Extensive Contact Lens Wear Modulates Expression of miRNA-320 and miRNA-423-5p in the Human Corneal Epithelium: Possible Biomarkers of Corneal Health and Environmental Impact
Abstract
1. Introduction
2. Materials and Methods
2.1. Corneal Epithelium Collection
2.2. Real-Time PCR for miRNAs
2.2.1. microRNA Extraction
2.2.2. Reverse Transcriptase Reactions
2.2.3. Real-Time PCR
2.2.4. Target Prediction Tools
2.3. Statistical Analysis
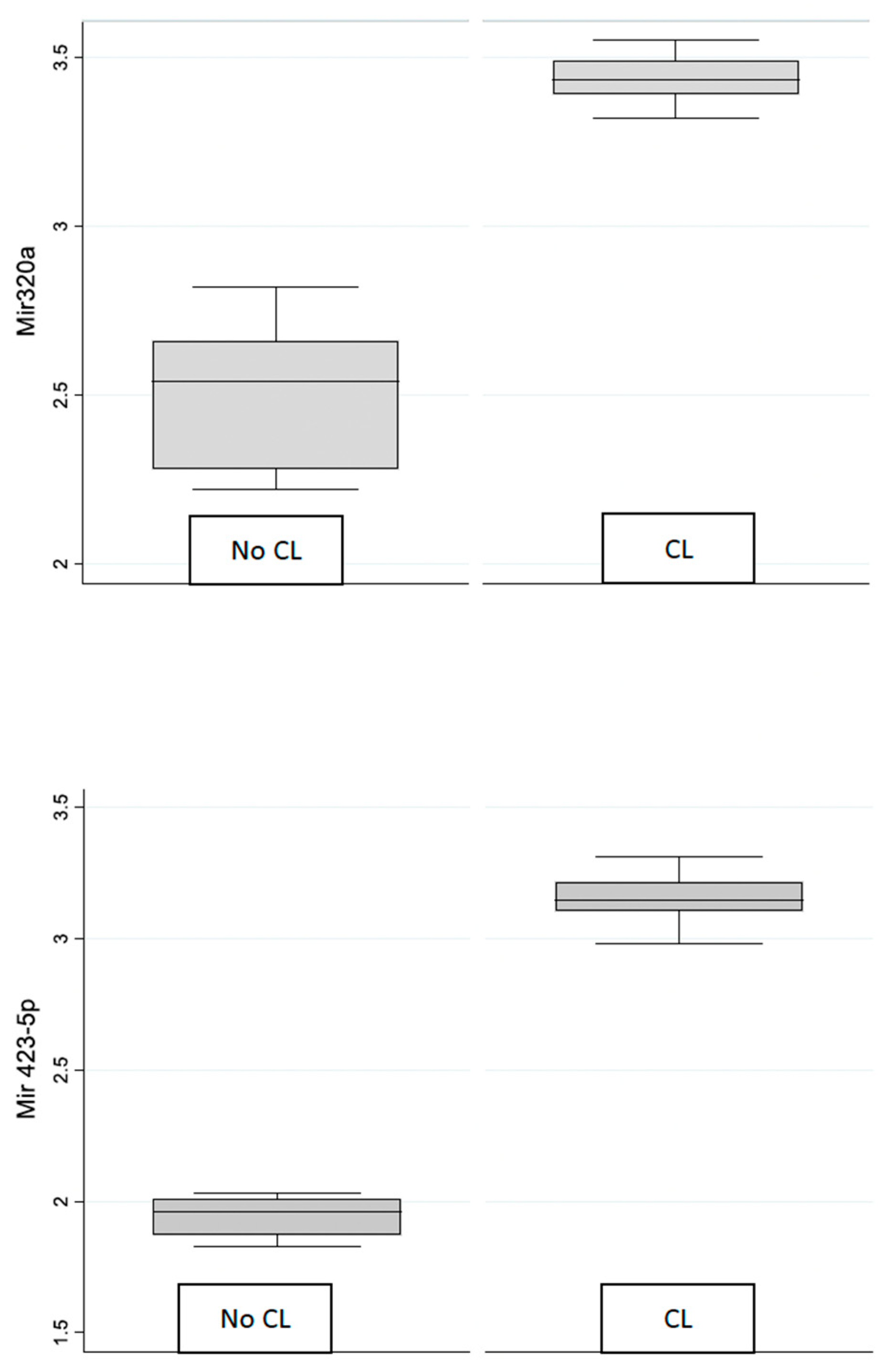
3. Results
Expression of miR-320 and miR 423-5p
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krol, J.; Loedige, I.; Filipowicz, W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010, 11, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Zan on-Moreno, V.; García-Medina, J.J.; Bendala-Tufanisco, E.; Vinuesa-Silva, I.; del Castillo, F.B. MicroRNAs as potential biomarkers of eye diseases. Arch. la Soc. Espanola Oftalmol. 2015, 90, 604–605. [Google Scholar]
- Rassi, D.M.; De Paiva, C.S.; Dias, L.C.; Módulo, C.M.; Adriano, L.; Fantucci, M.Z.; Rocha, E.M. Review: MicroRNAS in ocular surface and dry eye diseases. Ocul. Surf. 2017, 15, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Aguennouz, M.; Polito, F.; Visalli, M.; Vita, G.; Raffa, G.; Oteri, R.; Ghazi, B.; Scalia, G.; Angileri, F.F.; Barresi, V.; et al. MicroRNA-10 and -221 modulate differential expression of Hippo signaling pathway in human astroglial tumors. Cancer Treat. Res. Commun. 2020, 24, 100203. [Google Scholar] [CrossRef] [PubMed]
- Conti, A.; Romeo, S.G.; Cama, A.; La Torre, D.; Barresi, V.; Pezzino, G.; Tomasello, C.; Cardali, S.; Angileri, F.F.; Polito, F.; et al. MiRNA expression profiling in human gliomas: Upregulated miR-363 increases cell survival and proliferation. Tumor Biol. 2017, 37, 14035–14048. [Google Scholar] [CrossRef] [PubMed]
- Xu, S. MicroRNA expression in the eyes and their significance in relation to functions. Prog. Retin. Eye Res. 2009, 28, 87–116. [Google Scholar] [CrossRef] [PubMed]
- Han, R. MicroRNA-146a negatively regulates inflammation via the IRAK1/TRAF6/NF-κB signaling pathway in dry eye. Sci. Rep. 2023, 13, 11192. [Google Scholar] [CrossRef]
- Liao, C.H.; Tseng, C.-L.; Lin, S.-L.; Liang, C.-L.; Juo, S.-H.H. MicroRNA Therapy for Dry Eye Disease. J. Ocul. Pharmacol. Ther. 2022, 38, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ryan, D.G.; Getsios, S.; Oliveira-Fernandes, M.; Fatima, A.; Lavker, R.M. MicroRNA-184 antagonizes microRNA-205 to maintain SHIP2 levels in epithelia. Proc. Natl. Acad. Sci. USA 2008, 105, 19300–19305. [Google Scholar] [CrossRef]
- Konsta, O.D.; Thabet, Y.; Le Dantec, C.; Brooks, W.H.; Tzioufas, A.G.; Pers, J.O.; Renaudineau, Y. The contribution of epigenetics in Sj€ogren’s Syndrome. Front. Genet. 2014, 5, 71. [Google Scholar] [CrossRef]
- Le Discorde, M.; Moreau, P.; Sabatier, P.; Legeais, J.M.; Carosella, E.D. Expression of HLA-G in human cornea, an immune-privileged tissue. Hum. Immunol. 2003, 64, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Mo, Z.; Lyu, D.; Zhang, L.; Qin, Z.; Tang, Q.; Yin, H.; Xu, P.; Wu, L.; Lou, X.; et al. Air pollution and outpatient visits for conjunctivitis: A case-crossover study in Hangzhou, China. Environ. Pollut. 2017, 231, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Altinors, D.D.; Akca, S.; Akova, Y.A.; Bilezikci, B.; Goto, E.; Dogru, M.; Tsubota, K. Smoking associated with damage to the lipid layer of the ocular surface. Am. J. Ophthalmol. 2006, 141, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, H.; Huang, C.; Li, Z.; Li, W.; Zhang, X.; Liu, Z. Impact of chronic smoking on meibomian gland dysfunction. PLoS ONE 2016, 11, e0168763. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.K.; Dogru, M.; Wakamatsu, T.; Ibrahim, O.; Matsumoto, Y.; Kojima, T.; Sato, E.A.; Ogawa, J.; Schnider, C.; Negishi, K.; et al. Passive cigarette smoke exposure and soft contact lens wear. Optom. Vis. Sci. 2010, 87, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Xu, Y.; Zhang, H.; Xu, P.; Ye, J. Cigarette smoke induces ROS mediated autophagy impairment in human corneal epithelial cells. Environ. Pollut. 2019, 245, 389–397. [Google Scholar] [CrossRef]
- Kageyama, S.; Sou, Y.S.; Uemura, T.; Kametaka, S.; Saito, T.; Ishimura, R.; Kouno, T.; Bedford, L.; Mayer, R.J.; Lee, M.-S.; et al. Proteasome dysfunction activates autophagy the Keap1- Nrf2 pathway. J. Biol. Chem. 2014, 289, 24944–24955. [Google Scholar] [CrossRef]
- Karnati, R.; Talla, V.; Peterson, K.; Laurie, G.W. Lacritin and other autophagy associated proteins in ocular surface health. Exp. Eye Res. 2016, 144, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Jamali, Z.; Taheri-Anganeh, M.; Shabaninejad, Z.; Keshavarzi, A.; Taghizadeh, H.; Razavi, Z.S. Autophagy regulation by microRNAs: Novel insights into osteosarcoma therapy. IUBMB Life 2020, 72, 1306–1321. [Google Scholar] [CrossRef]
- Li, Y.; Tang, X.; He, Q.; Yang, X.; Ren, X.; Wen, X.; Zhang, J.; Wang, Y.; Liu, N.; Ma, J. Overexpression of Mitochondria Mediator Gene TRIAP1 by miR-320b Loss Is Associated with Progression in Nasopharyngeal Carcinoma. PLoS Genet. 2016, 12, e1006183. [Google Scholar] [CrossRef]
- Zhou, H.; Peng, C.; Huang, D.-S.; Liu, L.; Guan, P. microRNA Expression Profiling Based on Microarray Approach in Human Diabetic Retinopathy: A Systematic Review and Meta-Analysis. DNA Cell Biol. 2020, 39, 441–450. [Google Scholar] [CrossRef]
- Sapkota, K.; Franco, S.; Lira, M. Daily versus monthly disposable contact lens: Which is better for ocular surface physiology and comfort? Contact Lens Anterior Eye 2018, 41, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Clayton, J.A. Dry Eye. N. Engl. J. Med. 2018, 378, 2212–2223. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.-S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Miljanovi’c, B.; Dana, R.; Sullivan, D.A.; Schaumberg, D.A. Impact of dry eye syndrome on vision-related quality of life. Am. J. Ophthalmol. 2007, 143, 409–415. [Google Scholar] [CrossRef]
- Kojima, T. Contact Lens-Associated Dry Eye Disease: Recent Advances Worldwide and in Japan. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES102–DES108. [Google Scholar] [CrossRef]
- Uchino, M.; Schaumberg, D.A.; Dogru, M.; Uchino, Y.; Fukagawa, K.; Shimmura, S.; Satoh, T.; Takebayashi, T.; Tsubota, K. Prevalence of dry eye disease among Japanese visual display terminal users. Ophthalmology 2008, 115, 1982–1988. [Google Scholar] [CrossRef]
- Uchino, M.; Dogru, M.; Uchino, Y.; Fukagawa, K.; Shimmura, S.; Takebayashi, T.; Schaumberg, D.A.; Tsubota, K. Japan Ministry of Health study on prevalence of dry eye disease among Japanese high school students. Am. J. Ophthalmol. 2008, 146, 925–929. [Google Scholar] [CrossRef]
- Aragona, P.; Ferreri, G.; Micali, A.; Puzzolo, D. Morphological changes of the conjunctival epithelium in contact lens wearers evaluated by impression cytology. Eye 1998, 12, 461–466. [Google Scholar] [CrossRef]
- Xu, J.; Chen, P.; Yu, C.; Liu, Y.; Hu, S.; Di, G. In vivo Confocal Microscopic Evaluation of Corneal Dendritic Cell Density and Subbasal Nerve Parameters in Dry Eye Patients: A Systematic Review and Meta-analysis. Front. Med. 2021, 7, 578233. [Google Scholar] [CrossRef]
- Chien, K.H.; Chen, S.J.; Liu, J.H.; Woung, L.C.; Chen, J.T.; Liang, C.M.; Chiou, S.-H.; Tsai, C.-Y.; Cheng, C.-K.; Hu, C.-C.; et al. Correlation of microRNA-145 levels and clinical severity of pterygia. Ocul. Surf. 2013, 11, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.E.; Bradley, D.T.; Campbell, M.; Lechner, J.; Dash, D.P.; Simpson, D.A.; Willoughby, C.E. Mutation altering the miR-184 seed region causes familial keratoconus with cataract. Am. J. Hum. Genet. 2011, 89, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Bykhovskaya, Y.; Seldin, M.F.; Liu, Y.; Ransom, M.; Li, X.; Rabinowitz, Y.S. Independent origin of c.57 C > T mutation in MIR184 associated with inherited corneal and lens abnormalities. Ophthalmic Genet. 2015, 36, 95–97. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Osae, E.A.; Jones, L.; Nichols, J.J. The impact of contact lenses on meibomian gland morphology. Ocul. Surf. 2022, 24, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jacobs, D.S. Contact Lenses for Ocular Surface Disease. Eye Contact Lens 2022, 48, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.; Cazzanelli, G.; Rasul, S.; Hitchinson, B.; Hu, Y.; Coombes, R.C.; Raguz, S.; Yagüe, E. Apoptosis inhibitor TRIAP1 is a novel effector of drug resistance. Oncol. Rep. 2015, 34, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Potting, C.; Tatsuta, T.; König, T.; Haag, M.; Wai, T.; Aaltonen, M.J.; Langer, T. TRIAP1/PRELI complexes prevent apoptosis by mediating intramitochondrial transport of phosphatidic acid. Cell Metab. 2013, 18, 287–295. [Google Scholar] [CrossRef]
- Ohtsuka, M.; Arase, H.; Takeuchi, A.; Yamasaki, S.; Shiina, R.; Suenaga, T.; Sakurai, D.; Yokosuka, T.; Arase, N.; Iwashima, M.; et al. NFAM1, an immunoreceptor tyrosine-based activation motif-bearing molecule that regulates B cell development and signaling. Proc. Natl. Acad. Sci. USA 2004, 101, 8126–8131. [Google Scholar] [CrossRef] [PubMed]
- Bushati, N.; Cohen, S.M. microRNA functions. Annu. Rev. Cell Dev. Biol. 2007, 23, 175–205. [Google Scholar] [CrossRef]
- Juchem, K.W.; Gounder, A.P.; Gao, J.P.; Seccareccia, E.; Yeddula, N.; Huffmaster, N.J.; Côté-Martin, A.; Fogal, S.E.; Souza, D.; Wang, S.S.; et al. NFAM1 Promotes Pro-Inflammatory Cytokine Production in Mouse and Human Monocytes. Front. Immunol. 2022, 12, 773445. [Google Scholar] [CrossRef]
- Zhao, P.; Sun, S.; Zhai, Y.; Tian, Q.; Zhou, T.; Li, J. miR-423-5p inhibits the proliferation and metastasis of glioblastoma cells by targeting phospholipase C beta 1. Int. J. Clin. Exp. Pathol. 2019, 12, 2941–2950. [Google Scholar] [PubMed]
| CL | Control | |
|---|---|---|
| N | 20 | 19 |
| Age (years) | 30.4 ± 8.83 | 37.89 ± 8.87 |
| Male/Female n (%) | 5 (25%)/15 (75%) | 10 (52.63%)/9 (47.37%) |
| Group | Variable | Mean | Std. Dev. | Conf. Interval 95% | |
|---|---|---|---|---|---|
| CL group | miR-320a | 3.44 | 0.07 | 3.41 | 3.47 |
| miR-4235p | 3.15 | 0.09 | 3.11 | 3.19 | |
| Control group | miR-320a | 2.50 | 0.20 | 2.41 | 2.60 |
| miR-4235p | 1.95 | 0.07 | 1.92 | 1.98 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roszkowska, A.M.; Aguennouz, M.; Aragona, E.; Gargano, R.; Oliverio, G.W.; Inferrera, L.; Aragona, P. Extensive Contact Lens Wear Modulates Expression of miRNA-320 and miRNA-423-5p in the Human Corneal Epithelium: Possible Biomarkers of Corneal Health and Environmental Impact. Genes 2024, 15, 816. https://doi.org/10.3390/genes15060816
Roszkowska AM, Aguennouz M, Aragona E, Gargano R, Oliverio GW, Inferrera L, Aragona P. Extensive Contact Lens Wear Modulates Expression of miRNA-320 and miRNA-423-5p in the Human Corneal Epithelium: Possible Biomarkers of Corneal Health and Environmental Impact. Genes. 2024; 15(6):816. https://doi.org/10.3390/genes15060816
Chicago/Turabian StyleRoszkowska, Anna M., M’hammed Aguennouz, Emanuela Aragona, Romana Gargano, Giovanni William Oliverio, Leandro Inferrera, and Pasquale Aragona. 2024. "Extensive Contact Lens Wear Modulates Expression of miRNA-320 and miRNA-423-5p in the Human Corneal Epithelium: Possible Biomarkers of Corneal Health and Environmental Impact" Genes 15, no. 6: 816. https://doi.org/10.3390/genes15060816
APA StyleRoszkowska, A. M., Aguennouz, M., Aragona, E., Gargano, R., Oliverio, G. W., Inferrera, L., & Aragona, P. (2024). Extensive Contact Lens Wear Modulates Expression of miRNA-320 and miRNA-423-5p in the Human Corneal Epithelium: Possible Biomarkers of Corneal Health and Environmental Impact. Genes, 15(6), 816. https://doi.org/10.3390/genes15060816







