Developing an Optimized Protocol for Regeneration and Transformation in Pepper
Abstract
:1. Introduction
2. Materials and Methods
2.1. Establishment of In Vitro Culture
Regeneration
2.2. Agrobacterium-Mediated Transformation
2.2.1. Preparation of Agrobacterium Inoculation
2.2.2. Molecular Characterization of Transgenic Plants
2.3. Statistical Analysis
3. Results
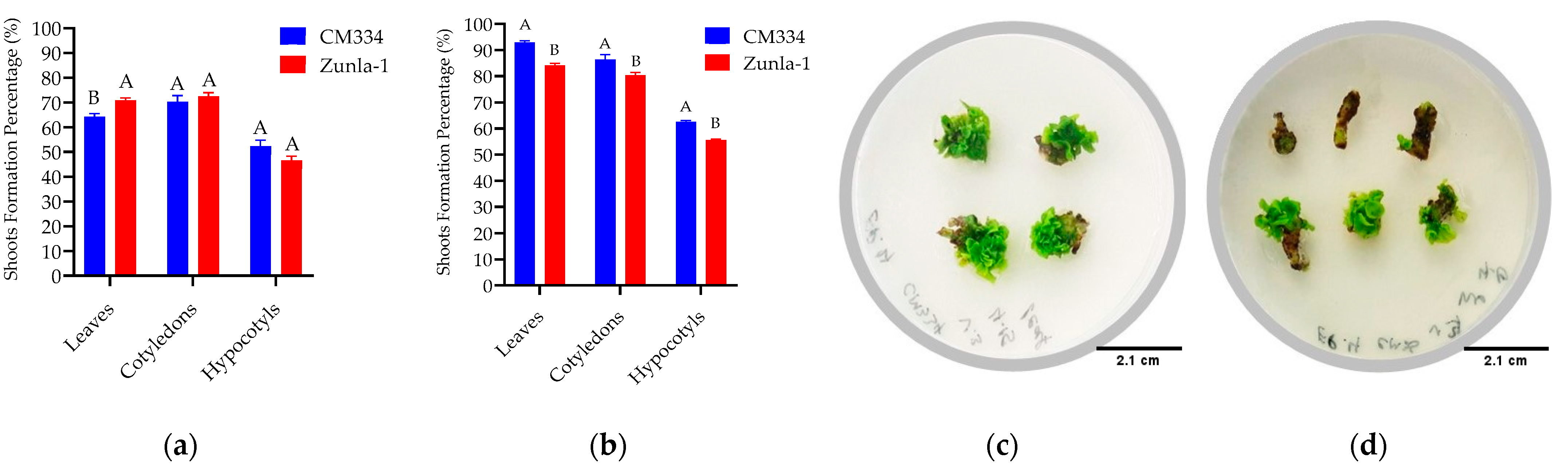
3.1. Regeneration Efficiency
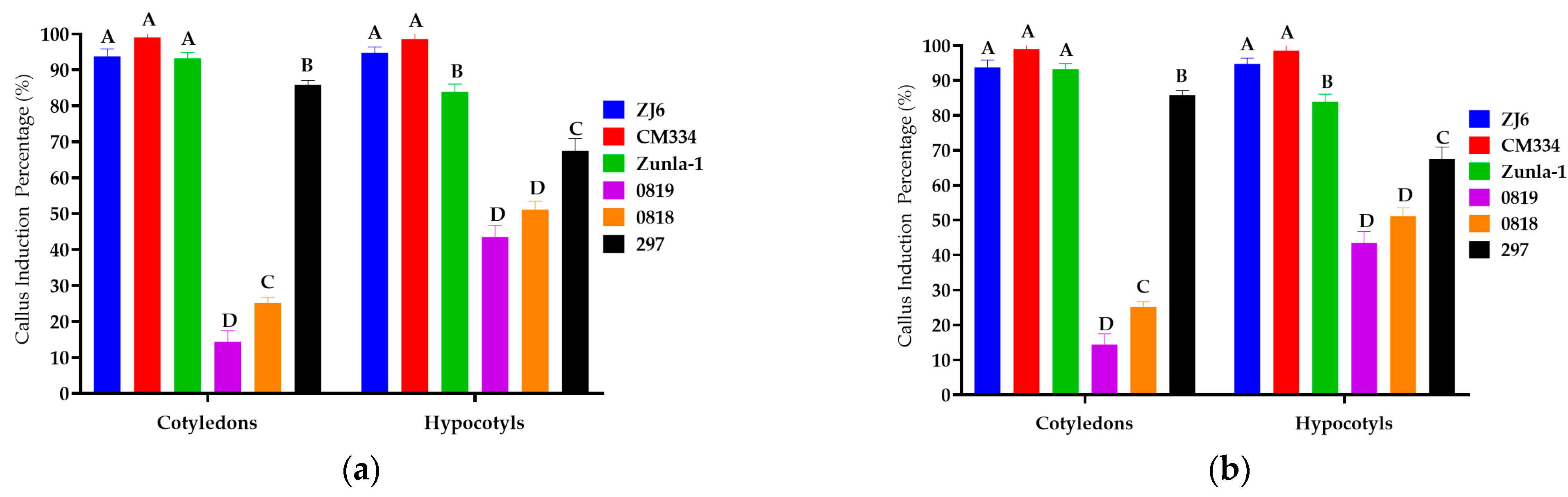
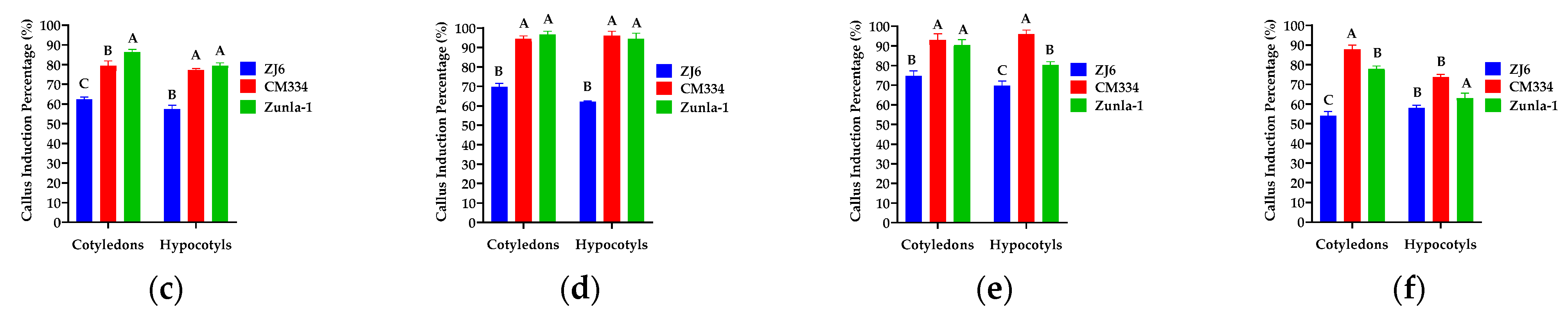
3.1.1. Callus Induction
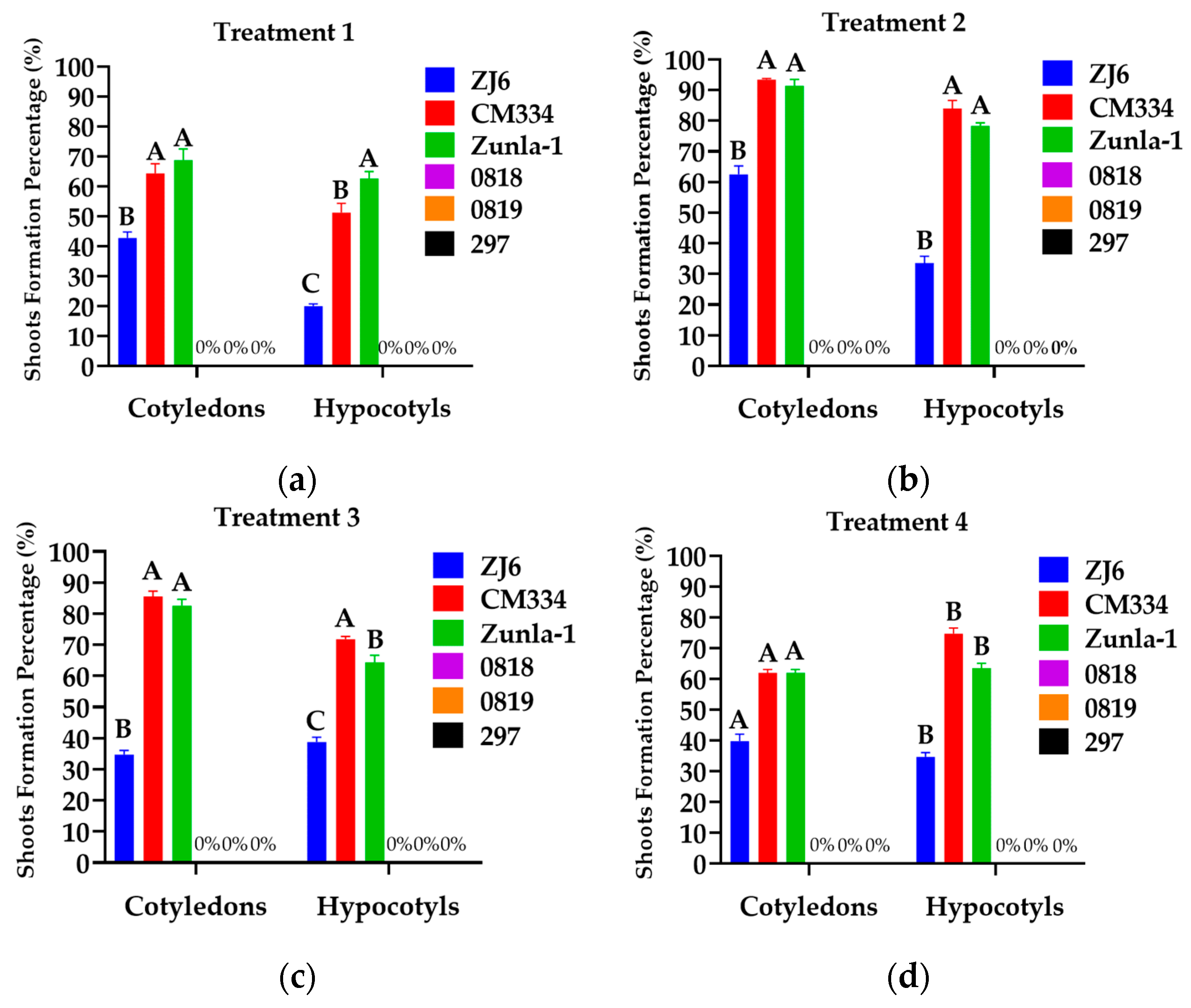
3.1.2. Shoot Regeneration
3.1.3. Shoot Elongation
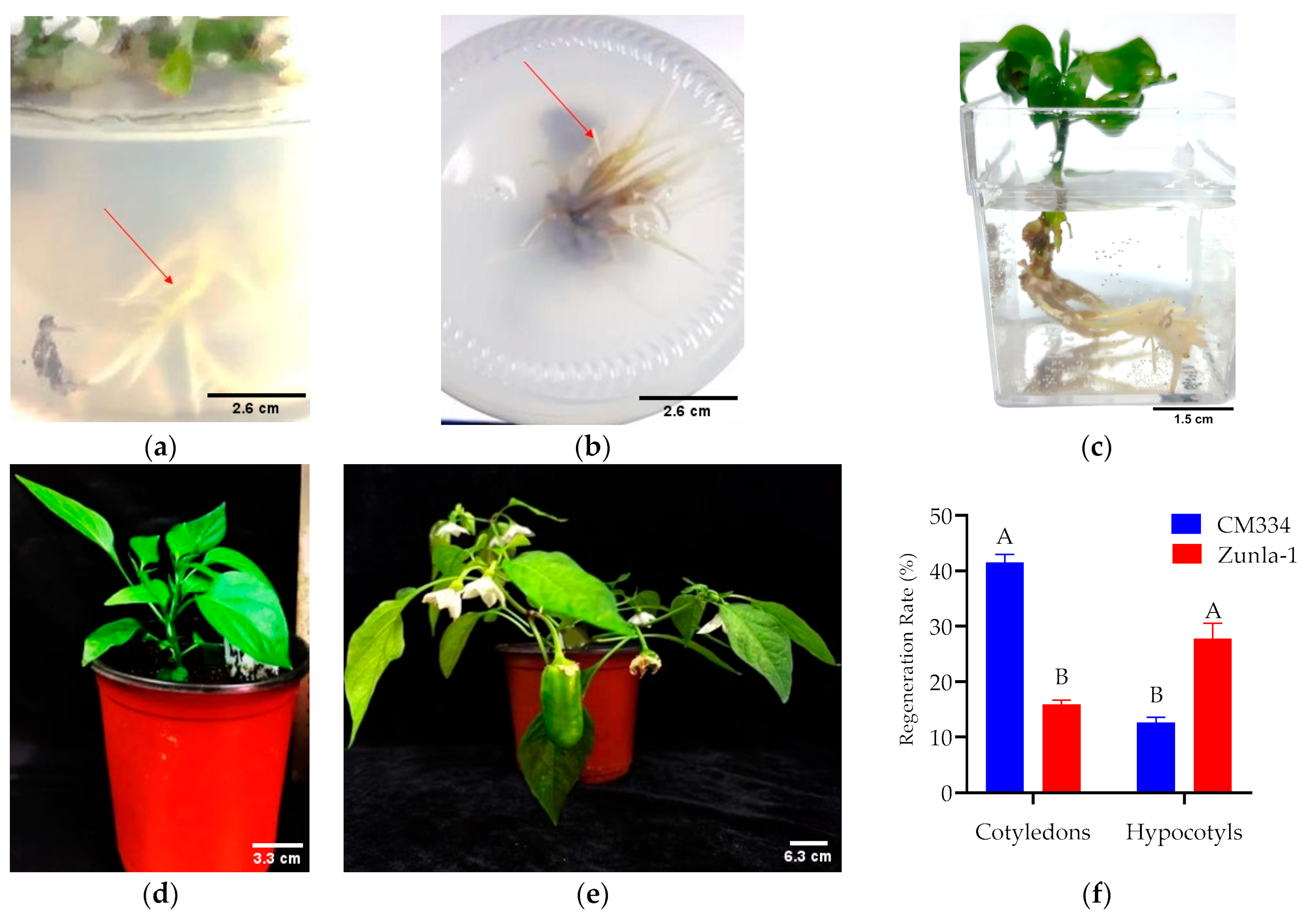
3.1.4. Rooting and Acclimatization
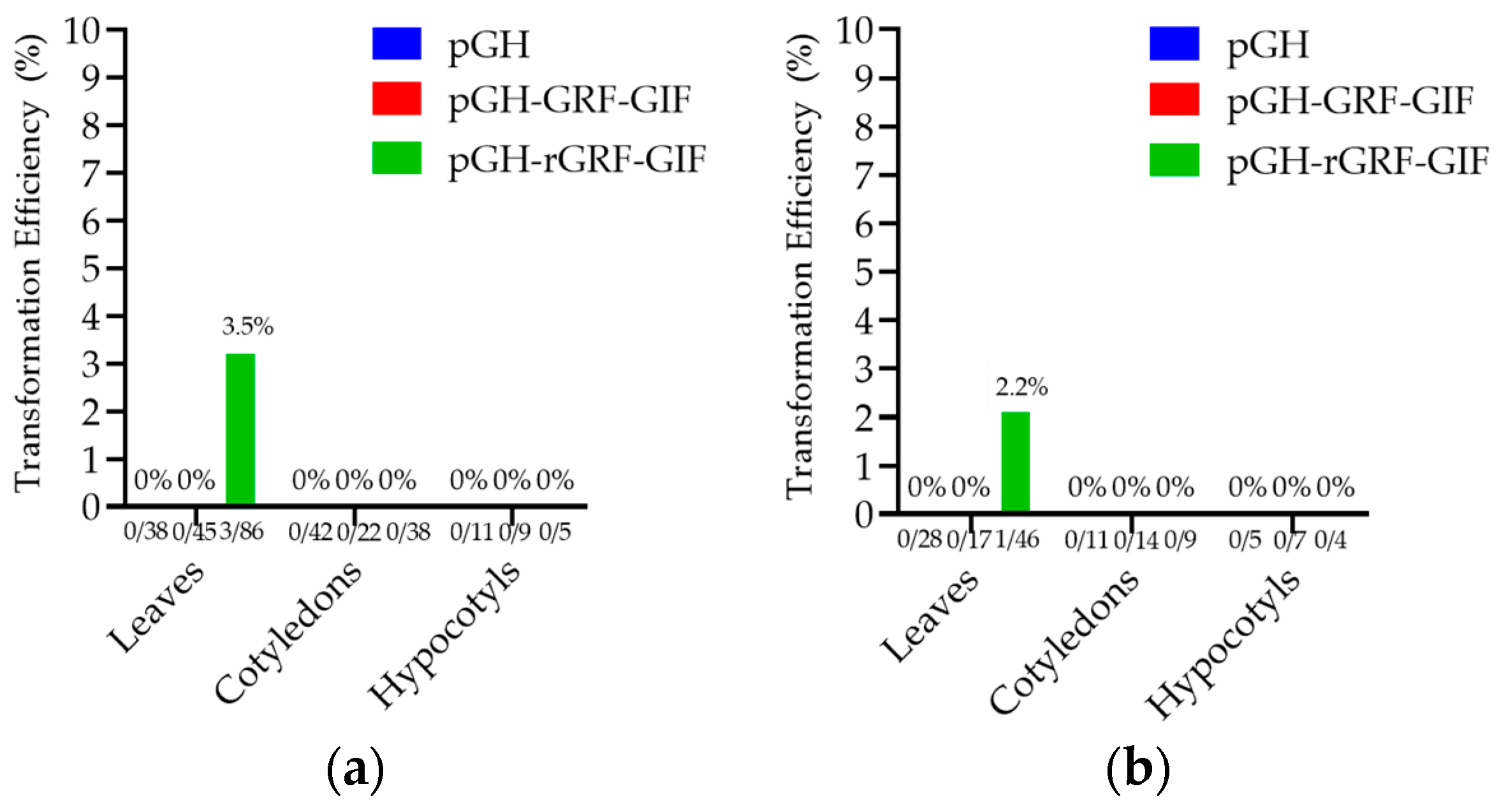
3.2. Transformation
GFP Expression and PCR Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, V.K.; Srivastava, A.; Mangal, M. Recent Trends in Sweet Pepper Breeding. In Accelerated Plant Breeding; Gosal, S., Wani, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; Volume 2, pp. 417–444. [Google Scholar]
- Pérez-Grajales, M.; Martínez-Damián, M.T.; Cruz-Alvarez, O.; Potrero-Andrade, S.M.; Peña-Lomelí, A.; González-Hernández, V.A.; Villegas-Monter, A. Content of capsaicinoids and physicochemical characteristics of manzano hot pepper grown in greenhouse. Not. Bot. Horti Agrobot. 2019, 47, 119–127. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Ahmed, Z. Influence of Plant Densities on Productivity of Bell Pepper (Capsicum annuum L.) under Greenhouse in High Altitude Cold Desert of Ladakh. In Proceedings of the International Symposium on Medicinal and Nutraceutical Plants 756, Macon, GA, USA, 8 November 2007; pp. 309–314. [Google Scholar]
- Pawar, S.S.; Bharude, N.V.; Sonone, S.S.; Deshmukh, R.S.; Raut, A.K.; Umarkar, A.R. Chillies as Food, Spice and Medicine: A Perspective. Int. J. Pharm. Biol. Sci. 2011, 1, 311–318. [Google Scholar]
- Kumar, R.V.; Sharma, V.K.; Chattopadhyay, B.; Chakraborty, S. An Improved Plant Regeneration and Agrobacterium-Mediated Transformation of Red Pepper (Capsicum annuum L.). Physiol. Mol. Biol. Plants 2012, 18, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Loyola-Vargas, V.M. The History of Somatic Embryogenesis. In Somatic Embryogenesis: Fundamental Aspects and Applications; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 11–22. ISBN 978-3-319-33704-3. [Google Scholar]
- Martínez-López, M.; García-Pérez, A.; Gimeno-Páez, E.; Prohens, J.; Vilanova, S.; García-Fortea, E. Screening of Suitable Plant Regeneration Protocols for Several Capsicum spp. Through Direct Organogenesis. Horticulturae 2021, 7, 261. [Google Scholar] [CrossRef]
- Bhatia, S.; Bera, T. Somatic Embryogenesis and Organogenesis. Mod. Appl. Plant Biotechnol. Pharm. Sci. 2015, 6, 209–230. [Google Scholar]
- Ochoa-Alejo, N. Somatic Embryogenesis in Capsicum spp. In Somatic Embryogenesis: Fundamental Aspects and Applications; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 233–240. ISBN 978-3-319-33704-3. [Google Scholar]
- Tan, J.; Lin, L.; Luo, H.; Zhou, S.; Zhu, Y.; Wang, X.; Miao, L.; Wang, H.; Zhang, P. Recent Progress in the Regeneration and Genetic Transformation System of Cucumber. Appl. Sci. 2022, 12, 7180. [Google Scholar] [CrossRef]
- Aboshama, H.; Amer, E.; Abu Hussein, I. An Efficient Protocol for Direct Organogenesis of Pepper (Capsicum annuum)التکوين العضوى المباشر فى نبات الفلفل. J. Plant Prod. 2020, 11, 727–732. [Google Scholar]
- Grozeva, S.; Todorova, V. In Vitro Regeneration in Pepper (Capsicum annuum L.) and Characterization of Plant-Regenerants. Electron. J. Biol. 2015, 11, 17–22. [Google Scholar]
- Pishbin, N.; Mousavi, A.; Kalatejari, S.; Shariatpanahi, M.; Jahromi, B.B. The Effect of Plant Growth Regulators and Different Types of Explants on in Vitro Regeneration of Sweet Pepper (Capsicum annuum L.). Int. J. Biosci. 2014, 5, 139–146. [Google Scholar]
- İzgü, T.; İlbi, H.; Mendi, Y.Y. Optimization of Plant Regeneration in Different Pepper (Capsicum annuum L.) Lines. Turk. J. Agric.-Food Sci. Technol. 2020, 8, 471–477. [Google Scholar] [CrossRef]
- David, M.H.K.; Tony, K.; Thierry, H.D.; Crescent, B.; Luc, H.J.; Bernard, G.C. Response of Pepper (Capsicum annuum) Cultivars from Benin for Somatic Embryogenesis from Callus. Issues Biol. Sci. Pharm. Res. 2021, 9, 48–53. [Google Scholar]
- Swamy, S.; Krupakar, A.; Chandran, D.S.; Koshy, E.P. Direct Regeneration Protocols of Five Capsicum annuum L. Varieties. Afr. J. Biotechnol. 2014, 13, 307–312. [Google Scholar]
- Verma, S.; Dhiman, K.; Srivastava, D.K. Efficient in Vitro Regeneration from Cotyledon Explants in Bell Pepper (Capsicum annuum L. Cv. California Wonder). Int. J. Adv. Biotechnol. Res. 2013, 4, 391–396. [Google Scholar]
- Rustgi, S.; Luo, H. (Eds.) Biolistic DNA Delivery in Plants: Methods and Protocols; In Methods in Molecular Biology; Springer: New York, NY, USA, 2020; Volume 2124, ISBN 978-1-07-160355-0. [Google Scholar]
- Rehman, F.; Gong, H.; Bao, Y.; Zeng, S.; Huang, H.; Wang, Y. CRISPR Gene Editing of Major Domestication Traits Accelerating Breeding for Solanaceae Crops Improvement. Plant Mol. Biol. 2022, 108, 157–173. [Google Scholar] [CrossRef]
- Chee, M.J.Y.; Lycett, G.W.; Chin, C.F. Development of a Direct Transformation Method by GFP Screening and in Vitro Whole Plant Regeneration of Capsicum frutescens L. Electron. J. Biotechnol. 2018, 34, 51–58. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.-B.; Jeon, H.-J.; Kim, H. Agrobacterium-mediated Capsicum annuum Gene Editing in Two Cultivars, Hot Pepper CM334 and Bell Pepper Dempsey. Int. J. Mol. Sci. 2021, 22, 3921. [Google Scholar] [CrossRef]
- Chai, L.; Du, C.; Fan, H.; Liu, C.; Si, Y. Improved Agrobacterium Tumefaciens-Mediated Transformation of Cucumber via Modified Use of Antibiotics and Acetosyringone. Res. Sq. 2020; preprint (Version 1). [Google Scholar] [CrossRef]
- Kota, S.; Lakkam, R.; Kasula, K.; Narra, M.; Qiang, H.; Rao Allini, V.; Zanmin, H.; Abbagani, S. Construction of a Species-Specific Vector for Improved Plastid Transformation Efficiency in Capsicum annuum L. 3 Biotech 2019, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Heidmann, I.; De Lange, B.; Lambalk, J.; Angenent, G.C.; Boutilier, K. Efficient Sweet Pepper Transformation Mediated by the BABY BOOM Transcription Factor. Plant Cell Rep. 2011, 30, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Islam, M.N. Efficient Regeneration System for Popular Chili Variety of Bangladesh. Int. J. Adv. Res. Bot. 2017, 6, 6–12. [Google Scholar]
- Mahto, B.K.; Sharma, P.; Rajam, M.V.; Reddy, P.M.; Dhar-Ray, S. An Efficient Method for Agrobacterium-mediated Genetic Transformation of Chili Pepper (Capsicum annuum L.). Indian J. Plant Physiol. 2018, 23, 573–581. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Xiao, L.; He, Y.; Liu, M.; Wang, J.; Tian, S.; Zhang, X.; Yuan, L. Highly Efficient, Genotype-independent Transformation and Gene Editing in Watermelon (Citrullus lanatus) Using a Chimeric ClGRF4-GIF1 Gene. J. Integr. Plant Biol. 2021, 63, 2038–2042. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J. Isolation of Plant DNA from Fresh Tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics, a Biometrical Approach, 2nd ed.; McGraw-Hill Kogakusha, Ltd.: Tokyo, Japan, 1981; p. 633. [Google Scholar]
- Hyde, C.L.; Phillips, G.C. Silver Nitrate Promotes Shoot Development and Plant Regeneration of Chili Pepper (Capsicum annuum L.) via organogenesis. In Vitro Cell. Dev. Biol.-Plant 1996, 32, 72–80. [Google Scholar] [CrossRef]
- Kumar, V.; Parvatam, G.; Ravishankar, G.A. Agno3: A Potential Regulator of Ethylene Activity and Plant Growth Modulator. Electron. J. Biotechnol. 2009, 12, 8–9. [Google Scholar] [CrossRef]
- Kraft, K.H.; Brown, C.H.; Nabhan, G.P.; Luedeling, E.; Luna Ruiz, J.D.J.; Coppens d’Eeckenbrugge, G.; Hijmans, R.J.; Gepts, P. Multiple Lines of Evidence for the Origin of Domesticated Chili Pepper, Capsicum annuum , in Mexico. Proc. Natl. Acad. Sci. USA 2014, 111, 6165–6170. [Google Scholar] [CrossRef] [PubMed]
- Chiou, K.L.; Hastorf, C.A. A Systematic Approach to Species–Level Identification of Chile Pepper (Capsicum spp.) Seeds: Establishing the Groundwork for Tracking the Domestication and Movement of Chile Peppers through the Americas and Beyond. Econ. Bot. 2014, 68, 316–336. [Google Scholar] [CrossRef]
- Kothari, S.L.; Joshi, A.; Kachhwaha, S.; Ochoa-Alejo, N. Chili Peppers—A Review on Tissue Culture and Transgenesis. Biotechnol. Adv. 2010, 28, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Grozeva, S.; Rodeva, V.; Todorova, V. In Vitro Shoot Organogenesis in Bulgarian Sweet Pepper (Capsicum annuum L.) Varieties. Electron. J. Biol. 2012, 8, 39–44. [Google Scholar]
- Dabauza, M.; Peña, L. High Efficiency Organogenesis in Sweet Pepper (Capsicum annuum L.) Tissues from Different Seedling Explants. Plant Growth Regul. 2001, 33, 221–229. [Google Scholar] [CrossRef]
- Ashrafuzzaman, M.; Hossain, M.M.; Ismail, M.R.; Haque, M.S.; Shahidullah, S.M.; Uz-zaman, S. Regeneration Potential of Seedling Explants of Chili (Capsicum annuum). Afr. J. Biotechnol. 2009, 8, 591–596. [Google Scholar]
- Ayzenshtat, D.; Kumar, M.; Zemach, H.; Forotan, Z.; Faigenbom, A.; Bocobza, S. Morphological and Transcriptional Analyses of Regeneration Events in Pepper Plants (Capsicum annuum) Expose Patterns of Shoot Apical Meristem Formation. J. Plant Growth Regul. 2023, 42, 7474–7487. [Google Scholar] [CrossRef]
- Bhutia, K.L.; Meetei, N.G.T.; Khanna, V.K. In Vitro Regeneration of Dalle Khursani, an Important Chili Cultivar of Sikkim, Using Various Explants. Agrotechnology 2016, 5, 142. [Google Scholar]
- Kasula, K.; Prasad, S.; Umate, P.; Gadidasu, K.; Abbagani, S. Efficient TDZ and IAA-Assisted Plant Regeneration from Cotyledon and Leaf Explants of Capsicum annuum L. One-Step Protocol for Shoot Bud Differentiation and Elongation. Int. J. Plant Dev. Biol. 2008, 2, 114–117. [Google Scholar]
- Desai, K.; Solanki, B.; Mankad, A.; Pandya, H. Genetic Transformation of Plants. Int. J. Sci. Res. Rev. 2019, 8, 1792–1806. [Google Scholar]
- Pratiwi, R.A.; Surya, M.I. Agrobacterium-mediated Transformation. Genet. Transform. Crops 2020, 2, 912. [Google Scholar] [CrossRef]
- Li, D.; Zhao, K.; Xie, B.; Zhang, B.; Luo, K. Establishment of a Highly Efficient Transformation System for Pepper (Capsicum annuum L.). Plant Cell Rep. 2003, 21, 785–788. [Google Scholar] [CrossRef]
- Maligeppagol, M.; Manjula, R.; Navale, P.M.; Babu, K.P.; Kumbar, B.M.; Laxman, R.H. Genetic Transformation of Chili (Capsicum annuum L.) with Dreb1A Transcription Factor Known to Impart Drought Tolerance. Indian J. Biotechnol. 2016, 15, 17–24. [Google Scholar]
- Mishra, R.; Mohanty, J.N.; Mahanty, B.; Joshi, R.K. A Single Transcript CRISPR/Cas9 Mediated Mutagenesis of CaERF28 Confers Anthracnose Resistance in Chili Pepper (Capsicum annuum L.). Planta 2021, 254, 5. [Google Scholar] [CrossRef]
- Humara, J.M.; López, M.; Ordás, R.J. Agrobacterium Tumefaciens -Mediated Transformation of Pinus pinea L. Cotyledons: An Assessment of Factors Influencing the Efficiency of uidA Gene Transfer. Plant Cell Rep. 1999, 19, 51–58. [Google Scholar] [CrossRef]
- Debernardi, J.M.; Tricoli, D.M.; Ercoli, M.F.; Hayta, S.; Ronald, P.; Palatnik, J.F.; Dubcovsky, J. A GRF-GIF chimeric protein improves the regeneration efficiency of transgenic plants. Nat. Biotechnol. 2020, 38, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, G.; Cheng, C.; Lei, L.; Sun, J.; Xu, Y.; Deng, C.; Dai, Z.; Yang, Z.; Chen, X.; et al. Establishment of an Agrobacterium-mediated genetic transformation and CRISPR/Cas9-mediated targeted mutagenesis in hemp (Cannabis Sativa L.). Plant Biotechnol. J. 2021, 19, 1979–1987. [Google Scholar] [CrossRef] [PubMed]







| Compounds | SGM | CIM I | CIM II | CIM III | CIM IV | CIM V | CIM VI | SFM I | SFM II | SFM III | SFM IV | SEM I | SEM II | RM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MS (g/L) | 4.43 | 4.43 | 4.43 | 4.43 | 4.43 | 4.43 | 4.43 | 4.43 | 4.43 | 4.43 | 4.43 | 4.43 | 4.43 | 4.43 |
| Sucrose (g/L) | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Agar (g/L) | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| BAP (mg/L) | - | 5 | 5 | 6 | - | 5 | 5 | 5 | 5 | 6 | 8 | 5 | 5 | 5 |
| IAA (mg/L) | - | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | - |
| NAA (mg/L) | - | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | - |
| ZR (mg/L) | - | - | 2 | 2 | 2 | 4 | - | 2 | 2 | 2 | 2 | 2 | 2 | - |
| ZT (mg/L) | - | 2 | - | - | - | - | - | - | - | - | - | - | - | - |
| Inositol (mg/L) | - | - | - | - | - | - | - | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | - |
| GA3 (mg/L) | - | - | - | - | - | - | - | - | - | - | - | 0.5 | 1 | - |
| AgNO3 (mg/L) | - | - | - | - | - | - | - | 5 | 5 | 5 | - | - | - | |
| IBA (mg/L) | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 |
| Compounds | SGM | PCM I | PCM II | CCM I | CCM II | SM I | SM II | SFM I | SFM II |
|---|---|---|---|---|---|---|---|---|---|
| MS (g/L) | 4.43 | 4.43 | 4.43 | 4.43 | 4.43 | 4.43 | 4.43 | 4.43 | 4.43 |
| Sucrose (g/L) | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Agar (g/L) | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| BAP (mg/L) | - | 5 | - | 5 | - | 5 | - | 5 | - |
| TDZ (mg/L) | - | 4 | - | 4 | - | 4 | - | 4 | |
| IAA (mg/L) | - | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| NAA (mg/L) | - | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| ZR (mg/L) | - | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Inositol (mg/L) | - | - | - | - | - | - | - | 0.1 | 0.1 |
| AgNO3 (mg/L) | - | - | - | - | - | - | - | 5 | 5 |
| Acetosyringone (mg/L) | - | - | - | 20 | 20 | - | - | - | - |
| Dithiothretol (mg/L) | - | - | - | 150 | 150 | - | - | - | - |
| Hygromycin (mg/L) | - | - | - | - | - | 30 | 30 | - | - |
| Cefotaxime (mg/L) | - | - | - | - | - | 300 | 300 | 300 | 300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shams, S.; Naeem, B.; Ma, L.; Li, R.; Zhang, Z.; Cao, Y.; Yu, H.; Feng, X.; Qiu, Y.; Wu, H.; et al. Developing an Optimized Protocol for Regeneration and Transformation in Pepper. Genes 2024, 15, 1018. https://doi.org/10.3390/genes15081018
Shams S, Naeem B, Ma L, Li R, Zhang Z, Cao Y, Yu H, Feng X, Qiu Y, Wu H, et al. Developing an Optimized Protocol for Regeneration and Transformation in Pepper. Genes. 2024; 15(8):1018. https://doi.org/10.3390/genes15081018
Chicago/Turabian StyleShams, Shamsullah, Beenish Naeem, Lingling Ma, Rongxuan Li, Zhenghai Zhang, Yacong Cao, Hailong Yu, Xigang Feng, Yinhui Qiu, Huamao Wu, and et al. 2024. "Developing an Optimized Protocol for Regeneration and Transformation in Pepper" Genes 15, no. 8: 1018. https://doi.org/10.3390/genes15081018






