Differential Gene Expression in Contrasting Common Bean Cultivars for Drought Tolerance during an Extended Dry Period
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Material and Induction of Water Deficit in Plants
2.2. Isolation and Analysis of Total RNA and RNASeq
2.3. Analysis and Annotation of the Sequences
2.4. Validation of Genes via RT-qPCR and Statistical Analyses
3. Results
3.1. Sequence Alignment, Functional Annotation, and Analysis of Differential Expression
3.2. Water Deficit Responsive Genes
3.3. Gene Expression Analysis
4. Discussion
4.1. Regulatory Genes Involved in Signaling Cascades and Transcriptional Control
4.1.1. Phytohormone Signaling Pathways
4.1.2. Signaling and Signal Transduction
4.1.3. Transcription Regulatory Factors
4.2. Genes That Promote the Protection of Membranes and Water and Ion Uptake/Transport Proteins
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nelson, G.C.; Valin, H.; Sands, R.D.; Havlík, P.; Ahammad, H.; Deryng, D.; Elliott, J.; Fujimori, S.; Hasegawa, T.; Heyhoe, E.; et al. Climate Change Effects on Agriculture: Economic Responses to Biophysical Shocks. Proc. Natl. Acad. Sci. USA 2014, 111, 3274–3279. [Google Scholar] [CrossRef]
- Li, Y.; Ye, W.; Wang, M.; Yan, X. Climate Change and Drought: A Risk Assessment of Crop-Yield Impacts. Clim. Res. 2009, 39, 31–46. [Google Scholar] [CrossRef]
- Ortiz-Bobea, A.; Ault, T.R.; Carrillo, C.M.; Chambers, R.G.; Lobell, D.B. Anthropogenic Climate Change Has Slowed Global Agricultural Productivity Growth. Nat. Clim. Change 2021, 11, 306–312. [Google Scholar] [CrossRef]
- Uebersax, M.A.; Cichy, K.A.; Gomez, F.E.; Porch, T.G.; Heitholt, J.; Osorno, J.M.; Kamfwa, K.; Snapp, S.S.; Bales, S. Dry Beans (Phaseolus vulgaris L.) as a Vital Component of Sustainable Agriculture and Food Security—A Review. Legume Sci. 2023, 5, e155. [Google Scholar] [CrossRef]
- Pujolà, M.; Farreras, A.; Casañas, F. Protein and Starch Content of Raw, Soaked and Cooked Beans (Phaseolus vulgaris L.). Food Chem. 2007, 102, 1034–1041. [Google Scholar] [CrossRef]
- Kotue, T.C.; Marlyne, J.M.; Wirba, L.Y.; Amalene, S.R.H.; Nkenmeni, D.C.; Kwuimgoin, I.; Djote, W.N.B.; Kansci, G.; Fokou, E.; Fokam, D.P. Nutritional Properties and Nutrients Chemical Analysis of Common Beans Seed. MOJ Biol. Med. 2018, 3, 41–47. [Google Scholar] [CrossRef]
- Smith, M.R.; Veneklaas, E.; Polania, J.; Rao, I.M.; Beebe, S.E.; Merchant, A. Field Drought Conditions Impact Yield but Not Nutritional Quality of the Seed in Common Bean (Phaseolus vulgaris L.). PLoS ONE 2019, 14, e0217099. [Google Scholar] [CrossRef] [PubMed]
- Khatun, M.; Sarkar, S.; Era, F.M.; Islam, A.K.M.M.; Anwar, M.P.; Fahad, S.; Datta, R.; Islam, A.K.M.A. Drought Stress in Grain Legumes: Effects, Tolerance Mechanisms and Management. Agronomy 2021, 11, 2374. [Google Scholar] [CrossRef]
- UNCCD. Global Drought Snapshot 2023—The Need for Proactive Action; United Nations Convention to Combat Desertification: Bonn, Germany, 2023; 40p. [Google Scholar]
- Salazar, C.; Hernández, C.; Pino, M.T. Plant Water Stress: Associations between Ethylene and Abscisic Acid Response. Chil. J. Agric. Res. 2015, 75, 71–79. [Google Scholar] [CrossRef]
- Sourour, A.; Afef, O.; Mounir, R.; Mongi, B.Y. A Review: Morphological, Physiological, Biochemical and Molecular Plant Responses to Water Deficit Stress. Int. J. Eng. Sci. 2017, 6, 1–4. [Google Scholar] [CrossRef]
- Martínez, J.P.; Silva, H.; Ledent, J.F.; Pinto, M. Effect of Drought Stress on the Osmotic Adjustment, Cell Wall Elasticity and Cell Volume of Six Cultivars of Common Beans (Phaseolus vulgaris L.). Eur. J. Agron. 2007, 26, 30–38. [Google Scholar] [CrossRef]
- Mathobo, R.; Marais, D.; Steyn, J.M. The Effect of Drought Stress on Yield, Leaf Gaseous Exchange and Chlorophyll Fluorescence of Dry Beans (Phaseolus vulgaris L.). Agric. Water Manag. 2017, 180, 118–125. [Google Scholar] [CrossRef]
- Ghanbari, A.A.; Mousavi, S.H.; Gorji, A.M.; Rao, I. Effects of Water Stress on Leaves and Seeds of Bean (Phaseolus vulgaris L.). Turk. J. Field Crops 2013, 18, 73–77. [Google Scholar]
- Hummel, M.; Hallahan, B.F.; Brychkova, G.; Ramirez-Villegas, J.; Guwela, V.; Chataika, B.; Curley, E.; Mckeown, P.C.; Morrison, L.; Talsma, F.; et al. Reduction in Nutritional Quality and Growing Area Suitability of Common Bean under Climate Change Induced Drought Stress in Africa. Sci. Rep. 2018, 8, 16187. [Google Scholar] [CrossRef]
- Peña-Valdivia, C.B.; Sánchez-Urdaneta, A.B.; Rangel, J.M.; Muñoz, J.J.; García-Nava, R.; Velázquez, R.C. Anatomical Root Variations in Response to Water Deficit: Wild and Domesticated Common Bean (Phaseolus vulgaris L.). Biol. Res. 2010, 43, 417–427. [Google Scholar] [CrossRef]
- Papathanasiou, F.; Ninou, E.; Mylonas, I.; Baxevanos, D.; Papadopoulou, F.; Avdikos, I.; Sistanis, I.; Koskosidis, A.; Vlachostergios, D.N.; Stefanou, S.; et al. The Evaluation of Common Bean (Phaseolus vulgaris L.) Genotypes under Water Stress Based on Physiological and Agronomic Parameters. Plants 2022, 11, 2432. [Google Scholar] [CrossRef]
- Widuri, L.I.; Lakitan, B.; Sodikin, E.; Hasmeda, M.; Meihana, M.; Kartika, K.; Siaga, E. Shoot and Root Growth in Common Bean (Phaseolus vulgaris L.) Exposed to Gradual Drought Stress. AGRIVITA J. Agric. Sci. 2018, 40, 442–452. [Google Scholar] [CrossRef]
- Manzi, M.; Lado, J.; Rodrigo, M.J.; Zacarías, L.; Arbona, V.; Gómez-Cadenas, A. Root ABA Accumulation in Long-Term Water-Stressed Plants Is Sustained by Hormone Transport from Aerial Organs. Plant Cell Physiol. 2015, 56, 2457–2466. [Google Scholar] [CrossRef]
- Tombesi, S.; Nardini, A.; Frioni, T.; Soccolini, M.; Zadra, C.; Farinelli, D.; Poni, S.; Palliotti, A. Stomatal Closure Is Induced by Hydraulic Signals and Maintained by ABA in Drought-Stressed Grapevine. Sci. Rep. 2015, 5, 12449. [Google Scholar] [CrossRef]
- Figueiredo, M.V.B.; Burity, H.A.; Martínez, C.R.; Chanway, C.P. Alleviation of Drought Stress in the Common Bean (Phaseolus vulgaris L.) by Co-Inoculation with Paenibacillus polymyxa and Rhizobium tropici. Appl. Soil Ecol. 2008, 40, 182–188. [Google Scholar] [CrossRef]
- Ilyas, M.; Nisar, M.; Khan, N.; Hazrat, A.; Khan, A.H.; Hayat, K.; Fahad, S.; Khan, A.; Ullah, A. Drought Tolerance Strategies in Plants: A Mechanistic Approach. J. Plant Growth Regul. 2021, 40, 926–944. [Google Scholar] [CrossRef]
- Zadražnik, T.; Hollung, K.; Egge-Jacobsen, W.; Meglič, V.; Šuštar-Vozlič, J. Differential Proteomic Analysis of Drought Stress Response in Leaves of Common Bean (Phaseolus vulgaris L.). J. Proteom. 2013, 78, 254–272. [Google Scholar] [CrossRef]
- Kusvuran, S.; Dasgan, H.Y. Effects of Drought Stress on Physiological and Biochemical Changes in Phaseolus vulgaris L. Legume Res. 2017, 40, 55–62. [Google Scholar] [CrossRef]
- Androcioli, L.G.; Zeffa, D.M.; Alves, D.S.; Tomaz, J.P.; Moda-Cirino, V. Effect of Water Deficit on Morphoagronomic and Physiological Traits of Common Bean Genotypes with Contrasting Drought Tolerance. Water 2020, 12, 217. [Google Scholar] [CrossRef]
- Recchia, G.H.; Caldas, D.G.G.; Beraldo, A.L.A.; Silva, M.J.; Tsai, S.M. Transcriptional Analysis of Drought-Induced Genes in the Roots of a Tolerant Genotype of the Common Bean (Phaseolus vulgaris L.). Int. J. Mol. Sci. 2013, 14, 7155–7179. [Google Scholar] [CrossRef]
- Zargar, S.M.; Nagar, P.; Deshmukh, R.; Nazir, M.; Wani, A.A.; Masoodi, K.Z.; Agrawal, G.K.; Rakwal, R. Aquaporins as Potential Drought Tolerance Inducing Proteins: Towards Instigating Stress Tolerance. J. Proteom. 2017, 169, 233–238. [Google Scholar] [CrossRef]
- Patel, J.; Mishra, A. Plant Aquaporins Alleviate Drought Tolerance in Plants by Modulating Cellular Biochemistry, Root-Architecture, and Photosynthesis. Physiol. Plant. 2021, 172, 1030–1044. [Google Scholar] [CrossRef]
- Zupin, M.; Sedlar, A.; Kidrič, M.; Meglič, V. Drought-Induced Expression of Aquaporin Genes in Leaves of Two Common Bean Cultivars Differing in Tolerance to Drought Stress. J. Plant Res. 2017, 130, 735–745. [Google Scholar] [CrossRef]
- Chen, J.B.; Wang, S.M.; Jing, R.L.; Mao, X.G. Cloning the PvP5CS Gene from Common Bean (Phaseolus vulgaris) and Its Expression Patterns under Abiotic Stresses. J. Plant Physiol. 2009, 166, 12–19. [Google Scholar] [CrossRef]
- Cortés, A.J.; This, D.; Chavarro, C.; Madriñán, S.; Blair, M.W. Nucleotide Diversity Patterns at the Drought-Related DREB2 Encoding Genes in Wild and Cultivated Common Bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 2012, 125, 1069–1085. [Google Scholar] [CrossRef] [PubMed]
- Müller, B.S.F.; Sakamoto, T.; Silveira, R.D.D.; Zambussi-Carvalho, P.F.; Pereira, M.; Pappas, G.J., Jr.; Costa, M.M.C.; Guimarães, C.M.; Pereira, W.J.; Brondani, C.; et al. Differentially Expressed Genes during Flowering and Grain Filling in Common Bean (Phaseolus vulgaris) Grown under Drought Stress Conditions. Plant Mol. Biol. Report. 2014, 32, 438–451. [Google Scholar] [CrossRef]
- Saeidi, K.; Zare, N.; Baghizadeh, A.; Asghari-Zakaria, R. Phaseolus vulgaris Genome Possesses CAMTA Genes, and phavuCAMTA1 Contributes to the Drought Tolerance. J. Genet. 2019, 98, 31. [Google Scholar] [CrossRef]
- Ling, L.; Zhang, W.; An, Y.; Du, B.; Wang, D.; Guo, C. Genome-Wide Analysis of the TCP Transcription Factor Genes in Five Legume Genomes and Their Response to Salt and Drought Stresses. Funct. Integr. Genom. 2020, 20, 537–550. [Google Scholar] [CrossRef]
- Aygören, A.S.; Aydinyurt, R.; Uçar, S.; Kasapoğlu, A.G.; Yaprak, E.; Öner, B.M.; Muslu, S.; Isiyel, M.; İlhan, E.; Aydin, M.; et al. Genome-Wide Analysis and Characterization of the PIF Gene Family under Salt and Drought Stress in Common Beans (Phaseolus vulgaris L.). Türkiye Tarımsal Araştırmalar Derg. 2022, 9, 274–285. [Google Scholar] [CrossRef]
- CIAT. CIAT Annual Report; Centro Internacional de Agricultura Tropical: Cali, CO, USA, 1983. [Google Scholar]
- Collart, M.A.; Oliviero, S. Preparation of Yeast RNA. Curr. Protoc. Mol. Biol. 1993, 13, 13.12.1–13.12.5. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2017. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 12 October 2017).
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-Length Transcriptome Assembly from RNA-Seq Data without a Reference Genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Vlasova, A.; Capella-Gutiérrez, S.; Rendón-Anaya, M.; Hernández-Oñate, M.; Minoche, A.E.; Erb, I.; Câmara, F.; Prieto-Barja, P.; Corvelo, A.; Sanseverino, W.; et al. Genome and Transcriptome Analysis of the Mesoamerican Common Bean and the Role of Gene Duplications in Establishing Tissue and Temporal Specialization of Genes. Genome Biol. 2016, 17, 32. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Bardou, P.; Mariette, J.; Escudié, F.; Djemiel, C.; Klopp, C. jvenn: An Interactive Venn Diagram Viewer. BMC Bioinform. 2014, 15, 293. [Google Scholar] [CrossRef] [PubMed]
- Kolberg, L.; Raudvere, U.; Kuzmin, I.; Adler, P.; Vilo, J.; Peterson, H. g:Profiler—Interoperable Web Service for Functional Enrichment Analysis and Gene Identifier Mapping (2023 Update). Nucleic Acids Res. 2023, 51, W207–W212. [Google Scholar] [CrossRef]
- Pereira, W.J.; Bassinello, P.Z.; Vianello, R.P. Caracterização de Genes de Referência com Amplo Espectro de Uso em Feijoeiro Comum para Validação de Dados de Expressão Gênica. In Proceedings of the Congresso Brasileiro de Melhoramento de Plantas, Uberlândia, Brazil, 4–8 August 2013; pp. 76–79. [Google Scholar]
- Ferreira, D.F. Sisvar: A Computer Statistical Analysis System. Ciência Agrotecnologia 2011, 35, 1039–1042. [Google Scholar] [CrossRef]
- Joshi, R.; Singh, B.; Bohra, A.; Chinnusamy, V. Salt Stress Signalling Pathways: Specificity and Crosstalk. In Managing Salinity Tolerance in Plants: Molecular and Genomic Perspectives; Wani, S.H., Hossain, M.A., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 51–78. [Google Scholar] [CrossRef]
- Aguado, A.; Capote, N.; Romero, F.; Dodd, I.C.; Colmenero-Flores, J.M. Physiological and Gene Expression Responses of Sunflower (Helianthus annuus L.) Plants Differ According to Irrigation Placement. Plant Sci. 2014, 227, 37–44. [Google Scholar] [CrossRef][Green Version]
- Lu, P.; Magwanga, R.O.; Guo, X.; Kirungu, J.N.; Lu, H.; Cai, X.; Zhou, Z.; Wei, Y.; Wang, X.; Zhang, Z.; et al. Genome-Wide Analysis of Multidrug and Toxic Compound Extrusion (MATE) Family in Gossypium raimondii and Gossypium arboreum and Its Expression Analysis under Salt, Cadmium, and Drought Stress. G3 2018, 8, 2483–2500. [Google Scholar] [CrossRef]
- Lim, C.W.; Baek, W.; Jung, J.; Kim, J.H.; Lee, S.C. Function of ABA in Stomatal Defense Against Biotic and Drought Stresses. Int. J. Mol. Sci. 2015, 16, 15251–15270. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhang, M.; Jia, J.; Zhao, X.; Huang, X.; Ji, E.; Ni, L.; Jiang, M. An Atypical Late Embryogenesis Abundant Protein OsLEA5 Plays a Positive Role in ABA-Induced Antioxidant Defense in Oryza sativa L. Plant Cell Physiol. 2018, 59, 916–929. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Huang, Y.; Tang, N.; Xiong, L. Over-Expression of a LEA Gene in Rice Improves Drought Resistance under the Field Conditions. Theor. Appl. Genet. 2007, 115, 35–46. [Google Scholar] [CrossRef]
- Shiraku, M.L.; Magwanga, R.O.; Zhang, Y.; Hou, Y.; Kirungu, J.N.; Mehari, T.G.; Xu, Y.; Wang, Y.; Wang, K.; Cai, X.; et al. Late Embryogenesis Abundant Gene LEA3 (Gh_A08G0694) Enhances Drought and Salt Stress Tolerance in Cotton. Int. J. Biol. Macromol. 2022, 207, 700–714. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Ma, S.; Zong, W.; Yang, N.; Lv, Y.; Yan, C.; Guo, Z.; Li, J.; Li, X.; Xiang, Y.; et al. MODD Mediates Deactivation and Degradation of OsbZIP46 to Negatively Regulate ABA Signaling and Drought Resistance in Rice. Plant Cell 2016, 28, 2161–2177. [Google Scholar] [CrossRef] [PubMed]
- Hong-Bo, S.; Zong-Suo, L.; Ming-An, S. LEA Proteins in Higher Plants: Structure, Function, Gene Expression and Regulation. Colloids Surf. B Biointerfaces 2005, 45, 131–135. [Google Scholar] [CrossRef]
- Gundaraniya, S.A.; Ambalam, P.S.; Budhwar, R.; Padhiyar, S.M.; Tomar, R.S. Transcriptome Analysis Provides Insights into the Stress Response in Cultivated Peanut (Arachis hypogaea L.) Subjected to Drought-Stress. Mol. Biol. Rep. 2023, 50, 6691–6701. [Google Scholar] [CrossRef]
- Bies-Ethève, N.; Gaubier-Comella, P.; Debures, A.; Lasserre, E.; Jobet, E.; Raynal, M.; Cooke, R.; Delseny, M. Inventory, Evolution and Expression Profiling Diversity of the LEA (Late Embryogenesis Abundant) Protein Gene Family in Arabidopsis thaliana. Plant Mol. Biol. 2008, 67, 107–124. [Google Scholar] [CrossRef]
- Candat, A.; Paszkiewicz, G.; Neveu, M.; Gautier, R.; Logan, D.C.; Avelange-Macherel, M.H.; Macherel, D. The Ubiquitous Distribution of Late Embryogenesis Abundant Proteins across Cell Compartments in Arabidopsis Offers Tailored Protection Against Abiotic Stress. Plant Cell 2014, 26, 3148–3166. [Google Scholar] [CrossRef] [PubMed]
- Geng, W.; Wang, Y.; Zhang, J.; Liu, Z.; Chen, X.; Qin, L.; Yang, L.; Tang, H. Genome-Wide Identification and Expression Analyses of Late Embryogenesis Abundant (LEA) Gene Family in Tobacco (Nicotiana tabacum L.) Reveal Their Function in Abiotic Stress Responses. Gene 2022, 836, 146665. [Google Scholar] [CrossRef] [PubMed]
- Kavar, T.; Maras, M.; Kidrič, M.; Šuštar-Vozlič, J.; Meglič, V. Identification of Genes Involved in the Response of Leaves of Phaseolus vulgaris to Drought Stress. Mol. Breed. 2008, 21, 159–172. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Zhang, B.; Yi, J.; Yang, Y.; Kong, C.; Lei, C.; Gong, M. The Role of the Late Embryogenesis-Abundant (LEA) Protein Family in Development and the Abiotic Stress Response: A Comprehensive Expression Analysis of Potato (Solanum tuberosum). Genes 2019, 10, 148. [Google Scholar] [CrossRef]
- Mertens, J.; Aliyu, H.; Cowan, D.A. LEA Proteins and the Evolution of the WHy Domain. Appl. Environ. Microbiol. 2018, 84, e00539-18. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ropero, F.; Van Der Vegt, N.F. Direct Osmolyte-Macromolecule Interactions Confer Entropic Stability to Folded States. J. Phys. Chem. 2014, 118, 7327–7334. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhou, H.; Shi, X.; Yu, B.; Zhou, Y.; Chen, S.L.; Wang, Y.; Peng, Y.; Meyer, R.C.; Smeekens, S.C.; et al. The ABI4-Induced Arabidopsis ANAC060 Transcription Factor Attenuates ABA Signaling and Renders Seedlings Sugar Insensitive When Present in the Nucleus. PLoS Genet. 2014, 10, e1004213. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, Q.; Wu, J.; Wang, Z.Q.; Geng, Y.J.; Li, J.; Zhang, Y.; Li, S. Arabidopsis Calcineurin B-Like-Interacting Protein Kinase 8 and Its Functional Homolog in Tomato Negatively Regulates ABA-Mediated Stomatal Movement and Drought Tolerance. Plant Cell Environ. 2024. ahead to print. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Chen, N.; Song, L.; Wang, D.; Cai, H.; Yao, L.; Li, X.; Guo, C. Alfalfa (Medicago sativa L.) MsCML46 Gene Encoding Calmodulin-Like Protein Confers Tolerance to Abiotic Stress in Tobacco. Plant Cell Rep. 2021, 40, 1907–1922. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Li, J.; Yang, W.; Ci, J.; Ren, X.; Wang, W.; Wang, Y.; Jiang, L.; Yang, W. Identification and Expression Analysis Revealed Drought Stress-Responsive Calmodulin and Calmodulin-Like Genes in Maize. J. Plants Interact. 2022, 17, 450–461. [Google Scholar] [CrossRef]
- Meer, L.; Mumtaz, S.; Labbo, A.M.; Khan, M.J.; Sadiq, I. Genome-Wide Identification and Expression Analysis of Calmodulin-Binding Transcription Activator Genes in Banana under Drought Stress. Sci. Hortic. 2019, 244, 10–14. [Google Scholar] [CrossRef]
- Rahman, H.; Ramanathan, V.; Nallathambi, J.; Duraialagaraja, S.; Muthurajan, R. Over-Expression of a NAC 67 Transcription Factor from Finger Millet (Eleusine coracana L.) Confers Tolerance Against Salinity and Drought Stress in Rice. BMC Biotechnol. 2016, 16, 35. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cheng, X.; Liu, X.; Wu, H.; Bi, H.; Xu, H. The Wheat MYB Transcription Factor TaMYB31 Is Involved in Drought Stress Responses in Arabidopsis. Front. Plant Sci. 2018, 9, 1426. [Google Scholar] [CrossRef]
- Zhou, Y.; Zeng, L.; Chen, R.; Wang, Y.; Song, J. Genome-Wide Identification and Characterization of Stress-Associated Protein (SAP) Gene Family Encoding A20/AN1 Zinc-Finger Proteins in Medicago truncatula. Arch. Biol. Sci. 2018, 70, 87–98. [Google Scholar] [CrossRef]
- Sharma, G.; Giri, J.; Tyagi, A.K. Rice OsiSAP7 Negatively Regulates ABA Stress Signalling and Imparts Sensitivity to Water-Deficit Stress in Arabidopsis. Plant Sci. 2015, 237, 80–92. [Google Scholar] [CrossRef]
- Saad, R.B.; Safi, H.; Hsouna, A.B.; Brini, F.; Romdhane, W.B. Functional Domain Analysis of LmSAP Protein Reveals the Crucial Role of the Zinc-Finger A20 Domain in Abiotic Stress Tolerance. Protoplasma 2019, 256, 1333–1344. [Google Scholar] [CrossRef]
- Qiu, Y.; Yu, D. Over-Expression of the Stress-Induced OsWRKY45 Enhances Disease Resistance and Drought Tolerance in Arabidopsis. Environ. Exp. Bot. 2009, 65, 35–47. [Google Scholar] [CrossRef]
- Tao, Z.; Kou, Y.; Liu, H.; Li, X.; Xiao, J.; Wang, S. OsWRKY45 Alleles Play Different Roles in Abscisic Acid Signalling and Salt Stress Tolerance but Similar Roles in Drought and Cold Tolerance in Rice. J. Exp. Bot. 2011, 62, 4863–4874. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Zhao, Y.; Wang, Y.; Lin, Y.; Peng, X.; Li, Q.; Chang, Y.; Jiang, H.; Xiang, Y.; Cheng, B. Overexpression of a Maize Wrky58 Gene Enhances Drought and Salt Tolerance in Transgenic Rice. Plant Cell Tissue Organ Cult. 2014, 119, 565–577. [Google Scholar] [CrossRef]
- Kiranmai, K.; Rao, G.L.; Pandurangaiah, M.; Nareshkumar, A.; Reddy, V.A.; Lokesh, U.; Venkatesh, B.; Johnson, A.M.A.; Sudhakar, C. A Novel WRKY Transcription Factor, MuWRKY3 (Macrotyloma uniflorum Lam. Verdc.) Enhances Drought Stress Tolerance in Transgenic Groundnut (Arachis hypogaea L.) Plants. Front. Plant Sci. 2018, 9, 346. [Google Scholar] [CrossRef]
- Venkatesh, B.; Vennapusa, A.R.; Kumar, N.J.; Jayamma, N.; Reddy, B.M.; Johnson, A.M.A.; Madhusudan, K.V.; Pandurangaiah, M.; Kiranmai, K.; Sudhakar, C. Co-Expression of Stress-Responsive Regulatory Genes, MuNAC4, MuWRKY3 and MuMYB96 Associated with Resistant-Traits Improves Drought Adaptation in Transgenic Groundnut (Arachis hypogaea L.) Plants. Front. Plant Sci. 2022, 13, 1055851. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, B.; Li, X.; Wei, Y.; He, C.; Shi, H. MaWRKY80 Positively Regulates Plant Drought Stress Resistance through Modulation of Abscisic Acid and Redox Metabolism. Plant Physiol. Biochem. 2020, 156, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, Y.; Ma, J.; Su, S.; Chen, L.; Cheng, Y.; Buter, S.; Zhao, X.; Yi, L.; Lu, Z. Analyzing the Diversity of MYB Family Response Strategies to Drought Stress in Different Flax Varieties Based on Transcriptome Data. Plants 2024, 13, 710. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Suh, M.C. Advances in the understanding of Cuticular Waxes in Arabidopsis thaliana and Crop Species. Plant Cell Rep. 2015, 34, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Brosché, M.; Lehtonen, M.T.; Amiryousefi, A.; Xu, E.; Punkkinen, M.; Valkonen, J.P.; Fujii, H.; Overmyer, K. Dissecting Abscisic Acid Signaling Pathways Involved in Cuticle Formation. Mol. Plant 2016, 9, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Jäger, K.; Fábián, A.; Eitel, G.; Szabó, L.; Deák, C.; Barnabás, B.; Papp, I. A Morpho-Physiological Approach Differentiates Bread Wheat Cultivars of Contrasting Tolerance under Cyclic Water Stress. J. Plant Physiol. 2014, 171, 1256–1266. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Feng, P.; Zhang, X.; Xie, Q.; Chen, G.; Zhou, S.; Hu, Z. Silencing of SlMYB50 Affects Tolerance to Drought and Salt Stress in Tomato. Plant Physiol. Biochem. 2022, 193, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Yin, Y.; Chen, F.; Xu, Y.; Dixon, R.A. A Bioinformatic Analysis of NAC Genes for Plant Cell Wall Development in Relation to Lignocellulosic Bioenergy Production. Bioenergy Res. 2009, 2, 217–232. [Google Scholar] [CrossRef]
- Wu, Y.; Hou, J.; Yu, F.; Nguyen, S.T.T.; Mccurdy, D.W. Transcript Profiling Identifies NAC-Domain Genes Involved in Regulating Wall Ingrowth Deposition in Phloem Parenchyma Transfer Cells of Arabidopsis thaliana. Front. Plant Sci. 2018, 9, 341. [Google Scholar] [CrossRef]
- Liu, J.; Qiao, Y.; Li, C.; Hou, B. The NAC Transcription Factors Play Core Roles in Flowering and Ripening Fundamental to Fruit Yield and Quality. Front. Plant Sci. 2023, 14, 1095967. [Google Scholar] [CrossRef]
- Shao, H.; Wang, H.; Tang, X. NAC Transcription Factors in Plant Multiple Abiotic Stress Responses: Progress and Prospects. Front. Plant Sci. 2015, 6, 902. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Peng, Z.; Xu, P.; Tang, G.; Ma, C.; Zhu, J.; Shan, L.; Wan, S. Genome-Wide Identification of NAC Transcription Factors and Their Functional Prediction of Abiotic Stress Response in Peanut. Front. Genet. 2021, 12, 630292. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, F.; Yao, Z.; Zhao, X.; Chu, G.; Ye, J. Comprehensive Genomic Characterisation of the NAC Transcription Factor Family and Its Response to Drought Stress in Eucommia ulmoides. PeerJ 2023, 11, e16298. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, L.; Wang, S. Comprehensive Analysis and Discovery of Drought-Related NAC Transcription Factors in Common Bean. BMC Plant Biol. 2016, 16, 193. [Google Scholar] [CrossRef] [PubMed]
- Negi, S.; Tak, H.; Ganapathi, T.R. A Banana NAC Transcription Factor (MusaSNAC1) Impart Drought Tolerance by Modulating Stomatal Closure and H2O2 Content. Plant Mol. Biol. 2018, 96, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.L.; Yue, X.F.; Min, Z.; Wang, X.H.; Fang, Y.L.; Zhang, J.X. VvNAC17, a Novel Stress-Responsive Grapevine (Vitis vinifera L.) NAC Transcription Factor, Increases Sensitivity to Abscisic Acid and enhances Salinity, Freezing, and Drought Tolerance in Transgenic Arabidopsis. Plant Physiol. Biochem. 2020, 46, 98–111. [Google Scholar] [CrossRef]
- So, H.A.; Lee, J.H. NAC Transcription Factors from Soybean (Glycine max L.) Differentially Regulated by Abiotic Stress. J. Plant Biol. 2019, 62, 147–160. [Google Scholar] [CrossRef]
- Shekoofa, A.; Sinclair, T.R. Aquaporin Activity to Improve Crop Drought Tolerance. Cells 2018, 7, 123. [Google Scholar] [CrossRef]
- Pou, A.; Medrano, H.; Flexas, J.; Tyerman, S.D. A Putative Role for TIP and PIP Aquaporins in Dynamics of Leaf Hydraulic and Stomatal Conductances in Grapevine under Water Stress and Re-Watering. Plant Cell Environ. 2013, 36, 828–843. [Google Scholar] [CrossRef]
- Li, H.; Jiang, H.; Bu, Q.; Zhao, Q.; Sun, J.; Xie, Q.; Li, C. The Arabidopsis RING Finger E3 Ligase RHA2b Acts Additively with RHA2a in Regulating Abscisic Acid Signaling and Drought Response. Plant Physiol. 2011, 156, 550–563. [Google Scholar] [CrossRef]
- Zhang, C.; Hou, Y.; Hao, Q.; Chen, H.; Chen, L.; Yuan, S.; Shan, Z.; Zhang, X.; Yang, Z.; Qiu, D.; et al. Genome-Wide Survey of the Soybean GATA Transcription Factor Gene Family and Expression Analysis under Low Nitrogen Stress. PLoS ONE 2015, 10, e0125174. [Google Scholar] [CrossRef]
- Miricescu, A.; Goslin, K.; Graciet, E. Ubiquitylation in Plants: Signaling Hub for the Integration of Environmental Signals. J. Exp. Bot. 2018, 69, 4511–4527. [Google Scholar] [CrossRef]
- Xu, F.Q.; Xue, H.W. The Ubiquitin-Proteasome System in Plant Responses to Environments. Plant Cell Environ. 2019, 42, 2931–2944. [Google Scholar] [CrossRef]
- Yu, S.G.; Kim, J.H.; Cho, N.H.; Oh, T.R.; Kim, W.T. Arabidopsis RING E3 Ubiquitin Ligase JUL1 Participates in ABA-Mediated Microtubule Depolymerization, Stomatal Closure, and Tolerance Response to Drought Stress. Plant J. 2020, 103, 824–842. [Google Scholar] [CrossRef]
- Jing, Q.; Chen, A.; Lv, Z.; Dong, Z.; Wang, L.; Meng, X.; Feng, Y.; Wan, Y.; Su, C.; Cui, Y.; et al. Systematic Analysis of Galactinol Synthase and Raffinose Synthase Gene Families in Potato and Their Expression Patterns in Development and Abiotic Stress Responses. Genes 2023, 14, 1344. [Google Scholar] [CrossRef]
- Knaupp, M.; Mishra, K.B.; Nedbal, L.; Heyer, A.G. Evidence for a Role of Raffinose in Stabilizing Photosystem II during Freeze–Thaw Cycles. Planta 2011, 234, 477–486. [Google Scholar] [CrossRef]
- Salvi, P.; Saxena, S.C.; Petla, B.P.; Kamble, N.U.; Kaur, H.; Verma, P.; Rao, V.; Ghosh, S.; Majee, M. Differentially Expressed Galactinol Synthase(s) in Chickpea Are Implicated in Seed Vigor and Longevity by Limiting the Age Induced ROS Accumulation. Sci. Rep. 2016, 6, 35088. [Google Scholar] [CrossRef]
- Nishizawa-Yokoi, A.; Yabuta, Y.; Shigeoka, S. The Contribution of Carbohydrates Including Raffinose Family Oligosaccharides and Sugar Alcohols to Protection of Plant Cells from Oxidative Damage. Plant Signal. Behav. 2008, 3, 1016–1018. [Google Scholar] [CrossRef]
- Qi, J.; Song, C.P.; Wang, B.; Zhou, J.; Kangasjarvi, J.; Zhu, J.K.; Gong, Z. Reactive Oxygen Species Signaling and Stomatal Movement in Plant Responses to Drought Stress and Pathogen Attack. Int. J. Plant Biol. 2018, 60, 805–826. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, M.Q.; Yu, D. Oxygen Enriched Co-Combustion Characteristics of Herbaceous Biomass and Bituminous Coal. chim. Acta 2013, 569, 17–24. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, D.; Cao, L.; Zhang, W.; Zheng, H.; Liu, Z.; Han, S.; Dong, Y.; Zhu, F.; Liu, H.; et al. Functions and Regulatory Framework of ZmNST3 in Maize under Lodging and Drought Stress. Plant Cell Environ. 2020, 43, 2272–2286. [Google Scholar] [CrossRef]
- Zhang, Y.; He, J.; Xiao, Y.; Zhang, Y.; Liu, Y.; Wan, S.; Liu, L.; Dong, Y.; Liu, H.; Yu, Y. CsGSTU8, a Glutathione S-Transferase from Camellia sinensis, Is Regulated by CsWRKY48 and Plays a Positive Role in Drought Tolerance. Front. Plant Sci. 2021, 12, 795919. [Google Scholar] [CrossRef]
- Molla, M.R.; Ali, M.R.; Hasanuzzaman, M.; Al-Mamun, M.H.; Ahmed, A.; Nazim-Ud-Dowla, M.A.N.; Rohman, M.M. Exogenous Proline and Betaine-Induced Upregulation of Glutathione Transferase and Glyoxalase I in Lentil (Lens culinaris) under Drought Stress. Not. Bot. Horti Agrobot. Cluj-Napoca 2014, 42, 73–80. [Google Scholar] [CrossRef]
- Mizutani, M.; Sato, F. Unusual P450 Reactions in Plant Secondary Metabolism. Arch. Biochem. Biophys. Res. Commun. 2011, 507, 194–203. [Google Scholar] [CrossRef]
- Yan, Q.; Cui, X.; Lin, S.; Gan, S.; Xing, H.; Dou, D. GmCYP82A3, a Soybean Cytochrome P450 Family Gene Involved in the Jasmonic Acid and Ethylene Signaling Pathway, Enhances Plant Resistance to Biotic and Abiotic Stresses. PLoS ONE 2016, 11, e0162253. [Google Scholar] [CrossRef]
- Rao, M.J.; Xu, Y.; Tang, X.; Huang, Y.; Liu, J.; Deng, X.; Xu, Q. CsCYT75B1, a Citrus Cytochrome P450 Gene, Is Involved in Accumulation of Antioxidant Flavonoids and Induces Drought Tolerance in Transgenic Arabidopsis. Antioxidants 2020, 9, 161. [Google Scholar] [CrossRef]
- Wei, K.; Chen, H. Global Identification, Structural Analysis and Expression Characterization of Cytochrome P450 Monooxygenase Superfamily in Rice. BMC Genom. 2018, 19, 35. [Google Scholar] [CrossRef]
- Laloi, C.; Mestres-Ortega, D.; Marco, Y.; Meyer, Y.; Reichheld, J.P. The Arabidopsis cytosolic thioredoxin h5 Gene Induction by Oxidative Stress and Its W-Box-Mediated Response to Pathogen Elicitor. Plant Physiol. 2004, 134, 1006–1016. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Fisiologia Vegetal, 3rd ed.; Artmed: Guelph, ON, Canada, 2004; 719p. [Google Scholar]
- Holmgren, A. Thioredoxin. Annu. Rev. Biochem. 1985, 54, 237–271. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Pereira, P.; Daloso, D.M.; Gago, J.; Nunes-Nesi, A.; Araújo, W.L. On the Role of the Plant Mitochondrial Thioredoxin System during Abiotic Stress. Plant Signal. Behav. 2019, 14, 1592536. [Google Scholar] [CrossRef] [PubMed]
- Zagorchev, L.; Teofanova, D.; Odjakova, M. Ascorbate–Glutathione Cycle: Controlling the Redox Environment for Drought Tolerance. In Drought Stress Tolerance in Plants, Volume 1; Hossain, M., Wani, S., Bhattacharjee, S., Burritt, D., Tran, L.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
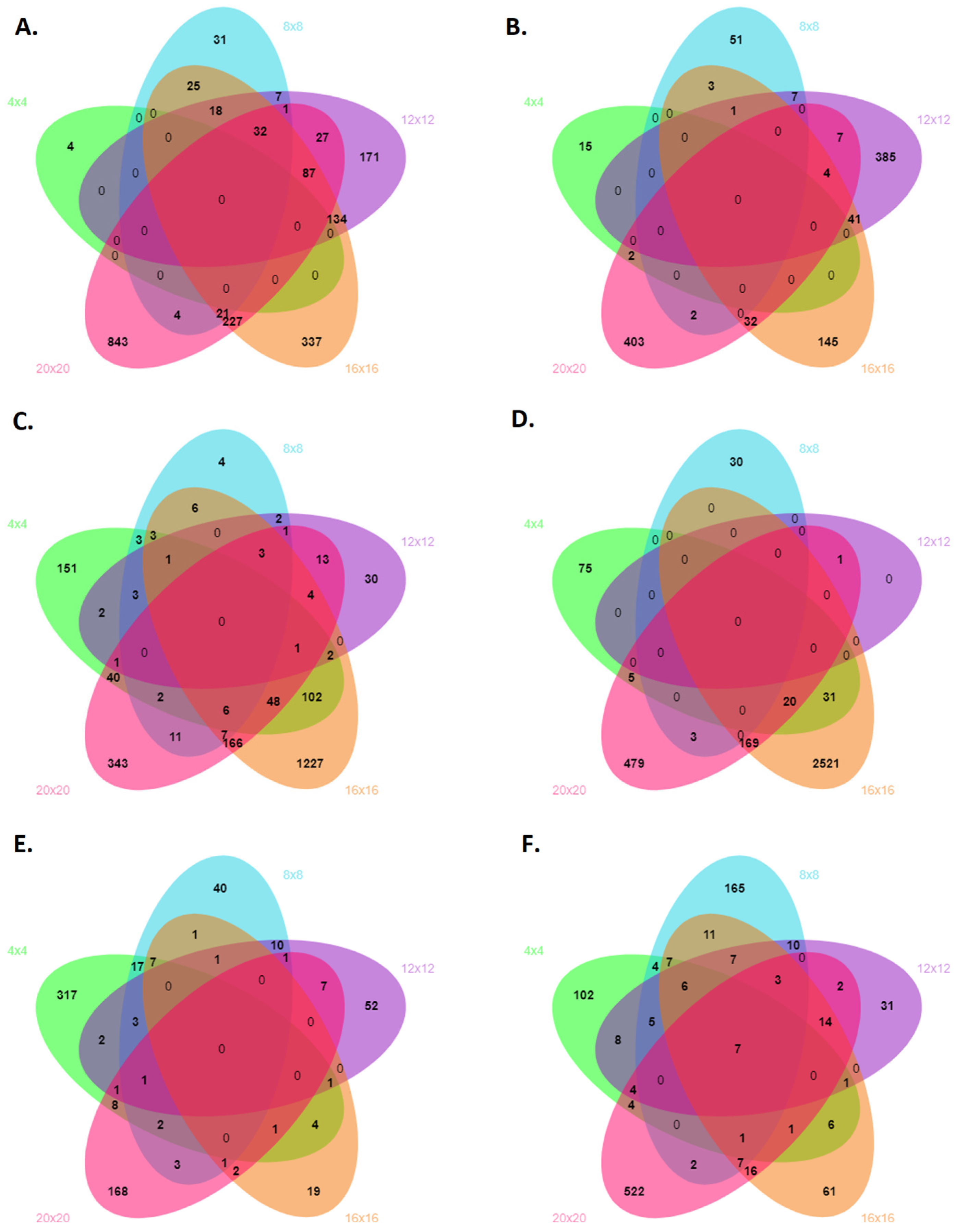
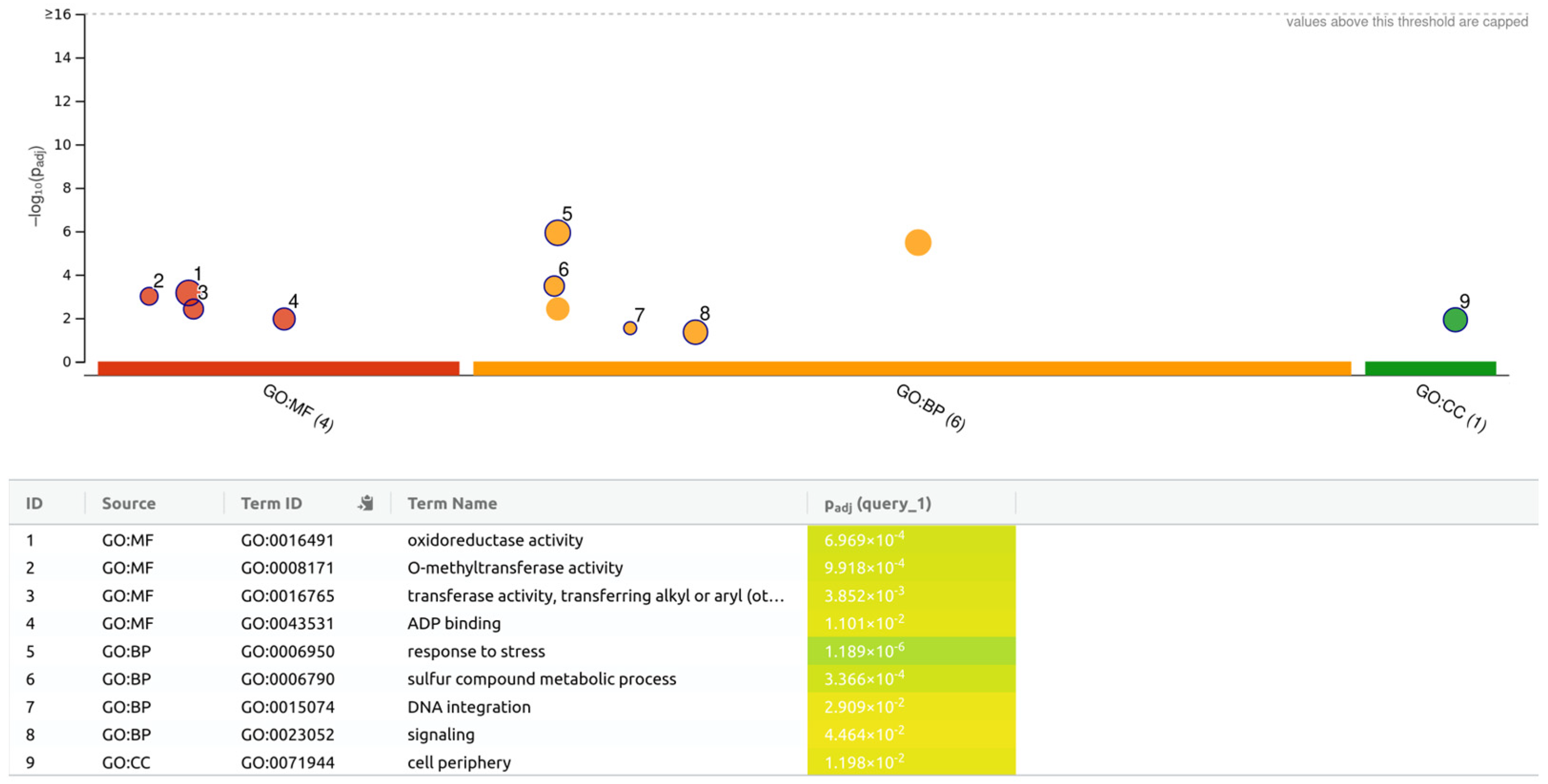
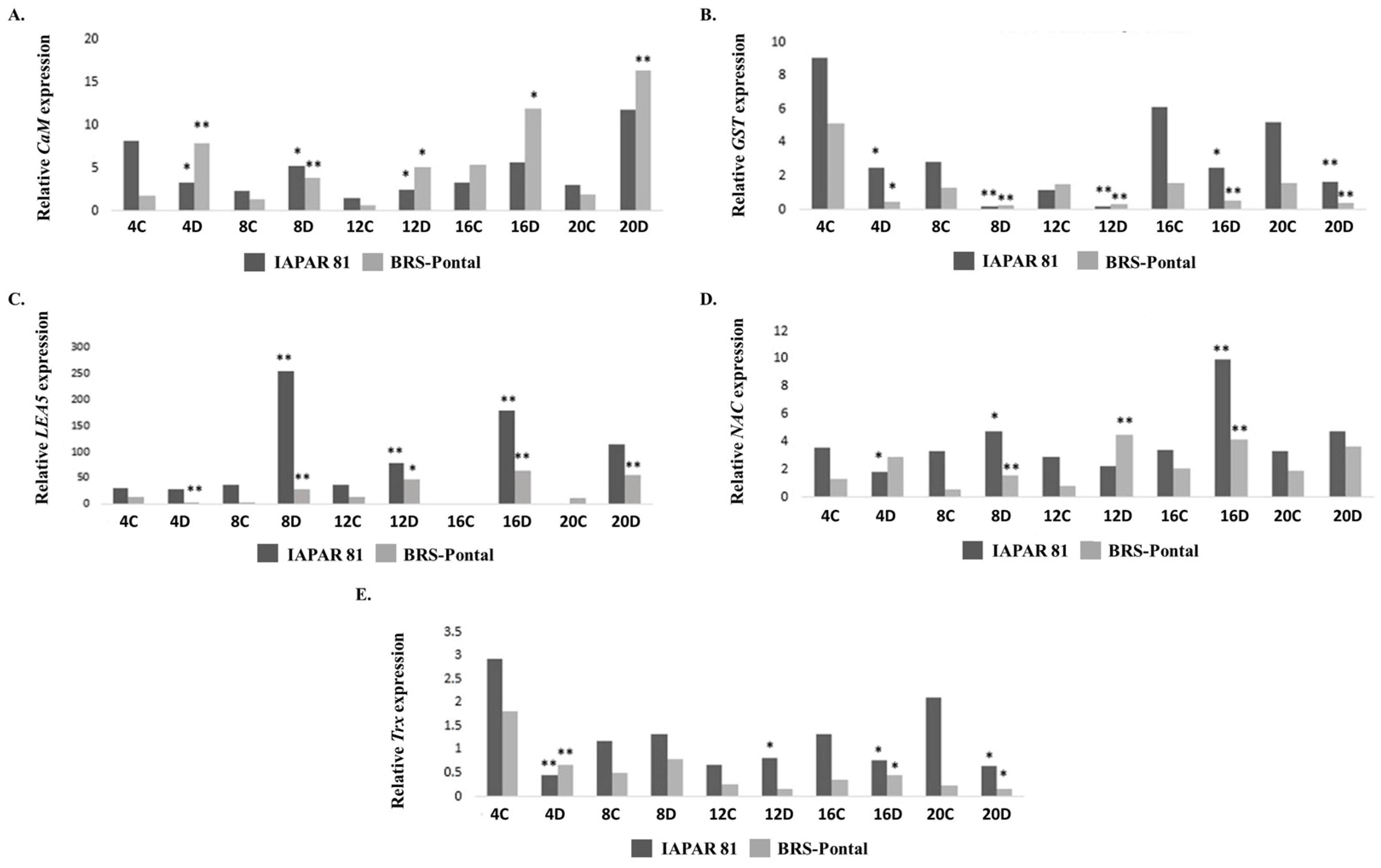
| Cultivar | Number of DEGs | 4 Days | 8 Days | 12 Days | 16 Days | 20 Days | Total |
|---|---|---|---|---|---|---|---|
| BRS-Pontal | Annotated | 21 | 203 | 922 | 1107 | 692 | 2945 |
| Upregulated | 17 | 64 | 445 | 226 | 450 | 1202 | |
| Downregulated | 4 | 139 | 477 | 881 | 242 | 1743 | |
| IAPAR 81 | Annotated | 496 | 85 | 64 | 4317 | 1323 | 6285 |
| Upregulated | 131 | 33 | 1 | 2741 | 677 | 3583 | |
| Downregulated | 365 | 52 | 63 | 1576 | 646 | 2702 | |
| BRS-Pontal vs. IAPAR 81 | Annotated | 520 | 322 | 177 | 185 | 778 | 1982 |
| Upregulated | 156 | 235 | 98 | 148 | 583 | 1220 | |
| Downregulated | 364 | 87 | 79 | 37 | 195 | 762 |
| Regulation at (Fold Change) | ||||||
|---|---|---|---|---|---|---|
| Cultivar | DEG | 4 Days | 8 Days | 12 Days | 16 Days | 20 Days |
| BRS-Pontal | Bifunctional Inhibitor/Plant Lipid Transfer Protein/Seed Storage Helical Domain-Containing Protein (V7CA38) | - | −2.8 | −3.0 | −2.5 | - |
| Peroxidase (V7BP88) | - | - | −3.5 | −3.4 | −3.6 | |
| Non-Specific Serine/Threonine Protein Kinase (V7CDN2) | - | 4.5 | 3.1 | 4.3 | 3.2 | |
| Cysteine-Rich Transmembrane Cystm Domain-Containing Protein (V7BXH8) | - | 2.1 | 2.5 | 3.7 | 5.4 | |
| NAC Domain-Containing Protein 72-Like Isoform 1 (T2DP29) | - | 2.4 | 2.3 | 2.6 | 5.3 | |
| Elongation Factor 1-Alpha(V5N8W1) | - | 6.1 | 5.2 | 5.6 | 6.2 | |
| ADF-H Domain-Containing Protein (V7BFY8) | - | 4.0 | 7.2 | 5.5 | 3.4 | |
| IAPAR 81 | RACK (B7T1N8) | 6.9 | 5.7 | 5.7 | 4.8 | - |
| TR-Type G Domain-Containing Protein (V7BXI7) | 5.4 | 5.6 | 4.9 | - | - | |
| Elongation Factor 1-Alpha(Fragment)(A7l3u9) | 6.3 | 4.1 | 5.2 | - | - | |
| Fatty Acid Hydroxylase Domain-Containing Protein (V7AP25) | 7.2 | 9.0 | 7.7 | - | - | |
| Pectinesterase (V7BKF6) | 4.1 | 3.6 | - | - | 3.9 | |
| Glucan Endo-1,3-Beta-D-Glucosidase (V7CVL7) | −6.3 | - | - | −8.4 | −2.3 | |
| Microtubule-Associated Protein 70-5 (V7BRH0) | −3.1 | - | - | −2.8 | −5.5 | |
| Peroxidase (V7BP88) | −2.9 | - | - | −5.8 | −5.8 | |
| Asparagine Synthetase [Glutamine-Hydrolyzing] (A9XS73) | −2.1 | - | - | −3.9 | −2.6 | |
| Hexosyltransferase (V7BA81) | −2.1 | - | - | −2.0 | −2.3 | |
| BRS-Pontal vs. IAPAR 81 | DUF4005 Domain-Containing Protein (V7C9T5) | 4.9 | 3.6 | 4.2 | - | 2.2 |
| Aminotransferase-Like Plant Mobile Domain-Containing Protein (V7C5U2) | 2.4 | - | - | 2.3 | 2.2 | |
| TIR Domain-Containing Protein (V7AKT3) | 4.5 | 7.3 | - | 5.8 | - | |
| LRGB-Like Protein (V7CJ50) | 3.4 | 2.0 | - | 3.0 | - | |
| NADP-Dependent Oxidoreductase Domain-Containing Protein (V7APC5) | 4.2 | 3.4 | - | 3.0 | - | |
| FAS1 Domain-Containing Protein (V7BYV1) | −12.8 | −7.6 | −10.4 | −9.2 | - | |
| O-Methyltransferase Domain-Containing Protein (V7C4M2) | −3.8 | −2.7 | −2.3 | −2.7 | - | |
| DUF4283 Domain-Containing Protein (V7CB55) | −9.7 | −8.2 | −9.0 | −7.9 | −7.3 | |
| Calmodulin Binding Protein (Fragment) (V7BIM0) | −6.8 | −5.3 | −5.3 | −5.2 | −5.5 | |
| NB-ARC Domain-Containing Protein (V7AF13) | −4.2 | −5.7 | −4.9 | −5.1 | −3.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponce, T.P.; Bugança, M.d.S.; da Silva, V.S.; de Souza, R.F.; Moda-Cirino, V.; Tomaz, J.P. Differential Gene Expression in Contrasting Common Bean Cultivars for Drought Tolerance during an Extended Dry Period. Genes 2024, 15, 935. https://doi.org/10.3390/genes15070935
Ponce TP, Bugança MdS, da Silva VS, de Souza RF, Moda-Cirino V, Tomaz JP. Differential Gene Expression in Contrasting Common Bean Cultivars for Drought Tolerance during an Extended Dry Period. Genes. 2024; 15(7):935. https://doi.org/10.3390/genes15070935
Chicago/Turabian StylePonce, Talita Pijus, Michely da Silva Bugança, Victória Stern da Silva, Rogério Fernandes de Souza, Vânia Moda-Cirino, and Juarez Pires Tomaz. 2024. "Differential Gene Expression in Contrasting Common Bean Cultivars for Drought Tolerance during an Extended Dry Period" Genes 15, no. 7: 935. https://doi.org/10.3390/genes15070935
APA StylePonce, T. P., Bugança, M. d. S., da Silva, V. S., de Souza, R. F., Moda-Cirino, V., & Tomaz, J. P. (2024). Differential Gene Expression in Contrasting Common Bean Cultivars for Drought Tolerance during an Extended Dry Period. Genes, 15(7), 935. https://doi.org/10.3390/genes15070935






