Screening of CIMMYT and South Asian Bread Wheat Germplasm Reveals Marker–Trait Associations for Seedling Resistance to Septoria Nodorum Blotch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Disease Screening
2.3. Statistical Analysis
2.4. Genotyping
2.5. Population Structure and Principal Coordinate Analysis
2.6. Kinship and Linkage Disequilibrium Analysis
2.7. GWAS Analysis for SNB
3. Results
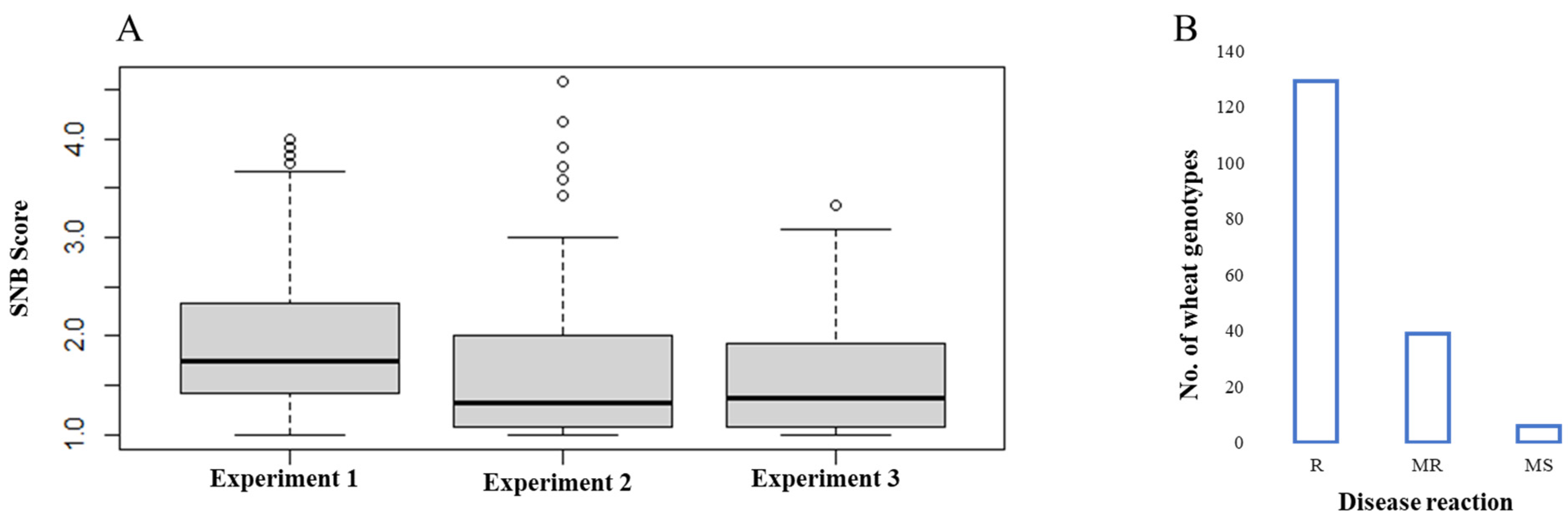
3.1. Phenotypic Evaluation
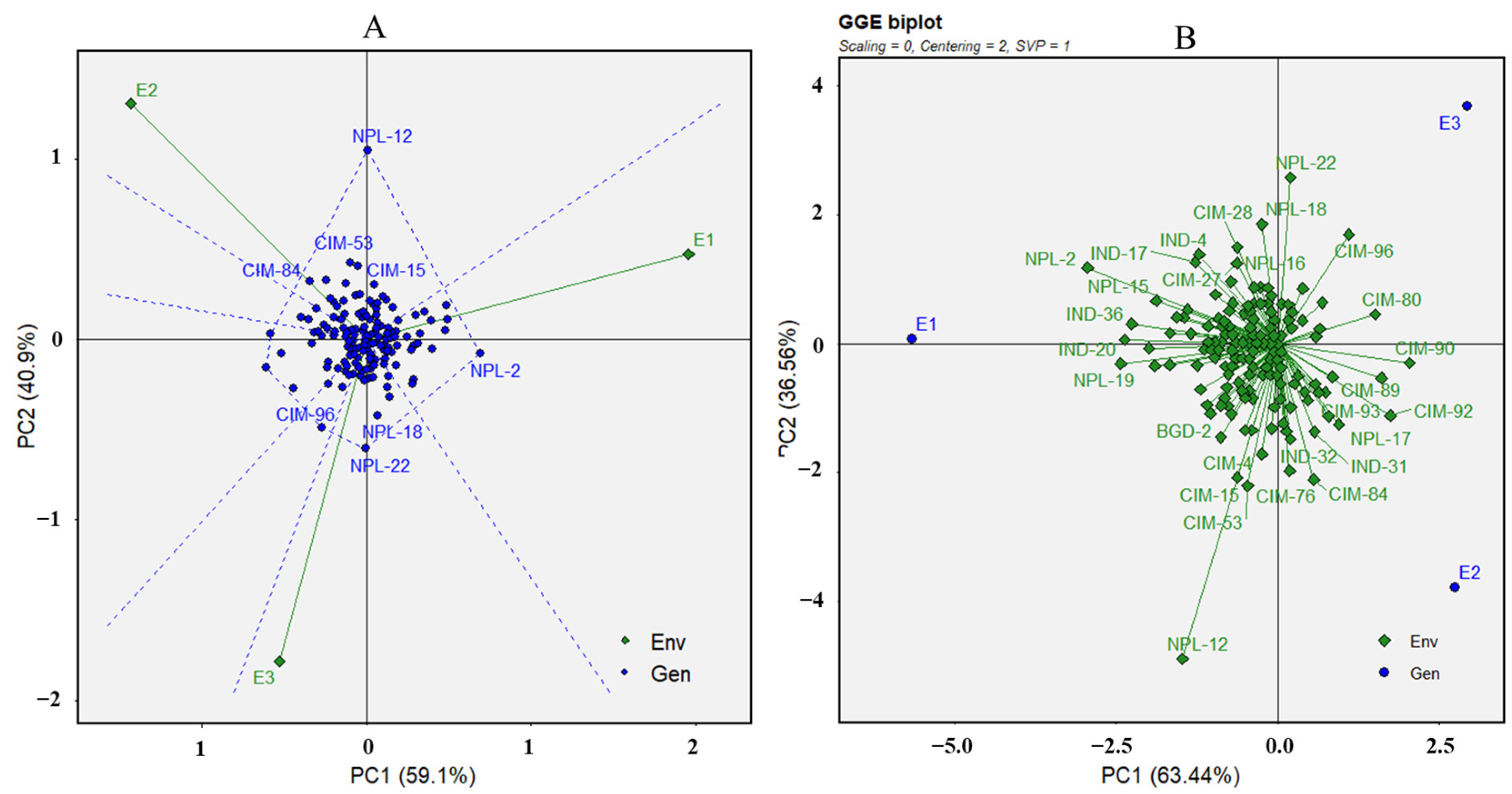
3.2. Population Structure and Principal Coordinate Analysis
3.3. Kinship and Linkage Disequilibrium Analysis
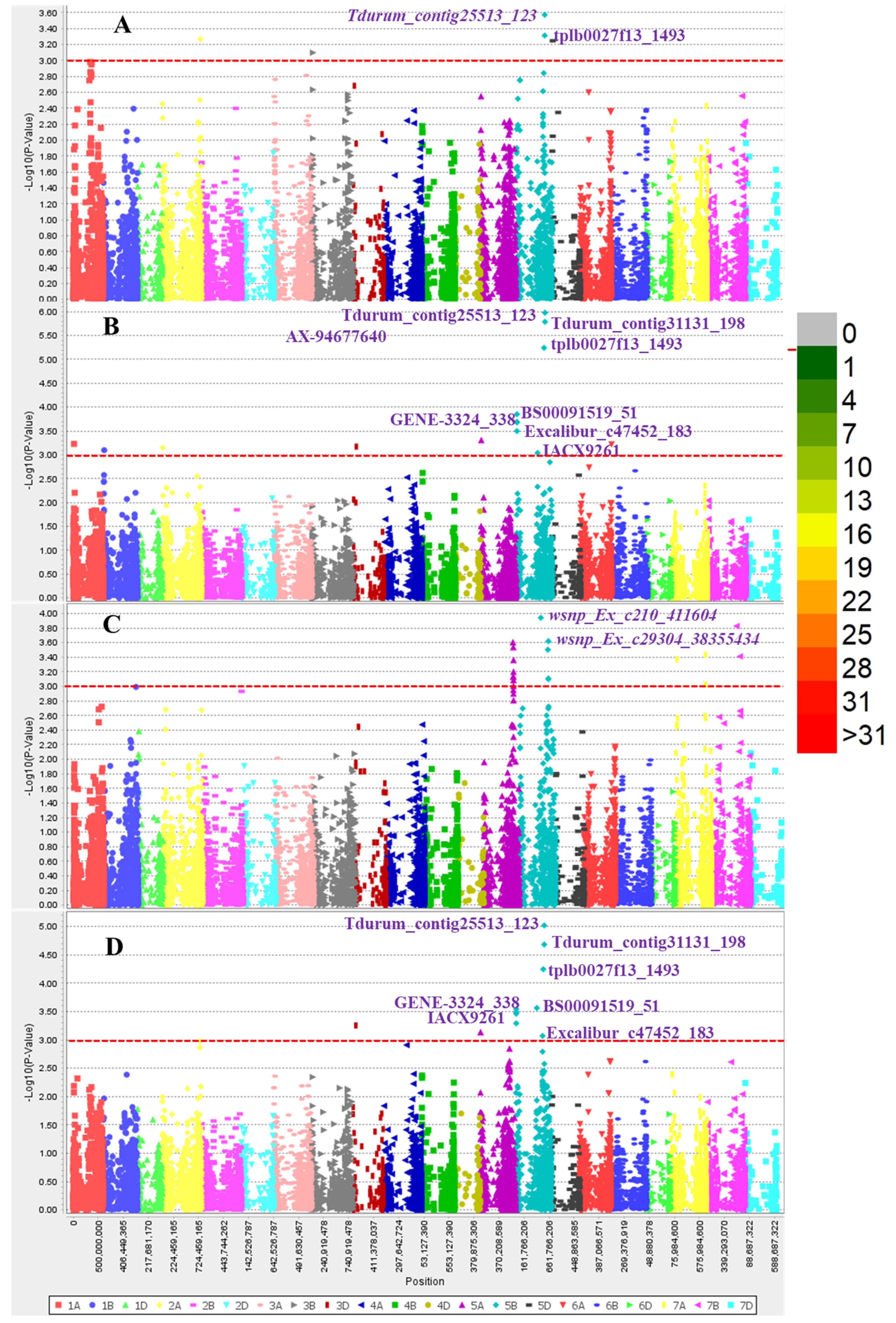
3.4. Marker Trait Association (MTA) for SNB
3.5. Candidate Genes for the Significant MTAs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| FarmCPU | Fixed and random model Circulating Probability Unification |
| GWAS | Genome-Wide Association Study |
| GLM | General Linear Model |
| MLM | Multiple Linear Model |
| MTA | Marker Traits Association |
| QTL | Quantitative Trait Loci |
| rMVP | Memory-Efficient, Visualize-Enhanced, Parallel-Accelerated GWAS Tool |
| SNB | Septoria Nodorum Blotch |
| SNP | Single Nucleotide Polymorphism |
References
- Ruud, A.K.; Lillemo, M. Diseases affecting wheat: Septoria nodorum blotch. In Burleigh Dodds Series in Agricultural Science; Burleigh Dodds Science Publishing Limited: Cambridge, UK, 2018; pp. 109–144. [Google Scholar] [CrossRef]
- Bhathal, J.S.; Loughman, R.; Speijers, J. Yield reduction in wheat in relation to leaf disease from yellow (tan) spot and Septoria nodorum blotch. Eur. J. Plant Pathol. 2003, 109, 435–443. [Google Scholar] [CrossRef]
- Juliana, P.; Singh, R.P.; Singh, P.K.; Crossa, J.; Rutkoski, J.E.; Poland, J.A.; Bergstrom, G.C.; Sorrells, M.E. Comparison of models and whole-genome profiling approaches for genomic-enabled prediction of Septoria tritici blotch, Stagonospora nodorum blotch, and tan spot resistance in wheat. Plant Genome 2017, 10, plantgenome2016-08. [Google Scholar] [CrossRef] [PubMed]
- Katoch, S.; Rana, S.K.; Sharma, P.N. Application of PCR based diagnostics in the exploration of Parastagonospora nodorum prevalence in wheat growing regions of Himachal Pradesh. J. Plant Biochem. Biotechnol. 2019, 28, 169–175. [Google Scholar] [CrossRef]
- Navathe, S.; He, X.; Kamble, U.; Kumar, M.; Patial, M.; Singh, G.; Singh, G.P.; Joshi, A.K.; Singh, P.K. Assessment of Indian wheat germplasm for Septoria nodorum blotch and tan spot reveals new QTLs conferring resistance along with recessive alleles of Tsn1 and Snn3. Front. Plant Sci. 2023, 14, 1223959. [Google Scholar] [CrossRef] [PubMed]
- Ficke, A.; Cowger, G.C.; Bergstrom, G.C.; Guro, B. Understanding yield loss and pathogen biology to improve disease management: Septoria Nodorum Blotch—A case study in wheat. Plant Dis. 2017, 102, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Blixt, E.; Olson, Å.; Lindahl, B.; Djurle, A.; Yuen, J. Spatiotemporal variation in the fungal community associated with wheat leaves showing symptoms similar to Stagonospora nodorum blotch. Eur. J. Plant Pathol. 2010, 126, 373–386. [Google Scholar] [CrossRef]
- Friesen, T.L.; Holmes, D.J.; Bowden, R.L.; Faris, J.D. ToxA is present in the U.S. Bipolaris sorokiniana population and is a significant virulence factor on wheat harboring Tsn1. Plant Dis. 2018, 102, 2446–2452. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.C.; Ahren, D.; Simpfendorfer, S.; Milgate, A.; Solomon, P.S. The discovery of the virulence gene ToxA in the wheat and barley pathogen Bipolaris sorokiniana. Mol. Plant Pathol. 2018, 19, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Peters Haugrud, A.R.; Zhang, Z.; Richards, J.K.; Friesen, T.L.; Faris, J.D. Genetics of variable disease expression conferred by inverse gene-for-gene interactions in the wheat-Parastagonospora nodorum pathosystem. Plant Physiol. 2019, 180, 420–434. [Google Scholar] [CrossRef]
- Murphy, N.E.; Loughman, R.; Appels, R.; Lagudah, E.S.; Jones, M.G.K. Genetic variability in a collection of Stagonospora nodorum isolates from Western Australia. Aust. J. Agric. Res. 2000, 51, 679–684. [Google Scholar] [CrossRef]
- McDonald, B.A.; Linde, C. Pathogen population genetics, evolutionary potential, and durable resistance. Annu. Rev. Phytopathol. 2002, 40, 349–379 . [Google Scholar] [CrossRef] [PubMed]
- Stukenbrock, E.H.; Banke, S.; McDonald, B.A. Global migration patterns in the fungal wheat pathogen Phaeosphaeria nodorum. Mol. Ecol. 2006, 15, 2895–2904. [Google Scholar] [CrossRef]
- McDonald, M.C.; Razavi, M.; Friesen, T.L.; Brunner, P.C.; McDonald, B.A. Phylogenetic and population genetic analyses of Phaeosphaeria nodorum and its pathosystem parallels that of the wheat–tan spot system. Genome 2012, 49, 1265–1273. [Google Scholar] [CrossRef]
- Bostwick, D.E.; Ohm, H.W.; Shaner, G. Inheritance of Septoria-glume blotch resistance in wheat. Crop Sci. 1993, 33, 439–443. [Google Scholar] [CrossRef]
- Wicki, W.; Winzeler, M.; Schmid, J.E.; Stamp, P.; Messmer, M. Inheritance of resistance to leaf and glume blotch caused by Septoria nodorum Berk. in winter wheat. Theor. Appl. Genet. 1999, 99, 1265–1272. [Google Scholar] [CrossRef]
- Adhikari, T.B.; Jackson, E.W.; Gurung, S.; Hansen, J.M.; Bonman, J.M. Association mapping of quantitative resistance to Phaeosphaeria nodorum in spring wheat landraces from the USDA National Small Grains Collection. Phytopathology 2011, 101, 1301–1310. [Google Scholar] [CrossRef]
- Gurung, S.; Mamidi, S.; Bonman, J.M.; Xiong, M.; Brown-Guedira, G.; Adhikari, T.B. Genome-wide association study reveals novel quantitative trait loci associated with resistance to multiple leaf spot diseases of spring wheat. PLoS ONE 2014, 9, e108179. [Google Scholar] [CrossRef]
- Jighly, A.; Alagu, M.; Makdis, F.; Singh, M.; Singh, S.; Emebiri, L.C.; Ogbonnaya, F.C. Genomic regions conferring resistance to multiple fungal pathogens in synthetic hexaploid wheat. Mol. Breed. 2016, 36, 1–9. [Google Scholar] [CrossRef]
- Liu, Z.; El-Basyoni, I.; Kariyawasam, G.; Zhang, G.; Fritz, A.; Hansen, J.; Marais, F.; Friskop, A.; Chao, S.; Akhunov, E.; et al. Evaluation and association mapping of resistance to tan spot and Stagonospora nodorum blotch in adapted winter wheat germplasm. Plant Dis. 2015, 99, 1333–1341. [Google Scholar] [CrossRef]
- Ruud, A.K.; Dieseth, J.A.; Ficke, A.; Furuki, E.; Phan, H.T.T.; Oliver, R.P.; Tan, K.C.; Lillemo, M. Genome-wide association mapping of resistance to Septoria nodorum leaf blotch in a Nordic spring wheat collection. Plant Genome 2019, 12, 180105. [Google Scholar] [CrossRef]
- Breseghello, F.; Sorrells, M.E. Association analysis as a strategy for improvement of quantitative traits in plants. Crop Sci. 2006, 46, 1323–1330. [Google Scholar] [CrossRef]
- Gupta, P.K.; Kulwal, P.L.; Jaiswal, V. Association mapping in crop plants: Opportunities and challenges. Adv. Genet. 2014, 85, 109–147. [Google Scholar] [PubMed]
- Cockram, J.; Scuderi, A.; Barber, T.; Furuki, E.; Gardner, K.A.; Gosman, N.; Kowalczyk, R.; Phan, H.P.; Rose, G.A.; Tan, K.C.; et al. Fine-mapping the wheat Snn1 locus conferring sensitivity to the Parastagonospora nodorum necrotrophic effector SnTox1 using an eight founder multiparent advanced generation inter-cross population. G3 (Bethesda) 2015, 5, 2257–2266. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, R. Bandwagons I, too, have known. Theor. Appl. Genet. 2016, 129, 2323–2332. [Google Scholar] [CrossRef] [PubMed]
- Downie, R.C.; Bouvet, L.; Furuki, E.; Gosman, N.; Gardner, K.A.; Mackay, I.J.; Campos Mantello, C.; Mellers, G.; Phan, H.T.; Rose, G.A.; et al. Assessing European wheat sensitivities to Parastagonospora nodorum necrotrophic effectors and fine-mapping the Snn3-B1 locus conferring sensitivity to the effector SnTox3. Front. Plant Sci. 2018, 9, 881. [Google Scholar] [CrossRef]
- Lin, M.; Corsi, B.; Ficke, A.; Tan, K.C.; Cockram, J.; Lillemo, M. Genetic mapping using a wheat multi-founder population reveals a locus on chromosome 2A controlling resistance to both leaf and glume blotch caused by the necrotrophic fungal pathogen Parastagonospora nodorum. Theor. Appl. Genet. 2020, 133, 785–808. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Stadlmeier, M.; Mohler, V.; Tan, K.C.; Ficke, A.; Cockram, J.; Lillemo, M. Identification and cross-validation of genetic loci conferring resistance to Septoria nodorum blotch using a German multi-founder winter wheat population. Theor. Appl. Genet. 2021, 134, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Tommasini, L.; Schnurbusch, T.; Fossati, D.; Mascher, F.; Keller, B. Association mapping of Stagonospora nodorum blotch resistance in modern European winter wheat varieties. Theor. Appl. Genet. 2007, 115, 697–708. [Google Scholar] [CrossRef]
- Phan, H.T.; Rybak, K.; Furuki, E.; Breen, S.; Solomon, P.S.; Oliver, R.P.; Tan, K.C. Differential effector gene expression underpins epistasis in a plant fungal disease. Plant J. 2016, 87, 343–354. [Google Scholar] [CrossRef]
- Halder, J.; Zhang, J.; Ali, S.; Sidhu, J.S.; Gill, H.S.; Talukder, S.K.; Kleinjan, J.; Turnipseed, B.; Sehgal, S.K. Mining and genomic characterization of resistance to tan spot, Stagonospora nodorum blotch (SNB), and Fusarium head blight in Watkins core collection of wheat landraces. BMC Plant Biol. 2019, 19, 480. [Google Scholar] [CrossRef]
- Hu, W.; He, X.; Dreisigacker, S.; Sansaloni, C.P.; Juliana, P.; Singh, P.K. A wheat chromosome 5AL region confers seedling resistance to both tan spot and Septoria nodorum blotch in two mapping populations. Crop J. 2019, 7, 809–818. [Google Scholar] [CrossRef]
- Feng, J.; Ma, H.; Hughes, G.R. Genetics of resistance to Stagonospora nodorum blotch of hexaploid wheat. Crop Sci. 2004, 44, 2043–2048. [Google Scholar] [CrossRef]
- Singh, P.K.; Mergoum, M.; Adhikari, T.B.; Kianian, S.F.; Elias, E.M. Chromosomal location of genes for seedling resistance to tan spot and Stagonospora nodorum blotch in tetraploid wheat. Euphytica 2007, 155, 27–34. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, S.; Kharub, A.S.; Verma, R.P.S.; Singh, G.P. GGE Biplot Model for Environmental Delineation and Identification of Resistant Sources for Stripe Rust and Spot Blotch in India; Rabat, Morocco. 2017. Avaliable online: https://repo.mel.cgiar.org/handle/20.500.11766/8026 (accessed on 31 December 2023).
- Chattopadhyay, N.; Mandal, R.; Roy, A.; Bhattacharya, P.M.; Chowdhury, A.K. Correction to: Assessment of wheat genotypes based on genotype-by-environment interaction for durable resistance to spot blotch disease in hot spot. Cereal Res. Commun. 2022, 50, 103. [Google Scholar] [CrossRef]
- Wang, S.; Wong, D.; Forrest, K.; Allen, A.; Chao, S.; Huang, B.E.; Maccaferri, M.; Salvi, S.; Milner, S.G.; Cattivelli, L.; et al. Characterization of polyploid wheat genomic diversity using a high-density 90 000 single nucleotide polymorphism array. Plant Biotechnol. J. 2014, 12, 787–796. [Google Scholar] [CrossRef] [PubMed]
- AlTameemi, R.; Gill, H.S.; Ali, S.; Ayana, G.; Halder, J.; Sidhu, J.S.; Gill, U.S.; Turnipseed, B.; Hernandez, J.L.G.; Sehgal, S.K. Genome-wide association analysis permits characterization of Stagonospora nodorum blotch (SNB) resistance in hard winter wheat. Sci. Rep. 2021, 11, 12570. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Earl, D.A.; Vonholdt, B. Structure Harvester: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Ramasamy, R.K.; Ramasamy, S.; Bindroo, B.B.; Naik, V.G. STRUCTURE PLOT: A program for drawing elegant STRUCTURE bar plots in user friendly interface. Springerplus 2014, 3, 1–3. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Bradbury, P.; Zhang, Z.; Kroon, D.; Casstevens, T.; Ramdoss, Y.; Buckler, E. TASSEL: Software for association mapping of complex traits in diverse samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Zhang, H.; Tang, Z.; Xu, J.; Yin, D.; Zhang, Z.; Yuan, X.; Zhu, M.; Zhao, S.; Li, X.; et al. rMVP: A Memory-efficient, visualization-enhanced, and parallel-accelerated tool for genome-wide association study. Genom. Proteom. Bioinform. 2021, 19, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Ficke, A.; Dieseth, J.A.; Kim, M.O.; Lillemo, M. Bladsjukdommer i norsk hvete. Forekomst, betydning of tiltak. In Jord- og Plantekultur 2018/NIBIO BOK; Norwegian Institute of Bioeconomy Research: Ås, Norway, 2018; pp. 108–112. [Google Scholar]
- Thapa, R.; Brown-Guedira, G.; Ohm, H.W.; Mateos-Hernandez, M.; Wise, K.A.; Goodwin, S.B. Determining the order of resistance genes against Stagonospora nodorum blotch, Fusarium head blight and stem rust on wheat chromosome arm 3BS. BMC Res Notes 2016, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Phuke, R.M.; He, X.; Juliana, P.; Bishnoi, S.K.; Singh, G.P.; Kabir, M.R.; Roy, K.K.; Joshi, A.K.; Singh, R.P.; Singh, P.K. Association mapping of seedling resistance to tan spot (Pyrenophora tritici-repentis race 1) in CIMMYT and South Asian wheat germplasm. Front Plant Sci. 2020, 11, 1309. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Duveiller, E.; Singh, R.P. Evaluation of CIMMYT germplasm for resistance to leaf spotting diseases of wheat Czech. J. Genet. Plant Breed. 2011, 47, S102–S108. [Google Scholar] [CrossRef]
- Ortiz, R.; Sayre, K.D.; Govaerts, B.; Gupta, R.; Subbarao, G.V.; Ban, T.; Hodson, D.; Dixon, J.M.; Ortiz-Monasterio, J.I.; Reynolds, M. Climate change: Can wheat beat the heat? Agric. Ecosyst. Environ. 2008, 126, 46–58. [Google Scholar] [CrossRef]
- Chao, S.; Dubcovsky, J.; Dvorak, J.; Luo, M.-C.; Baenziger, S.P.; Matnyazov, R.; Clark, D.R.; Talbert, L.E.; Anderson, J.A.; Dreisigacker, S.; et al. Population- and genome-specific patterns of linkage disequilibrium and SNP variation in spring and winter wheat (Triticum aestivum L.). BMC Genom. 2010, 11, 727. [Google Scholar] [CrossRef] [PubMed]
- Bajgain, P.; Rouse, M.N.; Bulli, P.; Bhavani, S.; Gordon, T.; Wanyera, R.; Njau, P.N.; Legesse, W.; Anderson, J.A.; Pumphrey, M.O. Association mapping of North American spring wheat breeding germplasm reveals loci conferring resistance to Ug99 and other African stem rust races. BMC Plant Biol. 2015, 15, 249. [Google Scholar] [CrossRef]
- Francki, M.G.; Walker, E.; McMullan, C.J.; Morris, W.G. Evaluation of Septoria nodorum blotch (SNB) resistance in glumes of wheat (Triticum aestivum L.) and the genetic relationship with foliar disease response. Front Genet. 2021, 12, 681768. [Google Scholar] [CrossRef]
- Navathe, S.; Yadav, P.S.; Chand, R.; Mishra, V.K.; Vasistha, N.K.; Meher, P.K.; Joshi, A.K.; Gupta, P.K. ToxA-Tsn1 interaction for spot blotch susceptibility in Indian wheat: An example of inverse gene-for-gene relationship. Plant Dis. 2020, 104, 71–81. [Google Scholar] [CrossRef]
- Singh, R.P.; Singh, P.K.; Rutkoski, J.; Hodson, D.P.; He, X.; Jørgensen, L.N.; Hovmøller, M.S.; Huerta-Espino, J. Disease impact on wheat yield potential and prospects of genetic control. Annu. Rev. Phytopathol. 2016, 54, 303–322. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.; He, X.; Gahtyari, N.C.; Mahapatra, S.; Singh, P.K. Managing spot blotch disease in wheat: Conventional to molecular aspects. Front Plant Sci. 2023, 14, 1098648. [Google Scholar] [CrossRef] [PubMed]


| Source | Df | MS | p-Value |
|---|---|---|---|
| Experiment | 2 | 18.38816 | p < 0.001 |
| Replication | 6 | 0.128591 | p > 0.05 |
| Genotype | 173 | 3.042647 | p < 0.001 |
| Genotype × Experiment | 346 | 0.553166 | p < 0.001 |
| Residual | 1038 | 0.092841 |
| Exp. | Marker | Chr | Position | p-Value | R2 (%) |
|---|---|---|---|---|---|
| Exp2 | AX-94677640 | 5B | 6,838,168 | 2 × 10−4 | 8.96 |
| Mean | AX-94677640 | 5B | 6,838,168 | 3 × 10−4 | 8.48 |
| Exp2 | AX-94864577 | 5B | 6,974,754 | 1 × 10−4 | 9.68 |
| Mean | AX-94864577 | 5B | 6,974,754 | 3 × 10−4 | 8.63 |
| Mean | AX-94978939 | 3D | 52,806,848 | 6 × 10−4 | 7.65 |
| Exp2 | AX-94978939 | 3D | 52,806,848 | 7 × 10−4 | 7.35 |
| Exp2 | BS00091519_51 | 5B | 6,648,517 | 2 × 10−4 | 8.80 |
| Mean | BS00091519_51 | 5B | 6,648,517 | 3 × 10−4 | 8.44 |
| Exp2 | Excalibur_c47452_183 | 5B | 6,654,116 | 3 × 10−4 | 8.22 |
| Mean | Excalibur_c47452_183 | 5B | 6,654,116 | 5 × 10−4 | 7.83 |
| Exp2 | GENE-3324_338 | 5B | 3,521,225 | 5 × 10−4 | 7.76 |
| Mean | GENE-3324_338 | 5B | 3,521,225 | 5 × 10−4 | 7.40 |
| Exp2 | IACX9261 | 5B | 546,703,936 | 6 × 10−4 | 13.30 |
| Mean | IACX9261 | 5B | 546,703,936 | 6 × 10−4 | 10.31 |
| Exp2 | Tdurum_contig25513_123 | 5B | 565,753,503 | 2 × 10−6 | 15.23 |
| Mean | Tdurum_contig25513_123 | 5B | 565,753,503 | 1 × 10−5 | 12.93 |
| Exp1 | Tdurum_contig25513_123 | 5B | 565,753,503 | 3 × 10−4 | 8.59 |
| Exp3 | Tdurum_contig31131_198 | 5B | 417,443,545 | 1 × 10−4 | 11.34 |
| Mean | Tdurum_contig31131_198 | 5B | 417,443,545 | 3 × 10−4 | 9.49 |
| Exp2 | Tdurum_contig31131_198 | 5B | 417,443,545 | 9 × 10−4 | 7.86 |
| Exp2 | tplb0027f13_1493 | 5B | 565,754,215 | 1 × 10−6 | 15.67 |
| Mean | tplb0027f13_1493 | 5B | 565,754,215 | 2 × 10−5 | 11.77 |
| Exp1 | tplb0027f13_1493 | 5B | 565,754,215 | 2 × 10−4 | 7.68 |
| Exp3 | wsnp_Ex_c210_411604 | 5A | 584,054,461 | 8 × 10−4 | 7.50 |
| Exp3 | wsnp_Ex_c29304_38355434 | 5A | 584,451,511 | 1 × 10−3 | 7.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandal, R.; He, X.; Singh, G.; Kabir, M.R.; Joshi, A.K.; Singh, P.K. Screening of CIMMYT and South Asian Bread Wheat Germplasm Reveals Marker–Trait Associations for Seedling Resistance to Septoria Nodorum Blotch. Genes 2024, 15, 890. https://doi.org/10.3390/genes15070890
Mandal R, He X, Singh G, Kabir MR, Joshi AK, Singh PK. Screening of CIMMYT and South Asian Bread Wheat Germplasm Reveals Marker–Trait Associations for Seedling Resistance to Septoria Nodorum Blotch. Genes. 2024; 15(7):890. https://doi.org/10.3390/genes15070890
Chicago/Turabian StyleMandal, Rupsanatan, Xinyao He, Gyanendra Singh, Muhammad Rezaul Kabir, Arun Kumar Joshi, and Pawan Kumar Singh. 2024. "Screening of CIMMYT and South Asian Bread Wheat Germplasm Reveals Marker–Trait Associations for Seedling Resistance to Septoria Nodorum Blotch" Genes 15, no. 7: 890. https://doi.org/10.3390/genes15070890





