Molecular Cloning, Characterization, and Function of Insulin-Related Peptide 1 (IRP1) in the Haliotis discus hanna
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Sample Collection
2.2. RNA Extraction and cDNA Preparation
2.3. Hdh-MIRP1 ORF Verification and Sequence Analysis
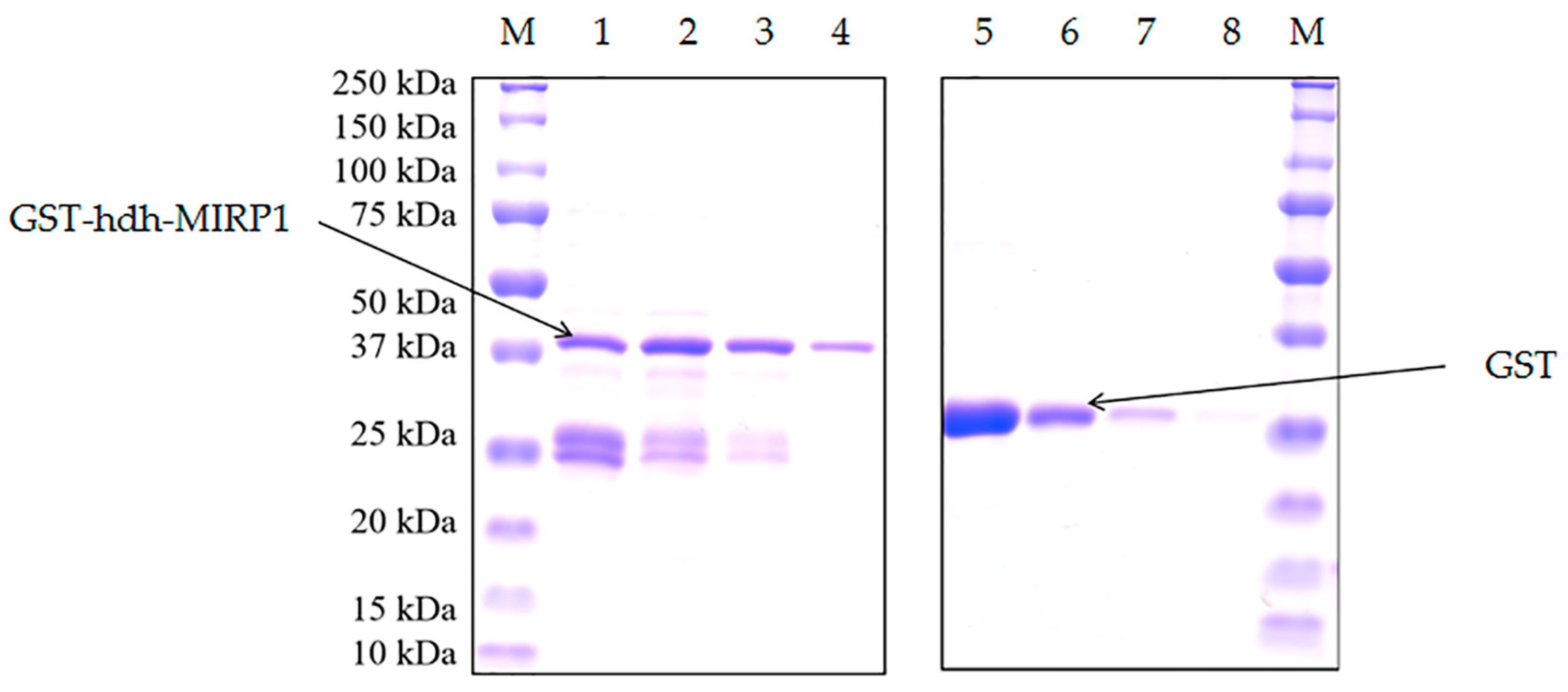
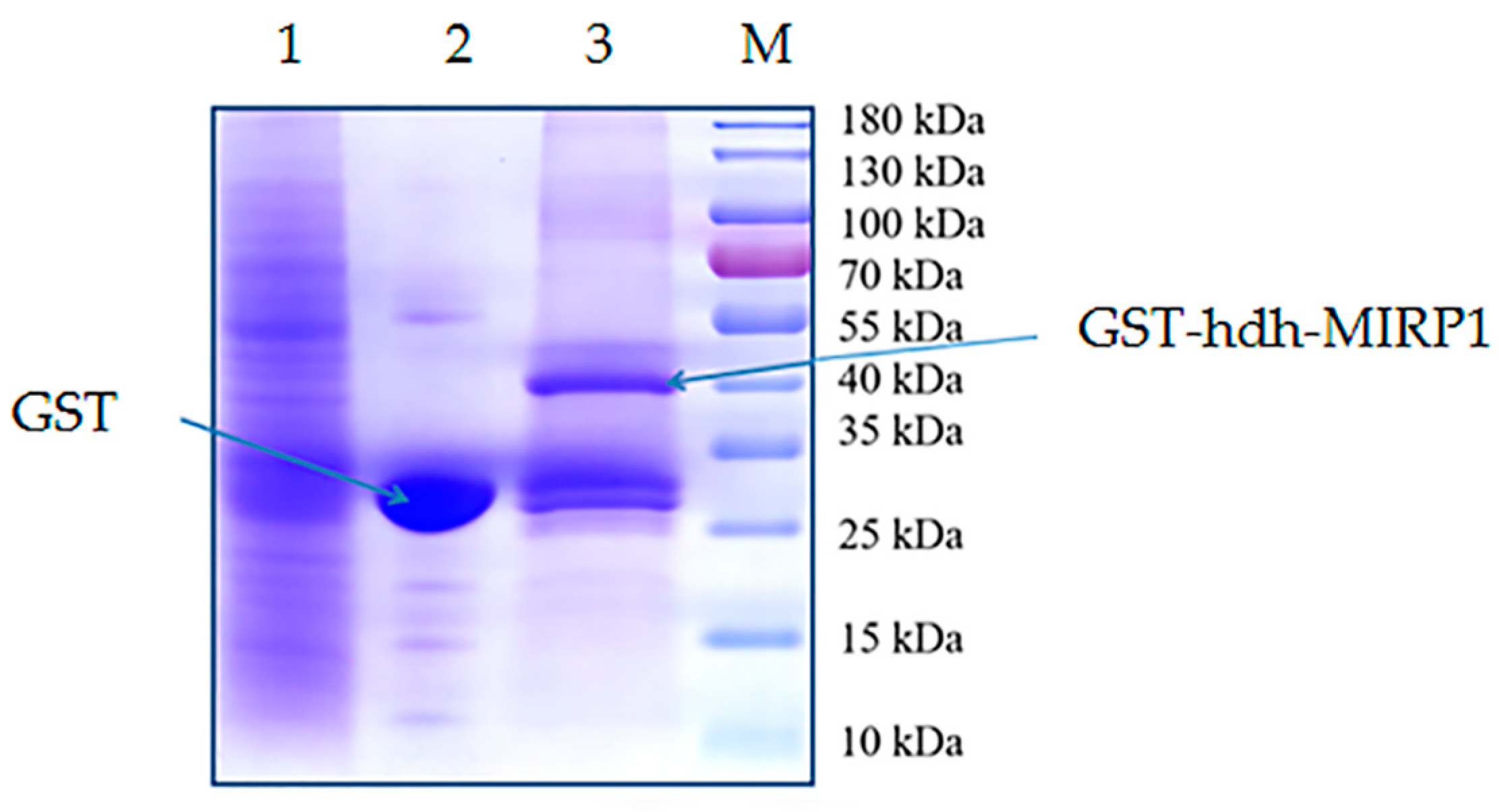
2.4. Expression of Recombinant Proteins, GST Pull-Down, and Identification of hdh-MIRP1-Interacting Protein
2.5. Gene Expression Profiles via RT-PCR
2.6. Immunofluorescence
2.7. Sequence and SNP Analysis
2.8. Statistical Analysis
3. Results
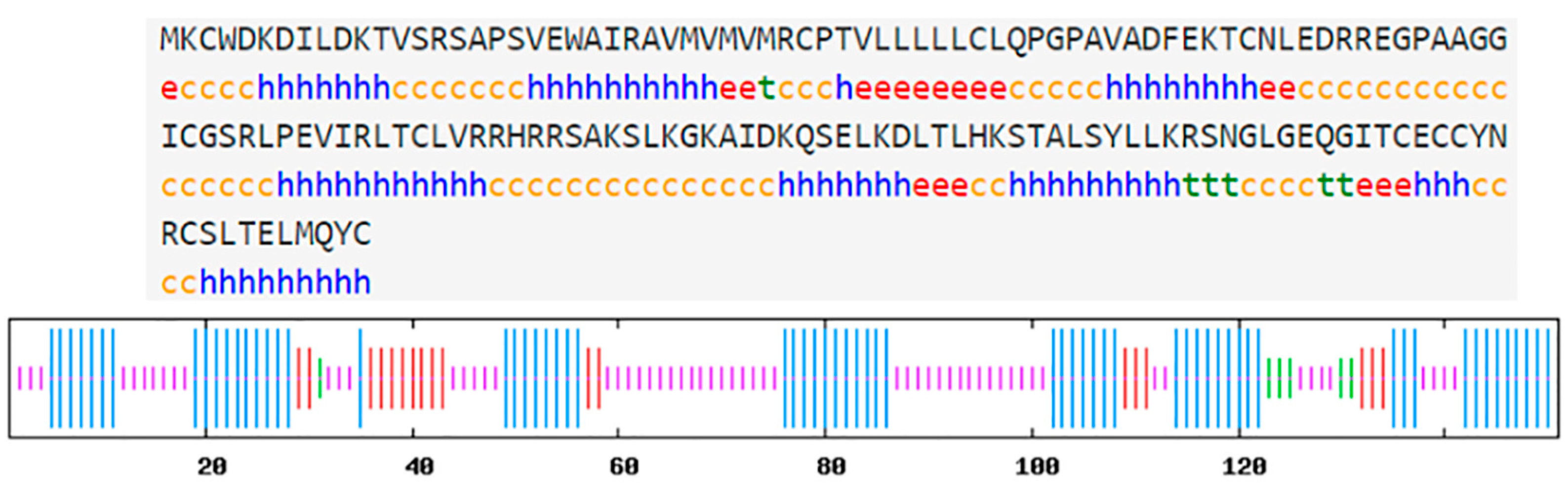
3.1. Characterization of hdh-MIRP1 Sequence
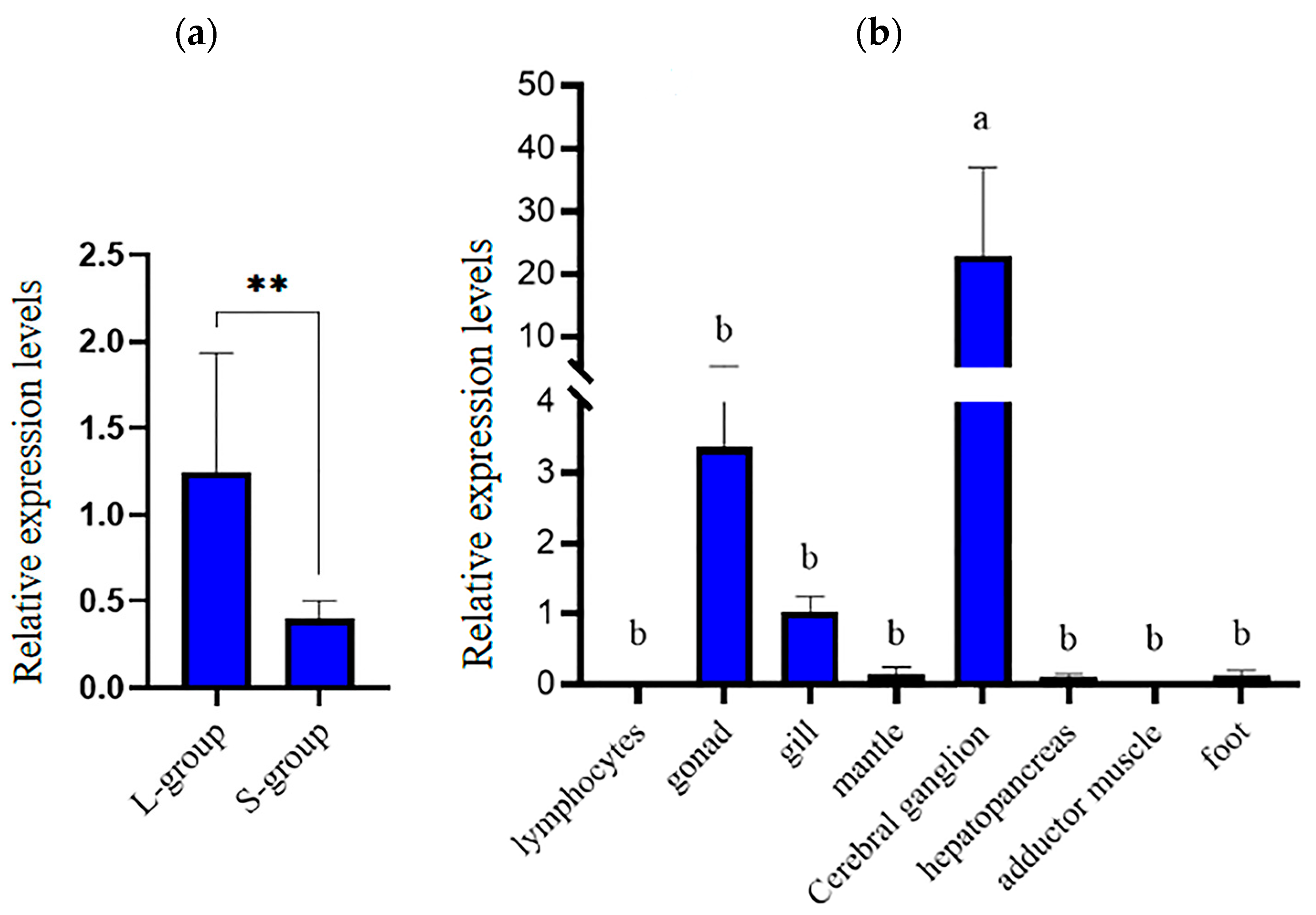
3.2. Expression Analysis of hdh-MIRP1
3.3. Growth-Linked SNP Loci in hdh-MIRP1
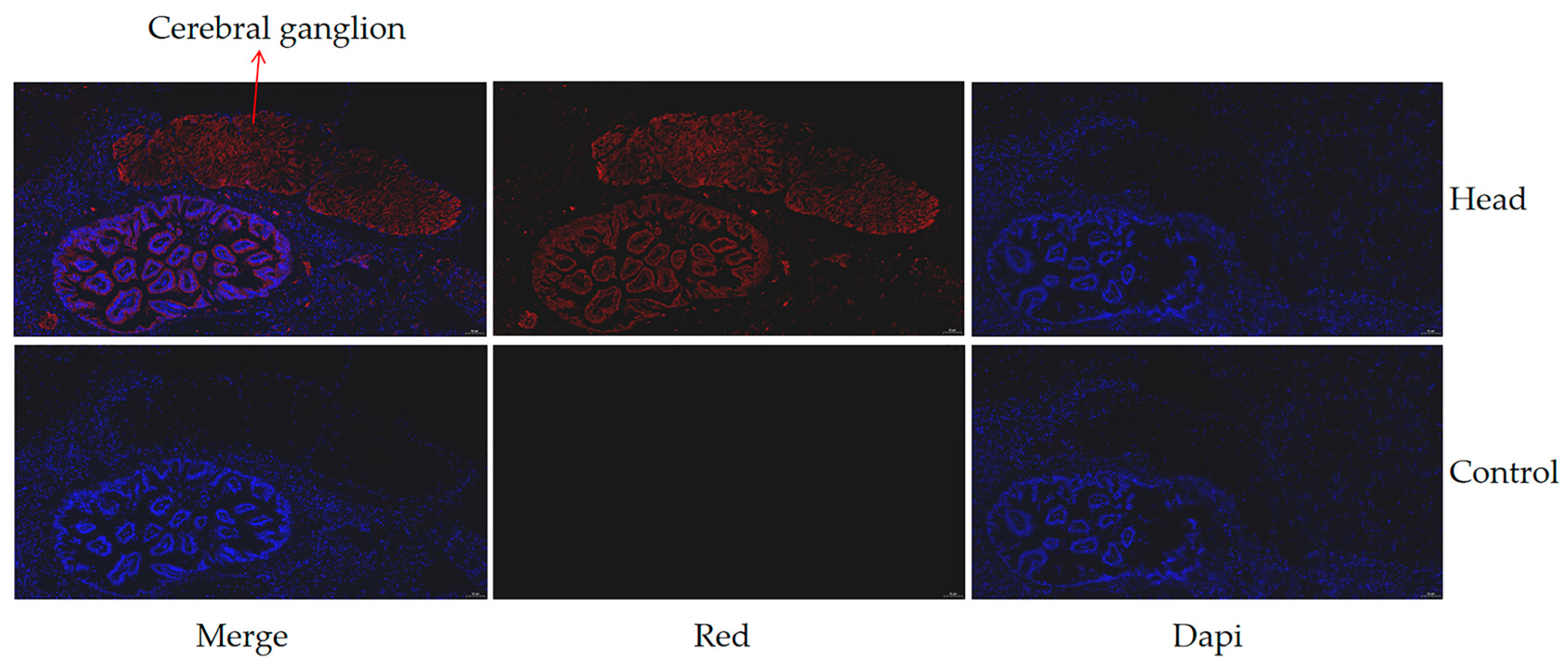
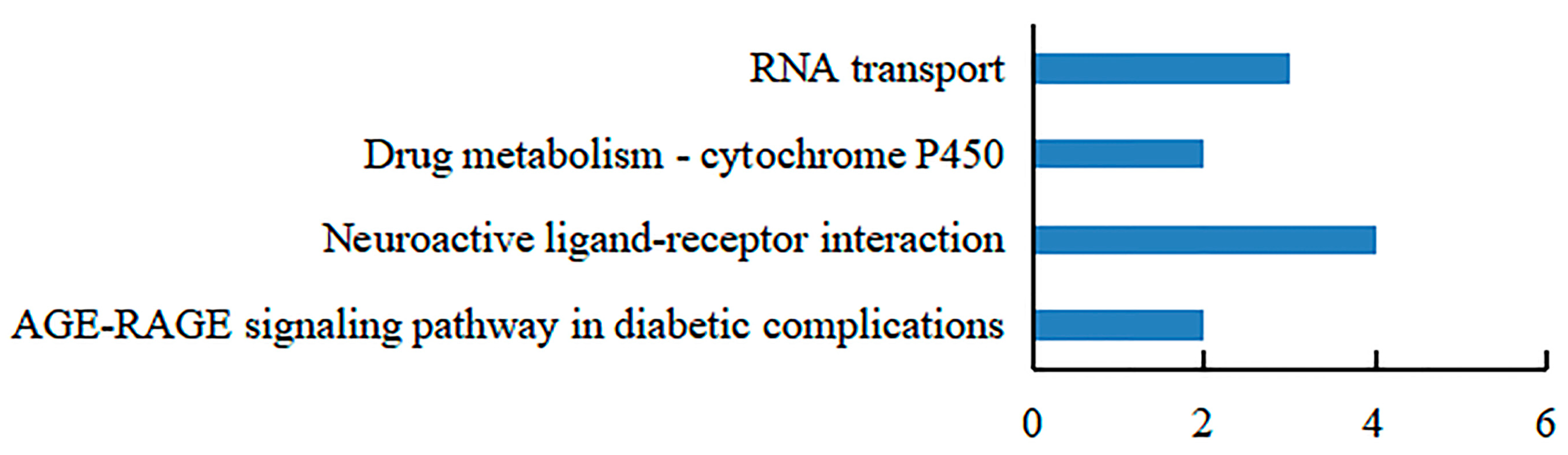
3.4. Confirmation of the Interacting Proteins with the hdh-MIRP1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, X.; Ke, C.; You, W. Estimates of correlations for shell morphological traits on body weight of interspecific hybrid abalone (Haliotis discus hannai and Haliotis gigantea). J. Shellfish Res. 2013, 32, 115–118. [Google Scholar] [CrossRef]
- Wang, D.; Wu, F.X. China Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2022. [Google Scholar]
- Dong, Y. Transcriptome Analysis using 454 Pyrosequencing and Cloning and Expression of Growth-Related Genes for the Blood Clam Tegillarca granosa (Linnaeus, 1758). Ph.D. Thesis, Ocean University of China, Qingdao, China, 2012. [Google Scholar]
- Hao, R.; Zheng, Z.; Du, X.; Wang, Q.; Li, J.; Deng, Y.; Chen, W. Molecular cloning and characteristics analysis of Pmtgfbr1 from Pinctada fucata martensii. Biotechnol. Rep. Amst. 2018, 19, e00262. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, C.; Qi, H.; Meng, J.; Wang, W.; Wu, F.; Li, L.; Zhang, G. A genome-wide association study to identify the genes associated with shell growth and shape-related traits in Crassostrea gigas. Aquaculture 2021, 543, 736926. [Google Scholar] [CrossRef]
- Liu, G. The Roles of BMP/Smad Pathway and Tyrosinase in the Early Development of the Pacific Oyster Crassostrea gigas. Master’s Thesis, Institute of Oceanology, The University of Chinese Academy of Sciences, Qingdao, China, 2013. [Google Scholar]
- He, P.R. IRR and FGFR Genes in Haliotis diversicolor: Molecular Cloning, Expression Analysis and Identification of SNPs Associated with Growth Traits. Master’s Thesis, Xiamen University, Xiamen, China, 2015. [Google Scholar]
- Ai, J.L. Molecular Cloning, Expression Analysis of the Gene MSTN, BMP-2 and SNPs Association with Growth Traits in Haliotis diversicolor Supertexta. Master’s Thesis, Ocean University of Guangdong, Zhanjiang, China, 2015. [Google Scholar]
- Carrera-Naipil, C.; Valenzuela-Muñoz, V.; Valdés, J.A.; Molina, A.; Gallardo-Escárate, C. RNA interference in Haliotis rufescens myostatin evidences upregulation of insulin signaling pathway. Agri Gene 2016, 1, 93–99. [Google Scholar] [CrossRef]
- York, P.S.; Cummins, S.F.; Lucas, T.; Blomberg, S.P.; Degnan, S.M.; Degnan, B.M. Differential expression of neuropeptides correlates with growth rate in cultivated Haliotis asinina (Vetigastropoda: Mollusca). Aquaculture 2012, 334, 159–168. [Google Scholar] [CrossRef]
- Wang, F. Research of Development Transcriptome Library and Function of Insulin-like Peptide Genes in Sinonovacula constricta. Master’s Thesis, Ocean University of Shanghai, Shanghai, China, 2016. [Google Scholar]
- Castillo, J.; Codina, M.; Martínez, M.L.; Navarro, I.; Gutiérrez, J. Metabolic and mitogenic effects of IGF-I and insulin on muscle cells of rainbow trout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R935–R941. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, X.; Xu, Q.; Yan, J.; Yu, F.; Wang, F.; Xiao, J.; Luo, Y.; Zhong, H. Nonadditive and allele-specific expression of insulin-like growth factor 1 in Nile tilapia (Oreochromis niloticus, ♀) × blue tilapia (O. aureus, ♂) hybrids. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2019, 232, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Semaniuk, U.; Piskovatska, V.; Strilbytska, O.; Strutynska, T.; Burdyliuk, N.; Vaiserman, A.; Bubalo, V.; Storey, K.B.; Lushchak, O. Drosophila insulin-like peptides: From expression to functions–a review. Entomol. Exp. Appl. 2020, 169, 195–208. [Google Scholar] [CrossRef]
- Smit, A.B.; Spijker, S.; Van Minnen, J.; Burke, J.F.; De Winter, F.; Van Elk, R.; Geraerts, W.P. Expression and characterization of molluscan insulin-related peptide VII from the mollusc Lymnaea stagnalis. Neuroscience 1996, 70, 589–596. [Google Scholar] [CrossRef]
- Zhang, H.; He, M. The role of a new insulin-like peptide in the pearl oyster Pinctada fucata martensii. Sci. Rep. 2020, 10, 433. [Google Scholar] [CrossRef]
- Shipilov, V.N.; Shpakov, A.O.; Rusakov, Y.I. Pleiotropic action of insulin-like peptides of mollusk, Anodonta cygnea. Ann. N. Y. Acad. Sci. 2005, 1040, 464–465. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.; Wang, F.; Zhao, H.; Wang, Z.; Xie, S.; Li, J. Identification, expression, and innate immune responses of two insulin-like peptide genes in the razor clam Sinonovacula constricta. Fish Shellfish Immunol. 2016, 51, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Cherif-Feildel, M.; Heude Berthelin, C.; Adeline, B.; Rivière, G.; Favrel, P.; Kellner, K. Molecular evolution and functional characterisation of insulin related peptides in molluscs: Contributions of Crassostrea gigas genomic and transcriptomic-wide screening. Gen. Comp. Endocrinol. 2019, 271, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, H.; Zhang, F.; Ren, L.; Tian, J.; Li, Q.; Liu, S. Identification, characterization, and expression profiles of insulin-like peptides suggest their critical roles in growth regulation of the Pacific oyster, Crassostrea gigas. Gene 2021, 769, 145244. [Google Scholar] [CrossRef] [PubMed]
- Cong, R.; Li, Q.; Kong, L. Polymorphism in the insulin-related peptide gene and its association with growth traits in the Pacific oyster Crassostrea gigas. Biochem. Syst. Ecol. 2013, 46, 36–43. [Google Scholar] [CrossRef]
- Chung, J.S. An insulin-like growth factor found in hepatopancreas implicates carbohydrate metabolism of the blue crab Callinectes sapidus. Gen. Comp. Endocrinol. 2014, 199, 56–64. [Google Scholar] [CrossRef]
- Feng, J.; Li, D.; Liu, L.; Tang, Y.; Du, R. Interaction of the small GTP-binding protein (Rab7) with β-actin in Litopenaeus vannamei and its role in white spot syndrome virus infection. Fish Shellfish Immunol. 2019, 88, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.M. Expression Characteristics and Preliminary Functional Analysis of Three Genes in the Insulin-like Growth Factor System of Razor Clam Sinonovacula constricta. Ph.D. Thesis, Ocean University of Shanghai, Shanghai, China, 2018. [Google Scholar]
- Chen, X.T. Characterization of the Insulin-like Peptides Gene in Bombyx Mori (BmILP). Master’s Thesis, Jiangsu University, Zhenjiang, China, 2016. [Google Scholar]
- Kang, S.M.; Tark, D.; Song, B.M.; Lee, G.H.; Yang, J.H.; Han, H.J.; Yim, S.K. Evaluation of Antiviral Effect against SARS-CoV-2 Propagation by Crude Polysaccharides from Seaweed and Abalone Viscera In Vitro. Mar. Drugs 2022, 20, 296. [Google Scholar] [CrossRef] [PubMed]
- Veenstra, J.A. Neurohormones and neuropeptides encoded by the genome of Lottia gigantea, with reference to other mollusks and insects. Gen. Comp. Endocrinol. 2010, 167, 86–103. [Google Scholar] [CrossRef]
- Smit, A.B.; van Kesteren, R.E.; Li, K.W.; Van Minnen, J.; Spijker, S.; Van Heerikhuizen, H.; Geraerts, W.P. Towards understanding the role of insulin in the brain: Lessons from insulin-related signaling systems in the invertebrate brain. Prog. Neurobiol. 1998, 54, 35–54. [Google Scholar] [CrossRef]
- Hamano, K.; Awaji, M.; Usuki, H. cDNA structure of an insulin-related peptide in the Pacific oyster and seasonal changes in the gene expression. J. Endocrinol. 2005, 187, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Brogiolo, W.; Stocker, H.; Ikeya, T.; Rintelen, F.; Fernandez, R.; Hafen, E. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr. Biol. 2001, 11, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Claeys, I.; Simonet, G.; Poels, J.; Van Loy, T.; Vercammen, L.; De Loof, A.; Vanden Broeck, J. Insulin-related peptides and their conserved signal transduction pathway. Peptides 2002, 23, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Loffing-Cueni, D.; Schmid, A.C.; Graf, H.; Reinecke, M. IGF-I in the bony fish Cottus scorpius: cDNA, expression and differential localization in brain and islets. Mol. Cell. Endocrinol. 1998, 141, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, S.; Fu, C.; Li, T.; Cui, X. Molecular and functional analysis of the insulin-like peptides gene in the oriental river prawn Macrobrachium nipponense. Gen. Comp. Endocrinol. 2019, 280, 209–214. [Google Scholar] [CrossRef]
- Heyland, A.; Vue, Z.; Voolstra, C.R.; Medina, M.; Moroz, L.L. Developmental transcriptome of Aplysia californica. J. Exp. Zool. B Mol. Dev. Evol. 2011, 316b, 113–134. [Google Scholar] [CrossRef] [PubMed]
- Geraerts, W.P.; Smit, A.B.; Li, K.W.; Hordijk, P.L. The Light Green Cells of Lymnaea: A neuroendocrine model system for stimulus-induced expression of multiple peptide genes in a single cell type. Experientia 1992, 48, 464–473. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.; Zhang, W.; Zhang, Y. Proteins interacting with chicken NOS2 were screened by GST pull-down combined with mass spectrometry. China Poult. 2020, 42, 19–25. [Google Scholar] [CrossRef]
- Wang, X.L. Cloning, Expression and Functional Characterization of Superoxide Dismutase Multigene Family in Nibea albiflora. Master’s Thesis, Jimei University, Xiamen, China, 2018. [Google Scholar]
- Guzmán-Rodríguez, F.; Alatorre-Santamaría, S.; Gómez-Ruiz, L.; Rodríguez-Serrano, G.; García-Garibay, M.; Cruz-Guerrero, A. Employment of fucosidases for the synthesis of fucosylated oligosaccharides with biological potential. Biotechnol. Appl. Biochem. 2019, 66, 172–191. [Google Scholar] [CrossRef]
- Bévort, M.; Leffers, H. Down regulation of ribosomal protein mRNAs during neuronal differentiation of human NTERA2 cells. Differentiation 2000, 66, 81–92. [Google Scholar] [CrossRef]
- Chen, F.W.; Ioannou, Y.A. Ribosomal proteins in cell proliferation and apoptosis. Int. Rev. Immunol. 1999, 18, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.Y. Isolation, Identification, Expression and Function Studies of Growth Performance Candidate Genes in Litopenaeus vannamei. Ph.D Thesis, Northwest A & F University, Yangling, China, 2014. [Google Scholar]
- Xu, X.; Xiong, X.; Sun, Y. The role of ribosomal proteins in the regulation of cell proliferation, tumorigenesis, and genomic integrity. Sci. China Life Sci. 2016, 59, 656–672. [Google Scholar] [CrossRef]
- Zhu, J.; Thompson, C.B. Metabolic regulation of cell growth and proliferation. Nat. Rev. Mol. Cell Biol. 2019, 20, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Deck, C.A.; Gary Anderson, W.; Walsh, P.J. Effects of glucose and insulin administration on glucose transporter expression in the North Pacific spiny dogfish (Squalus suckleyi). Gen. Comp. Endocrinol. 2017, 247, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Hong, Y. Regulation of hemolymph trehalose level by an insulin-like peptide through diel feeding rhythm of the beet armyworm, Spodoptera exigua. Peptides 2015, 68, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Navarro, I.; Rojas, P.; Capilla, E.; Albalat, A.; Castillo, J.; Montserrat, N.; Codina, M.; Gutiérrez, J. Insights into insulin and glucagon responses in fish. Fish Physiol. Biochem. 2002, 27, 205–216. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Zhang, X.; Yuan, J.; Zhang, X.; Yang, M.; Li, F. Gene structure and expression analysis of insulin-like peptide in the Pacific white shrimp Litopenaeus vannamei. Prog. Fish. Sci. 2024, 5, 105–117. [Google Scholar] [CrossRef]
- Mohd Nor, N.A.; Budin, S.B.; Zainalabidin, S.; Jalil, J.; Sapian, S.; Jubaidi, F.F.; Mohamad Anuar, N.N. The Role of Polyphenol in Modulating Associated Genes in Diabetes-Induced Vascular Disorders. Int. J. Mol. Sci. 2022, 23, 6396. [Google Scholar] [CrossRef]





| Primer | Sequence (5′-3′) |
|---|---|
| hdh-MIRP1-F | CATGAAGTGTTGGGACAAAGA |
| hdh-MIRP1-R | GATTGGTTGTGACGTCAGCAG |
| hdh-MIRP1-yh-F | CGCGGATCCATGAAGTGTTGGGACAAAG |
| hdh-MIRP1-yh-R | CCGCTCGAGGCAGTACTGCATAAGCTC |
| hdh-MIRP1-qF | AGCAGTGATGGTGATGGTGA |
| hdh-MIRP1-qR | CCGCCGGTCCTCCAGATT |
| β-actin-qF | GGTATCCTCACCCTCAAGT |
| β-actin-qR | GGGTCATCTTTTCACGGTTG |
| Locus | Genotype | Sample Number | Shell Length (mm) | Shell Width (mm) | Total Weight (g) | Muscle Weight (g) | Muscle Weight/Total Weight |
|---|---|---|---|---|---|---|---|
| T-213G | TT | 203 | 73.99 ± 9.65 a | 49.61 ± 6.14 a | 41.50 ± 15.83 a | 16.99 ± 0.53 a | 0.4011 ± 0.0030 a |
| GT | 16 | 71.73 ± 9.85 a | 50.87 ± 7.00 a | 42.81 ± 16.18 a | 18.23 ± 1.95 a | 0.4155 ± 0.0109 a | |
| T-351C | TT | 35 | 72.95 ± 9.49 a | 49.44 ± 6.39 a | 40.39 ± 15.61 a | 16.85 ± 7.87 a | 0.4063 ± 0.0412 ab |
| TC | 96 | 74.21 ± 9.99 a | 50.28 ± 6.31 a | 43.09 ± 16.67 a | 17.95 ± 7.81 a | 0.4086 ± 0.0428 a | |
| CC | 86 | 73.75 ± 9.54 a | 49.12 ± 6.02 a | 40.14 ± 14.93 a | 16.20 ± 7.07 a | 0.3957 ± 0.0411 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Zhou, M.; Chen, J.; Ke, C. Molecular Cloning, Characterization, and Function of Insulin-Related Peptide 1 (IRP1) in the Haliotis discus hanna. Genes 2024, 15, 960. https://doi.org/10.3390/genes15070960
Huang J, Zhou M, Chen J, Ke C. Molecular Cloning, Characterization, and Function of Insulin-Related Peptide 1 (IRP1) in the Haliotis discus hanna. Genes. 2024; 15(7):960. https://doi.org/10.3390/genes15070960
Chicago/Turabian StyleHuang, Jianfang, Mingcan Zhou, Jianming Chen, and Caihuan Ke. 2024. "Molecular Cloning, Characterization, and Function of Insulin-Related Peptide 1 (IRP1) in the Haliotis discus hanna" Genes 15, no. 7: 960. https://doi.org/10.3390/genes15070960




