Genome-Wide Identification and Expression Analysis of Nitrate Transporter (NRT) Gene Family in Eucalyptus grandis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Identification and Characterization of NRT Genes in E. grandis
2.3. Chromosomal Localization and Phylogenetic Analysis of EgNRT Proteins
2.4. Gene Structure and Conserved Motif Analysis of EgNRT Proteins
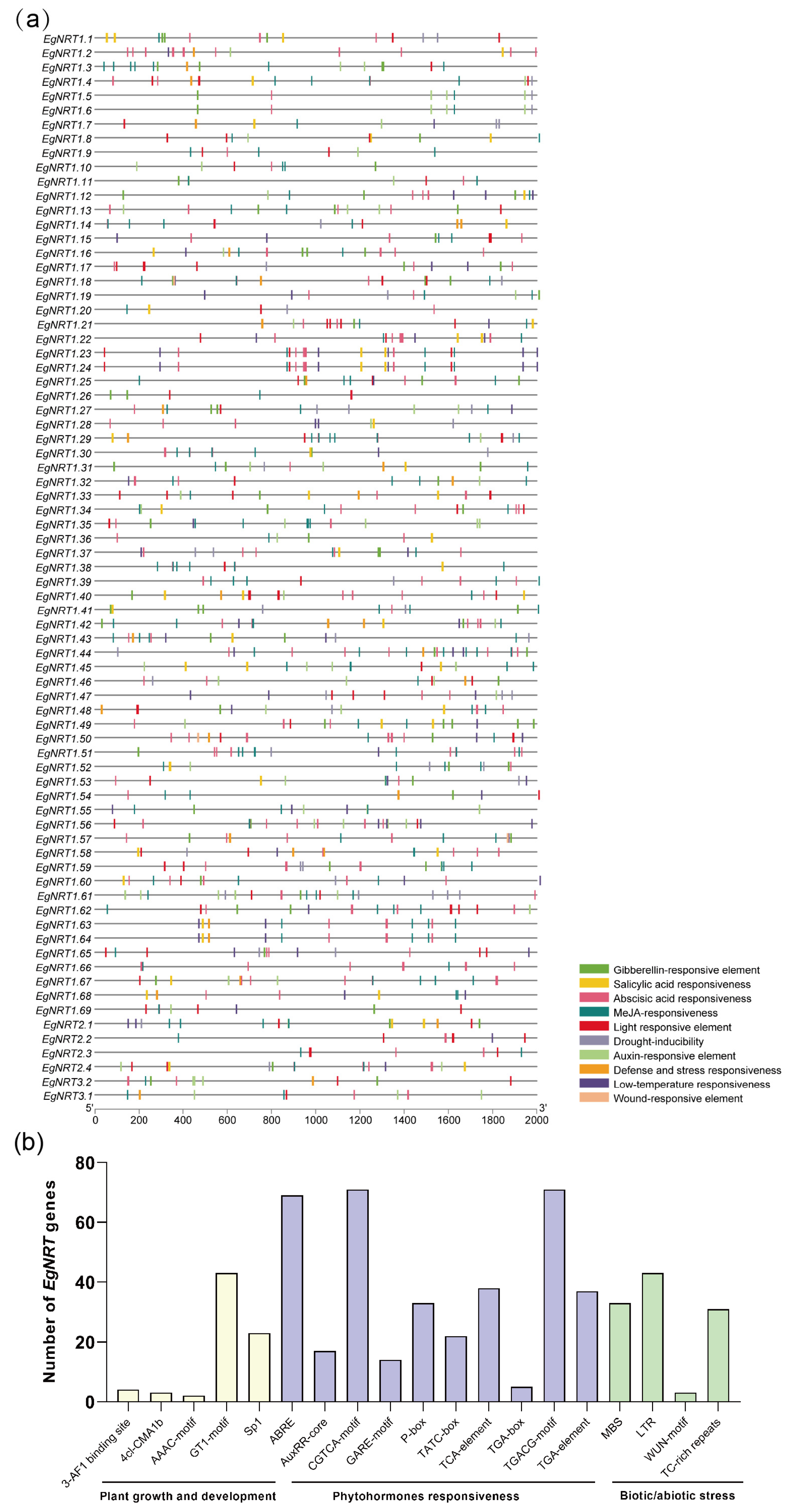
2.5. Cis-Element Analysis of EgNRT Gene Promoters
2.6. RNA Sequencing
2.7. Real Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.8. Statistical Analysis
3. Results
3.1. Genome-Wide Characterization of NRT Genes in E. grandis
3.2. Phylogenetic and Structure Analysis of the EgNRT Genes
3.3. Cis-Element Analysis of EgNRT Genes Promoters
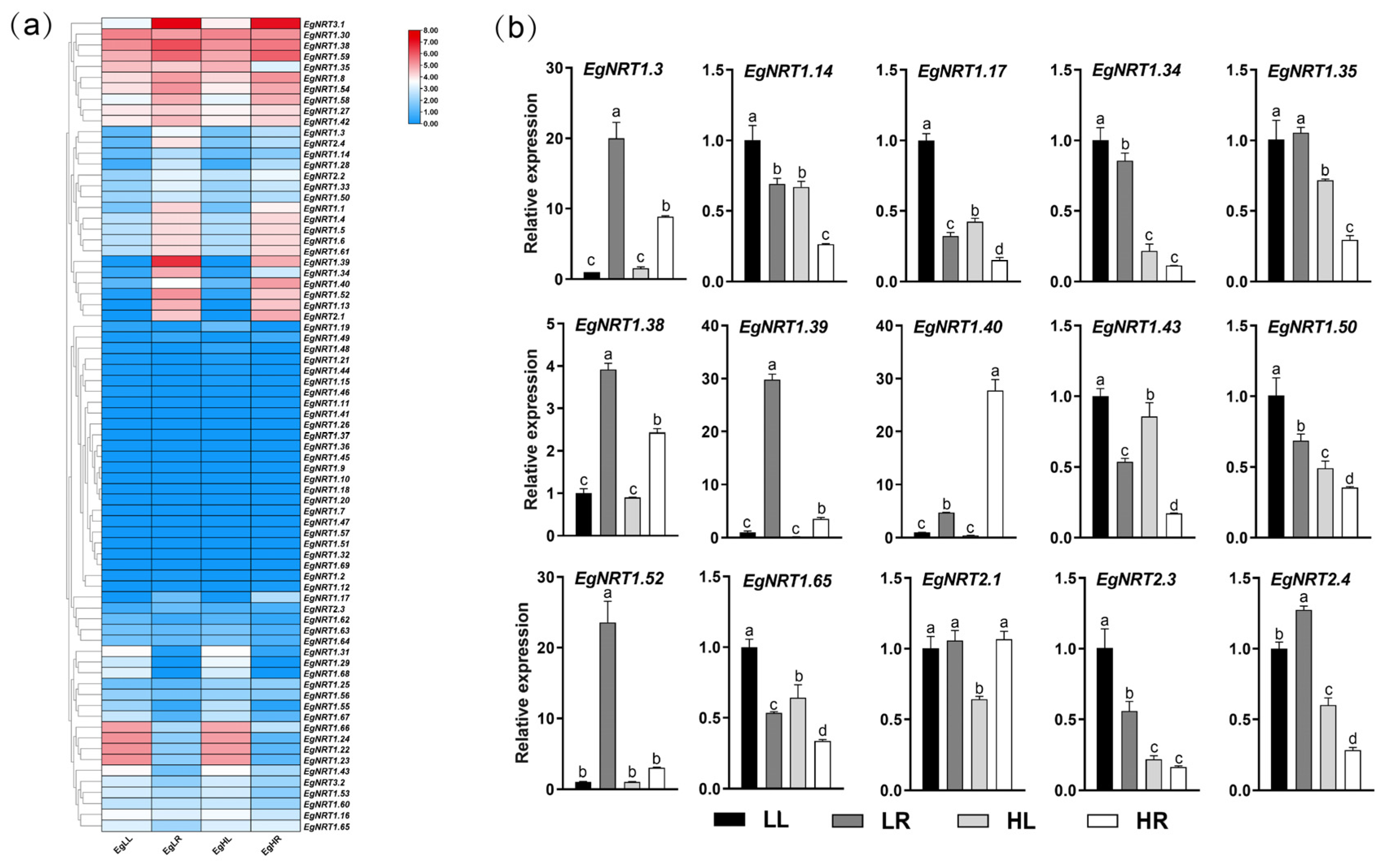
3.4. RNA-Seq Analysis of Root and Leaf in E. grandis under Low and High N Supply
3.5. Expression Profiles of EgNRT Genes under Low and High N Supply
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garnett, T.; Plett, D.; Heuer, S.; Okamoto, M. Genetic approaches to enhancing nitrogen-use efficiency (NUE) in cereals: Challenges and future directions. Funct. Plant Biol. 2015, 42, 921–941. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Beeckman, T.; Xu, G.H. Plant nitrogen nutrition: Sensing and signaling. Curr. Opin. Plant Biol. 2017, 39, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Hsu, P.K.; Tsay, Y.F. Uptake, allocation and signaling of nitrate. Trends Plant Sci. 2012, 17, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Gojon, A.; Krouk, G.; Perrine, W.F.; Laugier, E. Nitrate transceptor(s) in plants. J. Exp. Bot. 2011, 62, 2299–2308. [Google Scholar] [CrossRef] [PubMed]
- Migocka, M.; Warzybok, A.; Kłobus, G. The genomic organization and transcriptional pattern of genes encoding nitrate transporters 1 (NRT1) in cucumber. Plant Soil 2012, 364, 254–260. [Google Scholar] [CrossRef]
- Ding, M.C.; He, M.; Zhang, W.L.; Han, Y.; Zhang, X.Y.; Zhang, X.L.; Zhu, Y.L.; Wang, Y.; Liu, L.W.; Xu, L. Genome-wide identification and expression analysis of RsNRT gene family reveals their potential roles in response to low-nitrogen condition in radish (Raphanus sativus L.). Sci. Hortic. 2023, 321, 112273. [Google Scholar] [CrossRef]
- Plett, D.; Toubia, J.; Garnett, T.; Tester, M.; Kaiser, N.B.; Baumann, U. Dichotomy in the NRT gene families of dicots and grass species. PLoS ONE 2010, 5, e15289. [Google Scholar] [CrossRef]
- Jia, L.H.; Hu, D.S.; Wang, J.B.; Liang, Y.Y.; Li, F.; Wang, Y.; Han, Y.L. Genome-wide identification and functional analysis of nitrate transporter genes (NPF, NRT2 and NRT3) in Maize. Int. J. Mol. Sci. 2023, 24, 12941. [Google Scholar] [CrossRef]
- Bai, H.; Euring, D.; Volmer, K.; Janz, D.; Polle, A. The nitrate transporter (NRT) gene family in poplar. PLoS ONE 2013, 8, e72126. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, P.F.; Liu, P.; Song, Y.P.; Zhang, D.Q. Genetic effects and expression patterns of the nitrate transporter (NRT) gene family in Populus tomentosa. Front. Plant Sci. 2021, 12, 661635. [Google Scholar] [CrossRef]
- Li, W.M.; Yan, M.K.; Hu, B.Y.; Priyadarshani, S.V.G.N.; Hou, Z.M.; Ojolo, P.S.; Xiong, J.J.; Zhao, H.M.; Qin, Y. Characterization and the expression analysis of nitrate transporter (NRT) gene family in pineapple. Trop. Plant Biol. 2018, 11, 177–191. [Google Scholar] [CrossRef]
- Guo, F.Q.; Wang, R.C.; Crawford, M.N. The Arabidopsis dual-affinity nitrate transporter gene AtNRT1.1 (CHL1) is regulated by auxin in both shoots and roots. J. Exp. Bot. 2002, 53, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.Q.; Young, J.; Crawford, M.N. The nitrate transporter AtNRT1.1 (CHL1) functions in stomatal opening and contributes to drought susceptibility in Arabidopsis. Plant Cell 2003, 15, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Li, W.B.; Wang, Y.; Okamoto, M.; Crawford, N.M.; Siddiqi, M.Y.; Glass, D.M.A. Dissection of the AtNRT2.1:AtNRT2.2 inducible high-affinity nitrate transporter gene cluster. Plant Physiol. 2007, 143, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Kiba, T.; Kudo, T.; Kojima, M.; Sakakibara, H. Hormonal control of nitrogen acquisition: Roles of auxin, abscisic acid, and cytokinin. J. Exp. Bot. 2011, 62, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.M.; Fan, X.R.; Yan, M.; Liu, X.Q.; Miller, J.A.; Xu, G.H. Multiple roles of nitrate transport accessory protein NAR2 in plants. Plant Signal. Behav. 2011, 6, 1286–1289. [Google Scholar] [CrossRef]
- Okamoto, M.; Kumar, A.; Li, W.; Wang, Y.; Siddiqi, M.Y.; Crawford, N.M.; Glass, A.D.M. High-affinity nitrate transport in roots of Arabidopsis depends on expression of the NAR2-like gene AtNRT3.1. Plant Physiol. 2006, 140, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Kotur, Z.; Mackenzie, N.; Ramesh, S.; Tyerman, S.D.; Kaiser, B.N.; Glass, A.D. Nitrate transport capacity of the Arabidopsis thaliana NRT2 family members and their interactions with AtNAR2.1. New Phytol. 2012, 194, 724–731. [Google Scholar] [CrossRef]
- Yan, M.; Fan, X.R.; Feng, H.M.; Miller, A.J.; Shen, Q.R.; Xu, G.H. Rice OsNAR2.1 interacts with OsNRT2.1, OsNRT2.2 and OsNRT2.3a nitrate transporters to provide uptake over high and low concentration ranges. Plant Cell Environ. 2011, 34, 1360–1372. [Google Scholar] [CrossRef]
- Marília, G.S.C.; Paulo, M.; Tiago, S.B. Insights into temperature modulation of the Eucalyptus globulus and Eucalyptus grandis antioxidant and lignification subproteomes. Phytochemistry 2017, 137, 15–23. [Google Scholar]
- Myburg, A.; Grattapaglia, D.; Tuskan, G. The genome of Eucalyptus grandis. Nature 2014, 510, 356–362. [Google Scholar] [CrossRef]
- Krouk, G. Hormones and nitrate: A two-way connection. Plant Mol. Biol. 2016, 91, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Guan, P.Z. Dancing with Hormones: A current perspective of nitrate signaling and regulation in Arabidopsis. Front. Plant Sci. 2017, 8, 1697. [Google Scholar] [CrossRef] [PubMed]
- Landrein, B.; Formosa-Jordan, P.; Malivert, A.; Schuster, C.; Melnyk, W.C.; Yang, W.B.; Turnbull, C.; Meyerowitz, M.E.; Locke, C.W.J.; Jönsson, H. Nitrate modulates stem cell dynamics in Arabidopsis shoot meristems through cytokinins. Proc. Natl. Acad. Sci. USA 2018, 115, 201718670. [Google Scholar] [CrossRef] [PubMed]
- Mounier, E.; Pervent, M.; Ljung, K.; Gojon, A.; Nacry, P. Auxin-mediated nitrate signalling by NRT1.1 participates in the adaptive response of Arabidopsis root architecture to the spatial heterogeneity of nitrate availability. Plant Cell Environ. 2014, 37, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Vega, A.; O’Brien, J.A.; Gutierrez, R.A. Nitrate and hormonal signaling crosstalk for plant growth and development. Curr. Opin. Plant Biol. 2019, 52, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Vidmar, J.J.; Glass, A.D.M. Regulation of NRT1 and NRT2 gene families of Arabidopsis thaliana: Responses to nitrate provision. Plant Cell Physiol. 2003, 44, 304–317. [Google Scholar] [CrossRef]
- Nazoa, P.; Vidmar, J.J.; Tranbarger, T.J.; Mouline, K.; Damiani, I.; Tillard, P.; Zhuo, D.; Glass, D.M.A.; Touraine, B. Regulation of the nitrate transporter gene AtNRT2.1 in Arabidopsis thaliana: Responses to nitrate, amino acids and developmental stage. Plant Mol. Biol. 2003, 52, 689–703. [Google Scholar] [CrossRef]
- Alvarez, J.M.; Vidal, E.A.; Gutiérrez, R.A. Integration of local and systemic signaling pathways for plant N responses. Curr. Opin. Plant Biol. 2012, 15, 185–191. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, Y.N.; Yu, M.; Su, B.D.; Gong, W.; Baluška, F.; Komis, G.; Šamaj, J.; Shan, X.Y.; Li, J.X. Phosphorylation-mediated dynamics of nitrate transceptor NRT1.1 regulate auxin flux and nitrate signaling in lateral root growth. Plant Physiol. 2019, 181, 480–498. [Google Scholar] [CrossRef]
- Lezhneva, L.; Kiba, T.; Feriabourrelier, A.B.; Lafouge, F.; Boutet-Mercey, S.; Zoufan, P.; Sakakibara, H.; Daniel-Vedele, F.; Krapp, A. The Arabidopsis nitrate transporter NRT2.5 plays a role in nitrate acquisition and remobilization in nitrogen-starved plants. Plant J. 2015, 80, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.S.; Song, A.P.; Zhang, X.X.; Wang, H.B.; Li, T.; Chen, Y.; Jiang, J.F.; Chen, F.D.; Chen, S.M. Cloning of chrysanthemum high-affinity nitrate transporter family (CmNRT2) and characterization of CmNRT2.1. Sci. Rep. 2016, 6, 23462. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2020, 49, D458–D460. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, R.H.; Frank, H.M.; He, Y.H.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, B.; Gao, S.H.; Lercher, J.M.; Hu, S.N.; Chen, W.H. Evolview v3: A webserver for visualization, annotation, and management of phylogenetic trees. Nucleic Acids Res. 2019, 47, W270–W275. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.F.; Li, J.J.; Zhang, Y.; Zhang, X.N.; Liu, H.L.; Qin, Z.H.; Chen, B.W. Transcriptome analysis identifies genes involved in the somatic embryogenesis of Eucalyptus. BMC Genom. 2020, 21, 803. [Google Scholar] [CrossRef]
- Van, B.M.; Proost, S.; Wischnitzki, E.; Movahedi, S.; Scheerlinck, C.; Van, P.Y.; Vandepoele, K. Dissecting plant genomes with the PLAZA comparative genomics platform. Plant Physiol. 2012, 158, 590–600. [Google Scholar]




| Gene Name | Gene ID | Chromosome | Start | End | Length (aa) | Molecular Weight (kDa) | Theoretical pI | Instability Index | Gravy |
|---|---|---|---|---|---|---|---|---|---|
| EgNRT1.1 | KAK3410094.1 | Chr10 | 26,452,832 | 26,456,837 | 600 | 66.50 | 8.99 | 41.06 | 0.167 |
| EgNRT1.2 | KAK3417242.1 | Chr8 | 42,098,203 | 42,099,747 | 479 | 53.77 | 9.66 | 34.99 | 0.258 |
| EgNRT1.3 | KAK3417244.1 | Chr8 | 42,112,816 | 42,118,538 | 592 | 66.43 | 9.28 | 31.56 | 0.228 |
| EgNRT1.4 | KAK3426575.1 | Chr6 | 42,080,525 | 42,083,773 | 571 | 62.29 | 6.90 | 39.33 | 0.410 |
| EgNRT1.5 | KAK3426576.1 | Chr6 | 42,080,525 | 42,089,987 | 571 | 62.25 | 6.83 | 40.74 | 0.413 |
| EgNRT1.6 | KAK3426577.1 | Chr6 | 42,086,125 | 42,089,987 | 571 | 61.94 | 6.83 | 40.73 | 0.424 |
| EgNRT1.7 | KAK3431236.1 | Chr5 | 49,790,727 | 49,794,988 | 572 | 63.81 | 9.15 | 34.49 | 0.283 |
| EgNRT1.8 | KAK3441088.1 | Chr2 | 21,269,805 | 21,274,259 | 593 | 65.43 | 9.03 | 35.09 | 0.285 |
| EgNRT1.9 | KAK3444550.1 | Chr1 | 8,907,636 | 8,908,499 | 261 | 29.19 | 8.99 | 32.01 | 0.183 |
| EgNRT1.10 | KAK3444551.1 | Chr1 | 8,912,563 | 8,913,917 | 326 | 36.52 | 8.21 | 37.83 | 0.082 |
| EgNRT1.11 | KAK3444552.1 | Chr1 | 8,962,732 | 8,964,089 | 326 | 36.59 | 8.79 | 39.60 | −0.012 |
| EgNRT1.12 | KAK3415186.1 | Chr8 | 9,882,107 | 9,884,471 | 527 | 58.26 | 9.60 | 28.56 | 0.308 |
| EgNRT1.13 | KAK3418198.1 | Chr8 | 55,988,616 | 55,991,440 | 587 | 65.54 | 8.96 | 37.33 | 0.271 |
| EgNRT1.14 | KAK3419514.1 | Chr7 | 828,051 | 832,508 | 608 | 67.37 | 9.06 | 45.91 | 0.187 |
| EgNRT1.15 | KAK3421958.1 | Chr7 | 46,265,830 | 46,267,324 | 343 | 37.87 | 8.94 | 45.76 | 0.372 |
| EgNRT1.16 | KAK3421969.1 | Chr7 | 46,339,254 | 46,341,108 | 442 | 48.17 | 9.25 | 36.47 | 0.333 |
| EgNRT1.17 | KAK3423094.1 | Chr6 | 77,443 | 80,673 | 596 | 65.65 | 9.11 | 31.77 | 0.292 |
| EgNRT1.18 | KAK3431109.1 | Chr5 | 47,014,489 | 47,017,668 | 599 | 66.28 | 9.01 | 32.54 | 0.492 |
| EgNRT1.19 | KAK3440113.1 | Chr2 | 4,017,890 | 4,021,277 | 577 | 63.27 | 9.12 | 42.37 | 0.407 |
| EgNRT1.20 | KAK3440606.1 | Chr2 | 9,819,211 | 9,821,092 | 395 | 43.55 | 8.51 | 30.53 | 0.475 |
| EgNRT1.21 | KAK3441424.1 | Chr2 | 28,576,333 | 28,578,626 | 527 | 58.26 | 8.38 | 36.16 | 0.433 |
| EgNRT1.22 | KAK3404455.1 | Chr11 | 9,062,109 | 9,066,244 | 595 | 65.80 | 8.82 | 24.58 | 0.234 |
| EgNRT1.23 | KAK3404456.1 | Chr11 | 9,062,616 | 9,066,244 | 546 | 60.81 | 8.90 | 25.43 | 0.243 |
| EgNRT1.24 | KAK3404457.1 | Chr11 | 9,062,446 | 9,066,244 | 546 | 60.81 | 8.90 | 25.43 | 0.243 |
| EgNRT1.25 | KAK3422698.1 | Chr7 | 52,269,257 | 52,274,320 | 584 | 65.32 | 8.99 | 34.99 | 0.254 |
| EgNRT1.26 | KAK3425311.1 | Chr6 | 28,102,756 | 28,106,418 | 602 | 66.84 | 9.56 | 34.21 | 0.211 |
| EgNRT1.27 | KAK3426574.1 | Chr6 | 42,076,124 | 42,080,091 | 579 | 62.82 | 6.03 | 37.39 | 0.336 |
| EgNRT1.28 | KAK3427971.1 | Chr6 | 51,701,358 | 51,705,252 | 536 | 59.13 | 8.02 | 42.99 | 0.253 |
| EgNRT1.29 | KAK3421328.1 | Chr7 | 38,221,725 | 38,225,022 | 649 | 71.60 | 9.02 | 36.52 | 0.252 |
| EgNRT1.30 | KAK3421701.1 | Chr7 | 44,006,688 | 44,012,020 | 582 | 64.52 | 8.96 | 32.12 | 0.260 |
| EgNRT1.31 | KAK3439365.1 | Chr3 | 79,521,298 | 79,525,885 | 576 | 63.02 | 9.04 | 31.88 | 0.358 |
| EgNRT1.32 | KAK3405187.1 | Chr11 | 17,678,784 | 17,681,533 | 598 | 66.32 | 9.35 | 34.37 | 0.077 |
| EgNRT1.33 | KAK3406913.1 | Chr11 | 37,923,342 | 37,928,314 | 595 | 66.16 | 9.01 | 39.35 | 0.274 |
| EgNRT1.34 | KAK3409078.1 | Chr10 | 12,946,580 | 12,949,871 | 563 | 61.71 | 9.07 | 34.88 | 0.401 |
| EgNRT1.35 | KAK3409870.1 | Chr10 | 24,836,710 | 24,840,859 | 570 | 63.65 | 8.69 | 24.92 | 0.198 |
| EgNRT1.36 | KAK3417246.1 | Chr8 | 42,197,856 | 42,202,878 | 595 | 65.56 | 9.17 | 31.11 | 0.287 |
| EgNRT1.37 | KAK3417247.1 | Chr8 | 42,217,236 | 42,223,672 | 596 | 65.61 | 9.26 | 33.40 | 0.286 |
| EgNRT1.38 | KAK3421394.1 | Chr7 | 39,895,137 | 39,900,397 | 581 | 64.11 | 5.63 | 31.42 | 0.271 |
| EgNRT1.39 | KAK3430197.1 | Chr5 | 21,446,821 | 21,449,295 | 543 | 60.23 | 5.78 | 32.58 | 0.170 |
| EgNRT1.40 | KAK3434719.1 | Chr4 | 35,140,519 | 35,145,774 | 597 | 66.46 | 6.76 | 32.68 | 0.153 |
| EgNRT1.41 | KAK3442420.1 | Chr2 | 45,896,146 | 45,898,996 | 637 | 70.20 | 6.05 | 29.04 | 0.303 |
| EgNRT1.42 | KAK3442480.1 | Chr2 | 46,229,753 | 46,235,956 | 584 | 64.35 | 5.09 | 32.84 | 0.297 |
| EgNRT1.43 | KAK3442482.1 | Chr2 | 46,237,521 | 46,244,731 | 428 | 47.59 | 6.15 | 38.10 | 0.212 |
| EgNRT1.44 | KAK3443531.1 | Chr2 | 56,169,871 | 56,174,670 | 584 | 64.21 | 7.79 | 28.69 | 0.328 |
| EgNRT1.45 | KAK3444549.1 | Chr1 | 8,900,093 | 8,901,821 | 523 | 58.40 | 8.37 | 39.65 | 0.375 |
| EgNRT1.46 | KAK3404606.1 | Chr11 | 11,376,427 | 11,378,235 | 525 | 57.69 | 9.20 | 39.38 | 0.447 |
| EgNRT1.47 | KAK3408803.1 | Chr10 | 10,440,073 | 10,443,789 | 582 | 63.44 | 8.90 | 41.94 | 0.360 |
| EgNRT1.48 | KAK3408804.1 | Chr10 | 10,462,388 | 10,465,144 | 544 | 59.73 | 8.80 | 36.80 | 0.347 |
| EgNRT1.49 | KAK3408805.1 | Chr10 | 10,487,412 | 10,490,375 | 545 | 59.40 | 9.09 | 35.42 | 0.287 |
| EgNRT1.50 | KAK3410139.1 | Chr10 | 26,946,074 | 26,949,565 | 528 | 59.27 | 8.18 | 45.59 | 0.324 |
| EgNRT1.51 | KAK3440094.1 | Chr2 | 3,808,628 | 3,811,132 | 617 | 68.95 | 9.40 | 46.36 | 0.183 |
| EgNRT1.52 | KAK3442757.1 | Chr2 | 50,546,560 | 50,549,464 | 452 | 50.09 | 8.43 | 29.64 | 0.146 |
| EgNRT1.53 | KAK3443072.1 | Chr2 | 52,890,529 | 52,894,671 | 646 | 70.57 | 9.21 | 38.27 | 0.277 |
| EgNRT1.54 | KAK3446521.1 | Chr1 | 37,349,479 | 37,352,842 | 627 | 69.13 | 8.39 | 36.76 | 0.162 |
| EgNRT1.55 | KAK3409854.1 | Chr10 | 24,732,599 | 24,735,325 | 570 | 63.96 | 8.85 | 28.49 | 0.249 |
| EgNRT1.56 | KAK3445120.1 | Chr1 | 20,129,841 | 20,134,001 | 571 | 63.91 | 8.12 | 24.92 | 0.206 |
| EgNRT1.57 | KAK3406767.1 | Chr11 | 36,353,120 | 36,355,616 | 606 | 67.99 | 9.11 | 38.27 | 0.182 |
| EgNRT1.58 | KAK3406769.1 | Chr11 | 36,380,891 | 36,384,668 | 589 | 64.90 | 9.28 | 34.47 | 0.345 |
| EgNRT1.59 | KAK3406773.1 | Chr11 | 36,422,757 | 36,425,873 | 617 | 68.93 | 8.59 | 35.14 | 0.176 |
| EgNRT1.60 | KAK3406774.1 | Chr11 | 36,444,862 | 36,448,073 | 597 | 66.05 | 9.21 | 38.63 | 0.253 |
| EgNRT1.61 | KAK3426578.1 | Chr6 | 42,086,125 | 42,089,987 | 404 | 44.60 | 6.74 | 39.95 | 0.460 |
| EgNRT1.62 | KAK3440091.1 | Chr2 | 3,792,284 | 3,794,705 | 600 | 66.73 | 9.31 | 35.27 | 0.270 |
| EgNRT1.63 | KAK3440092.1 | Chr2 | 3,808,628 | 3,811,132 | 600 | 66.84 | 9.28 | 34.88 | 0.251 |
| EgNRT1.64 | KAK3440093.1 | Chr2 | 3,803,834 | 3,806,399 | 575 | 64.00 | 9.30 | 35.81 | 0.202 |
| EgNRT1.65 | KAK3426647.1 | Chr6 | 42,459,508 | 42,462,203 | 533 | 58.96 | 8.86 | 43.17 | 0.205 |
| EgNRT1.66 | KAK3435815.1 | Chr3 | 8,569,729 | 8,573,136 | 537 | 58.92 | 7.50 | 37.84 | 0.447 |
| EgNRT1.67 | KAK3435907.1 | Chr3 | 11,297,439 | 11,301,408 | 632 | 69.37 | 5.98 | 41.14 | 0.402 |
| EgNRT1.68 | KAK3439164.1 | Chr3 | 76,588,303 | 76,591,154 | 586 | 64.67 | 9.29 | 36.47 | 0.256 |
| EgNRT1.69 | KAK3439166.1 | Chr3 | 76,595,223 | 76,597,726 | 574 | 63.13 | 9.11 | 43.35 | 0.360 |
| EgNRT2.1 | KAK3408717.1 | Chr10 | 9,727,832 | 9,729,792 | 530 | 57.47 | 8.86 | 39.08 | 0.324 |
| EgNRT2.2 | KAK3416771.1 | Chr8 | 33,614,452 | 33,616,983 | 508 | 54.83 | 8.51 | 35.42 | 0.390 |
| EgNRT2.3 | KAK3410216.1 | Chr10 | 27,433,602 | 27,437,118 | 378 | 39.93 | 6.29 | 33.94 | 0.580 |
| EgNRT2.4 | KAK3443186.1 | Chr2 | 53,783,851 | 53,786,418 | 503 | 54.02 | 8.82 | 34.37 | 0.407 |
| EgNRT3.1 | KAK3439874.1 | Chr2 | 1,786,661 | 1,788,242 | 205 | 22.29 | 9.21 | 38.46 | −0.092 |
| EgNRT3.2 | KAK3439873.1 | Chr2 | 1,769,131 | 1,772,134 | 320 | 35.90 | 6.26 | 38.76 | −0.238 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Yang, D.; Hu, Y.; Xu, J.; Lu, Z. Genome-Wide Identification and Expression Analysis of Nitrate Transporter (NRT) Gene Family in Eucalyptus grandis. Genes 2024, 15, 930. https://doi.org/10.3390/genes15070930
Li G, Yang D, Hu Y, Xu J, Lu Z. Genome-Wide Identification and Expression Analysis of Nitrate Transporter (NRT) Gene Family in Eucalyptus grandis. Genes. 2024; 15(7):930. https://doi.org/10.3390/genes15070930
Chicago/Turabian StyleLi, Guangyou, Deming Yang, Yang Hu, Jianmin Xu, and Zhaohua Lu. 2024. "Genome-Wide Identification and Expression Analysis of Nitrate Transporter (NRT) Gene Family in Eucalyptus grandis" Genes 15, no. 7: 930. https://doi.org/10.3390/genes15070930




