Intermolecular Gene Conversion for the Equalization of Genome Copies in the Polyploid Haloarchaeon Haloferax volcanii: Identification of Important Proteins
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Media, and Growth Conditions
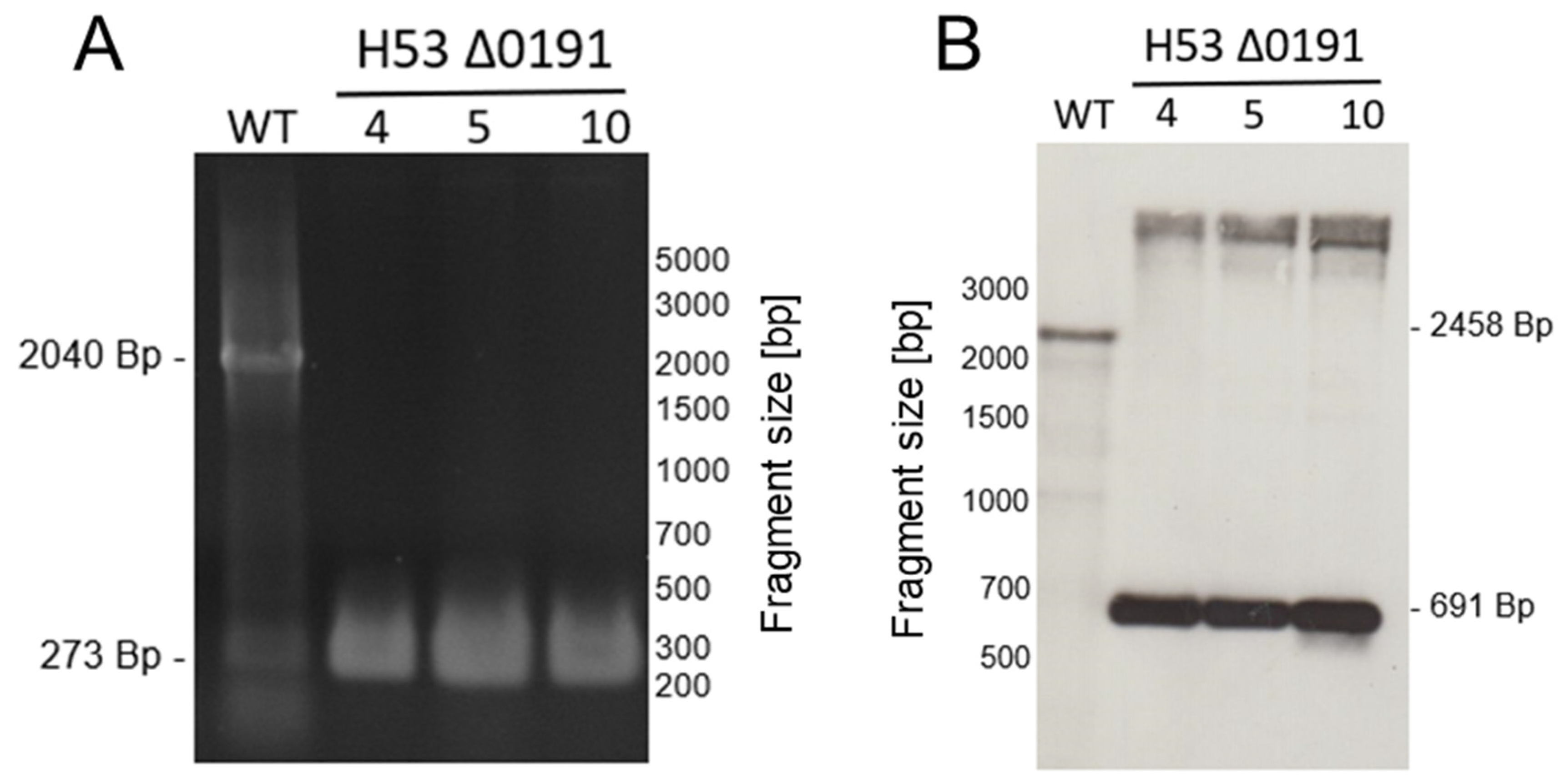
2.2. Generation of In-Frame Deletion Mutants
2.3. Growth Analyses
2.4. Analyses of Gene Conversion Efficiencies
2.5. Bioinformatic Analyses
3. Results
3.1. Experimental Design for the Analysis of Unselected Gene Conversion
3.2. Overview of Generated and Analyzed In-Frame Deletion Mutants
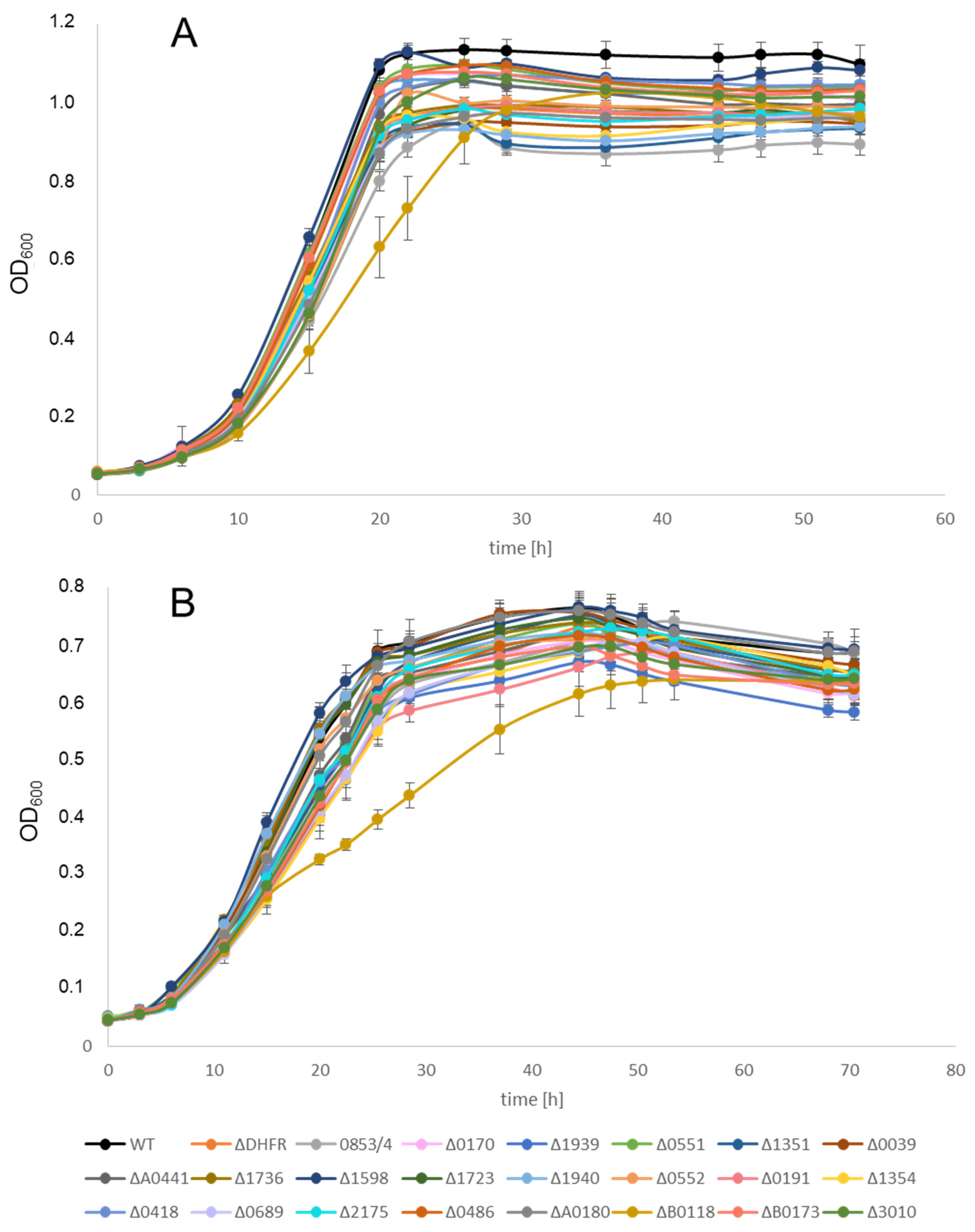
3.3. Growth Analysis of Deletion Mutants
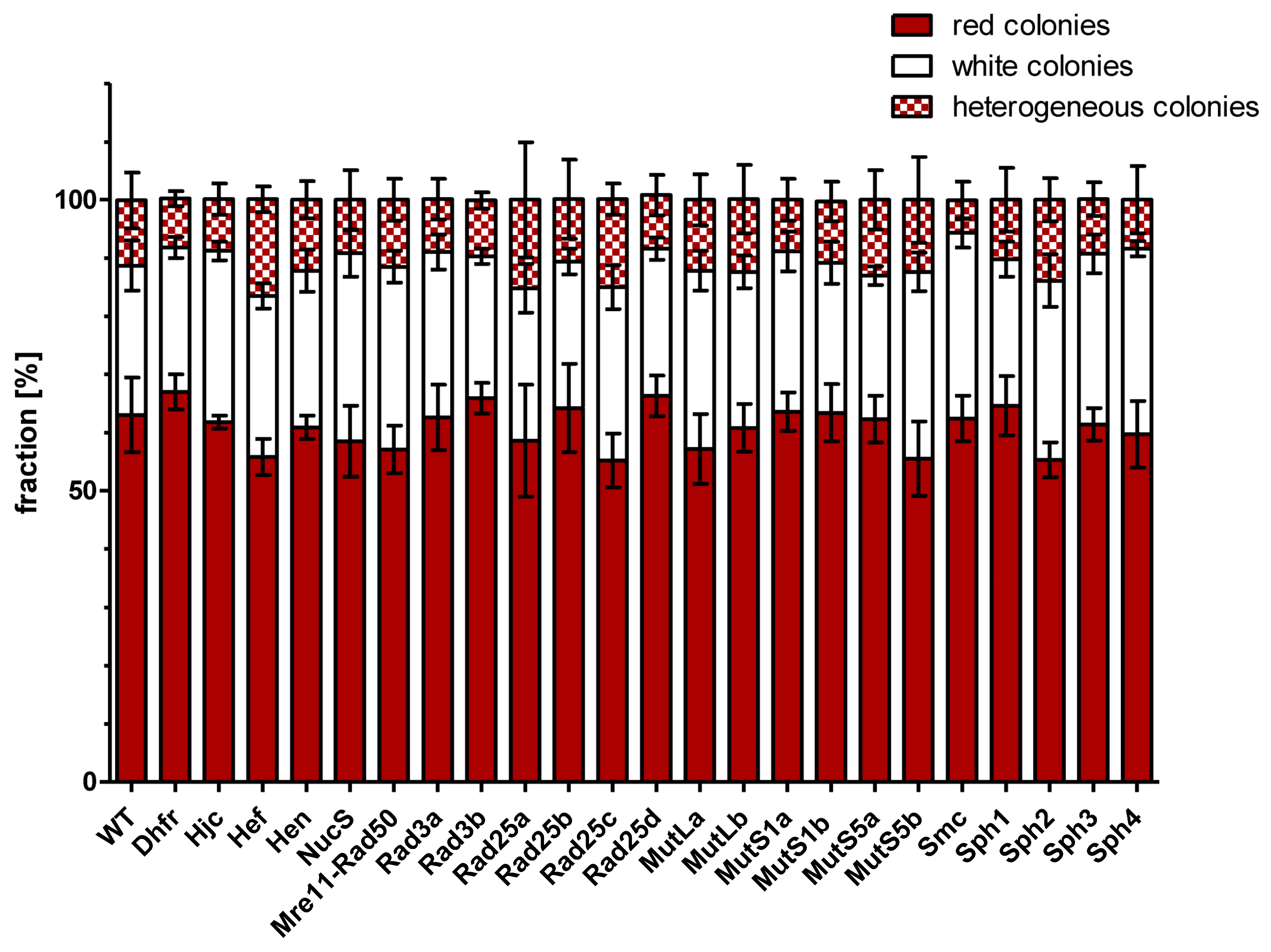
3.4. Gene Conversion Efficiencies of Wildtype and Mutants
3.5. Analysis of Gene Expression
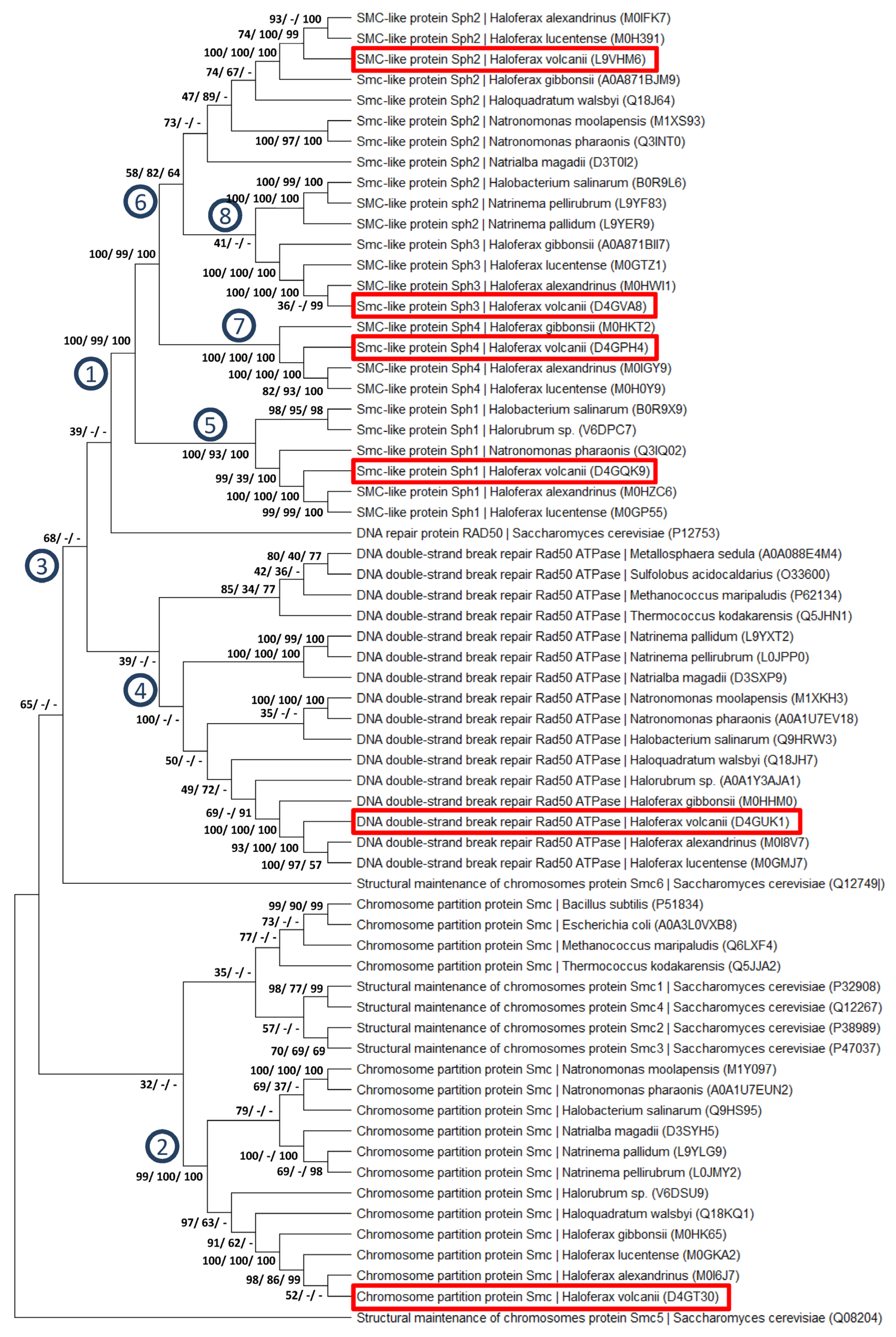
3.6. Bioinformatic Analyses of Paralogous Protein Families
3.7. Generation and Characterization of Mutants with Multiple Deletions
3.8. Further Characterization of the Sph-Deletion Mutants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fayos, I.; Frouin, J.; Meynard, D.; Vernet, A.; Herbert, L.; Guiderdoni, E. Manipulation of Meiotic Recombination to Hasten Crop Improvement. Biology 2022, 11, 369. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, M.D.; Zanders, S.E. Gene conversion generates evolutionary novelty that fuels genetic conflicts. Curr. Opin. Genet. Dev. 2019, 58–59, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Underwood, C.J.; Choi, K. Heterogeneous transposable elements as silencers, enhancers and targets of meiotic recombination. Chromosoma 2019, 128, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, J.A.; Innan, H. The Role of Gene Conversion between Transposable Elements in Rewiring Regulatory Networks. Genome Biol. Evol. 2019, 11, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Korunes, K.L.; Noor, M.A.F. Gene conversion and linkage: Effects on genome evolution and speciation. Mol. Ecol. 2017, 26, 351–364. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, R.; Morrison, L.J.; Hall, J.P.J. DNA Recombination Strategies during Antigenic Variation in the African Trypanosome. Microbiol. Spectr. 2015, 3, 409–435. [Google Scholar] [CrossRef] [PubMed]
- Duret, L.; Galtier, N. Biased gene conversion and the evolution of mammalian genomic landscapes. Annu. Rev. Genom. Hum. Genet. 2009, 10, 285–311. [Google Scholar] [CrossRef] [PubMed]
- Tang, E.S.; Martin, A. Immunoglobulin gene conversion: Synthesizing antibody diversification and DNA repair. DNA Repair 2007, 6, 1557–1571. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G.; Romero, D. Gene conversion and concerted evolution in bacterial genomes. FEMS Microbiol. Rev. 2005, 29, 169–183. [Google Scholar] [CrossRef]
- Foley, J. Mini-review: Strategies for Variation and Evolution of Bacterial Antigens. Comput. Struct. Biotechnol. J. 2015, 13, 407–416. [Google Scholar] [CrossRef]
- Vink, C.; Rudenko, G.; Seifert, H.S. Microbial antigenic variation mediated by homologous DNA recombination. FEMS Microbiol. Rev. 2012, 36, 917–948. [Google Scholar] [CrossRef] [PubMed]
- Espejo, R.T.; Plaza, N. Multiple Ribosomal RNA Operons in Bacteria; Their Concerted Evolution and Potential Consequences on the Rate of Evolution of Their 16S rRNA. Front. Microbiol. 2018, 9, 1232. [Google Scholar] [CrossRef] [PubMed]
- Liao, D. Gene conversion drives within genic sequences: Concerted evolution of ribosomal RNA genes in bacteria and archaea. J. Mol. Evol. 2000, 51, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Cuna, F.O.; Castellanos, M.; Romero, D. Biased Gene Conversion in Rhizobium etli Is Caused by Preferential Double-Strand Breaks on One of the Recombining Homologs. J. Bacteriol. 2016, 198, 591–599. [Google Scholar] [CrossRef]
- Paulsson, J.; El Karoui, M.; Lindell, M.; Hughes, D. The processive kinetics of gene conversion in bacteria. Mol. Microbiol. 2017, 104, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.; Zerulla, K.; Breuert, S.; Soppa, J. Gene conversion results in the equalization of genome copies in the polyploid haloarchaeon Haloferax volcanii. Mol. Microbiol. 2011, 80, 666–677. [Google Scholar] [CrossRef]
- Soppa, J. Evolutionary advantages of polyploidy in halophilic archaea. Biochem. Soc. Trans. 2013, 41, 339–343. [Google Scholar] [CrossRef]
- Wasser, D.; Borst, A.; Hammelmann, M.; Ludt, K.; Soppa, J. Characterization of Non-selected Intermolecular Gene Conversion in the Polyploid Haloarchaeon Haloferax volcanii. Front. Microbiol. 2021, 12, 680854. [Google Scholar] [CrossRef]
- Hildenbrand, C.; Stock, T.; Lange, C.; Rother, M.; Soppa, J. Genome copy numbers and gene conversion in methanogenic archaea. J. Bacteriol. 2011, 193, 734–743. [Google Scholar] [CrossRef]
- Ruhlman, T.A.; Zhang, J.; Blazier, J.C.; Sabir, J.S.M.; Jansen, R.K. Recombination-dependent replication and gene conversion homogenize repeat sequences and diversify plastid genome structure. Am. J. Bot. 2017, 104, 559–572. [Google Scholar] [CrossRef]
- Gong, L.; Olson, M.; Wendel, J.F. Cytonuclear evolution of rubisco in four allopolyploid lineages. Mol. Biol. Evol. 2014, 31, 2624–2636. [Google Scholar] [CrossRef]
- Hao, W.; Richardson, A.O.; Zheng, Y.; Palmer, J.D. Gorgeous mosaic of mitochondrial genes created by horizontal transfer and gene conversion. Proc. Natl. Acad. Sci. USA 2010, 107, 21576–21581. [Google Scholar] [CrossRef]
- Khakhlova, O.; Bock, R. Elimination of deleterious mutations in plastid genomes by gene conversion. Plant J. 2006, 46, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Dahal, S.; Dubey, S.; Raghavan, S.C. Homologous recombination-mediated repair of DNA double-strand breaks operates in mammalian mitochondria. Cell. Mol. Life Sci. 2018, 75, 1641–1655. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Hao, W. Horizontal transfer and gene conversion as an important driving force in shaping the landscape of mitochondrial introns. G3 Genes Genomes Genet. 2014, 4, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.R.; Keeling, P.J. Gene conversion shapes linear mitochondrial genome architecture. Genome Biol. Evol. 2013, 5, 905–912. [Google Scholar] [CrossRef]
- Suh, M.H.; Pulakat, L.; Gavini, N. Isolation and characterization of nifDK:kanamycin and nitrogen fixation proficient Azotobacter vinelandii strain, and its implication on the status of multiple chromosomes in Azotobacter. Genetica 2000, 110, 101–107. [Google Scholar] [CrossRef]
- Nodop, A.; Pietsch, D.; Höcker, R.; Becker, A.; Pistorius, E.K.; Forchhammer, K.; Michel, K.-P. Transcript profiling reveals new insights into the acclimation of the mesophilic fresh-water cyanobacterium Synechococcus elongatus PCC 7942 to iron starvation. Plant Physiol. 2008, 147, 747–763. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takahama, K.; Matsuoka, M.; Nagahama, K.; Ogawa, T. Construction and analysis of a recombinant cyanobacterium expressing a chromosomally inserted gene for an ethylene-forming enzyme at the psbAI locus. J. Biosci. Bioeng. 2003, 95, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Spence, E.; Bailey, S.; Nenninger, A.; Møller, S.G.; Robinson, C. A homolog of Albino3/OxaI is essential for thylakoid biogenesis in the cyanobacterium Synechocystis sp. PCC6803. J. Biol. Chem. 2004, 279, 55792–55800. [Google Scholar] [CrossRef]
- Allers, T.; Ngo, H.-P.; Mevarech, M.; Lloyd, R.G. Development of additional selectable markers for the halophilic archaeon Haloferax volcanii based on the leuB and trpA genes. Appl. Environ. Microbiol. 2004, 70, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Nagy, C.; Thiel, K.; Mulaku, E.; Mustila, H.; Tamagnini, P.; Aro, E.-M.; Pacheco, C.C.; Kallio, P. Comparison of alternative integration sites in the chromosome and the native plasmids of the cyanobacterium Synechocystis sp. PCC 6803 in respect to expression efficiency and copy number. Microb. Cell Fact. 2021, 20, 130. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, D.; Bizic-Ionescu, M.; de Maio, N.; Cypionka, H.; Grossart, H.-P. Community-like genome in single cells of the sulfur bacterium Achromatium oxaliferum. Nat. Commun. 2017, 8, 455. [Google Scholar] [CrossRef] [PubMed]
- Hartman, A.L.; Norais, C.; Badger, J.H.; Delmas, S.; Haldenby, S.; Madupu, R.; Robinson, J.; Khouri, H.; Ren, Q.; Lowe, T.M.; et al. The complete genome sequence of Haloferax volcanii DS2, a model archaeon. PLoS ONE 2010, 5, e9605. [Google Scholar] [CrossRef] [PubMed]
- Bitan-Banin, G.; Ortenberg, R.; Mevarech, M. Development of a gene knockout system for the halophilic archaeon Haloferax volcanii by use of the pyrE gene. J. Bacteriol. 2003, 185, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Jantzer, K.; Zerulla, K.; Soppa, J. Phenotyping in the archaea: Optimization of growth parameters and analysis of mutants of Haloferax volcanii. FEMS Microbiol. Lett. 2011, 322, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 1983, 166, 557–580. [Google Scholar] [CrossRef] [PubMed]
- Nagel, C.; Machulla, A.; Zahn, S.; Soppa, J. Several One-Domain Zinc Finger µ-Proteins of Haloferax Volcanii Are Important for Stress Adaptation, Biofilm Formation, and Swarming. Genes 2019, 10, 361. [Google Scholar] [CrossRef] [PubMed]
- Hammelmann, M.; Soppa, J. Optimized generation of vectors for the construction of Haloferax volcanii deletion mutants. J. Microbiol. Methods 2008, 75, 201–204. [Google Scholar] [CrossRef]
- Ludt, K.; Soppa, J. Influence of Origin Recognition Complex Proteins on the Copy Numbers of Three Chromosomes in Haloferax volcanii. J. Bacteriol. 2018, 200, e00161-18. [Google Scholar] [CrossRef]
- Rosenshine, I.; Tchelet, R.; Mevarech, M. The mechanism of DNA transfer in the mating system of an archaebacterium. Science 1989, 245, 1387–1389. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, F.; Broicher, A.; Gillich, T.; Klee, K.; Mejía, J.; Rampp, M.; Oesterhelt, D. Genome information management and integrated data analysis with HaloLex. Arch. Microbiol. 2008, 190, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Babski, J.; Haas, K.A.; Näther-Schindler, D.; Pfeiffer, F.; Förstner, K.U.; Hammelmann, M.; Hilker, R.; Becker, A.; Sharma, C.M.; Marchfelder, A.; et al. Genome-wide identification of transcriptional start sites in the haloarchaeon Haloferax volcanii based on differential RNA-Seq (dRNA-Seq). BMC Genom. 2016, 17, 629. [Google Scholar] [CrossRef] [PubMed]
- Laass, S.; Monzon, V.A.; Kliemt, J.; Hammelmann, M.; Pfeiffer, F.; Förstner, K.U.; Soppa, J. Characterization of the transcriptome of Haloferax volcanii, grown under four different conditions, with mixed RNA-Seq. PLoS ONE 2019, 14, e0215986. [Google Scholar] [CrossRef] [PubMed]
- Nicol, J.W.; Helt, G.A.; Blanchard, S.G.; Raja, A.; Loraine, A.E. The Integrated Genome Browser: Free software for distribution and exploration of genome-scale datasets. Bioinformatics 2009, 25, 2730–2731. [Google Scholar] [CrossRef]
- Jaschinski, K.; Babski, J.; Lehr, M.; Burmester, A.; Benz, J.; Heyer, R.; Dörr, M.; Marchfelder, A.; Soppa, J. Generation and phenotyping of a collection of sRNA gene deletion mutants of the haloarchaeon Haloferax volcanii. PLoS ONE 2014, 9, e90763. [Google Scholar] [CrossRef]
- Breuert, S.; Allers, T.; Spohn, G.; Soppa, J. Regulated polyploidy in halophilic archaea. PLoS ONE 2006, 1, e92. [Google Scholar] [CrossRef]
- Schiller, H.; Hong, Y.; Kouassi, J.; Rados, T.; Kwak, J.; DiLucido, A.; Safer, D.; Marchfelder, A.; Pfeiffer, F.; Bisson, A.; et al. Identification of structural and regulatory cell-shape determinants in Haloferax volcanii. Nat. Commun. 2024, 15, 1414. [Google Scholar] [CrossRef]
- de Silva, R.T.; Abdul-Halim, M.F.; Pittrich, D.A.; Brown, H.J.; Pohlschroder, M.; Duggin, I.G. Improved growth and morphological plasticity of Haloferax volcanii. Microbiology 2021, 167, 001012. [Google Scholar] [CrossRef]
- Dresser, A.R.; Hardy, P.-O.; Chaconas, G. Investigation of the genes involved in antigenic switching at the vlsE locus in Borrelia burgdorferi: An essential role for the RuvAB branch migrase. PLoS Pathog. 2009, 5, e1000680. [Google Scholar] [CrossRef]
- Xu, J.; Seifert, H.S. Analysis of Pilin Antigenic Variation in Neisseria meningitidis by Next-Generation Sequencing. J. Bacteriol. 2018, 200, e00465-18. [Google Scholar] [CrossRef]
- Jordan, P.W.; Klein, F.; Leach, D.R.F. Novel roles for selected genes in meiotic DNA processing. PLoS Genet. 2007, 3, e222. [Google Scholar] [CrossRef]
- Seitz, E.M.; Brockman, J.P.; Sandler, S.J.; Clark, A.J.; Kowalczykowski, S.C. RadA protein is an archaeal RecA protein homolog that catalyzes DNA strand exchange. Genes Dev. 1998, 12, 1248–1253. [Google Scholar] [CrossRef] [PubMed]
- RadB acts in homologous recombination in the archaeon Haloferax volcanii, consistent with a role as recombination mediator. DNA Repair 2017, 55, 7–16. [CrossRef]
- Castellanos, M.; Verhey, T.B.; Goldstein, M.; Chaconas, G. The Putative Endonuclease Activity of MutL Is Required for the Segmental Gene Conversion Events That Drive Antigenic Variation of the Lyme Disease Spirochete. Front. Microbiol. 2022, 13, 888494. [Google Scholar] [CrossRef]
- Verhey, T.B.; Castellanos, M.; Chaconas, G. Antigenic Variation in the Lyme Spirochete: Insights into Recombinational Switching with a Suggested Role for Error-Prone Repair. Cell Rep. 2018, 23, 2595–2605. [Google Scholar] [CrossRef] [PubMed]
- Naor, A.; Lazary, R.; Barzel, A.; Papke, R.T.; Gophna, U. In vivo characterization of the homing endonuclease within the polB gene in the halophilic archaeon Haloferax volcanii. PLoS ONE 2011, 6, e15833. [Google Scholar] [CrossRef][Green Version]
- Krishna, S.; Wagener, B.M.; Liu, H.P.; Lo, Y.-C.; Sterk, R.; Petrini, J.H.J.; Nickoloff, J.A. Mre11 and Ku regulation of double-strand break repair by gene conversion and break-induced replication. DNA Repair 2007, 6, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, M.; Sonoda, E.; Nojima, K.; Sale, J.E.; Takenaka, K.; Kikuchi, K.; Taniguchi, Y.; Nakamura, K.; Sumitomo, Y.; Bree, R.T.; et al. Genetic evidence for single-strand lesions initiating Nbs1-dependent homologous recombination in diversification of Ig v in chicken B lymphocytes. PLoS Genet. 2009, 5, e1000356. [Google Scholar] [CrossRef]
- Yabuki, M.; Fujii, M.M.; Maizels, N. The MRE11-RAD50-NBS1 complex accelerates somatic hypermutation and gene conversion of immunoglobulin variable regions. Nat. Immunol. 2005, 6, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Farah, J.A.; Cromie, G.; Steiner, W.W.; Smith, G.R. A novel recombination pathway initiated by the Mre11/Rad50/Nbs1 complex eliminates palindromes during meiosis in Schizosaccharomyces pombe. Genetics 2005, 169, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Delmas, S.; Duggin, I.G.; Allers, T. DNA damage induces nucleoid compaction via the Mre11-Rad50 complex in the archaeon Haloferax volcanii. Mol. Microbiol. 2013, 87, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Middleton, C.L.; Parker, J.L.; Richard, D.J.; White, M.F.; Bond, C.S. Substrate recognition and catalysis by the Holliday junction resolving enzyme Hje. Nucleic Acids Res. 2004, 32, 5442–5451. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nishino, T.; Komori, K.; Tsuchiya, D.; Ishino, Y.; Morikawa, K. Crystal structure of the archaeal holliday junction resolvase Hjc and implications for DNA recognition. Structure 2001, 9, 197–204. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nishino, T.; Komori, K.; Ishino, Y.; Morikawa, K. Dissection of the regional roles of the archaeal Holliday junction resolvase Hjc by structural and mutational analyses. J. Biol. Chem. 2001, 276, 35735–35740. [Google Scholar] [CrossRef] [PubMed]
- Bond, C.S.; Kvaratskhelia, M.; Richard, D.; White, M.F.; Hunter, W.N. Structure of Hjc, a Holliday junction resolvase, from Sulfolobus solfataricus. Proc. Natl. Acad. Sci. USA 2001, 98, 5509–5514. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Mayaka, J.B.; Zhong, Q.; Zhang, C.; Hou, G.; Ni, J.; Shen, Y. Phosphorylation of the Archaeal Holliday Junction Resolvase Hjc Inhibits Its Catalytic Activity and Facilitates DNA Repair in Sulfolobus islandicus REY15A. Front. Microbiol. 2019, 10, 1214. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Li, Y.; Zeng, C.; Song, T.; Yan, Z.; Ni, J.; She, Q.; Shen, Y. Genetic analysis of the Holliday junction resolvases Hje and Hjc in Sulfolobus islandicus. Extremophiles 2015, 19, 505–514. [Google Scholar] [CrossRef]
- Bolt, E.L.; Lloyd, R.G.; Sharples, G.J. Genetic analysis of an archaeal Holliday junction resolvase in Escherichia coli. J. Mol. Biol. 2001, 310, 577–589. [Google Scholar] [CrossRef]
- Komori, K.; Sakae, S.; Daiyasu, H.; Toh, H.; Morikawa, K.; Shinagawa, H.; Ishino, Y. Mutational analysis of the Pyrococcus furiosus holliday junction resolvase hjc revealed functionally important residues for dimer formation, junction DNA binding, and cleavage activities. J. Biol. Chem. 2000, 275, 40385–40391. [Google Scholar] [CrossRef]
- Komori, K.; Sakae, S.; Fujikane, R.; Morikawa, K.; Shinagawa, H.; Ishino, Y. Biochemical characterization of the hjc holliday junction resolvase of Pyrococcus furiosus. Nucleic Acids Res. 2000, 28, 4544–4551. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lestini, R.; Laptenok, S.P.; Kühn, J.; Hink, M.A.; Schanne-Klein, M.-C.; Liebl, U.; Myllykallio, H. Intracellular dynamics of archaeal FANCM homologue Hef in response to halted DNA replication. Nucleic Acids Res. 2013, 41, 10358–10370. [Google Scholar] [CrossRef] [PubMed]
- Fujikane, R.; Ishino, S.; Ishino, Y.; Forterre, P. Genetic analysis of DNA repair in the hyperthermophilic archaeon, Thermococcus kodakaraensis. Genes Genet. Syst. 2010, 85, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Nishino, T.; Komori, K.; Ishino, Y.; Morikawa, K. Structural and functional analyses of an archaeal XPF/Rad1/Mus81 nuclease: Asymmetric DNA binding and cleavage mechanisms. Structure 2005, 13, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Komori, K.; Hidaka, M.; Horiuchi, T.; Fujikane, R.; Shinagawa, H.; Ishino, Y. Cooperation of the N-terminal Helicase and C-terminal endonuclease activities of Archaeal Hef protein in processing stalled replication forks. J. Biol. Chem. 2004, 279, 53175–53185. [Google Scholar] [CrossRef]
- Lestini, R.; Delpech, F.; Myllykallio, H. DNA replication restart and cellular dynamics of Hef helicase/nuclease protein in Haloferax volcanii. Biochimie 2015, 118, 254–263. [Google Scholar] [CrossRef]
- Meetei, A.R.; Medhurst, A.L.; Ling, C.; Xue, Y.; Singh, T.R.; Bier, P.; Steltenpool, J.; Stone, S.; Dokal, I.; Mathew, C.G.; et al. A human ortholog of archaeal DNA repair protein Hef is defective in Fanconi anemia complementation group M. Nat. Genet. 2005, 37, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Kalb, R.; Neveling, K.; Nanda, I.; Schindler, D.; Hoehn, H. Fanconi anemia: Causes and consequences of genetic instability. Genome Dyn. 2006, 1, 218–242. [Google Scholar] [CrossRef]
- Rohleder, F.; Huang, J.; Xue, Y.; Kuper, J.; Round, A.; Seidman, M.; Wang, W.; Kisker, C. FANCM interacts with PCNA to promote replication traverse of DNA interstrand crosslinks. Nucleic Acids Res. 2016, 44, 3219–3232. [Google Scholar] [CrossRef]
- Lestini, R.; Duan, Z.; Allers, T. The archaeal Xpf/Mus81/FANCM homolog Hef and the Holliday junction resolvase Hjc define alternative pathways that are essential for cell viability in Haloferax volcanii. DNA Repair 2010, 9, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Zhang, L.; Wu, M.; Jiang, D.; Li, Z.; Yang, Z. Repair of Hypoxanthine in DNA Revealed by DNA Glycosylases and Endonucleases From Hyperthermophilic Archaea. Front. Microbiol. 2021, 12, 736915. [Google Scholar] [CrossRef] [PubMed]
- Cebrián-Sastre, E.; Martín-Blecua, I.; Gullón, S.; Blázquez, J.; Castañeda-García, A. Control of Genome Stability by EndoMS/NucS-Mediated Non-Canonical Mismatch Repair. Cells 2021, 10, 1314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, D.; Wu, M.; Yang, Z.; Oger, P.M. New Insights Into DNA Repair Revealed by NucS Endonucleases from Hyperthermophilic Archaea. Front. Microbiol. 2020, 11, 1263. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Huang, Q.; Ni, J.; Xiao, Y.; Yang, Y.; Shen, Y. Functional Analysis of the NucS/EndoMS of the Hyperthermophilic Archaeon Sulfolobus islandicus REY15A. Front. Microbiol. 2020, 11, 607431. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Nei, M.; Ma, H. The origins and early evolution of DNA mismatch repair genes--multiple horizontal gene transfers and co-evolution. Nucleic Acids Res. 2007, 35, 7591–7603. [Google Scholar] [CrossRef] [PubMed]
- Hofstatter, P.G.; Lahr, D.J.G. Complex Evolution of the Mismatch Repair System in Eukaryotes is Illuminated by Novel Archaeal Genomes. J. Mol. Evol. 2021, 89, 12–18. [Google Scholar] [CrossRef]
- Busch, C.R.; DiRuggiero, J. MutS and MutL are dispensable for maintenance of the genomic mutation rate in the halophilic archaeon Halobacterium salinarum NRC-1. PLoS ONE 2010, 5, e9045. [Google Scholar] [CrossRef] [PubMed]
- Ohshita, K.; Fukui, K.; Sato, M.; Morisawa, T.; Hakumai, Y.; Morono, Y.; Inagaki, F.; Yano, T.; Ashiuchi, M.; Wakamatsu, T. Archaeal MutS5 tightly binds to Holliday junction similarly to eukaryotic MutSγ. FEBS J. 2017, 284, 3470–3483. [Google Scholar] [CrossRef]
- Abdullah, M.F.F.; Hoffmann, E.R.; Cotton, V.E.; Borts, R.H. A role for the MutL homologue MLH2 in controlling heteroduplex formation and in regulating between two different crossover pathways in budding yeast. Cytogenet. Genome Res. 2004, 107, 180–190. [Google Scholar] [CrossRef]
- Alani, E.; Reenan, R.A.; Kolodner, R.D. Interaction between mismatch repair and genetic recombination in Saccharomyces cerevisiae. Genetics 1994, 137, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Hunter, N.; Borts, R.H. Mlh1 is unique among mismatch repair proteins in its ability to promote crossing-over during meiosis. Genes Dev. 1997, 11, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Prakash, L. Nucleotide excision repair in yeast. Mutat. Res. 2000, 451, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Soppa, J. Prokaryotic structural maintenance of chromosomes (SMC) proteins: Distribution, phylogeny, and comparison with MukBs and additional prokaryotic and eukaryotic coiled-coil proteins. Gene 2001, 278, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Hoencamp, C.; Rowland, B.D. Genome control by SMC complexes. Nat. Rev. Mol. Cell Biol. 2023, 24, 633–650. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, M.; Inagaki, Y. Ubiquity and Origins of Structural Maintenance of Chromosomes (SMC) Proteins in Eukaryotes. Genome Biol. Evol. 2021, 13, evab256. [Google Scholar] [CrossRef] [PubMed]
- Ruepp, A.; Wanner, G.; Soppa, J. A 71-kDa protein from Halobacterium salinarium belongs to a ubiquitous P-loop ATPase superfamily with head-rod-tail structure. Arch. Microbiol. 1998, 169, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, M.; Nakayama, T.; Inagaki, Y. A novel structural maintenance of chromosomes (SMC)-related protein family specific to Archaea. Front. Microbiol. 2022, 13, 913088. [Google Scholar] [CrossRef]
- Schramm, F.; Borst, A.; Linne, U.; Soppa, J. Elucidation of the Translation Initiation Factor Interaction Network of Haloferax volcanii Reveals Coupling of Transcription and Translation in Haloarchaea. Front. Microbiol. 2021, 12, 742806. [Google Scholar] [CrossRef]


| Protein | Gene | Annotated Function |
|---|---|---|
| HVO_ | ||
| Dhfr | ∆1279 | dihydrofolate reductase (control) |
| Hjc | ∆0170 | holliday junction resolvase |
| Hef | ∆3010 | ATP-dep. Helicase/nuclease |
| Hen | ∆0418 | homing endonuclease |
| NucS | ∆0486 | endonuclease |
| (RadA) 1 | (0104) | DNA repair + recombination protein |
| (RadB) 1 | (2383) | DNA repair + recombination protein |
| Mre11-Rad50 | ∆0853/4 | double strand break repair protein |
| Rad3a | ∆1351 | DNA repair helicase |
| Rad3b | ∆0039 | DNA repair helicase |
| Rad25a | ∆A0441 | DNA repair helicase |
| Rad25b | ∆1736 | DNA repair helicase |
| Rad25c | ∆1598 | DNA repair helicase |
| Rad25d | ∆1723 | DNA repair helicase |
| MutLa | ∆1939 | DNA mismatch repair protein |
| MutLb | ∆0551 | DNA mismatch repair protein |
| MutS1a | ∆1940 | DNA mismatch repair protein |
| MutS1b | ∆0552 | DNA mismatch repair protein |
| MutS5a | ∆0191 | DNA mismatch repair protein |
| MutS5b | ∆1354 | DNA mismatch repair protein |
| Smc | ∆0689 | chromosome segregation protein |
| Sph1 | ∆A0180 | SMC-like protein |
| Sph2 | ∆B0118 | SMC-like protein |
| Sph3 | ∆2175 | SMC-like protein |
| Sph4 | ∆B0173 | SMC-like protein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özer, H.; Wasser, D.; Sandner, L.; Soppa, J. Intermolecular Gene Conversion for the Equalization of Genome Copies in the Polyploid Haloarchaeon Haloferax volcanii: Identification of Important Proteins. Genes 2024, 15, 861. https://doi.org/10.3390/genes15070861
Özer H, Wasser D, Sandner L, Soppa J. Intermolecular Gene Conversion for the Equalization of Genome Copies in the Polyploid Haloarchaeon Haloferax volcanii: Identification of Important Proteins. Genes. 2024; 15(7):861. https://doi.org/10.3390/genes15070861
Chicago/Turabian StyleÖzer, Hanna, Daniel Wasser, Lara Sandner, and Jörg Soppa. 2024. "Intermolecular Gene Conversion for the Equalization of Genome Copies in the Polyploid Haloarchaeon Haloferax volcanii: Identification of Important Proteins" Genes 15, no. 7: 861. https://doi.org/10.3390/genes15070861
APA StyleÖzer, H., Wasser, D., Sandner, L., & Soppa, J. (2024). Intermolecular Gene Conversion for the Equalization of Genome Copies in the Polyploid Haloarchaeon Haloferax volcanii: Identification of Important Proteins. Genes, 15(7), 861. https://doi.org/10.3390/genes15070861






