ESC (b value) | 5.72 ± 1.77 | 6.03 ± 1.31 | 5.97 ± 1.33 |
| Eggshell glossiness (Gu) | 5.83 ± 1.48 | 6.17 ± 3.40 | 5.96 ± 2.32 |
| ESS (kg/cm2) | 3.618 ± 0.769 | 3.638 ± 0.774 | 3.643 ± 0.838 |
| EST (mm) | 0.388 ± 0.04 | 0.400 ± 0.034 | 0.393 ± 0.043 |
| MiRNA Type | Total Number of miRNAs | Number of miRNAs with Target Genes 1 | Number of Target Genes 2 |
|---|---|---|---|
| Known_miRNAs | 587 | 499 | 20,890 |
| Novel_miRNAs | 334 | 304 | 19,893 |
| Total | 921 | 803 | 22,158 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, K.; Li, D.; Jiang, X.; Du, Y.; Yu, M. Identification and Characterization of the miRNA Transcriptome Controlling Green Pigmentation of Chicken Eggshells. Genes 2024, 15, 811. https://doi.org/10.3390/genes15060811
Shi K, Li D, Jiang X, Du Y, Yu M. Identification and Characterization of the miRNA Transcriptome Controlling Green Pigmentation of Chicken Eggshells. Genes. 2024; 15(6):811. https://doi.org/10.3390/genes15060811
Chicago/Turabian StyleShi, Kai, Dongfeng Li, Xusheng Jiang, Yuesong Du, and Minli Yu. 2024. "Identification and Characterization of the miRNA Transcriptome Controlling Green Pigmentation of Chicken Eggshells" Genes 15, no. 6: 811. https://doi.org/10.3390/genes15060811






