Tracking Ovine Pulmonary Adenocarcinoma Development Using an Experimental Jaagsiekte Sheep Retrovirus Infection Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. JSRV Production and In Vitro Quantification
2.3. General Anaesthesia
2.4. Trans-Thoracic Ultrasound Scanning
2.5. Computed Tomography Scanning
2.6. Bronchoscopy
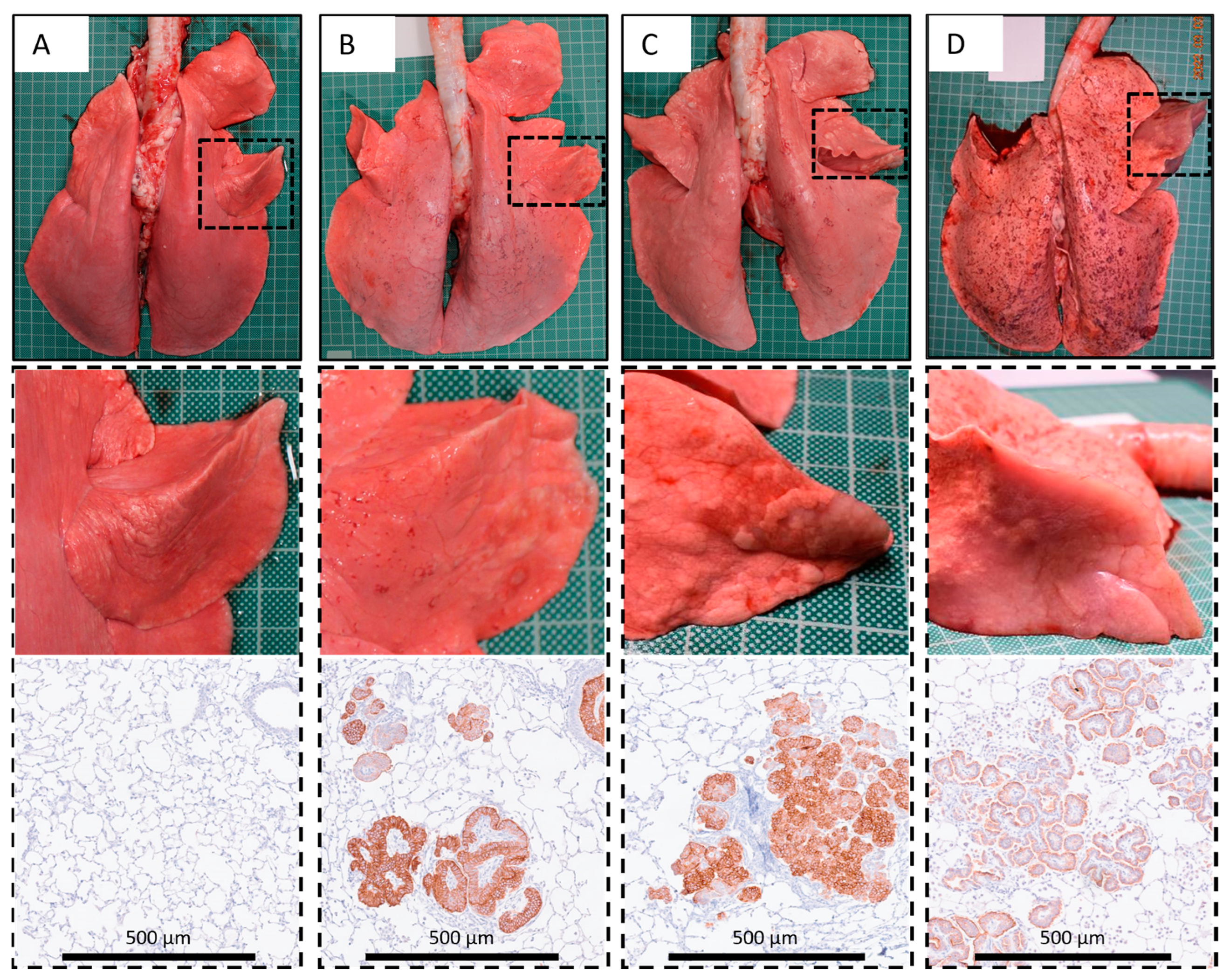
2.7. Post Mortem Examination
2.8. Histopathology and Immunohistochemistry
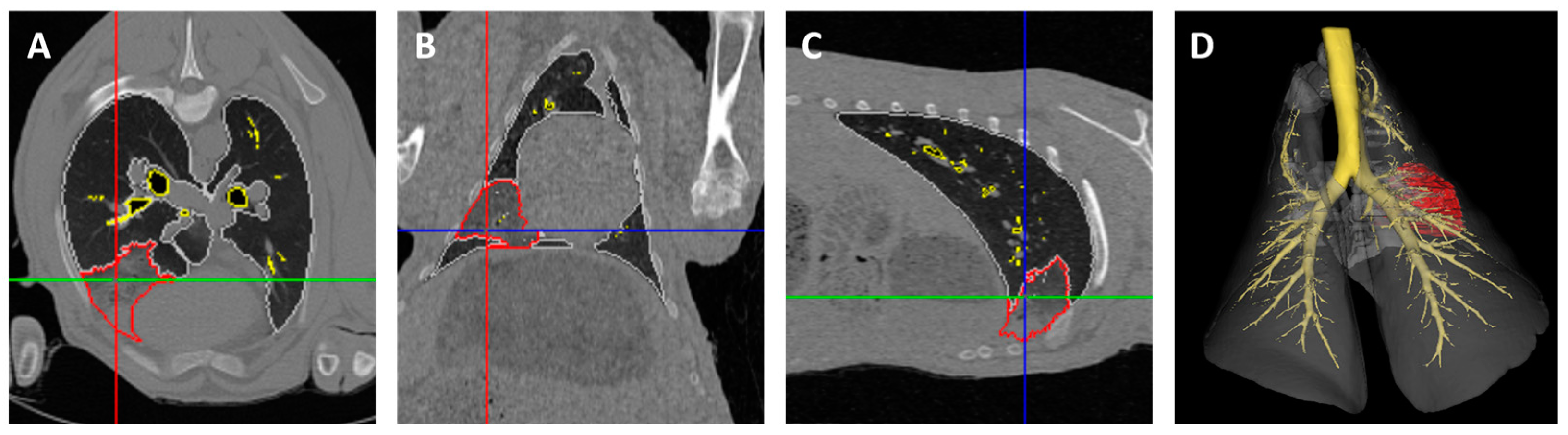
2.9. CT Image Analysis
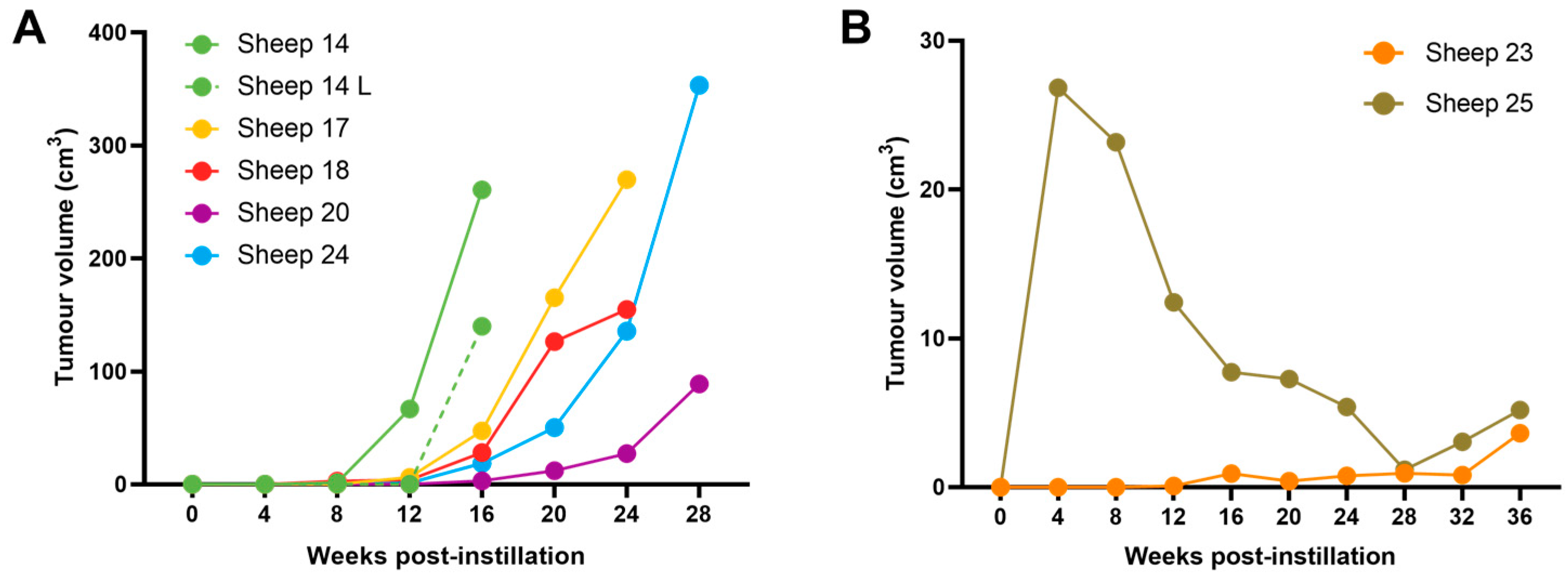
2.10. Statistical Analysis and Calculation of Tumour Volumes
3. Results
3.1. Local Bronchosopic Instillation Resulted in High Overall JSRV Infection and OPA Development Rates
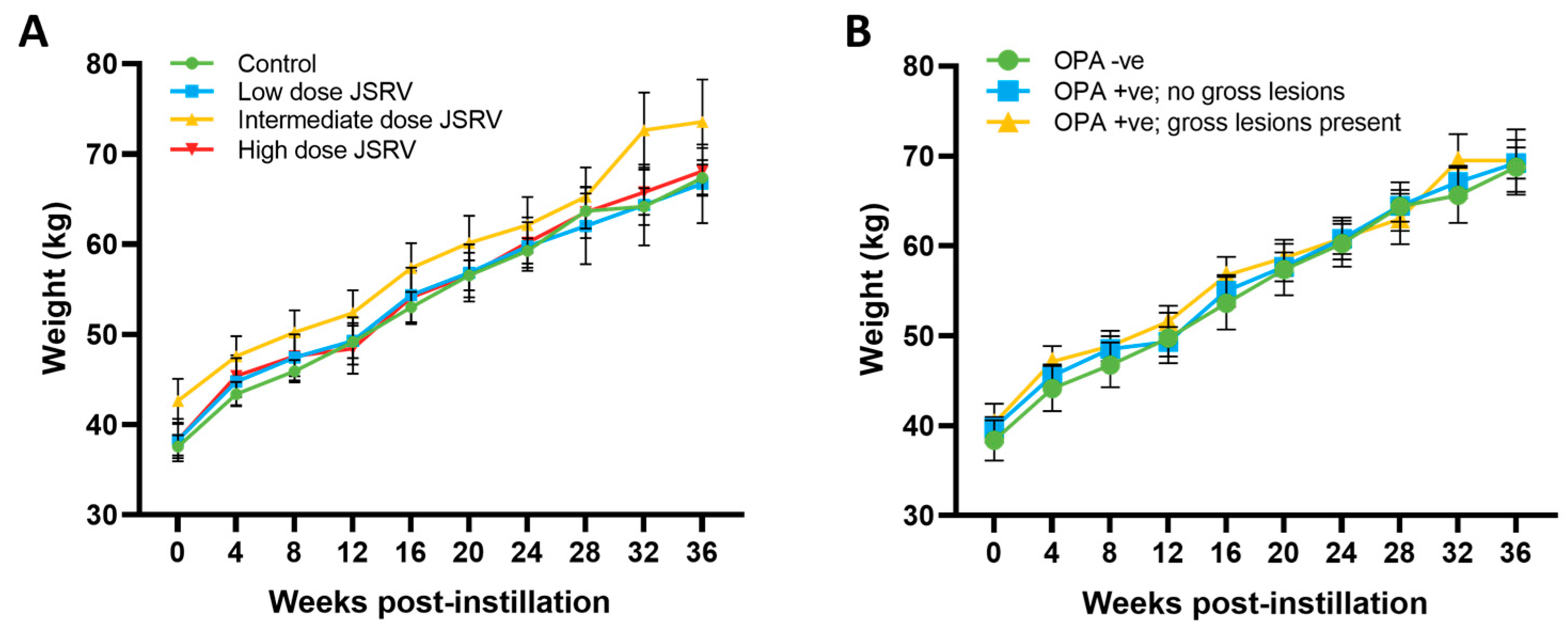
3.2. All Sheep Gained Weight and Remained Subclinical during the Study Period
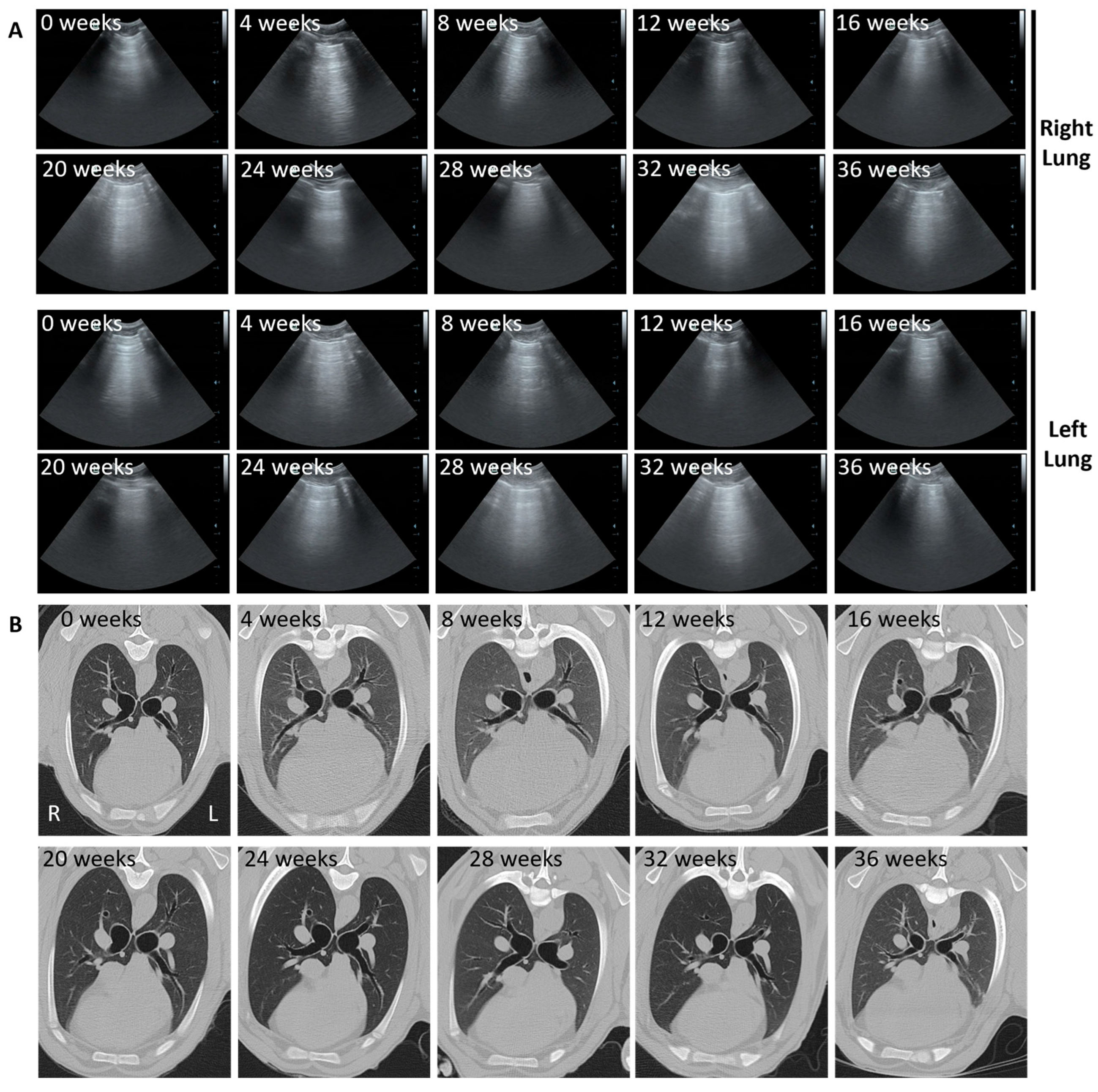
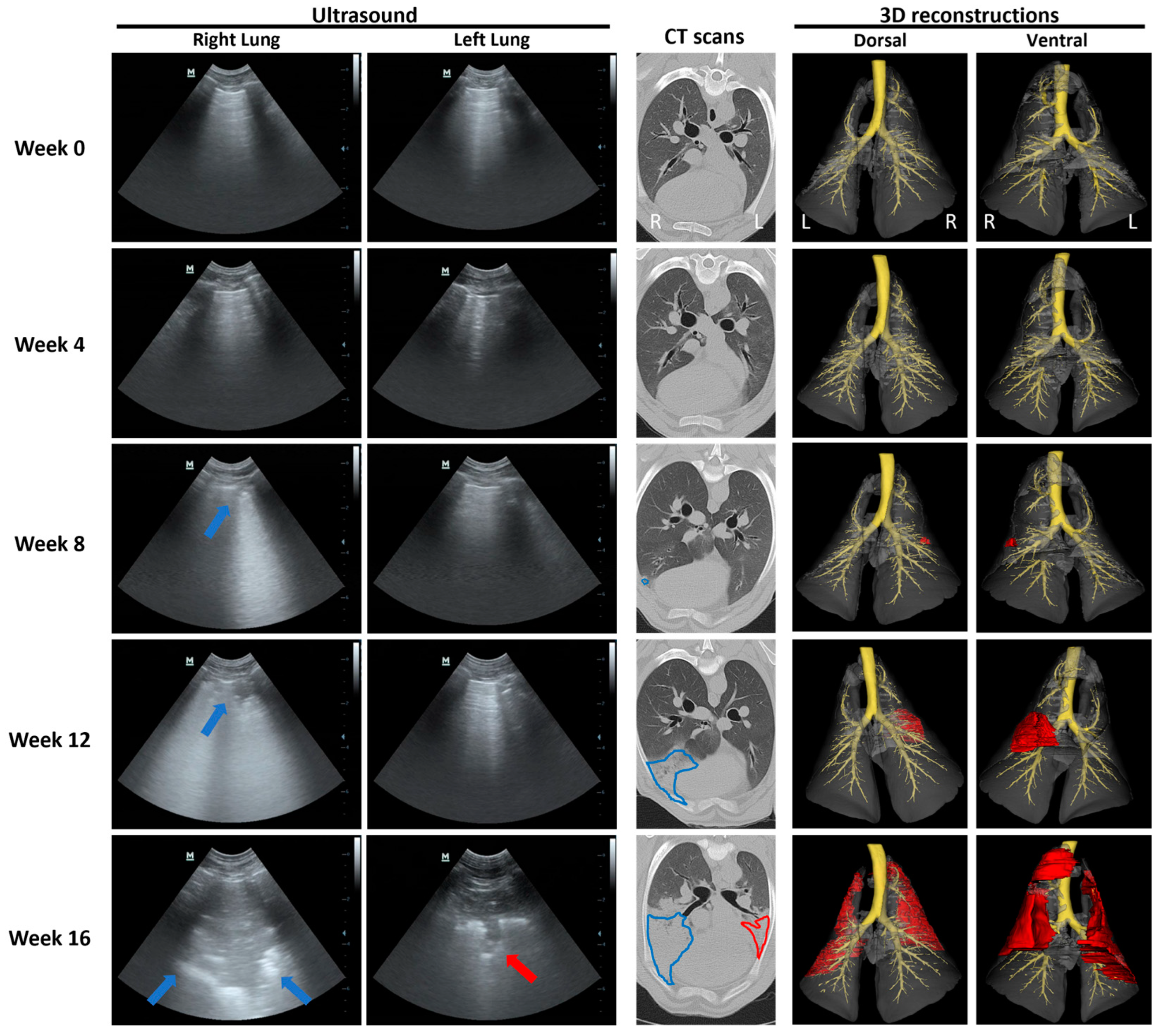
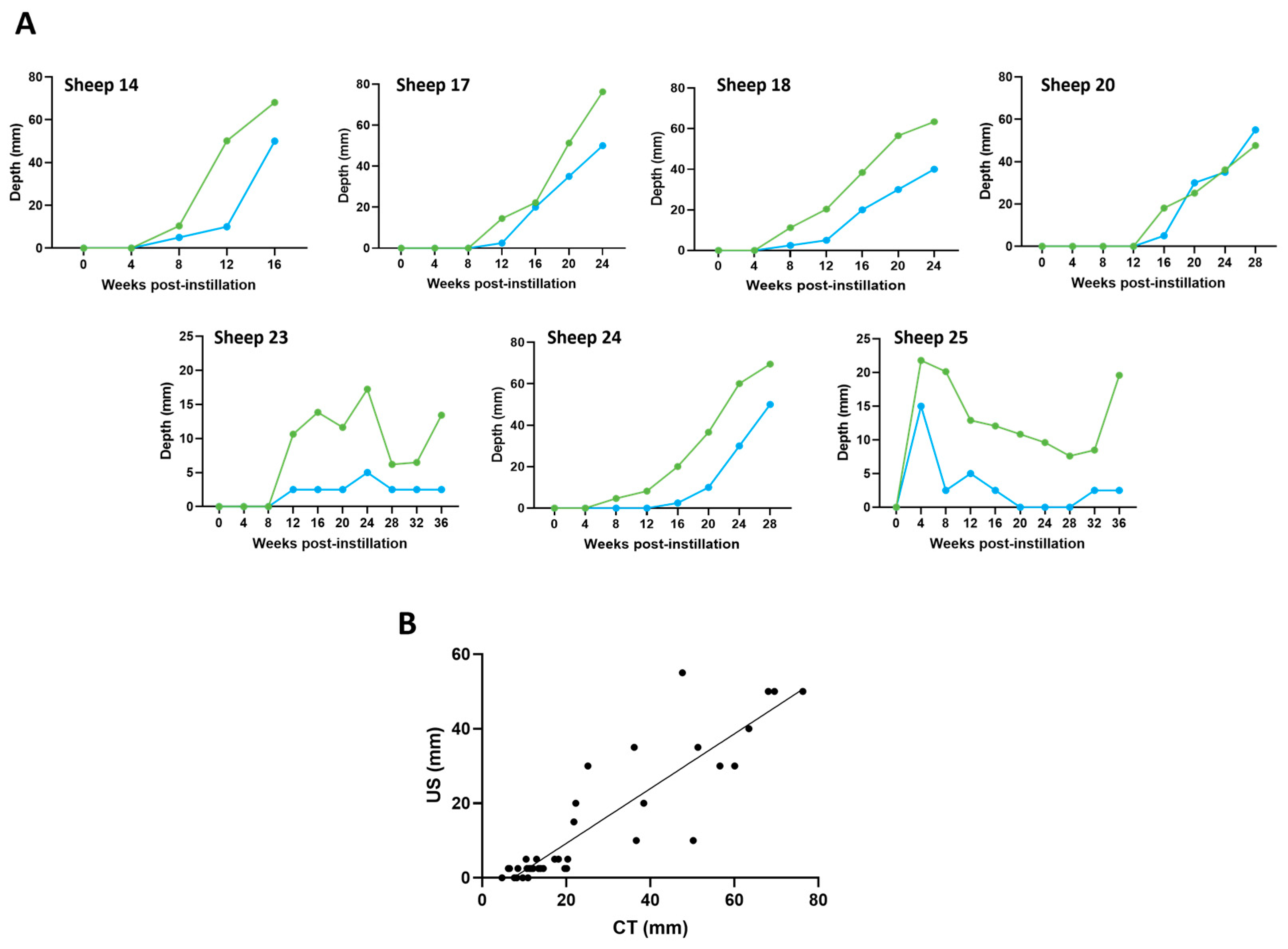
3.3. Ultrasound and CT Are Able to Identify Changes Consistent with OPA Diagnosis
3.4. OPA Tumour Growth Rates Are Variable but Can Be Rapid
3.5. Ultrasound and CT Are Highly Correlated in Their Ability to Track Tumour Development over Time
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeMartini, J.C.; York, D.F. Retrovirus-associated neoplasms of the respiratory system of sheep and goats: Ovine pulmonary carcinoma and enzootic nasal tumor. Vet. Clin. N. Am. Food Anim. Pract. 1997, 13, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Palmarini, M.; Fan, H.; Sharp, J.M. Sheep pulmonary adenomatosis: A unique model of retrovirus associated lung cancer. Trends Microbiol. 1997, 5, 478–483. [Google Scholar] [CrossRef]
- Griffiths, D.J.; Martineau, H.; Cousens, C. Pathology and pathogenesis of ovine pulmonary adenocarcinoma. J. Comp. Pathol. 2010, 142, 260–283. [Google Scholar] [CrossRef]
- Leroux, C.; Girard, N.; Cottin, V.; Greenland, T.; Mornex, J.-F.; Archer, F. Jaagsiekte Sheep Retrovirus (JSRV): From virus to lung cancer in sheep. Vet. Res. 2007, 38, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Fan, H. Jaagsiekte Sheep Retrovirus and Lung Cancer; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2003; Volume 275. [Google Scholar]
- Tustin, R. Ovine jaagsiekte. J. S. Afr. Vet. Assoc. 1969, 40, 3–23. [Google Scholar]
- Sharp, J.; DeMartini, J. Natural history of JSRV in sheep. In Jaagsiekte Sheep Retrovirus and Lung Cancer; Spring: Berlin/Heidelberg, Germany, 2003; pp. 55–79. [Google Scholar]
- Cousens, C.; Alleaume, C.; Bijsmans, E.; Martineau, H.M.; Finlayson, J.; Dagleish, M.P.; Griffiths, D.J. Jaagsiekte sheep retrovirus infection of lung slice cultures. Retrovirology 2015, 12, 31. [Google Scholar] [CrossRef]
- Archer, F.; Jacquier, E.; Lyon, M.; Chastang, J.; Cottin, V.; Mornex, J.F.; Leroux, C. Alveolar type II cells isolated from pulmonary adenocarcinoma: A model for JSRV expression in vitro. Am. J. Respir. Cell Mol. Biol. 2007, 36, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Wootton, S.K.; Halbert, C.L.; Miller, A.D. Sheep retrovirus structural protein induces lung tumours. Nature 2005, 434, 904–907. [Google Scholar] [CrossRef]
- Linnerth-Petrik, N.M.; Santry, L.A.; Yu, D.L.; Wootton, S.K. Adeno-associated virus vector mediated expression of an oncogenic retroviral envelope protein induces lung adenocarcinomas in immunocompetent mice. PLoS ONE 2012, 7, e51400. [Google Scholar] [CrossRef]
- Mestas, J.; Hughes, C.C. Of mice and not men: Differences between mouse and human immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef]
- Martin, W.B.; Scott, F.M.; Sharp, J.M.; Angus, K.W.; Norval, M. Experimental production of sheep pulmonary adenomatosis (Jaagsiekte). Nature 1976, 264, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Sharp, J.; Angus, K.; Gray, E.; Scott, F. Rapid transmission of sheep pulmonary adenomatosis (jaagsiekte) in young lambs. Arch. Virol. 1983, 78, 89–95. [Google Scholar] [CrossRef] [PubMed]
- DeMartini, J.C.; Rosadio, R.H.; Sharp, J.M.; Russell, H.I.; Lairmore, M.D. Experimental coinduction of type D retrovirus-associated pulmonary carcinoma and lentivirus-associated lymphoid interstitial pneumonia in lambs. J. Natl. Cancer Inst. 1987, 79, 167–177. [Google Scholar]
- Salvatori, D.; Gonzalez, L.; Dewar, P.; Cousens, C.; Heras, M.d.l.; Dalziel, R.G.; Sharp, J.M. Successful induction of ovine pulmonary adenocarcinoma in lambs of different ages and detection of viraemia during the preclinical period. J. Gen. Virol. 2004, 85, 3319–3324. [Google Scholar] [CrossRef] [PubMed]
- Martineau, H.M.; Cousens, C.; Imlach, S.; Dagleish, M.P.; Griffiths, D.J. Jaagsiekte sheep retrovirus infects multiple cell types in the ovine lung. J. Virol. 2011, 85, 3341–3355. [Google Scholar] [CrossRef] [PubMed]
- York, D.F.; Vigne, R.; Verwoerd, D.; Querat, G. Isolation, identification, and partial cDNA cloning of genomic RNA of jaagsiekte retrovirus, the etiological agent of sheep pulmonary adenomatosis. J. Virol. 1991, 65, 5061–5067. [Google Scholar] [CrossRef]
- York, D.; Vigne, R.; Verwoerd, D.; Querat, G. Nucleotide sequence of the jaagsiekte retrovirus, an exogenous and endogenous type D and B retrovirus of sheep and goats. J. Virol. 1992, 66, 4930–4939. [Google Scholar] [CrossRef] [PubMed]
- Palmarini, M.; Sharp, J.M.; de las Heras, M.; Fan, H. Jaagsiekte sheep retrovirus is necessary and sufficient to induce a contagious lung cancer in sheep. J. Virol. 1999, 73, 6964–6972. [Google Scholar] [CrossRef]
- DeMartini, J.C.; Bishop, J.V.; Allen, T.E.; Jassim, F.; Sharp, J.M.; de las Heras, M.; Voelker, D.R.; Carlson, J.O. Jaagsiekte sheep retrovirus proviral clone JSRVJS7, derived from the JS7 lung tumor cell line, induces ovine pulmonary carcinoma and is integrated into the surfactant protein A gene. J. Virol. 2001, 75, 4239–4246. [Google Scholar] [CrossRef]
- Ortín, A.; Minguijón, E.; Dewar, P.; García, M.; Ferrer, L.M.; Palmarini, M.; Gonzalez, L.; Sharp, J.M.; De las Heras, M. Lack of a specific immune response against a recombinant capsid protein of Jaagsiekte sheep retrovirus in sheep and goats naturally affected by enzootic nasal tumour or sheep pulmonary adenomatosis. Vet. Immunol. Immunopathol. 1998, 61, 229–237. [Google Scholar] [CrossRef]
- Sharp, J.; Herring, A. Sheep pulmonary adenomatosis: Demonstration of a protein which crossreacts with the major core proteins of Mason-Pfizer monkey virus and mouse mammary tumour virus. J. Gen. Virol. 1983, 64, 2323–2327. [Google Scholar] [CrossRef]
- Hudachek, S.F.; Kraft, S.L.; Thamm, D.H.; Bielefeldt-Ohmann, H.; DeMartini, J.C.; Miller, A.D.; Dernell, W.S. Lung Tumor Development and Spontaneous Regression in Lambs Coinfected With Jaagsiekte Sheep Retrovirus and Ovine Lentivirus. Vet. Pathol. 2010, 47, 148–162. [Google Scholar] [CrossRef]
- De las Heras, M.; Ortín, A.; Salvatori, D.; de Villareal, M.P.; Cousens, C.; Ferrer, L.M.; Cebrián, L.M.; de Jalón, J.A.G.; Gonzalez, L.; Sharp, J.M. A PCR technique for the detection of Jaagsiekte sheep retrovirus in the blood suitable for the screening of ovine pulmonary adenocarcinoma in field conditions. Res. Vet. Sci. 2005, 79, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Voigt, K.; Brügmann, M.; Huber, K.; Dewar, P.; Cousens, C.; Hall, M.; Sharp, J.; Ganter, M. PCR examination of bronchoalveolar lavage samples is a useful tool in pre-clinical diagnosis of ovine pulmonary adenocarcinoma (Jaagsiekte). Res. Vet. Sci. 2007, 83, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Lewis, F.; Brülisauer, F.; Cousens, C.; McKendrick, I.; Gunn, G. Diagnostic accuracy of PCR for Jaagsiekte sheep retrovirus using field data from 125 Scottish sheep flocks. Vet. J. 2011, 187, 104–108. [Google Scholar] [CrossRef]
- Cousens, C.; Scott, P. Assessment of transthoracic ultrasound diagnosis of ovine pulmonary adenocarcinoma in adult sheep. Vet. Rec. 2015, 177, 366–371. [Google Scholar] [CrossRef]
- De las Heras, M.; González, L.; Sharp, J.M. Pathology of ovine pulmonary adenocarcinoma. In Current Topics in Microbiology and Immunology; Spring: Berlin/Heidelberg, Germany, 2003; Volume 275, pp. 25–54. [Google Scholar] [CrossRef]
- Bai, J.; Zhu, R.; Stedman, K.; Cousens, C.; Carlson, J.; Sharp, J.; DeMartini, J. Unique long terminal repeat U3 sequences distinguish exogenous jaagsiekte sheep retroviruses associated with ovine pulmonary carcinoma from endogenous loci in the sheep genome. J. Virol. 1996, 70, 3159–3168. [Google Scholar] [CrossRef]
- Murgia, C.; Caporale, M.; Ceesay, O.; Di Francesco, G.; Ferri, N.; Varasano, V.; de las Heras, M.; Palmarini, M. Lung adenocarcinoma originates from retrovirus infection of proliferating type 2 pneumocytes during pulmonary post-natal development or tissue repair. PLoS Pathog. 2011, 7, e1002014. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.; Griffiths, D.; Cousens, C. Diagnosis and control of ovine pulmonary adenocarcinoma (Jaagsiekte). In Practice 2013, 35, 382–397. [Google Scholar] [CrossRef]
- Scott, P.; Cousens, C. Ultrasonography of ovine pulmonary adenocarcinoma. In Practice 2018, 40, 291–300. [Google Scholar] [CrossRef]
- Miller, A. Practical approach to lung ultrasound. BJA Educ. 2015, 16, 39–45. [Google Scholar] [CrossRef]
- Hare, W. The broncho-pulmonary segments in the sheep. J. Anat. 1955, 89, 387. [Google Scholar] [PubMed]
- Wootton, S.K.; Metzger, M.J.; Hudkins, K.L.; Alpers, C.E.; York, D.; DeMartini, J.C.; Miller, A.D. Lung cancer induced in mice by the envelope protein of jaagsiekte sheep retrovirus (JSRV) closely resembles lung cancer in sheep infected with JSRV. Retrovirology 2006, 3, 94. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M. A biomathematical approach to clinical tumor growth. Cancer 1961, 14, 1272–1294. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Caporale, M.; Centorame, P.; Giovannini, A.; Sacchini, F.; Di Ventura, M.; De las Heras, M.; Palmarini, M. Infection of lung epithelial cells and induction of pulmonary adenocarcinoma is not the most common outcome of naturally occurring JSRV infection during the commercial lifespan of sheep. Virology 2005, 338, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Cousens, C.; Maeda, N.; Murgia, C.; Dagleish, M.P.; Palmarini, M.; Fan, H. In vivo tumorigenesis by Jaagsiekte sheep retrovirus (JSRV) requires Y590 in Env TM, but not full-length orfX open reading frame. Virology 2007, 367, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Glendinning, L.; Wright, S.; Pollock, J.; Tennant, P.; Collie, D.; McLachlan, G. Variability of the sheep lung microbiota. Appl. Environ. Microbiol. 2016, 82, 3225–3238. [Google Scholar] [CrossRef]
- Caswell, J.L.; Williams, K.J. Chapter 5 Respiratory System. In Jubb, Kennedy & Palmer’s Pathology of Domestic Animals; Elsevier Ltd.: Philadelphia, PA, USA, 2006; Volume 2. [Google Scholar]
- Philbey, A.W.; Cousens, C.; Bishop, J.V.; Gill, C.A.; DeMartini, J.C.; Sharp, J.M. Multiclonal pattern of Jaagsiekte sheep retrovirus integration sites in ovine pulmonary adenocarcinoma. Virus Res. 2006, 117, 254–263. [Google Scholar] [CrossRef]
- Scott, P.R.; Dagleish, M.P.; Cousens, C. Development of superficial lung lesions monitored on farm by serial ultrasonographic examination in sheep with lesions confirmed as ovine pulmonary adenocarcinoma at necropsy. Ir. Vet. J. 2018, 71, 23. [Google Scholar] [CrossRef]
- Humann-Ziehank, E.; Renko, K.; Bruegmann, M.L.; Devi, V.R.; Hewicker-Trautwein, M.; Andreae, A.; Ganter, M. Long-term study of ovine pulmonary adenocarcinogenesis in sheep with marginal vs. sufficient nutritional selenium supply: Results from computed tomography, pathology, immunohistochemistry, JSRV-PCR and lung biochemistry. J. Trace Elem. Med. Biol. 2013, 27, 391–399. [Google Scholar] [CrossRef]
- Cousens, C.; Ewing, D.A.; McKendrick, I.J.; Todd, H.; Dagleish, M.P.; Scott, P.R. Efficacy of high-throughput transthoracic ultrasonographic screening for on-farm detection of ovine pulmonary adenocarcinoma. Vet. Rec. 2022, 191, e1797. [Google Scholar] [CrossRef] [PubMed]
- Scott, P. Thoracic ultrasonography as an adjunct to clinical examination in sheep. Small Rumin. Res. 2017, 152, 107–118. [Google Scholar] [CrossRef]
- Scott, P. Description of clinical cases of diseases of the thorax by means of ultrasonographic examination in sheep. Small Rumin. Res. 2017, 152, 128–131. [Google Scholar] [CrossRef]
- Mento, F.; Khan, U.; Faita, F.; Smargiassi, A.; Inchingolo, R.; Perrone, T.; Demi, L. State of the Art in Lung Ultrasound, Shifting from Qualitative to Quantitative Analyses. Ultrasound Med. Biol. 2022, 48, 2398–2416. [Google Scholar] [CrossRef]


| Phase | Drug | Manufacturer | Dose (mg/kg) | Route |
|---|---|---|---|---|
| Sedation | Medetomidine | ‘Medetor’; Virbac Ltd., Suffolk, UK | 0.005 | i.v. |
| in combination with | ||||
| Ketamine | ‘Ketamidor’; Chanelle Vet Ltd., Hungerford, UK | 0.5 | i.v. | |
| Induction | Propofol | ‘Propofol’; Fresenius Kabi, Cheshire, UK | To effect * (e.g., 2–5) | i.v. |
| Maintenance | Isoflurane | ‘Isofane’; Piramal Critical Care Ltd., West Drayton, UK | Inhaled | |
| Recovery | Atipamezole | ‘Atipam’; Dechra, Eurovet Animal Health BV, Bladel, The Netherlands | 0.025 | i.m. |
| Group | JSRV Low Dose | JSRV Intermediate Dose | JSRV High Dose | Control | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sheep No. | 14 | 18 | 17 | 13 | 16 | 15 | 24 | 20 | 23 | 21 | 22 | 19 | 25 | 26 | 27 | 28 | 29 | 30 | 1–6 | |
| Sex | M | F | F | M | F | M | M | F | M | F | M | F | M | M | M | F | F | F | M&F | |
| Months post-instil | 4 | 6 | 6 | 9 | 9 | 9 | 7 | 7 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | |
| IHC Sample Site | L1 | ++ | ++ | + | - | - | - | ++ | - | - | - | - | - | - | - | - | - | - | - | - |
| L2 | ++ | ++ | - | - | - | - | ++ | - | - | - | - | - | - | - | - | - | - | - | - | |
| L3 | ++ | ++ | ++ | + | + | - | ++ | ++ | + | ++ | + | - | ++ | + | + | + | + | + | - | |
| L4 | ++ | ++ | - | - | - | - | ++ | - | - | - | - | - | - | - | - | - | - | - | - | |
| L5 | ns | - | - | - | - | - | - | ++ | - | - | - | - | - | - | - | - | - | - | - | |
| L6 | ns | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| L7 | ns | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| L8 | ns | - | - | - | - | - | ++ | - | - | - | - | - | - | - | - | - | - | - | - | |
| L9 | + | ++ | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| L10 | - | ++ | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| OPA Status | ||||||||||||||||||||
| Advanced lesions | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||||
| Early lesions | ✓ | ✓ | ||||||||||||||||||
| No gross lesions | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Negative | ✓ | ✓ | ✓ | |||||||||||||||||
| Sheep No. | Tumour Volume Doubling Time (Days) |
|---|---|
| 14 | 7.39 |
| 17 | 15.37 |
| 18 | 19.56 |
| 20 | 17.20 |
| 24 | 14.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cousens, C.; Meehan, J.; Collie, D.; Wright, S.; Chang, Z.; Todd, H.; Moore, J.; Grant, L.; Daniel, C.R.; Tennant, P.; et al. Tracking Ovine Pulmonary Adenocarcinoma Development Using an Experimental Jaagsiekte Sheep Retrovirus Infection Model. Genes 2024, 15, 1019. https://doi.org/10.3390/genes15081019
Cousens C, Meehan J, Collie D, Wright S, Chang Z, Todd H, Moore J, Grant L, Daniel CR, Tennant P, et al. Tracking Ovine Pulmonary Adenocarcinoma Development Using an Experimental Jaagsiekte Sheep Retrovirus Infection Model. Genes. 2024; 15(8):1019. https://doi.org/10.3390/genes15081019
Chicago/Turabian StyleCousens, Chris, James Meehan, David Collie, Steven Wright, Ziyuan Chang, Helen Todd, Jo Moore, Lynn Grant, Carola R. Daniel, Peter Tennant, and et al. 2024. "Tracking Ovine Pulmonary Adenocarcinoma Development Using an Experimental Jaagsiekte Sheep Retrovirus Infection Model" Genes 15, no. 8: 1019. https://doi.org/10.3390/genes15081019





