Genome-Wide Identification and Characterization of CCT Gene Family from Microalgae to Legumes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Identification of CCT Proteins
2.2. Phylogenetic Analysis
2.3. Motif Composition and Gene Structure Analysis
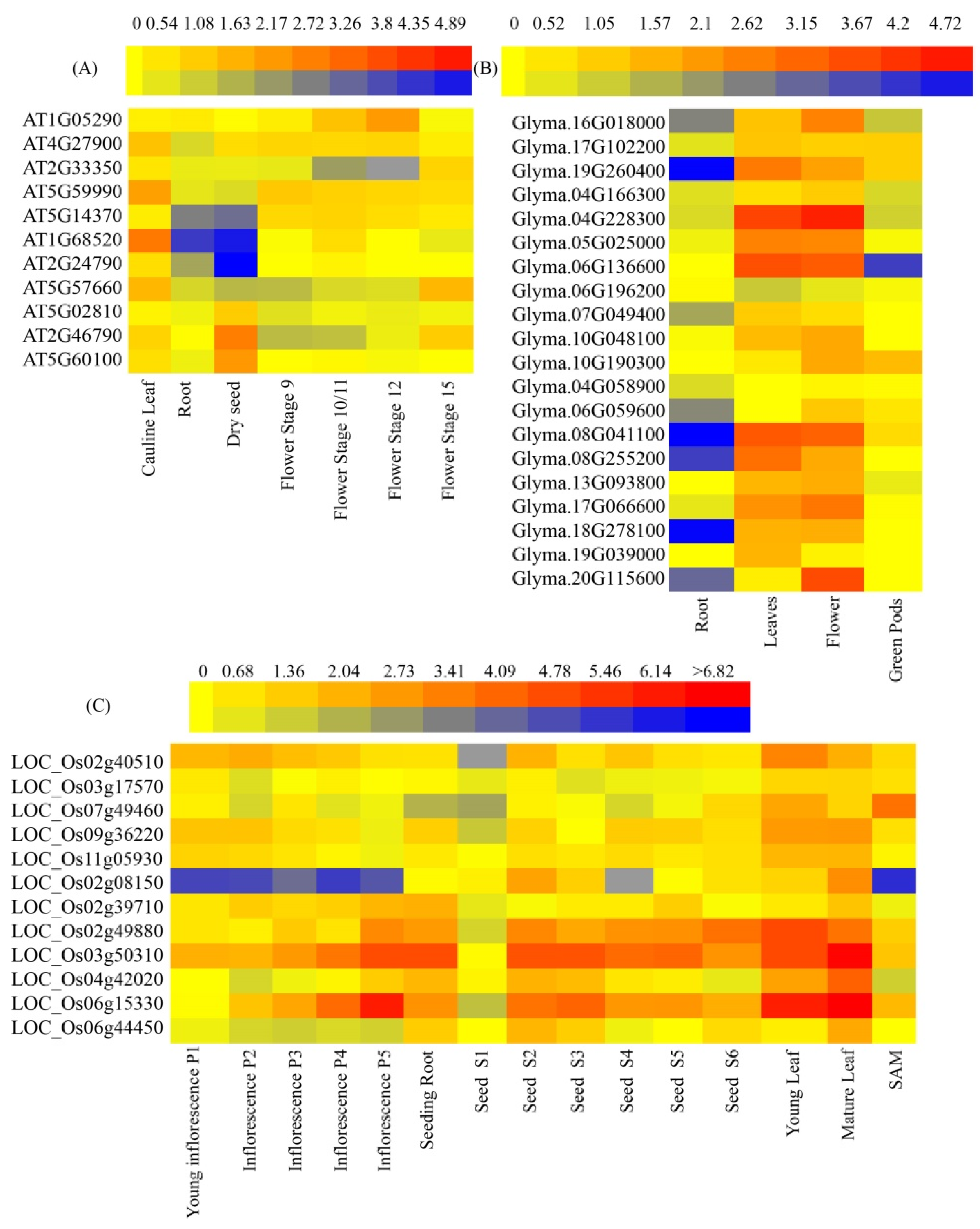
2.4. Gene Expression Analysis
2.5. Chromosome Location, Synteny Analysis, and Selective Force Analysis
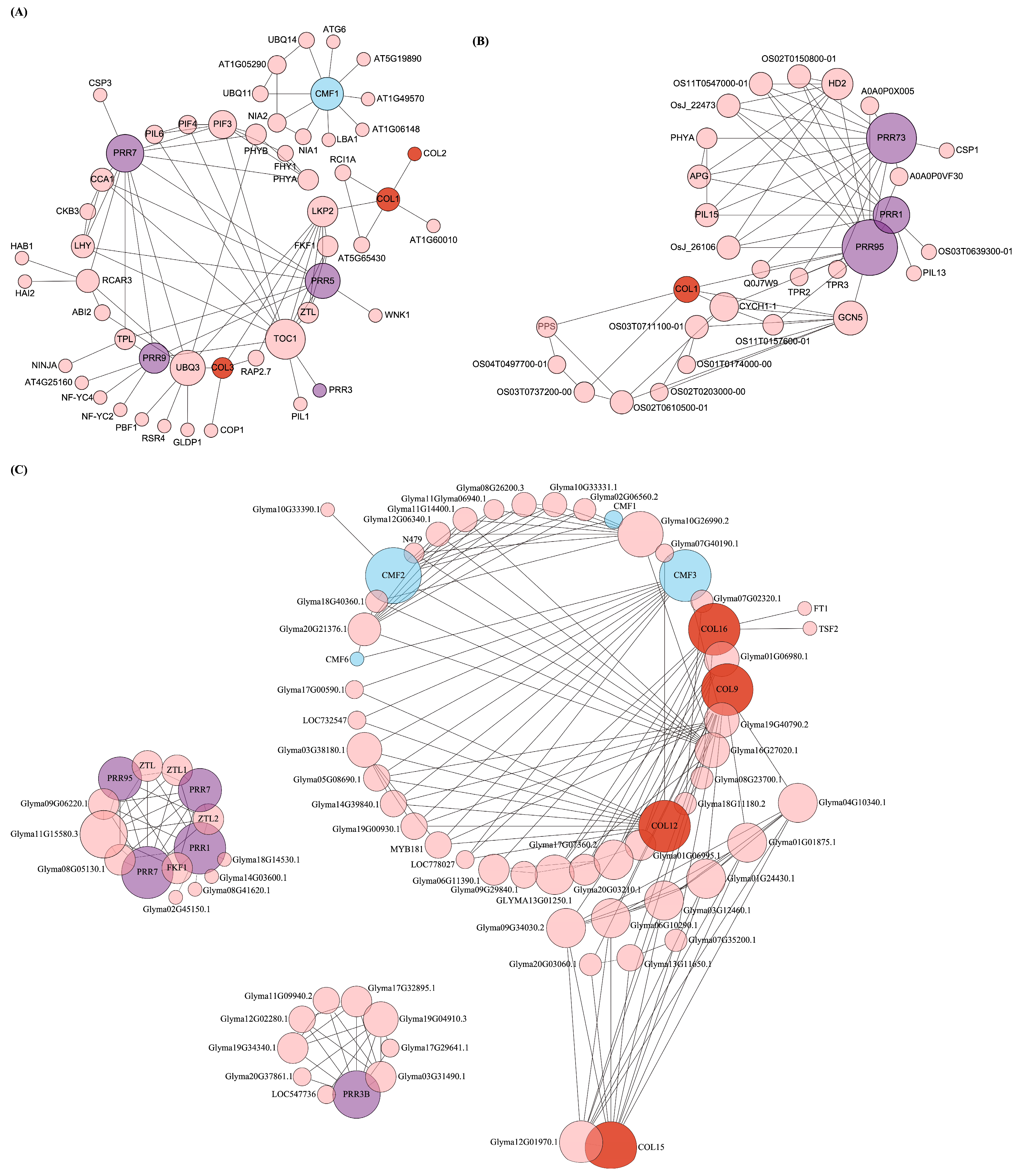
2.6. Protein–Protein Interaction Networks Analysis
3. Results
3.1. Comprehensive Identification and Distribution of CCT Genes in Plants
3.2. Gene Structure, Conserved Domain and Phylogenetic Relationship of CCT Genes
3.3. Expansion Pattern of CCT Genes and Collinearity Analysis
3.4. Expression Patterns of CCTs in Plants
3.5. Analysis of CCT Protein–Protein Interaction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, W.; Liu, H.; Zhou, X.; Li, Q.; Zhang, J.; Lu, L.; Liu, T.; Liu, H.; Zhang, C.; Zhang, Z.; et al. Natural variation in Ghd7.1 plays an important role in grain yield and adaptation in rice. Cell Res. 2013, 23, 969–971. [Google Scholar] [CrossRef]
- Liu, C.; Qu, X.; Zhou, Y.; Song, G.; Abiri, N.; Xiao, Y.; Liang, F.; Jiang, D.; Hu, Z.; Yang, D. OsPRR37 confers an expanded regulation of the diurnal rhythms of the transcriptome and photoperiodic flowering pathways in rice. Plant Cell Environ. 2018, 41, 630–645. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, M. CCT family genes in cereal crops: A current overview. Crop J. 2017, 5, 449–458. [Google Scholar] [CrossRef]
- Utterill, J.; Robson, F.; Lee, K.; Simon, R.; Coupland, G. The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell 1995, 80, 847–857. [Google Scholar] [CrossRef]
- Robson, F.; Costa, M.M.; Hepworth, S.R.; Vizir, I.; Piñeiro, M.; Reeves, P.H.; Putterill, J.; Coupland, G. Functional importance of conserved domains in the flowering-time gene CONSTANS demonstrated by analysis of mutant alleles and transgenic plants. Plant J. 2001, 28, 619–631. [Google Scholar] [CrossRef]
- Wenkel, S.; Turck, F.; Singer, K.; Gissot, L.; Le Gourrierec, J.; Samach, A.; Coupland, G. CONSTANS and the CCAAT box binding complex share a functionally important domain and interact to regulate flowering of Arabidopsis. Plant Cell 2006, 18, 2971–2984. [Google Scholar] [CrossRef] [PubMed]
- Turck, F.; Fornara, F.; Coupland, G. Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annu. Rev. Plant Biol. 2008, 59, 573–594. [Google Scholar] [CrossRef]
- Mizuno, T.; Nakamichi, N. Pseudo-Response Regulators (PRRs) or True Oscillator Components (TOCs). Plant Cell Physiol. 2005, 46, 677–685. [Google Scholar] [CrossRef]
- Matsushika, A.; Makino, S.; Kojima, M.; Mizuno, T. Circadian waves of expression of the APRR1/TOC1 family of pseudo-response regulators in Arabidopsis thaliana: Insight into the plant circadian clock. Plant Cell Physiol. 2000, 41, 1002–1012. [Google Scholar] [CrossRef]
- Yan, L.; Loukoianov, A.; Blechl, A.; Tranquilli, G.; Ramakrishna, W.; SanMiguel, P.; Bennetzen, J.L.; Echenique, V.; Dubcovsky, J. The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 2004, 303, 1640–1644. [Google Scholar] [CrossRef]
- Jin, M.; Liu, X.; Jia, W.; Liu, H.; Li, W.; Peng, Y.; Du, Y.; Wang, Y.; Yin, Y.; Zhang, X.; et al. ZmCOL3, a CCT gene represses flowering in maize by interfering with the circadian clock and activating expression of ZmCCT. J. Integr. Plant Biol. 2018, 60, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Q.; Dong, H.; He, Q.; Liang, L.; Tan, C.; Han, Z.; Yao, W.; Li, G.; Zhao, H.; et al. Three CCT domain-containing genes were identified to regulate heading date by candidate gene-based association mapping and transformation in rice. Sci. Rep. 2015, 5, 7663. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Yun, C.H.; Lee, J.H.; Jang, Y.H.; Park, H.Y.; Kim, J.K. OsCO3, a CONSTANS-LIKE gene, controls flowering by negatively regulating the expression of FT-like genes under SD conditions in rice. Planta 2008, 228, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Xing, Y.; Weng, X.; Zhao, Y.; Tang, W.; Wang, L.; Zhou, H.; Yu, S.; Xu, C.; Li, X.; et al. Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat. Genet. 2008, 40, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Jeong, D.H.; Lee, D.Y.; Yi, J.; Ryu, C.H.; Kim, S.L.; Jeong, H.J.; Choi, S.C.; Jin, P.; Yang, J.; et al. OsCOL4 is a constitutive flowering repressor upstream of Ehd1 and downstream of OsphyB. Plant J. 2010, 63, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shen, J.; Xu, Y.; Li, X.; Xiao, J.; Xiong, L. Ghd2, a CONSTANS-like gene, confers drought sensitivity through regulation of senescence in rice. J. Exp. Bot. 2016, 67, 5785–5798. [Google Scholar] [CrossRef] [PubMed]
- Strayer, C.; Oyama, T.; Schultz, T.F.; Raman, R.; Somers, D.E.; Más, P.; Panda, S.; Kreps, J.A.; Kay, S.A. Cloning of the Arabidopsis clock gene TOC1, an autoregulatory response regulator homolog. Science 2000, 289, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Alabadí, D.; Oyama, T.; Yanovsky, M.J.; Harmon, F.G.; Más, P.; Kay, S.A. Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science 2001, 293, 880–883. [Google Scholar] [CrossRef] [PubMed]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef]
- Matsushika, A.; Murakami, M.; Ito, S.; Nakamichi, N.; Yamashino, T.; Mizuno, T. Characterization of Circadian-associated pseudo-response regulators: I. Comparative studies on a series of transgenic lines misexpressing five distinctive PRR Genes in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2007, 71, 527–534. [Google Scholar] [CrossRef]
- Camacho, C. BLAST plus: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Marchler-Bauer, A.; Derbyshire, M.K.; Gonzales, N.R.; Lu, S.; Chitsaz, F.; Geer, L.Y.; Geer, R.C.; He, J.; Gwadz, M.; Hurwitz, D.I.; et al. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2015, 43, D222–D226. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A.; Coin, L.; Durbin, R.; Finn, R.D.; Hollich, V.; Griffiths-Jones, S.; Khanna, A.; Marshall, M.; Moxon, S.; Sonnhammer, E.L.; et al. The Pfam protein families database. Nucleic Acids Res. 2004, 32 (Suppl. S1), D138–D141. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Doerks, T.; Bork, P. SMART: Recent updates, new developments and status in 2015. Nucleic Acids Res. 2015, 43, D257–D260. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Higgins, D.G. Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinform. 2002, 32, 1792–1797. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Berglund, A.C.; Sjölund, E.; Ostlund, G.; Sonnhammer, E.L. InParanoid 6: Eukaryotic ortholog clusters with inparalogs. Nucleic Acids Res. 2008, 36, D263–D266. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Hurst, L.D. The Ka/Ks ratio: Diagnosing the form of sequence evolution. Trends Genet. 2002, 18, 486. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhou, X.; Li, Q.; Wang, L.; Xing, Y. CCT domain-containing genes in cereal crops: Flowering time and beyond. Theor. Appl. Genet. 2020, 133, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, J.L.; An, L.; Wu, T.; Yang, L.; Cheng, Y.S.; Nie, X.S.; Qin, Z.Q. Genome-wide analysis of the CCT gene family in Chinese white pear (Pyrus bretschneideri Rehd.) and characterization of PbPRR2 in response to varying light signals. BMC Plant Biol. 2022, 22, 81. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yi, D.; Yang, J.; Liu, X.; Pang, Y. Genome-Wide Identification, Expression Analysis and Functional Study of CCT Gene Family in Medicago truncatula. Plants 2020, 9, 513. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, V.; Fornara, F. Y flowering? Regulation and activity of CONSTANS and CCT-domain proteins in Arabidopsis and crop species. Biochim. Biophys. Acta Gene Regul. Mech. 2017, 1860, 655–660. [Google Scholar] [CrossRef]
- Farré, E.M. The brown clock: Circadian rhythms in stramenopiles. Physiol. Plant 2020, 169, 430–441. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Wang, J.; Sun, R.; Wu, J.; Liu, S.; Bai, Y.; Mun, J.H.; Bancroft, I.; Cheng, F.; et al. The Genome of the Mesopolyploid Crop Species Brassica rapa. Nat. Genet. 2011, 43, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Mandáková, T.; Wu, J.; Xie, Q.; Lysak, M.A.; Wang, X. Deciphering the Diploid Ancestral Genome of the Mesohexaploid Brassica rapa. Plant Cell 2013, 25, 1541–1554. [Google Scholar] [CrossRef]
- Li, K.; Ma, B.; Shen, J.; Zhao, S.; Ma, X.; Wang, Z.; Fan, Y.; Tang, Q.; Wei, D. The Evolution of the Expansin Gene Family in Brassica Species. Plant Physiol. Biochem. 2021, 167, 630–638. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, S.; He, F.; Zhu, J.; Hu, S.; Yu, J. How do variable substitution rates influence Ka and Ks calculations? Genom. Proteom. Bioinform. 2009, 7, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, T.; Sato, E.; Yamashino, T.; Mizuno, T. PRR5 (PSEUDO-RESPONSE REGULATOR 5) plays antagonistic roles to CCA1 (CIRCADIAN CLOCK-ASSOCIATED 1) in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2005, 69, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Yu, Y.; Liu, M.; Song, Y.; Li, H.; Sun, J.; Wang, Q.; Xie, Q.; Wang, L.; Xu, X. BBX19 fine-tunes the circadian rhythm by interacting with PSEUDO-RESPONSE REGULATOR proteins to facilitate their repressive effect on morning-phased clock genes. Plant Cell 2021, 33, 2602–2617. [Google Scholar] [CrossRef] [PubMed]
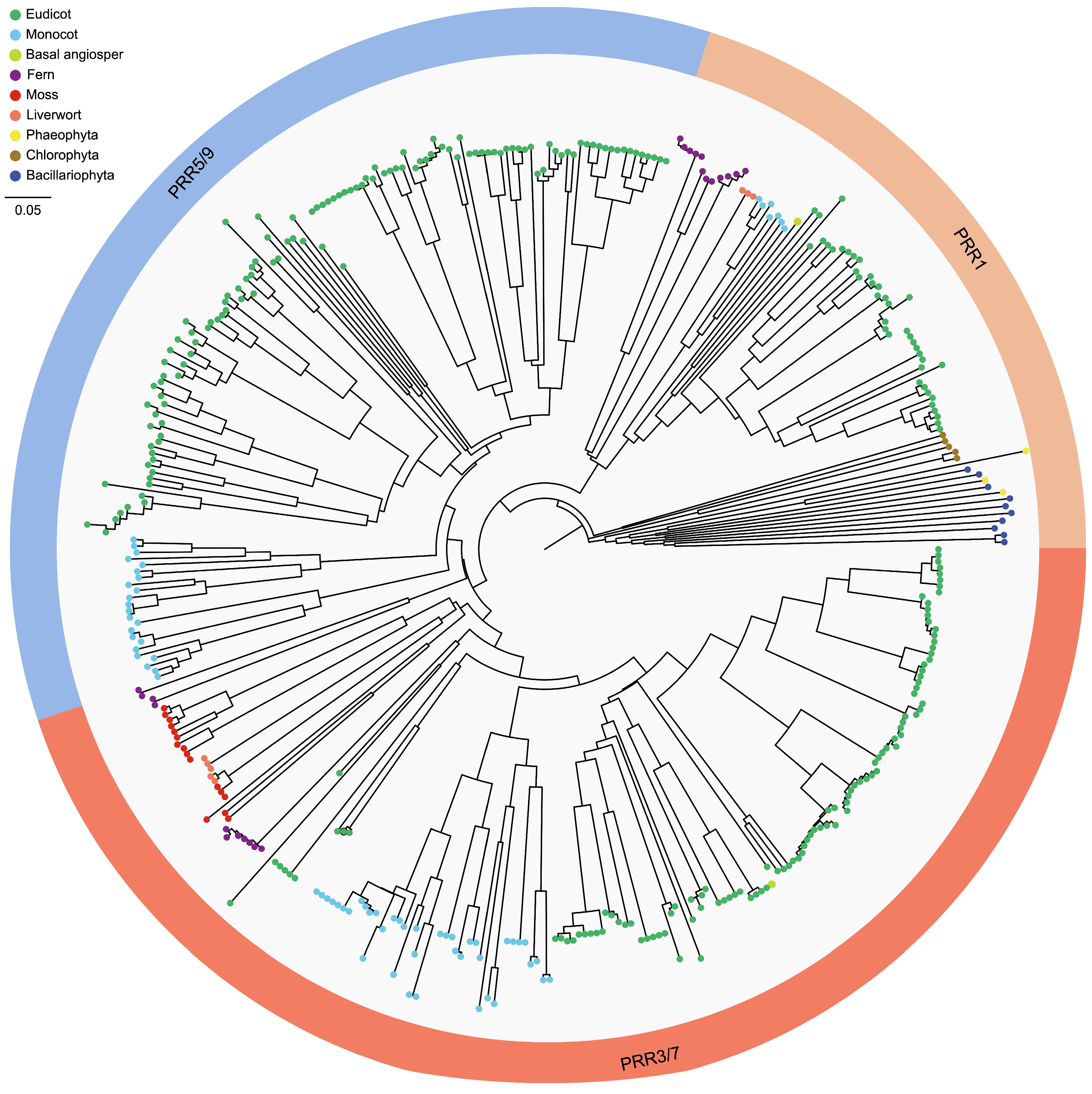
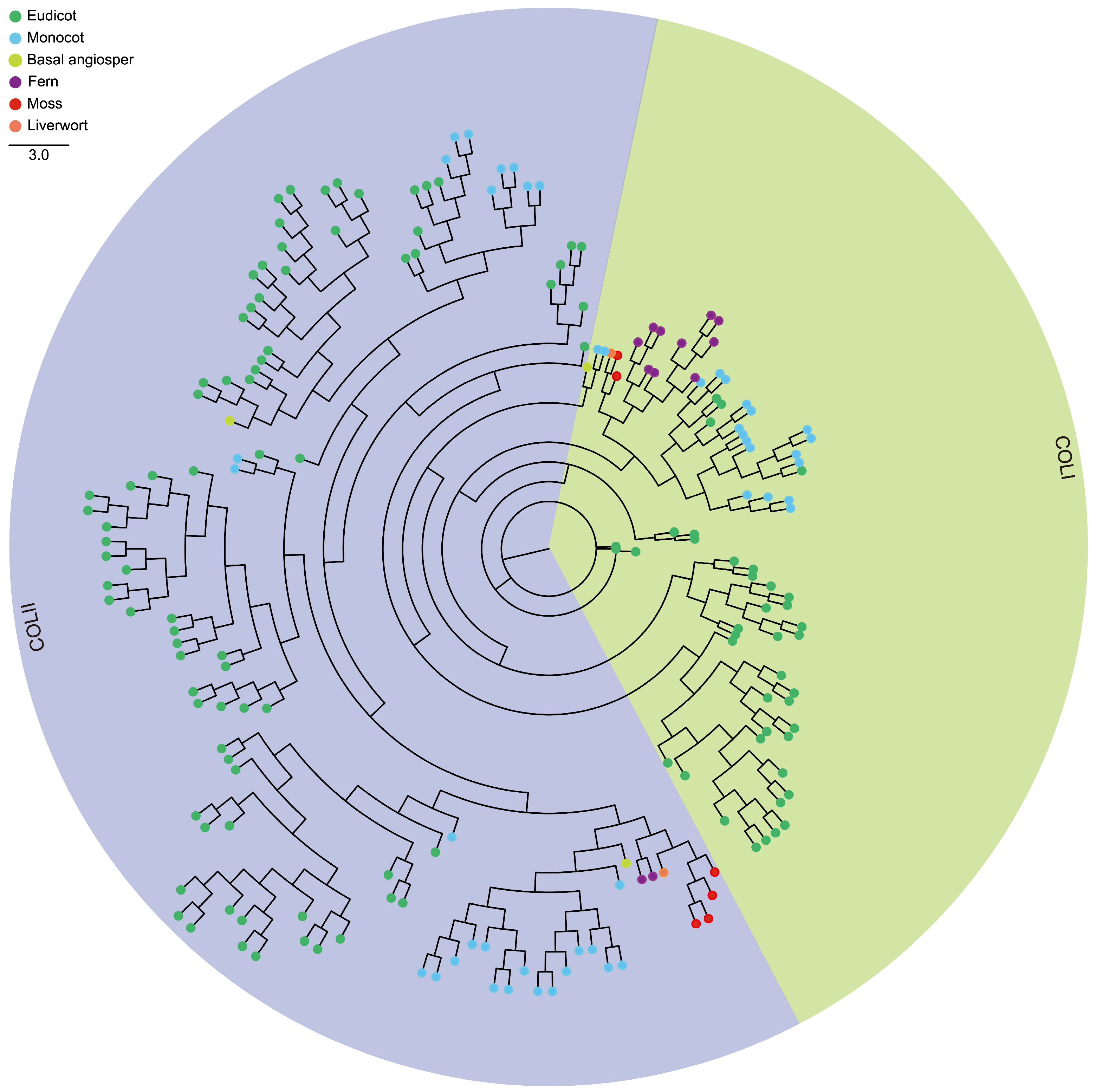
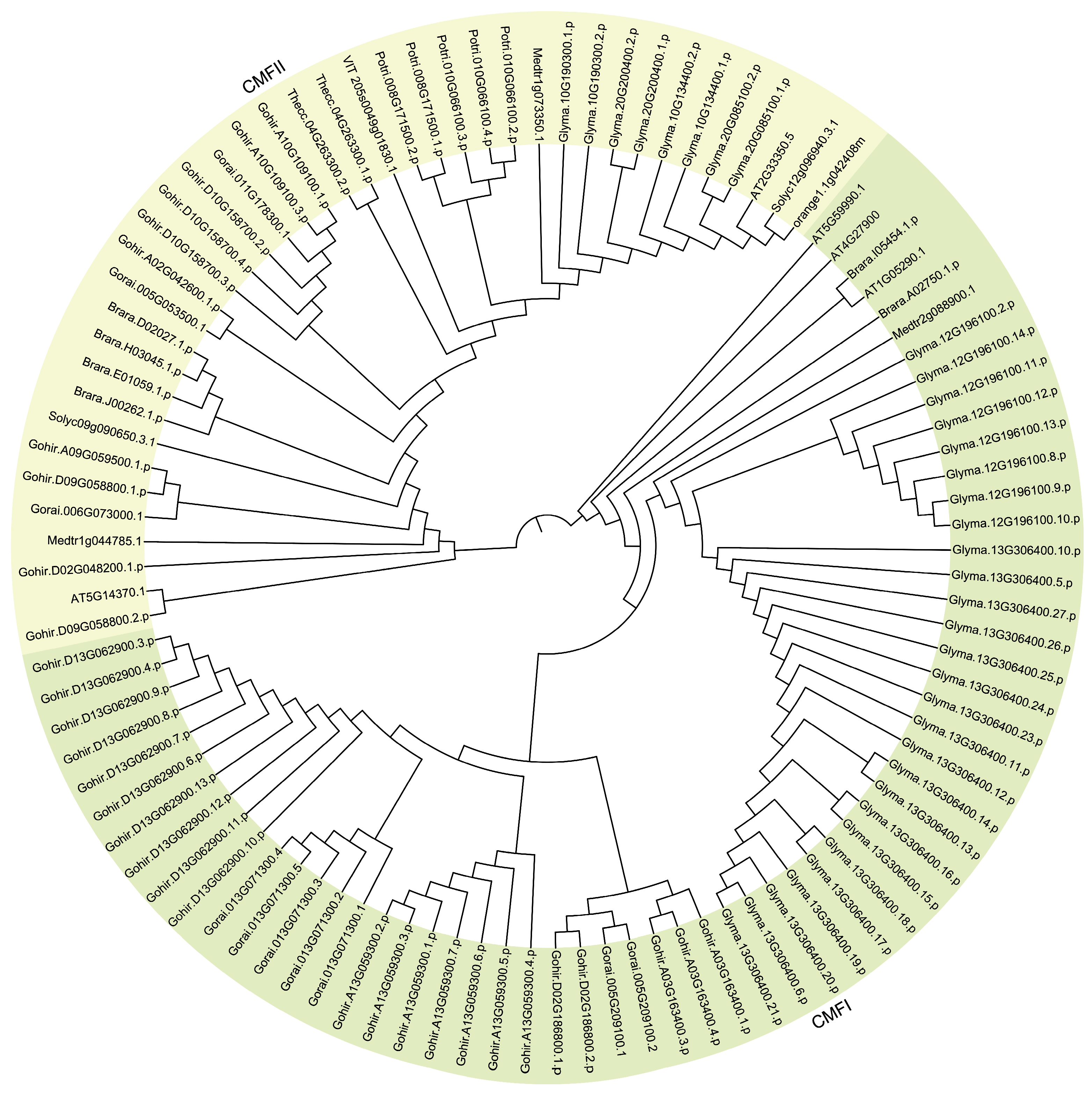
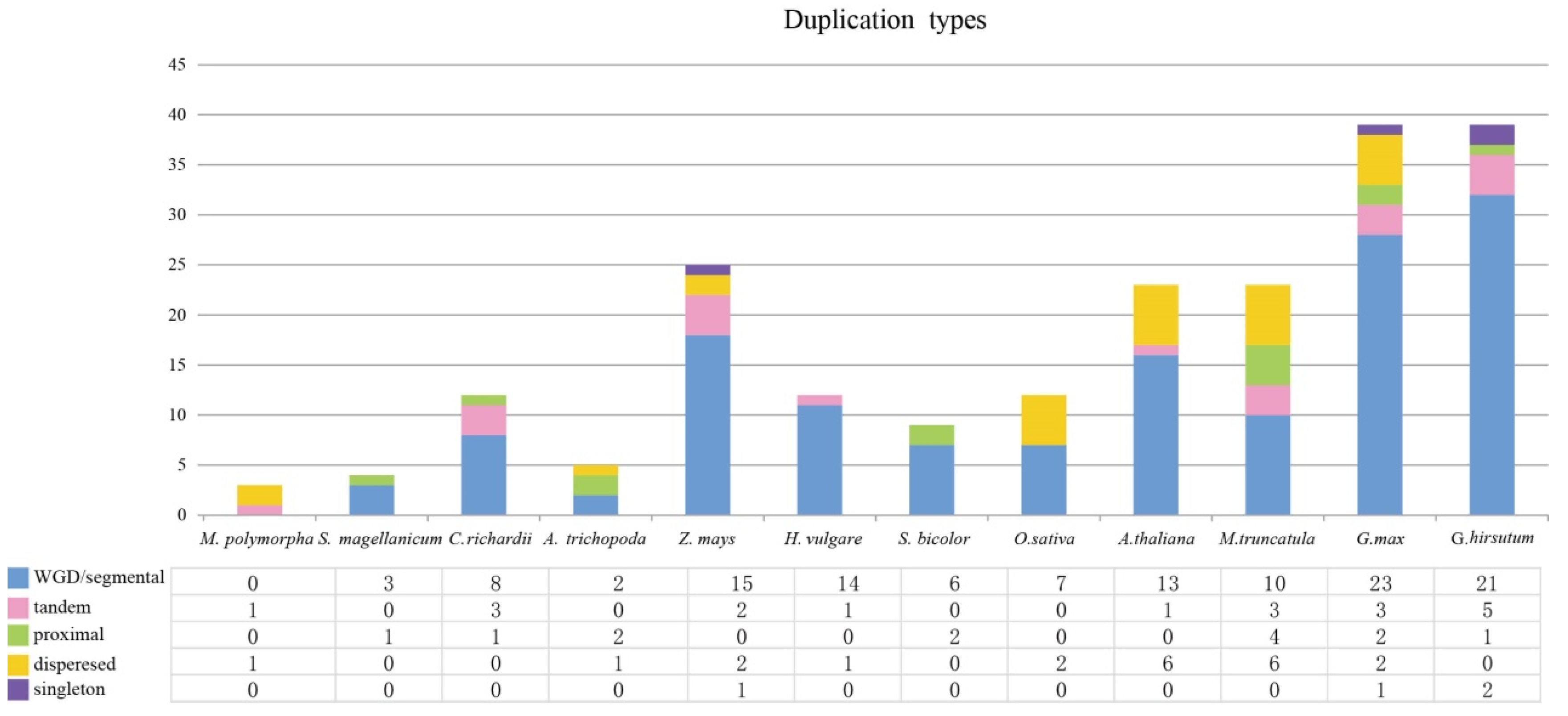


| Taxa | Species | Total | PRR | COL | CMF |
|---|---|---|---|---|---|
| Eudicot | Arabidopsis thaliana | 13 | 5 | 3 | 5 |
| Brassica rapa | 30 | 9 | 15 | 6 | |
| Citrus sinensis | 30 | 23 | 6 | 1 | |
| Gossypium darwinii | 48 | 22 | 16 | 10 | |
| Gossypium hirsutum | 117 | 58 | 26 | 33 | |
| Glycine max | 116 | 63 | 18 | 35 | |
| Medicago truncatula | 31 | 22 | 6 | 3 | |
| Populus trichocarpa | 38 | 23 | 10 | 5 | |
| Solanum lycopersicum | 17 | 6 | 9 | 2 | |
| Theobroma cacao | 27 | 18 | 7 | 2 | |
| Vitis vinifera | 28 | 21 | 6 | 1 | |
| Bacillariophyta | Cylindrotheca closterium | 1 | 1 | 0 | 0 |
| Mayamaea pseudoterrestris | 1 | 1 | 0 | 0 | |
| Nitzschia inconspicua | 4 | 4 | 0 | 0 | |
| Phaeodactylum tricornutum CCAP 1055/1 | 2 | 2 | 0 | 0 | |
| Seminavis robusta | 2 | 2 | 0 | 0 | |
| Chlorophyta | Chlamydomonas reinhardtii | 2 | 2 | 0 | 0 |
| Volvox cartei | 3 | 3 | 0 | 0 | |
| Phaeophyta | Ectocarpus siliculosus | 1 | 1 | 0 | 0 |
| Ectocarpus sp. CCAP 1310/34 | 2 | 2 | 0 | 0 | |
| Monocot | Hordeum vulgare | 20 | 7 | 13 | 0 |
| Oryza sativa | 21 | 14 | 7 | 0 | |
| Sorghum bicolor | 18 | 9 | 9 | 0 | |
| Triticum aestivum | 38 | 33 | 5 | 0 | |
| Zea mays | 17 | 7 | 10 | 0 | |
| Moss | Ceratodon purpureus | 7 | 5 | 2 | 0 |
| Sphagnum magellanicum | 17 | 13 | 4 | 0 | |
| Fern | Ceratopteris richardii | 34 | 23 | 11 | 0 |
| Liverwort | Marchantia polymorpha | 9 | 6 | 3 | 0 |
| Basal angiosperm | Amborella trichopoda | 6 | 3 | 3 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Yao, H.; Lan, Y.; Cao, Y.; Xu, Q.; Xu, H.; Qiao, D.; Cao, Y. Genome-Wide Identification and Characterization of CCT Gene Family from Microalgae to Legumes. Genes 2024, 15, 941. https://doi.org/10.3390/genes15070941
Xu Y, Yao H, Lan Y, Cao Y, Xu Q, Xu H, Qiao D, Cao Y. Genome-Wide Identification and Characterization of CCT Gene Family from Microalgae to Legumes. Genes. 2024; 15(7):941. https://doi.org/10.3390/genes15070941
Chicago/Turabian StyleXu, Yi, Huiying Yao, Yanhong Lan, Yu Cao, Qingrui Xu, Hui Xu, Dairong Qiao, and Yi Cao. 2024. "Genome-Wide Identification and Characterization of CCT Gene Family from Microalgae to Legumes" Genes 15, no. 7: 941. https://doi.org/10.3390/genes15070941





