Comparative Transcriptome Analysis of the Hypothalamic–Pituitary–Gonadal Axis of Jinhu Grouper (Epinephelus fuscoguttatus ♀ × Epinephelus tukula ♂) and Tiger Grouper (Epinephelus fuscoguttatus)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Fish and Sampling
2.3. RNA Extraction, Library Construction, and Sequencing
2.4. Analysis of Differentially Expressed Genes (DEGs)
2.5. Trend Analysis
2.6. Weighted Gene Co-Expression Network Construction
2.7. Experimental Validation with Quantitative Real-Time PCR (qRT-PCR)
3. Results
3.1. Transcriptome Overview
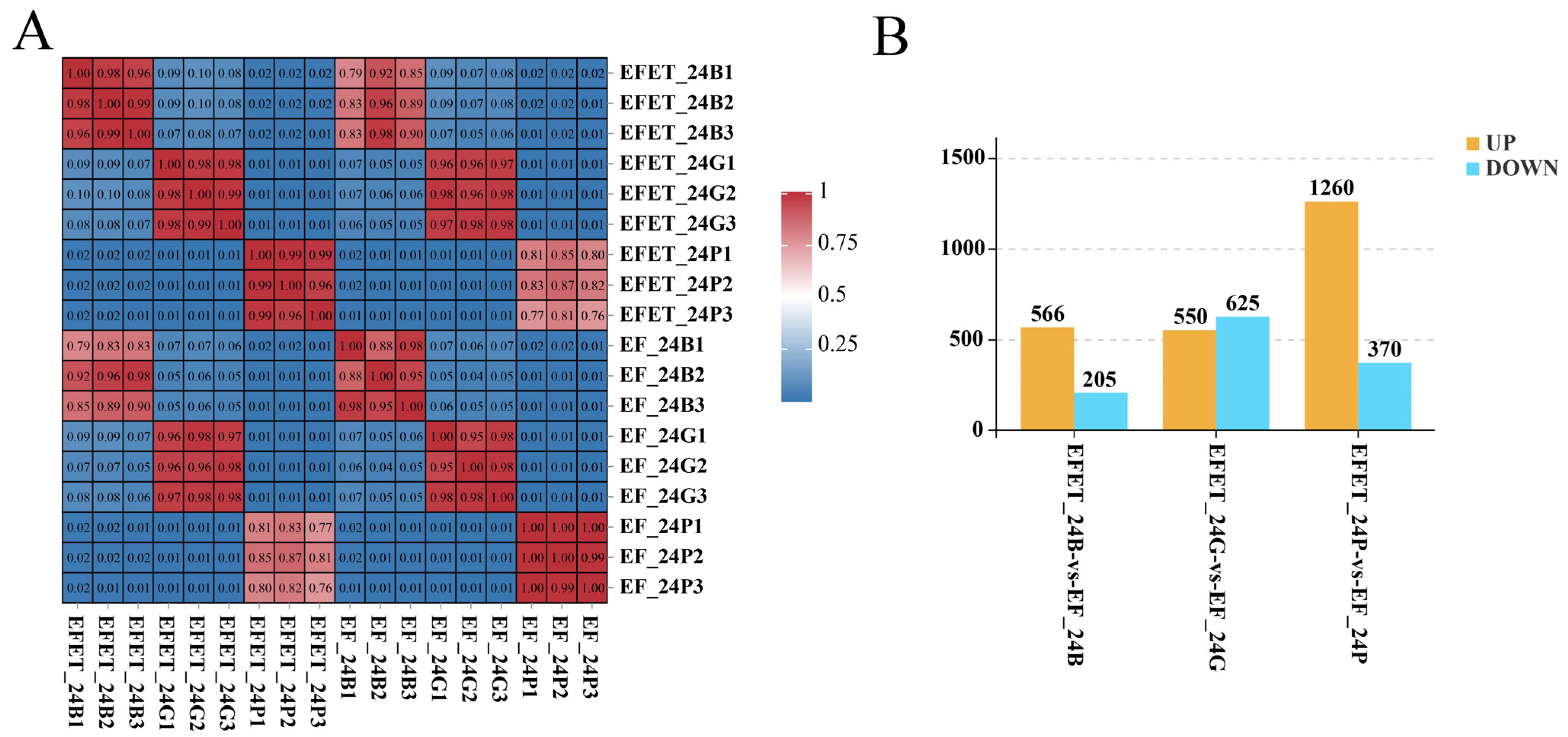
3.2. Sample Correlations and Identification of the DEGs
3.3. Functional Enrichment Analysis of DEGs
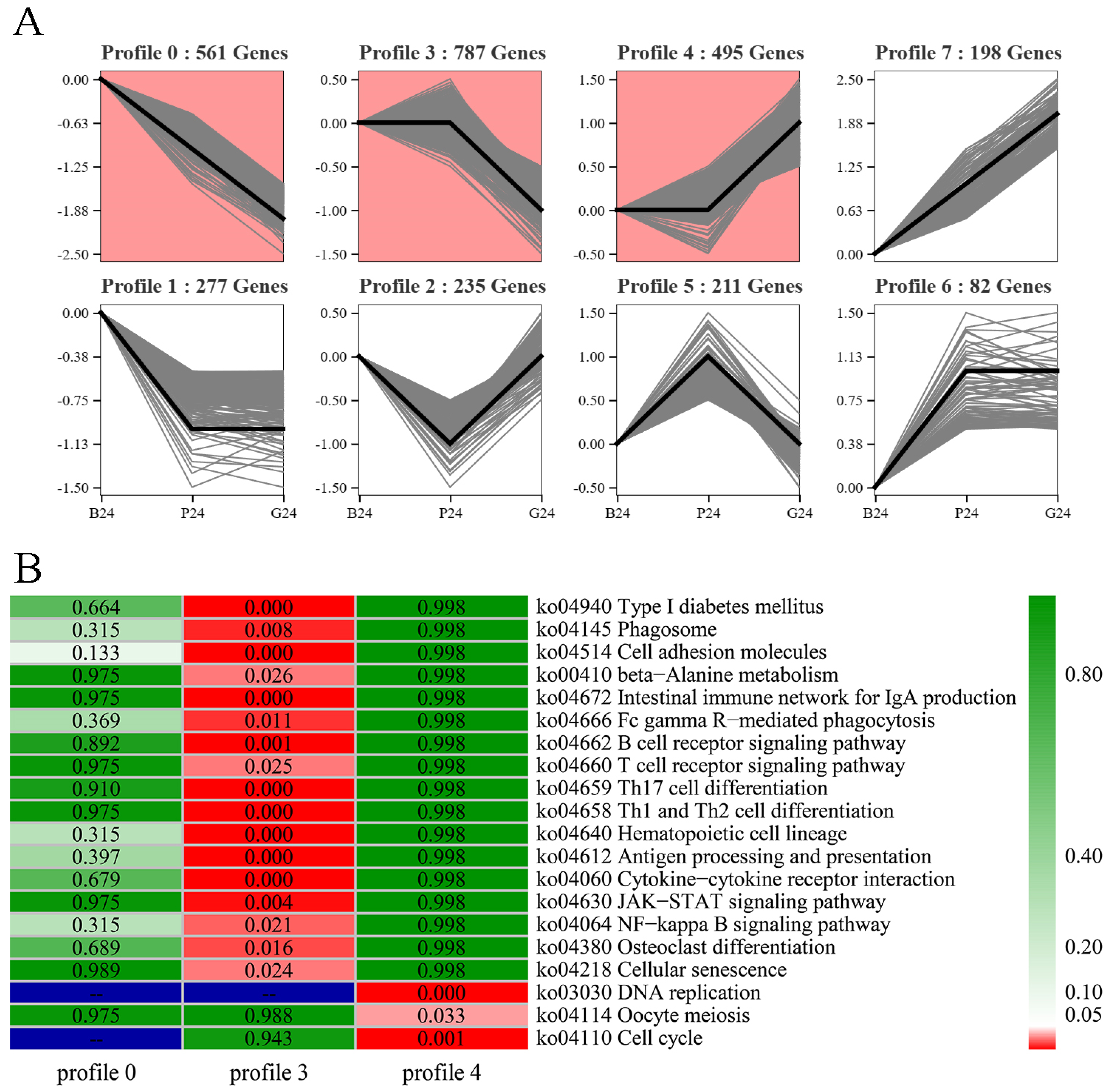
3.4. Trend Analysis of DEGs
3.5. Construction of a Gene Co-Expression Network
3.6. Functional Analysis of Target Modules
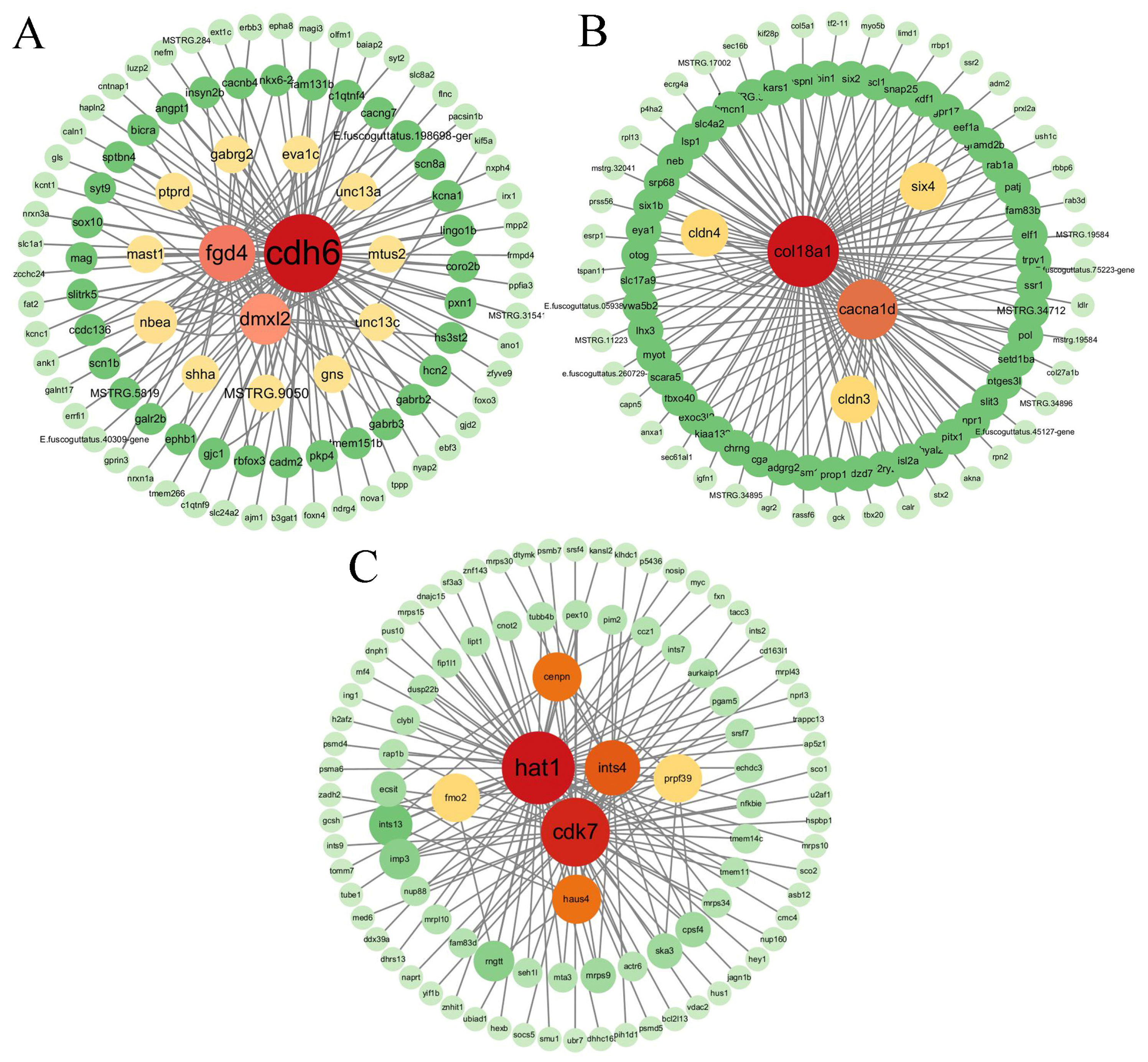
3.7. Network Construction and Gene Identification
3.8. qRT-PCR Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Luo, M.; Li, S.N.; Tao, M.; Ye, X.L.; Duan, W.; Zhang, C.; Qin, Q.B.; Xiao, J.; Liu, S.J. A comparative study of distant hybridization in plants and animals. Sci. China Life Sci. 2017, 61, 285–309. [Google Scholar] [CrossRef]
- Wang, S.; Tang, C.C.; Tao, M.; Qin, Q.B.; Zhang, C.; Luo, K.K.; Zhao, R.R.; Wang, J.; Ren, L.; Xiao, J.; et al. Establishment and application of distant hybridization technology in fish. Sci. China Life Sci. 2019, 62, 22–45. [Google Scholar] [CrossRef]
- Ou, M.; Zhao, J.; Luo, Q.; Hong, X.Y.; Zhu, X.P.; Liu, H.Y.; Chen, K.C. Characteristics of hybrids derived from Channa argus ♀ × Channa maculate ♂. Aquaculture 2018, 492, 349–356. [Google Scholar] [CrossRef]
- Li, M.H.; Robinson, E.H.; Manning, B.B.; Yant, D.R.; Chatakondi, N.G.; Bosworth, B.G.; Wolters, W.R. Comparison of the channel catfish, Ictalurus punctatus (NWAC103 Strain) and the channel × blue catfish, I. punctatus × I. furcatus, F1 hybrid for growth, feed efficiency, processing yield, and body composition. J. Appl. Aquac. 2004, 15, 63–71. [Google Scholar] [CrossRef]
- Pierre, S.; Gaillard, S.; Prévot-D’Alvise, N.; Aubert, J.; Rostaing-Capaillon, O.; Leung-Tack, D.; Grillasca, J.P. Grouper aquaculture: Asian success and Mediterranean trials. Aquat. Conserv. Mar. Freshw. Ecosyst. 2008, 18, 297–308. [Google Scholar] [CrossRef]
- Chen, C.; Kong, X.D.; Li, Y.L.; Song, Z.X.; Jia, R.J.; Yu, H.H.; Zhai, J.M.; Ma, W.H.; Pang, Z.F.; Liu, J.C. Embryonic and morphological development in the larva, juvenile, and young stages of Epinephelus fuscoguttatus (♀) × E. lanceolatus (♂). Prog. Fish. Sci. 2014, 35, 135–144. [Google Scholar] [CrossRef]
- Kiriyakit, A.; Gallardo, W.G.; Bart, A.N. Successful hybridization of groupers (Epinephelus coioides × Epinephelus lanceolatus) using cryopreserved sperm. Aquaculture 2011, 320, 106–112. [Google Scholar] [CrossRef]
- Tian, Y.S.; Chen, Z.F.; Duan, H.M.; Ma, W.H.; Tang, J.; Li, W.S.; Liu, J.C.; Hou, Y.X.; Sun, Z.X.; Pang, Z.F.; et al. The family line establishment ofthe hybrid Epinephelus moara (♀) × E. lanceolatus (♂) by using cryopreserved sperm and the related genetic effect analysis. J. Fish. China 2017, 38, 27–35. [Google Scholar] [CrossRef]
- Sugama, K.; Muzaki, A.; Permana, I.G.N.; Haryanti, H. Fluctuating asymmetry reflect the growth of hybrid grouper E. fuscoguttatus and Epinephelus polyphekadion. Indones. Aquac. J. 2014, 9, 97–102. [Google Scholar] [CrossRef]
- Tian, Y.S.; Tang, J.; Ma, W.H.; Cheng, M.L.; Li, Z.T.; Wu, Y.P.; Wang, X.M. Development and growth of hybrid offspring of brown grouper E. fuscoguttatus (♀) × blue speckled grouper E. tulcula (♂) using cryopreserved sperm. Prog. Fish. Sci. 2019, 40, 36–47. [Google Scholar] [CrossRef]
- Wu, Y.P.; Tian, Y.S.; Li, Z.T.; Zhang, J.J.; Li, Z.Q.; Cheng, M.L.; Wang, L.N.; Ma, W.H.; Pang, Z.F.; Zhai, J.M. Analysis on morphological difference between hybrid Epinephelus fuscoguttatus♀ × E. tukula♂ and its parents. J. Guangdong Ocean Univ. 2019, 39, 17–22. [Google Scholar] [CrossRef]
- Wu, Y.P.; Tian, Y.S.; Li, Z.T.; Zhang, J.J.; Li, L.L.; Wang, L.N.; Zhai, J.M. Karyotype analysis of hybrids of E. fuscoguttatus (♀) × E. tukula (♂). J. Guangdong Ocean Univ. 2021, 41, 119–123. [Google Scholar] [CrossRef]
- Wu, Y.P.; Tian, Y.S.; Wang, L.N.; Li, Z.T.; Zhang, J.J.; Li, L.L.; Li, B. Genetic diversity analysis of E. fuscoguttatus (♀) and E. tukula (♂) hybrids. Prog. Fish. Sci. 2021, 42, 25–32. [Google Scholar] [CrossRef]
- Chen, S.; Tian, Y.S.; Li, Z.T.; Liu, Y.; Li, Z.Q.; Duan, P.F.; Li, L.L.; Wang, X.Y.; Wang, L.N.; He, X.L.; et al. Heterosis in growth and low temperature tolerance in Jinhu grouper (Epinephelus fuscoguttatus ♀ × E. tukula ♂). Aquaculture 2023, 562, 738751. [Google Scholar] [CrossRef]
- Duan, P.F.; Tian, Y.S.; Li, Z.T.; Li, Z.Q.; Chen, S.; Li, L.L.; Wang, L.N.; Liu, Y.; Li, W.S.; Wang, X.M.; et al. Hypoxia tolerance of E. fuscoguttatus (♀) × E. tukula (♂) hybrids and E. fuscoguttatus. J. Fish. Sci. China 2022, 29, 220–233. [Google Scholar] [CrossRef]
- Duan, P.F.; Tian, Y.S.; Li, Z.T.; Chen, S.; Li, L.L.; Wang, X.Y.; Wang, L.N.; Liu, Y.; Zhai, J.M.; Li, W.S.; et al. Comparative transcriptome analysis of hybrid Jinhu grouper (E. fuscoguttatus ♀ × E. tukula ♂) and Epinephelus fuscoguttatus under temperature stress. Aquaculture 2023, 578, 740037. [Google Scholar] [CrossRef]
- Qiu, Y.S.; Ding, X.Y.; Li, Z.T.; Duan, P.F.; Wang, X.Y.; Li, L.L.; Wang, L.N.; Liu, Y.; Ma, W.H.; Pang, Z.F.; et al. Comparative analysis of ovarian development and sex steroid hormone levels in hybrid Jinhu grouper (E. fuscoguttatus ♀ × E. tukula ♂) and E. fuscoguttatus. J. Fish. Sci. China 2023, 30, 457–467. [Google Scholar] [CrossRef]
- Zhao, H.H.; He, Q.; Zhang, C.L.; Wang, Q.; Zhang, Y.; Yang, H.R.; Lin, F.M.; Li, Y.S.; Shi, H.R.; Zhang, H.F. A review on sex differentiation and sex change in hermaphroditic fish. J. Fish. China 2022, 46, 644–656. [Google Scholar] [CrossRef]
- Zhang, M.J.; Ouyang, H.; Xia, G.L. The signal pathway of gonadotrophins-induced mammalian oocyte meiotic resumption. Mol. Hum. Reprod. 2009, 15, 399–409. [Google Scholar] [CrossRef]
- Charni-Natan, M.; Aloni-Grinstein, R.; Osher, E.; Rotter, V. Liver and steroid hormones—Can a touch of p53 make a difference? Front. Endocrinol. 2019, 10, 374. [Google Scholar] [CrossRef]
- Nagahama, Y.; Yamashita, M. Regulation of oocyte maturation in fish. Dev. Growth Differ. 2008, 50, 195–219. [Google Scholar] [CrossRef]
- Luin, M.; Fui, C.F.; Senoo, S. Sexual maturation and gonad development in tiger grouper (Epinephelus fuscoguttatus) × giant grouper (E. lanceolatus) hybrid. J. Aquac. Res. Dev. 2013, 5, 213. [Google Scholar] [CrossRef]
- Li, Z.Q.; Chen, M.L.; Wang, L.N.; Li, Z.T.; Chen, S.; Li, L.L.; Wang, X.M.; Wang, Q.B.; Lin, H.W.; Li, B.; et al. Ploidy and gonadal development of Epinephelus moara♀ and Epinephelus moara♀ × Epinephelus lanceolatus♂. J. Guangdong Ocean Univ. 2021, 41, 134–141. [Google Scholar] [CrossRef]
- Muzaki, A.; Sembiring, S.B.M.; Permana, G.N.; Mahardika, K.; Haryanti. The development of the reproductive organs of the hybrid grouper cantik (Epinephelus fuscoguttatus × E. polyphekadion). IOP Conf. Ser. Earth Environ. Sci. 2021, 890, 012016. [Google Scholar] [CrossRef]
- Li, S.S.; Liu, Q.Y.; Xiao, L.; Tao, M.; Shu, H.; Zhang, H.F.; Lin, H.R.; Zhang, Y. Comparison of gonadal development in diploid and triploid hybrid groupers, Epinephelus coioides♀ × Epinephelus lanceolatus♂. J. World Aquac. Soc. 2018, 49, 328–337. [Google Scholar] [CrossRef]
- Liu, L.L.; Luo, M.; Chen, F.X.; Liu, J.Y. Effects of low salinity on osmoregulation, Na+/K+-ATPase activity and related gene expression of juvenile Epinephelus fuscoguttatus. Mar. Fish. 2022, 44, 315–327. [Google Scholar] [CrossRef]
- Toshiya, N.; Minoru, T. Gonadal Development in Fish. Sex Dev. 2014, 8, 252–261. [Google Scholar] [CrossRef]
- Muñoz-Cueto, J.A.; Zmora, N.; Paullada-Salmerón, J.A.; Marvel, M.; Mañanos, E.; Zohar, Y. The gonadotropin-releasing hormones: Lessons from fish. Gen. Comp. Endocrinol. 2020, 291, 113422. [Google Scholar] [CrossRef]
- Martin, L.J.; Tremblay, J.J. Glucocorticoids antagonize cAMP-induced Star transcription in Leydig cells through the orphan nuclear receptor NR4A1. J. Mol. Endocrinol. 2008, 41, 165–175. [Google Scholar] [CrossRef]
- Park, J.I.; Park, H.J.; Choi, H.S.; Lee, K.; Lee, W.K.; Chun, S.Y. Gonadotropin regulation of NGFI-B messenger ribonucleic acid expression during ovarian follicle development in the rat. Endocrinology 2001, 142, 3051–3059. [Google Scholar] [CrossRef]
- Martin, L.J.; Boucher, N.; Brousseau, C.; Tremblay, J.J. The orphan nuclear receptor NUR77 regulates hormone-induced StAR transcription in Leydig cells through cooperation with Ca2+/calmodulin-dependent protein kinase I. Mol. Endocrinol. 2008, 22, 2021–2037. [Google Scholar] [CrossRef]
- Dai, A.Y.; Yan, G.J.; He, Q.Y.; Jiang, Y.; Zhang, Q.; Fang, T.; Ding, L.J.; Sun, J.X.; Sun, H.X.; Hu, Y.L. Orphan Nuclear Receptor Nur77 Regulates Androgen Receptor Gene Expression in Mouse Ovary. PLoS ONE 2012, 7, e39950. [Google Scholar] [CrossRef]
- Sam, S.; Frohman, A.L. Normal physiology of hypothalamic pituitary regulation. Endocrinol. Metab. Clin. N. Am. 2008, 37, 1–22. [Google Scholar] [CrossRef]
- Das, S.; Majumder, S.; Mukherjee, D. Effect of phenol on ovarian secretion of 17β-estradiol in common carp Cyprinus carpio. Arch. Environ. Contam. Toxicol. 2013, 65, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.N.; Daly, S.C.J.; Pang, Y.F.; Anglade, I.; Kah, O.; Thomas, P.; Zhu, Y. Characterization and expression of the nuclear progestin receptor in zebrafish gonads and brain. Biol. Reprod. 2010, 82, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Graf, M.; Qi-Wen, E.R.T.; Sarusie, M.V.; Rajaei, F.; Winkler, C. Dmrt5 controls corticotrope and gonadotrope differentiation in the zebrafish pituitary. Mol. Endocrinol. 2015, 29, 187–199. [Google Scholar] [CrossRef]
- Makker, A.; Goel, M.M.; Mahdi, A.A. PI3K/PTEN/Akt and TSC/mTOR signaling pathways, ovarian dysfunction, and infertility: An update. J. Endocrinol. 2014, 53, 103–118. [Google Scholar] [CrossRef]
- He, F.X.; Jiang, D.N.; Huang, Y.Q.; Mustapha, U.F.; Yang, W.; Cui, X.F.; Tian, C.X.; Chen, H.P.; Shi, H.J.; Deng, S.P.; et al. Comparative transcriptome analysis of male and female gonads reveals sex-biased genes in spotted scat (Scatophagus argus). Fish Physiol. Biochem. 2019, 45, 1963–1980. [Google Scholar] [CrossRef]
- Zhong, J.; Ma, L.Y.; Wang, W.M. Phenotypic traits and gonadal development of hybrids between Misgurnus anguillicaudatus and Paramisgurnus dabryanus. Aquaculture 2020, 523, 735129. [Google Scholar] [CrossRef]
- Li, G.L.; Liu, X.C.; Zhang, Y.; Lin, H.R. Gonadal development, aromatase activity and P450 aromatase gene expression during sex inversion of protogynous red-spotted grouper Epinephelus akaara (Temminck and Schlegel) after implantation of the aromatase inhibitor, fadrozole. Aquac. Res. 2006, 37, 484–491. [Google Scholar] [CrossRef]
- Wong, T.T.; Ijiri, S.; Zohar, Y. Molecular biology of ovarian aromatase in sex reversal: Complementary DNA and 5’-flanking region isolation and differential expression of ovarian aromatase in the gilthead seabream (Sparus aurata). Biol. Reprod. 2006, 74, 857–864. [Google Scholar] [CrossRef]
- Li, G.L.; Liu, X.C.; Lin, H.R. Seasonal changes of serum sex steroids concentration and aromatase activity of gonad and brain in red-spotted grouper (Epinephelus akaara). Anim. Reprod. Sci. 2007, 99, 156–166. [Google Scholar] [CrossRef]
- Zhao, Z.X.; Li, S.L.; Li, S.L.; Wang, J.; Lin, H.; Fu, W.H. High expression of oncogene cadherin-6 correlates with tumor progression and a poor prognosis in gastric cancer. Cancer Cell Int. 2021, 21, 493. [Google Scholar] [CrossRef]
- Korla, P.K.; Chen, C.C.; Gracilla, D.E.; Lai, M.T.; Chen, C.M.; Chen, H.Y.; Hwang, T.; Chen, S.Y.; Sheu, J.J. Somatic mutational landscapes of adherens junctions and their functional consequences in cutaneous melanoma development. Theranostics 2020, 10, 12026–12043. [Google Scholar] [CrossRef]
- Pang, L.; Ren, F.; Xu, X.X.; Fu, L.Y.; Wang, T.F.; Guo, Z.Q. Construction and characterization of cadherin 6 (CDH6)-targeting chimeric antigen receptor (CAR) modified T cells. J. Environ. Pathol. Tox. 2022, 41, 55–71. [Google Scholar] [CrossRef]
- Aricescu, A.R.; McKinnell, I.W.; Halfter, W.; Stoker, A.W. Heparan sulfate proteoglycans are ligands for receptor protein tyrosine phosphatase σ. Mol. Cell. Biol. 2002, 22, 1881–1892. [Google Scholar] [CrossRef]
- Lin, Y.F.; Zhang, S.B.; Rehn, M.; Itäranta, P.; Tuukkanen, J.; Heljäsvaara, R.; Peltoketo, H.; Pihlajaniemi, T.; Vainio, S. Induced repatterning of type XVIII collagen expression in ureter bud from kidney to lung type: Association with sonic hedgehog and ectopic surfactant protein C. Development 2001, 128, 1573–1585. [Google Scholar] [CrossRef]
- Quélard, D.; Lavergne, E.; Hendaoui, I.; Elamaa, H.; Tiirola, U.; Heljäsvaara, R.; Pihlajaniemi, T.; Clément, B.; Musso, O. A cryptic frizzled module in cell surface collagen 18 inhibits Wnt/β-catenin signaling. PLoS ONE 2007, 3, e1878. [Google Scholar] [CrossRef]
- Liu, J.; He, B.; Yin, C.; Chang, Z.L.; Zhao, R.Q. Transcriptomic responses of porcine cumulus cells to heat exposure during oocytes in vitro maturation. Mol. Reprod. Dev. 2021, 88, 43–54. [Google Scholar] [CrossRef]
- Yin, C.; Liu, J.; He, B.; Jia, L.F.; Gong, Y.B.; Guo, H.D.; Zhao, R.Q. Heat stress induces distinct responses in porcine cumulus cells and oocytes associated with disrupted gap junction and trans-zonal projection colocalization. J. Cell. Physiol. 2019, 234, 4787–4798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, L.F.; Yu, X.; Liu, H.; Akhberdi, O.; Pan, J.; Zhu, X.D. A B-type histone acetyltransferase Hat1 regulates secondary metabolism, conidiation, and cell wall integrity in the taxol-producing fungus Pestalotiopsis microspora. J. Basic Microbiol. 2016, 56, 1380–1391. [Google Scholar] [CrossRef] [PubMed]
- Imhof, A.; Wolffe, A.P. Purification and properties of the Xenopus Hat1 acetyltransferase: Association with the 14-3-3 proteins in the oocyte nucleus. Biochemistry 1999, 38, 13085–13093. [Google Scholar] [CrossRef]
- Guo, B.C.; Zhang, S.N.; Wang, S.S.; Zhang, H.D.; Fang, J.S.; Kang, N.N.; Zhen, X.; Zhang, Y.; Zhou, J.D.; Yan, G.J.; et al. Decreased HAT1 expression in granulosa cells disturbs oocyte meiosis during mouse ovarian aging. Reprod. Biol. Endocrinol. 2023, 21, 103. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Y.; Duan, P.; Ding, X.; Li, Z.; Wang, X.; Li, L.; Liu, Y.; Wang, L.; Tian, Y. Comparative Transcriptome Analysis of the Hypothalamic–Pituitary–Gonadal Axis of Jinhu Grouper (Epinephelus fuscoguttatus ♀ × Epinephelus tukula ♂) and Tiger Grouper (Epinephelus fuscoguttatus). Genes 2024, 15, 929. https://doi.org/10.3390/genes15070929
Qiu Y, Duan P, Ding X, Li Z, Wang X, Li L, Liu Y, Wang L, Tian Y. Comparative Transcriptome Analysis of the Hypothalamic–Pituitary–Gonadal Axis of Jinhu Grouper (Epinephelus fuscoguttatus ♀ × Epinephelus tukula ♂) and Tiger Grouper (Epinephelus fuscoguttatus). Genes. 2024; 15(7):929. https://doi.org/10.3390/genes15070929
Chicago/Turabian StyleQiu, Yishu, Pengfei Duan, Xiaoyu Ding, Zhentong Li, Xinyi Wang, Linlin Li, Yang Liu, Linna Wang, and Yongsheng Tian. 2024. "Comparative Transcriptome Analysis of the Hypothalamic–Pituitary–Gonadal Axis of Jinhu Grouper (Epinephelus fuscoguttatus ♀ × Epinephelus tukula ♂) and Tiger Grouper (Epinephelus fuscoguttatus)" Genes 15, no. 7: 929. https://doi.org/10.3390/genes15070929




