Mustn1 in Skeletal Muscle: A Novel Regulator?
Abstract
:1. Skeletal Muscle
2. Mustn1
3. Clinical Involvement
4. Mustn1 in Skeletal Muscle
5. Future Research Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. 2000, 89, 465–471. [Google Scholar] [CrossRef]
- Miller, J.B.; Stockdale, F.E. Developmental origins of skeletal muscle fibers: Clonal analysis of myogenic cell lineages based on expression of fast and slow myosin heavy chains. Proc. Natl. Acad. Sci. USA 1986, 83, 3860–3864. [Google Scholar] [CrossRef]
- Cossu, G.; Biressi, S. Satellite cells, myoblasts and other occasional myogenic progenitors: Possible origin, phenotypic features and role in muscle regeneration. Semin. Cell Dev. Biol. 2005, 16, 623–631. [Google Scholar] [CrossRef]
- Ahmetov, I.I.; Vinogradova, O.L.; Williams, A.G. Gene polymorphisms and fiber-type composition of human skeletal muscle. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.A.; Morgan, J.L.; Kane, C.; Sharma, Y.; Heffner, C.S.; Lake, J.; Donahue, L.R. Mouse Gestation Length Is Genetically Determined. PLoS ONE 2010, 5, e12418. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, R.; Martin-Fairey, C.; Sojka, D.K.; Herzog, E.D.; Jungheim, E.S.; Stout, M.J.; Fay, J.C.; Mahendroo, M.; Reese, J.; Herington, J.L.; et al. Mouse models of preterm birth: Suggested assessment and reporting guidelines†. Biol. Reprod. 2018, 99, 922–937. [Google Scholar] [CrossRef] [PubMed]
- Deprez, A.; Orfi, Z.; Radu, A.; He, Y.; Ravizzoni Dartora, D.; Dort, J.; Dumont, N.A.; Nuyt, A.M. Transient neonatal exposure to hyperoxia, an experimental model of preterm birth, leads to skeletal muscle atrophy and fiber type switching. Clin. Sci. 2021, 135, 2589–2605. [Google Scholar] [CrossRef]
- Hawke, T.J.; Garry, D.J. Myogenic satellite cells: Physiology to molecular biology. J. Appl. Physiol. 2001, 91, 534–551. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Schiaffino, S. Muscle fiber type diversity revealed by anti-myosin heavy chain antibodies. FEBS J. 2018, 285, 3688–3694. [Google Scholar] [CrossRef]
- Lombardo, F.; Komatsu, D.; Hadjiargyrou, M. Molecular cloning and characterization of Mustang, a novel nuclear protein expressed during skeletal development and regeneration. FASEB J. 2004, 18, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Hadjiargyrou, M. Mustn1: A Developmentally Regulated Pan-Musculoskeletal Cell Marker and Regulatory Gene. Int. J. Mol. Sci. 2018, 19, 206. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hadjiargyrou, M. Identification and characterization of the Mustang promoter: Regulation by AP-1 during myogenic differentiation. Bone 2006, 39, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.P.; Moradi, J.; Coleman, S.K.; D’Souza, D.M.; Liu, C.; Kronenberg, M.S.; Rowe, D.W.; Hawke, T.J.; Hadjiargyrou, M. A novel GFP reporter mouse reveals Mustn1 expression in adult regenerating skeletal muscle, activted satellite cells and differentiating myoblasts. Acta Physiol. 2013, 208, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Bregua, P.; Chien, C.; Megias, M.; Du, S.; Rotllant, J. Promoter architecture and transcriptional regulation of musculoskeletal embryonic nuclear protein 1b (mustn1b) gene in zebrafish. Dev. Dyn. 2017, 246, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Gersch, R.; Hadjiargyrou, M. Mustn1 is expressed during chondrogenesis and is necessary for chondrocyte proliferation and differentiation in vitro. Bone 2009, 45, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Robert, G.; Hawke, T.; Hadjiargyrou, M. Silencing of Mustn1 inhibits myogenic fusion and differentiation. Am. J. Physiol. Cell Physiol. 2010, 298, C1100–C1108. [Google Scholar] [CrossRef] [PubMed]
- Gersch, R.; Kirmizitas, A.; Sobkow, L.; Sorrentino, G.; Thomsen, G.; Hadjiargyrou, M. Mustn1 is essential for craniofacial chondrogenesis during Xenopus development. Gene Expr. Patterns 2012, 12, 145–153. [Google Scholar] [CrossRef]
- Choksi, S.P.; Babu, D.; Lau, D.; Yu, X.; Roy, S. Systematic discovery of novel ciliary genes through functional genomics in the zebrafish. Dev. Camb. Engl. 2014, 141, 3410–3419. [Google Scholar] [CrossRef]
- Camarata, T.; Vasilyev, A.; Hadjiargyrou, M. Cloning of zebrafish Mustn1 orthologs and their expression during early development. Gene 2016, 593, 235–241. [Google Scholar] [CrossRef]
- Danzmann, R.G.; Kocmarek, A.L.; Norman, J.D.; Rexroad, C.E.; Palti, Y. Transcriptome profiling in fast versus slow-growing rainbow trout across seasonal gradients. BMC Genom. 2016, 17, 60. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Huang, W.; Zhang, X.; Ye, B.; Zhou, H.; Hou, S. Identification and characterization of genes related to the development of breast muscles in Pekin duck. Mol. Biol. Rep. 2012, 39, 7647–7655. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.S.; Gu, L.H.; Sun, Y.; Zhang, X.H.; Ye, B.G.; Liu, X.L.; Hou, S.S. Characterization of MUSTN1 gene and its relationship with skeletal muscle development at postnatal stages in Pekin ducks. Genet. Mol. Res. GMR 2015, 14, 4448–4460. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liang, W.; Li, X.; Zhang, Y.; Xu, Q.; Chen, G.; Zhang, H.; Chang, G. Characterization and expression of MUSTN1 gene from different duck breeds. Anim. Biotechnol. 2022, 33, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, Y.; Wang, Y.-G.; Zhao, X.-L.; Gilbert, E.R.; Liu, Y.-P.; Wang, Y.; Hu, Y.-D.; Zhu, Q. MUSTN1 mRNA Abundance and Protein Localization is Greatest in Muscle Tissues of Chinese Meat-Quality Chickens. Int. J. Mol. Sci. 2013, 14, 5545–5559. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.-W.; Hudson, N.; Seo, D.; Lee, S.; Khatri, B.; Lassiter, K.; Cook, D.; Piekarski, A.; Dridi, S.; Anthony, N.; et al. RNA sequencing for global gene expression associated with muscle growth in a single male modern broiler line compared to a foundational Barred Plymouth Rock chicken line. BMC Genom. 2017, 18, 82. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, M.; Shao, Y.; Nan, Y.; Blair, H.T.; Morris, S.T.; Zhao, Z.; Zhang, H. Characterization of muscle development and gene expression in early embryos of chicken, quail, and their hybrids. Gene 2021, 768, 145319. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Fang, W.; Yuan, M.; Sun, H.; Wang, J. Transcriptome Analysis of Leg Muscles and the Effects of ALOX5 on Proliferation and Differentiation of Myoblasts in Haiyang Yellow Chickens. Genes 2023, 14, 1213. [Google Scholar] [CrossRef]
- Kim, S.-S.; Kim, J.-R.; Moon, J.-K.; Choi, B.-H.; Kim, T.-H.; Kim, K.-S.; Kim, J.-J.; Lee, C.-K. Transcriptional alteration of p53 related processes as a key factor for skeletal muscle characteristics in Sus scrofa. Mol. Cells 2009, 28, 565–573. [Google Scholar] [CrossRef]
- Yu, J.; Yang, G.; Li, S.; Li, M.; Ji, C.; Liu, G.; Wang, Y.; Chen, N.; Lei, C.; Dang, R. Identification of Dezhou donkey muscle development-related genes and long non-coding RNA based on differential expression analysis. Anim. Biotechnol. 2023, 34, 2313–2323. [Google Scholar] [CrossRef]
- Rong, M.; Xing, X.; Zhang, R. Muscle Transcriptome Analysis of Mink at Different Growth Stages Using RNA-Seq. Biology 2024, 13, 283. [Google Scholar] [CrossRef] [PubMed]
- Kostek, M.C.; Chen, Y.-W.; Cuthbertson, D.J.; Shi, R.; Fedele, M.J.; Esser, K.A.; Rennie, M.J. Gene expression responses over 24 h to lengthening and shortening contractions in human muscle: Major changes in CSRP3, MUSTN1, SIX1, and FBXO32. Physiol. Genom. 2007, 31, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Blottner, D.; Capitanio, D.; Trautmann, G.; Furlan, S.; Gambara, G.; Moriggi, M.; Block, K.; Barbacini, P.; Torretta, E.; Py, G.; et al. Nitrosative Redox Homeostasis and Antioxidant Response Defense in Disused Vastus lateralis Muscle in Long-Term Bedrest (Toulouse Cocktail Study). Antioxidants 2021, 10, 378. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.W.; Chaput, C.D.; Brannen, J.; Probe, R.A.; Guleria, R.S.; Pan, J.; Baker, K.M.; VanBuren, V. Alcohol induced epigenetic perturbations during the inflammatory stage of fracture healing. Exp. Biol. Med. 2011, 236, 1389–1401. [Google Scholar] [CrossRef]
- Hadjiargyrou, M.; Zigomalas, A.; Haleem, A.; Komaatsu, D. Mustn1 Spatiotemporal Protein Expression During Skeletal Development and Regeneration. JMBR 2013, 28, SA0097. [Google Scholar]
- Salichos, L.; Thayavally, R.; Kloen, P.; Hadjiargyrou, M. Human nonunion tissues display differential gene expression in comparison to physiological fracture callus. Bone 2024, 183, 117091. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wu, C.; Wong, R.W.K.; M.Rabie, A.B. Identification of the chondrogenic pathway in the mandibular condylar cartilage. Front. Biosci. 2009, 14, 1932–1938. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.J.; Tew, S.R.; Vasieva, O.; Clegg, P.D.; Canty-Laird, E.G. A systems biology approach to defining regulatory mechanisms for cartilage and tendon cell phenotypes. Sci. Rep. 2016, 6, 33956. [Google Scholar] [CrossRef]
- Ducommun, S.; Jannig, P.R.; Cervenka, I.; Murgia, M.; Mittenbühler, M.J.; Chernogubova, E.; Dias, J.M.; Jude, B.; Correia, J.C.; Van Vranken, J.G.; et al. Mustn1 is a smooth muscle cell-secreted microprotein that modulates skeletal muscle extracellular matrix composition. Mol. Metab. 2024, 82, 101912. [Google Scholar] [CrossRef]
- Evans, P.L.; McMillin, S.L.; Weyrauch, L.A.; Witczak, C.A. Regulation of Skeletal Muscle Glucose Transport and Glucose Metabolism by Exercise Training. Nutrients 2019, 11, 2432. [Google Scholar] [CrossRef]
- Qin, H.; Jiao, W. Correlation of muscle mass and bone mineral density in the NHANES US general population, 2017–2018. Medicine 2022, 101, e30735. [Google Scholar] [CrossRef]
- Giudice, J.; Taylor, J.M. Muscle as a paracrine and endocrine organ. Curr. Opin. Pharmacol. 2017, 34, 49–55. [Google Scholar] [CrossRef] [PubMed]
- NIH Muscular Dystrophy|National Institute of Neurological Disorders and Stroke. Available online: https://www.ninds.nih.gov/health-information/disorders/muscular-dystrophy (accessed on 27 May 2024).
- Glaser, J.; Suzuki, M. Skeletal Muscle Fiber Types in Neuromuscular Diseases. In Muscle Cell and Tissue—Current Status of Research Field; IntechOpen: Rijeka, Croatia, 2018; ISBN 978-1-78984-006-3. [Google Scholar] [CrossRef]
- Mercuri, E.; Bönnemann, C.G.; Muntoni, F. Muscular dystrophies. Lancet 2019, 394, 2025–2038. [Google Scholar] [CrossRef] [PubMed]
- McNally, E.M.; Pytel, P. Muscle Diseases: The Muscular Dystrophies. Annu. Rev. Pathol. Mech. Dis. 2007, 2, 87–109. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Muntoni, F. Muscular dystrophies. Lancet 2013, 381, 845–860. [Google Scholar] [CrossRef]
- Theadom, A.; Rodrigues, M.; Roxburgh, R.; Balalla, S.; Higgins, C.; Bhattacharjee, R.; Jones, K.; Krishnamurthi, R.; Feigin, V. Prevalence of Muscular Dystrophies: A Systematic Literature Review. Neuroepidemiology 2014, 43, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Bushby, K.; Finkel, R.; Birnkrant, D.J.; Case, L.E.; Clemens, P.R.; Cripe, L.; Kaul, A.; Kinnett, K.; McDonald, C.; Pandya, S.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: Diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010, 9, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Muntoni, F. Muscular dystrophy: New challenges and review of the current clinical trials. Curr. Opin. Pediatr. 2013, 25, 701–707. [Google Scholar] [CrossRef]
- Turner, C.; Hilton-Jones, D. The myotonic dystrophies: Diagnosis and management. J. Neurol. Neurosurg. Psychiatry 2010, 81, 358–367. [Google Scholar] [CrossRef]
- Boyer, F.; Drame, M.; Morrone, I.; Novella, J.-L. Factors relating to carer burden for families of persons with muscular dystrophy. J. Rehabil. Med. 2006, 38, 309–315. [Google Scholar] [CrossRef]
- Graham, C.D.; Rose, M.R.; Grunfeld, E.A.; Kyle, S.D.; Weinman, J. A systematic review of quality of life in adults with muscle disease. J. Neurol. 2011, 258, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Magliano, L.; Patalano, M.; Sagliocchi, A.; Scutifero, M.; Zaccaro, A.; D’angelo, M.G.; Civati, F.; Brighina, E.; Vita, G.; Vita, G.L.; et al. Burden, professional support, and social network in families of children and young adults with muscular dystrophies. Muscle Nerve 2015, 52, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Chen, Z.; Yu, Y.; Zhang, N.; Jiang, H.; Zhang, G.; Zhang, Z.; Zhang, B. Current Pharmacological Strategies for Duchenne Muscular Dystrophy. Front. Cell Dev. Biol. 2021, 9, 689533. [Google Scholar] [CrossRef] [PubMed]
- Bushby, K.; Finkel, R.; Birnkrant, D.J.; Case, L.E.; Clemens, P.R.; Cripe, L.; Kaul, A.; Kinnett, K.; McDonald, C.; Pandya, S.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: Implementation of multidisciplinary care. Lancet Neurol. 2010, 9, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Matthews, E.; Brassington, R.; Kuntzer, T.; Jichi, F.; Manzur, A.Y. Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database Syst. Rev. 2016, 2016, CD003725. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.E.; Schnell, F.J.; Akana, C.; El-Husayni, S.H.; Desjardins, C.A.; Morgan, J.; Charleston, J.S.; Sardone, V.; Domingos, J.; Dickson, G.; et al. Increased dystrophin production with golodirsen in patients with Duchenne muscular dystrophy. Neurology 2020, 94, e2270–e2282. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.R.Q.; Maruyama, R.; Yokota, T. Eteplirsen in the treatment of Duchenne muscular dystrophy. Drug Des. Devel. Ther. 2017, 11, 533–545. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.M.; Campbell, C.; Torricelli, R.E.; Finkel, R.S.; Flanigan, K.M.; Goemans, N.; Heydemann, P.; Kaminska, A.; Kirschner, J.; Muntoni, F.; et al. Ataluren in patients with nonsense mutation Duchenne muscular dystrophy (ACT DMD): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Sienkiewicz, D.; Kulak, W.; Okurowska-Zawada, B.; Paszko-Patej, G.; Kawnik, K. Duchenne muscular dystrophy: Current cell therapies. Ther. Adv. Neurol. Disord. 2015, 8, 166–177. [Google Scholar] [CrossRef]
- Elangkovan, N.; Dickson, G. Gene Therapy for Duchenne Muscular Dystrophy. J. Neuromuscul. Dis. 2021, 8, S303–S316. [Google Scholar] [CrossRef]
- Balagopal, P.; Olney, R.; Darmaun, D.; Mougey, E.; Dokler, M.; Sieck, G.; Hammond, D. Oxandrolone enhances skeletal muscle myosin synthesis and alters global gene expression profile in Duchenne muscular dystrophy. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E530–E539. [Google Scholar] [CrossRef] [PubMed]
- Robriquet, F.; Lardenois, A.; Babarit, C.; Larcher, T.; Dubreil, L.; Leroux, I.; Zuber, C.; Ledevin, M.; Deschamps, J.-Y.; Fromes, Y.; et al. Differential Gene Expression Profiling of Dystrophic Dog Muscle after MuStem Cell Transplantation. PLoS ONE 2015, 10, e0123336. [Google Scholar] [CrossRef]
- Emery, A.E. Population frequencies of inherited neuromuscular diseases—A world survey. Neuromuscul. Disord. 1991, 1, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, W.R.; Alter, M.; Sung, J.H. Progressive proximal spinal and bulbar muscular atrophy of late onset: A sex-linked recessive trait. Neurology 1968, 18, 671. [Google Scholar] [CrossRef]
- La Spada, A.R.; Wilson, E.M.; Lubahn, D.B.; Harding, A.E.; Fischbeck, K.H. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature 1991, 352, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, L.; Echaniz-Laguna, A. Skeletal muscle in motor neuron diseases: Therapeutic target and delivery route for potential treatments. Curr. Drug Targets 2010, 11, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.; Ferrier, A.; Kothary, R. More than a bystander: The contributions of intrinsic skeletal muscle defects in motor neuron diseases. Front. Physiol. 2013, 4, 356. Available online: https://www.frontiersin.org/articles/10.3389/fphys.2013.00356 (accessed on 27 September 2023). [CrossRef]
- Halievski, K.; Mo, K.; Westwood, J.T.; Monks, D.A. Transcriptional Profile of Muscle following Acute Induction of Symptoms in a Mouse Model of Kennedy’s Disease/Spinobulbar Muscular Atrophy. PLoS ONE 2015, 10, e0118120. [Google Scholar] [CrossRef]
- Matre, P.R.; Mu, X.; Wu, J.; Danila, D.; Hall, M.A.; Kolonin, M.G.; Darabi, R.; Huard, J. CRISPR/Cas9-Based Dystrophin Restoration Reveals a Novel Role for Dystrophin in Bioenergetics and Stress Resistance of Muscle Progenitors. Stem Cells 2019, 37, 1615–1628. [Google Scholar] [CrossRef]
- McKenzie, M.J.; Goldfarb, A.H.; Kump, D.S. Gene Response of the Gastrocnemius and Soleus Muscles to an Acute Aerobic Run in Rats. J. Sports Sci. Med. 2011, 10, 385–392. [Google Scholar] [CrossRef]
- Jensen, J.H.; Conley, L.N.; Hedegaard, J.; Nielsen, M.; Young, J.F.; Oksbjerg, N.; Hornshøj, H.; Bendixen, C.; Thomsen, B. Gene expression profiling of porcine skeletal muscle in the early recovery phase following acute physical activity. Exp. Physiol. 2012, 97, 833–848. [Google Scholar] [CrossRef]
- Oh, S.L. Effect of Resistance Exercise Training on Mustn1 mRNA Expression in Rat Skeletal Muscle. Korean J. Sports Med. 2011, 29, 112–117. [Google Scholar] [CrossRef]
- Oh, S.L.; Oh, S.D. Effect of Resistance Training on Skeletal Muscle Gene Expression in Rats: A Beadarray Analysis. J. Life Sci. 2013, 23, 116–124. [Google Scholar] [CrossRef]
- Hu, Z.; Xu, H.; Lu, Y.; He, Q.; Yan, C.; Zhao, X.; Tian, Y.; Yang, C.; Zhang, Z.; Qiu, M.; et al. MUSTN1 is an indispensable factor in the proliferation, differentiation and apoptosis of skeletal muscle satellite cells in chicken. Exp. Cell Res. 2021, 407, 112833. [Google Scholar] [CrossRef]
- Kim, C.J.; Singh, C.; Lee, C.; DiMagno, K.; O’Donnell, M.; Kaczmarek, M.; Ahmed, A.; Salvo-Schaich, J.; Perez, A.; Letsou, W.; et al. Mustn1 ablation in skeletal muscle results in increased glucose tolerance concomitant with upregulated GLUT expression in male mice. Physiol. Rep. 2023, 11, e15674. [Google Scholar] [CrossRef]
- Kim, C.J.; Singh, C.; Kaczmarek, M.; O’Donnell, M.; Lee, C.; DiMagno, K.; Young, M.W.; Letsou, W.; Ramos, R.L.; Granatosky, M.C.; et al. Mustn1 ablation in skeletal muscle results in functional alterations. FASEB bioAdvances 2023, 5, 541–557. [Google Scholar] [CrossRef]
- Minchew, E.C.; Williamson, N.C.; Readyoff, A.T.; McClung, J.M.; Spangenburg, E.E. Isometric skeletal muscle contractile properties in common strains of male laboratory mice. Front. Physiol. 2022, 13, 937132. [Google Scholar] [CrossRef] [PubMed]
- Siriett, V.; Platt, L.; Salerno, M.S.; Ling, N.; Kambadur, R.; Sharma, M. Prolonged absence of myostatin reduces sarcopenia. J. Cell. Physiol. 2006, 209, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Handschin, C.; Chin, S.; Li, P.; Liu, F.; Maratos-Flier, E.; LeBrasseur, N.K.; Yan, Z.; Spiegelman, B.M. Skeletal Muscle Fiber-type Switching, Exercise Intolerance, and Myopathy in PGC-1α Muscle-specific Knock-out Animals*. J. Biol. Chem. 2007, 282, 30014–30021. [Google Scholar] [CrossRef]
- Kepser, L.-J.; Damar, F.; De Cicco, T.; Chaponnier, C.; Prószyński, T.J.; Pagenstecher, A.; Rust, M.B. CAP2 deficiency delays myofibril actin cytoskeleton differentiation and disturbs skeletal muscle architecture and function. Proc. Natl. Acad. Sci. USA 2019, 116, 8397–8402. [Google Scholar] [CrossRef]
- Lloyd, E.M.; Xu, H.; Murphy, R.M.; Grounds, M.D.; Pinniger, G.J. Dysferlin-deficiency has greater impact on function of slow muscles, compared with fast, in aged BLAJ mice. PLoS ONE 2019, 14, e0214908. [Google Scholar] [CrossRef] [PubMed]
- Pereyra, A.S.; Lin, C.-T.; Sanchez, D.M.; Laskin, J.; Spangenburg, E.E.; Neufer, P.D.; Fisher–Wellman, K.; Ellis, J.M. Skeletal muscle undergoes fiber type metabolic switch without myosin heavy chain switch in response to defective fatty acid oxidation. Mol. Metab. 2022, 59, 101456. [Google Scholar] [CrossRef] [PubMed]
- Mohammadabadi, M.; Bordbar, F.; Jensen, J.; Du, M.; Guo, W. Key Genes Regulating Skeletal Muscle Development and Growth in Farm Animals. Animals 2021, 11, 835. [Google Scholar] [CrossRef] [PubMed]
- Leduc-Gaudet, J.-P.; Franco-Romero, A.; Cefis, M.; Moamer, A.; Broering, F.E.; Milan, G.; Sartori, R.; Chaffer, T.J.; Dulac, M.; Marcangeli, V.; et al. MYTHO is a novel regulator of skeletal muscle autophagy and integrity. Nat. Commun. 2023, 14, 1199. [Google Scholar] [CrossRef] [PubMed]
- Beiner, J.M.; Jokl, P. Muscle Contusion Injuries: Current Treatment Options. J. Am. Acad. Orthop. Surg. 2001, 9, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Huard, J.; Li, Y.; Fu, F.H. Muscle injuries and repair: Current trends in research. J. Bone Jt. Surg. Am. 2002, 84, 822–832. [Google Scholar] [CrossRef]
- Murgia, M.; Toniolo, L.; Nagaraj, N.; Ciciliot, S.; Vindigni, V.; Schiaffino, S.; Reggiani, C.; Mann, M. Single Muscle Fiber Proteomics Reveals Fiber-Type-Specific Features of Human Muscle Aging. Cell Rep. 2017, 19, 2396–2409. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Costa, M.L.; Mermelstein, C.S.; Chagas, C.; Holtzer, S.; Holtzer, H. MyoD converts primary dermal fibroblasts, chondroblasts, smooth muscle, and retinal pigmented epithelial cells into striated mononucleated myoblasts and multinucleated myotubes. Proc. Natl. Acad. Sci. USA 1990, 87, 7988–7992. [Google Scholar] [CrossRef]
- Bar-Nur, O.; Gerli, M.F.M.; Di Stefano, B.; Almada, A.E.; Galvin, A.; Coffey, A.; Huebner, A.J.; Feige, P.; Verheul, C.; Cheung, P.; et al. Direct Reprogramming of Mouse Fibroblasts into Functional Skeletal Muscle Progenitors. Stem Cell Rep. 2018, 10, 1505–1521. [Google Scholar] [CrossRef]
- Xu, B.; Siehr, A.; Shen, W. Functional skeletal muscle constructs from transdifferentiated human fibroblasts. Sci. Rep. 2020, 10, 22047. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, B.; Ye, M.; Liang, P.; Zhangfang, Y.; Huang, J.; Liu, M.; Songyang, Z.; Ma, W. Ccndbp1 is a new positive regulator of skeletal myogenesis. J. Cell Sci. 2016, 129, 2767–2777. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; James, R.S.; Cox, V.M.; Tallis, J. The Effect of Increasing Age on the Concentric and Eccentric Contractile Properties of Isolated Mouse Soleus and Extensor Digitorum Longus Muscles. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Günther, S.; Looso, M.; Künne, C.; Krüger, M.; Kim, J.; Zhou, Y.; Braun, T. Prmt5 is a regulator of muscle stem cell expansion in adult mice. Nat. Commun. 2015, 6, 7140. [Google Scholar] [CrossRef] [PubMed]

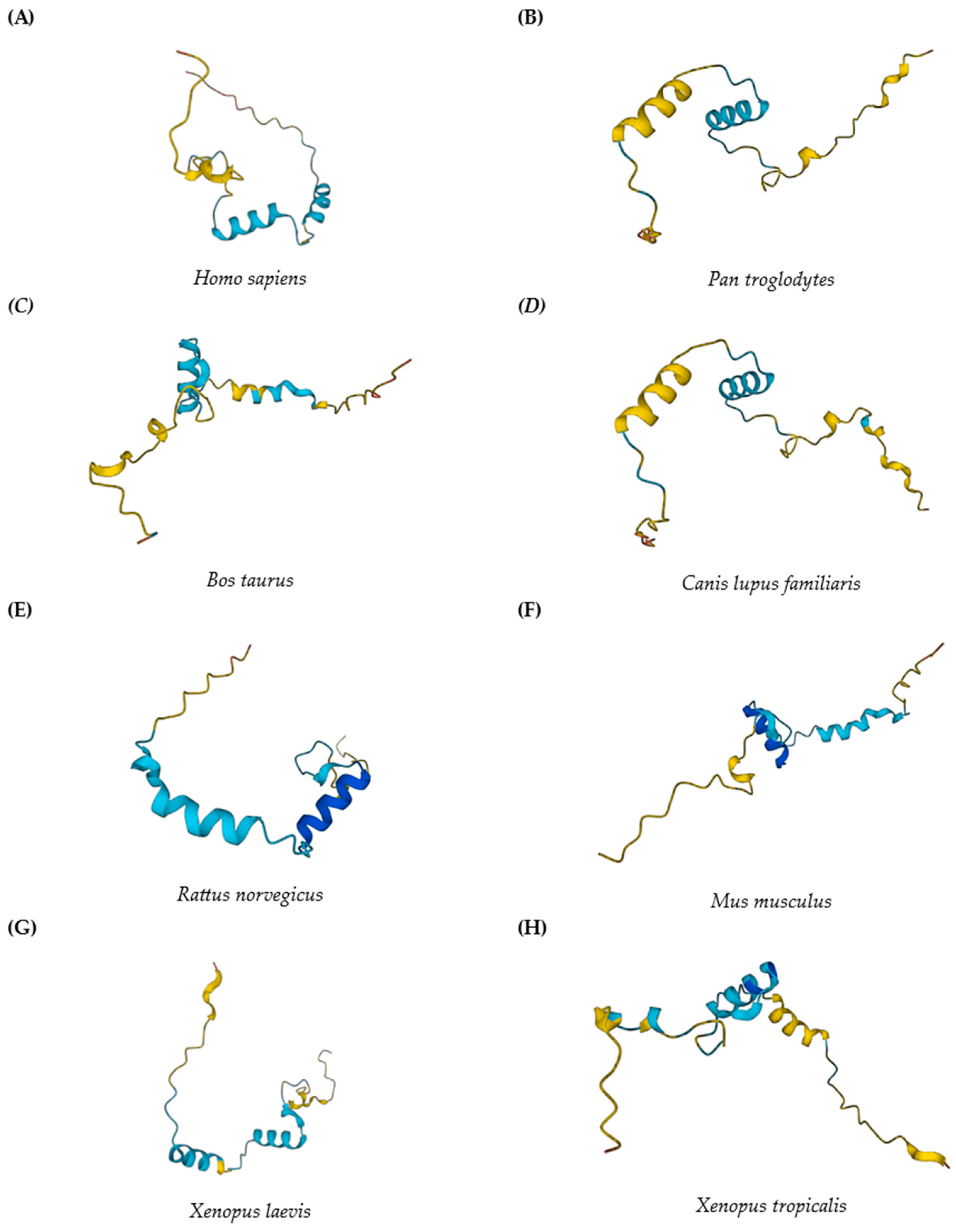
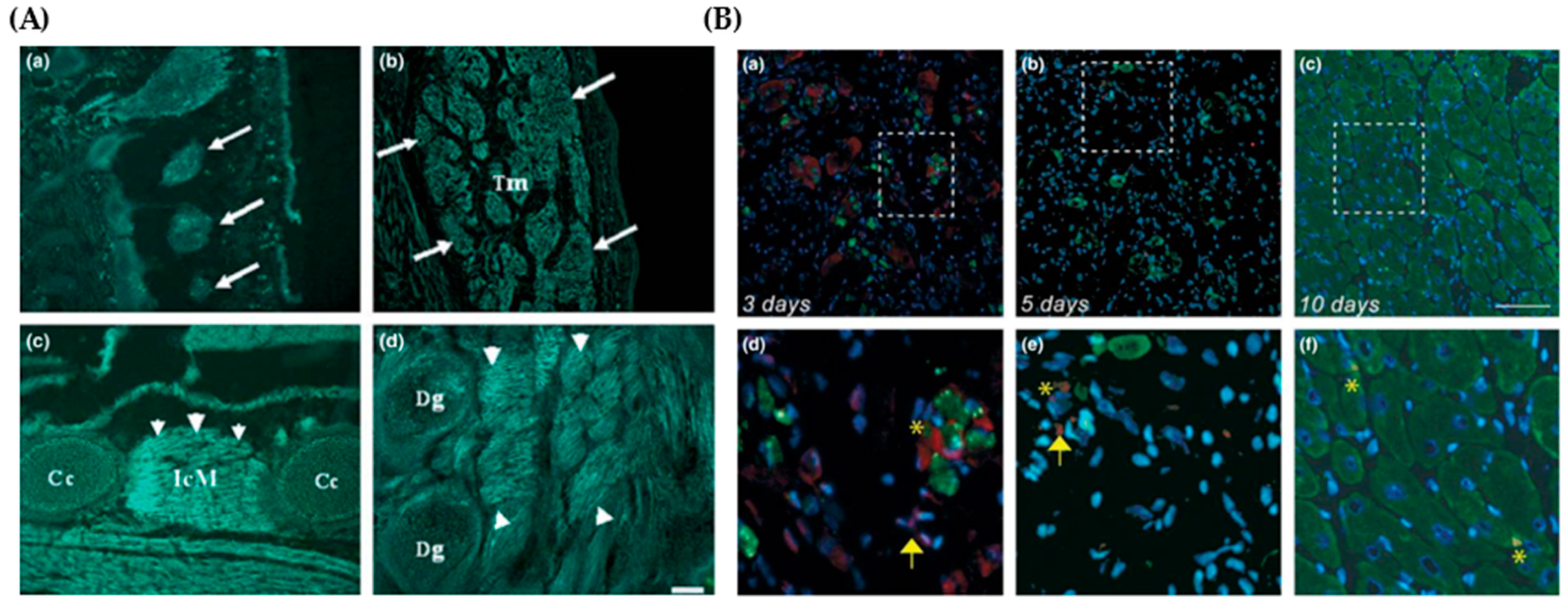
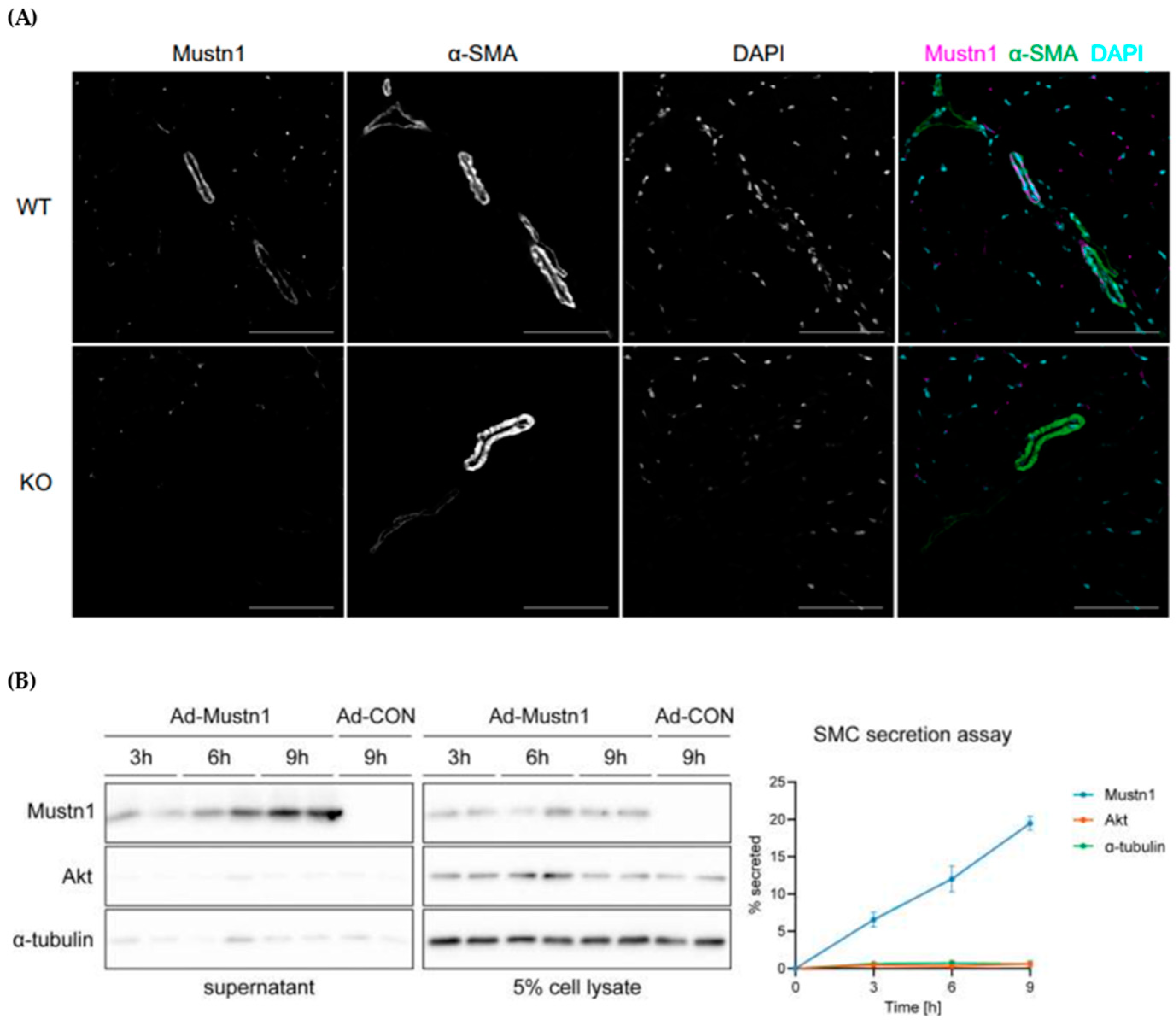
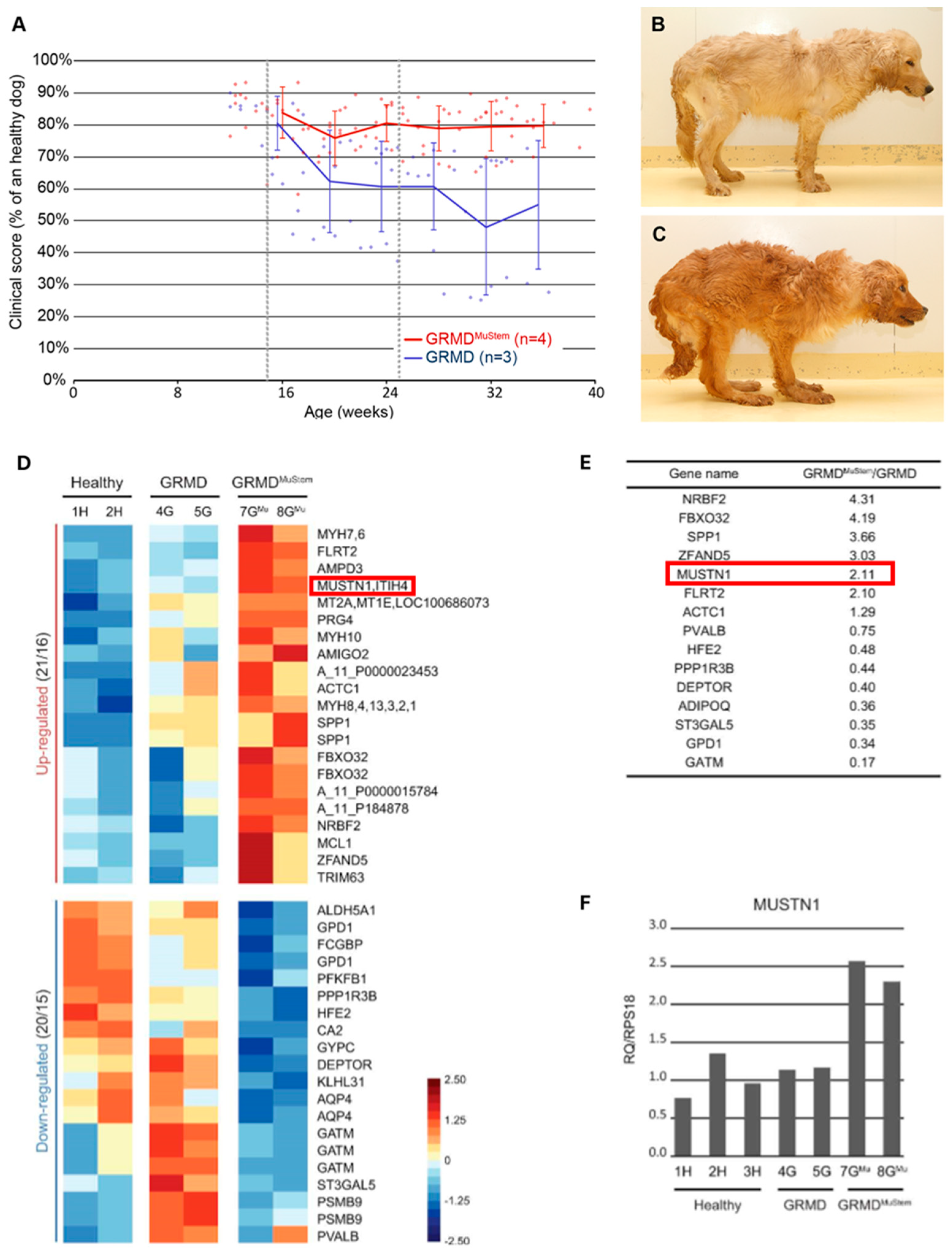
| Cell/Tissue | Approach | Observed Effects | Study |
|---|---|---|---|
| Myogenic cells (C212) | RNAi | Impaired myoblast differentiation, myofusion, and myotube formation with downregulation of differentiation and fusion factors | [17] |
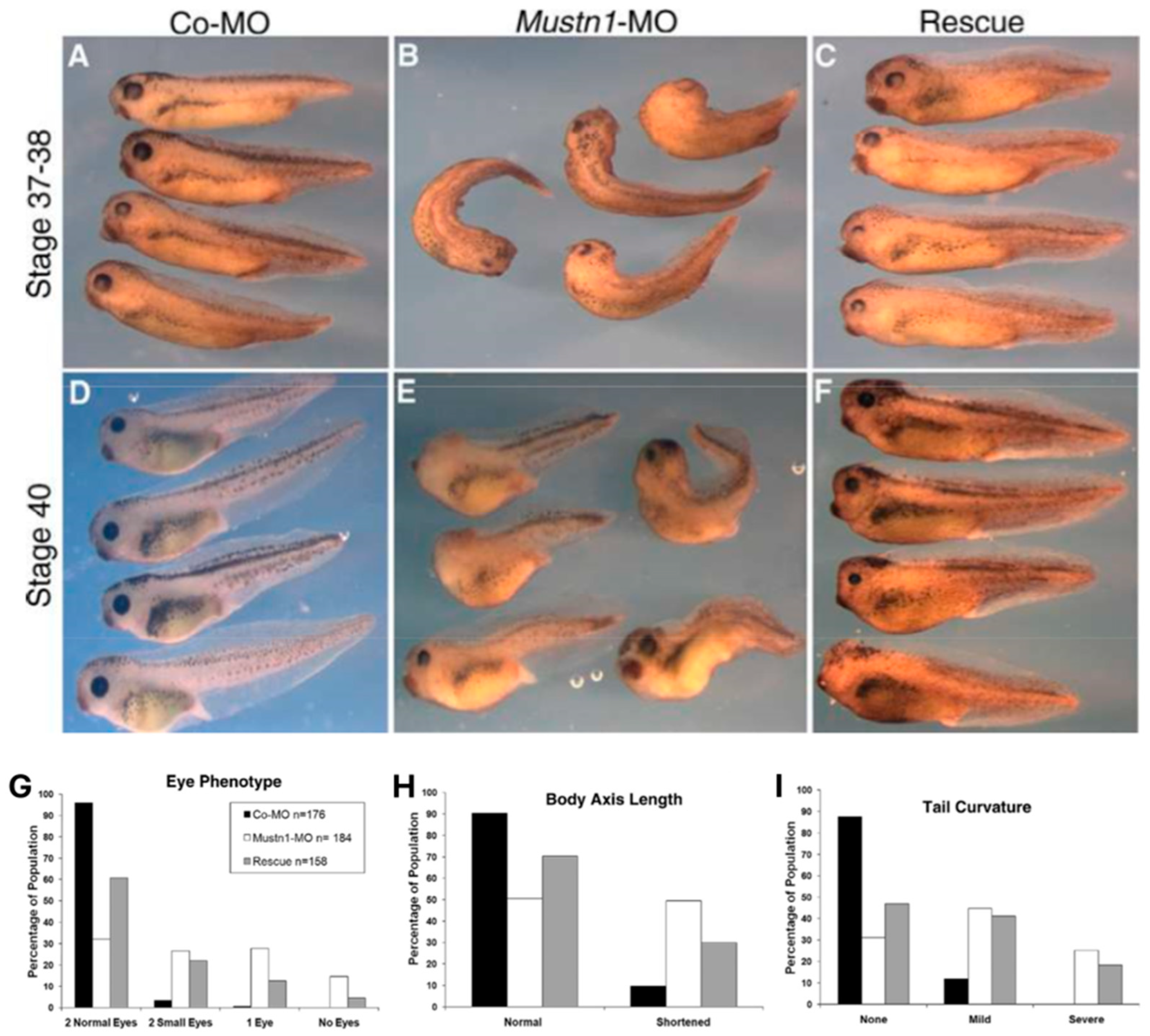
| Frog Embryo | Antisense MO | Small or absent eyes, shortened body axis, and tail curvature | [18] |
| Zebrafish embryo | Antisense MO | Curved body axis phenotype; otolith and left–right asymmetry defects Curling of cilia and disorganized γ-tubulin expression | [19] |
| Chicken Pax7 satellite cells | siRNA | Decreased relative expression, proliferation, differentiation, and myotube formation with downregulation of differentiation factors | [76] |
| Experiments | 2 Mo KO | 4 Mo KO |
|---|---|---|
| Weight | Decreased ** | ns |
| Glucose Metabolism | Higher tolerance **** | ns |
| Metabolism-related genes | MUP-1 ***, Scl25a10 *, OSTN * | MUP-1 * |
| Grip Strength (absolute) | Decreased ** | ns |
| Single Limb Force | ns | Increased hindlimb vertical * |
| Ex vivo: absolute force (soleus) | Increased 100 **, 150 ***, 300 *** Hz | Decreased 20 ** Hz |
| Ex vivo: specific force (soleus) | Decreased at 20 * Hz | Decreased 20 ***, 60 ** Hz |
| Ex vivo: fatigue (soleus) | Higher **** | Higher * |
| PCSA (soleus) | ns | Increased * |
| Muscle fiber composition (soleus) | ns | Decreased Type I * Decreased Type IIa * Increased Type IIb * |
| Experiments | Muscle | Observed Effects |
|---|---|---|
| Acute exercise (uphill treadmill) | Gastrocnemius | ns |
| Soleus | Mustn1 expression increased **** | |
| Tibialis anterior | ns | |
| Acute exercise (downhill treadmill) | Gastrocnemius | Mustn1 expression increased * |
| Soleus | Mustn1 expression increased * | |
| Tibialis anterior | ns | |
| Exercise training (free-wheel running) | Gastrocnemius | ns |
| Soleus | Mustn1 expression increased * | |
| Tibialis anterior | ns | |
| Hindlimb unloading/reloading | Gastrocnemius | Mustn1 expression increased **** 1 day of hindlimb reloading |
| Vasus lateralis (human) | Mustn1 expression decreased ** after 10 days of unloading Mustn1 expression increased ** after 21 days of active recovery | |
| Muscle injury | Gastrocnemius (Bacl2 injection) | Mustn1 expression increased **** 1 day post-injury |
| Tibialis anterior (cardiotoxin) | Collagen content increased * in female Mustn1 KO mice (not in males) 14 days after injury. | |
| Muscular dystrophy model | EDL | Mustn1 expression increased *** |
| Psoas | Mustn1 expression increased * | |
| Femoral artery ligation | Tibialis anterior | Mustn1 expression increased ** 14 days post-ligation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.J.; Hadjiargyrou, M. Mustn1 in Skeletal Muscle: A Novel Regulator? Genes 2024, 15, 829. https://doi.org/10.3390/genes15070829
Kim CJ, Hadjiargyrou M. Mustn1 in Skeletal Muscle: A Novel Regulator? Genes. 2024; 15(7):829. https://doi.org/10.3390/genes15070829
Chicago/Turabian StyleKim, Charles J., and Michael Hadjiargyrou. 2024. "Mustn1 in Skeletal Muscle: A Novel Regulator?" Genes 15, no. 7: 829. https://doi.org/10.3390/genes15070829
APA StyleKim, C. J., & Hadjiargyrou, M. (2024). Mustn1 in Skeletal Muscle: A Novel Regulator? Genes, 15(7), 829. https://doi.org/10.3390/genes15070829







