The First FISH-Confirmed Non-Canonical Telomeric Motif in Heteroptera: Cimex lectularius Linnaeus, 1758 and C. hemipterus (Fabricius, 1803) (Hemiptera, Cimicidae) Have a 10 bp Motif (TTAGGGATGG)n
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Chromosome Preparations and Fluorescence In Situ Hybridization with Probes for Telomeric Repeats
2.3. Microscopy
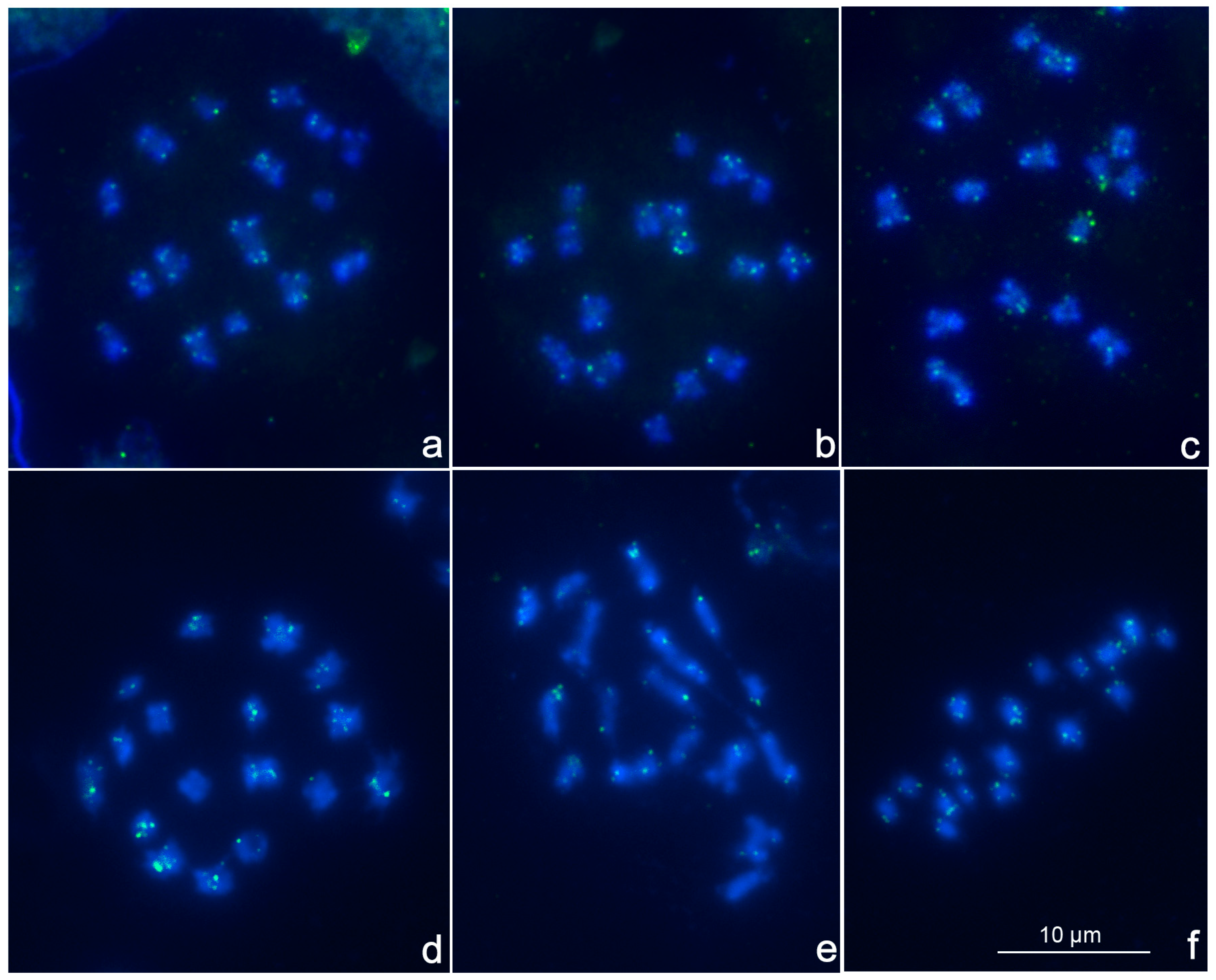
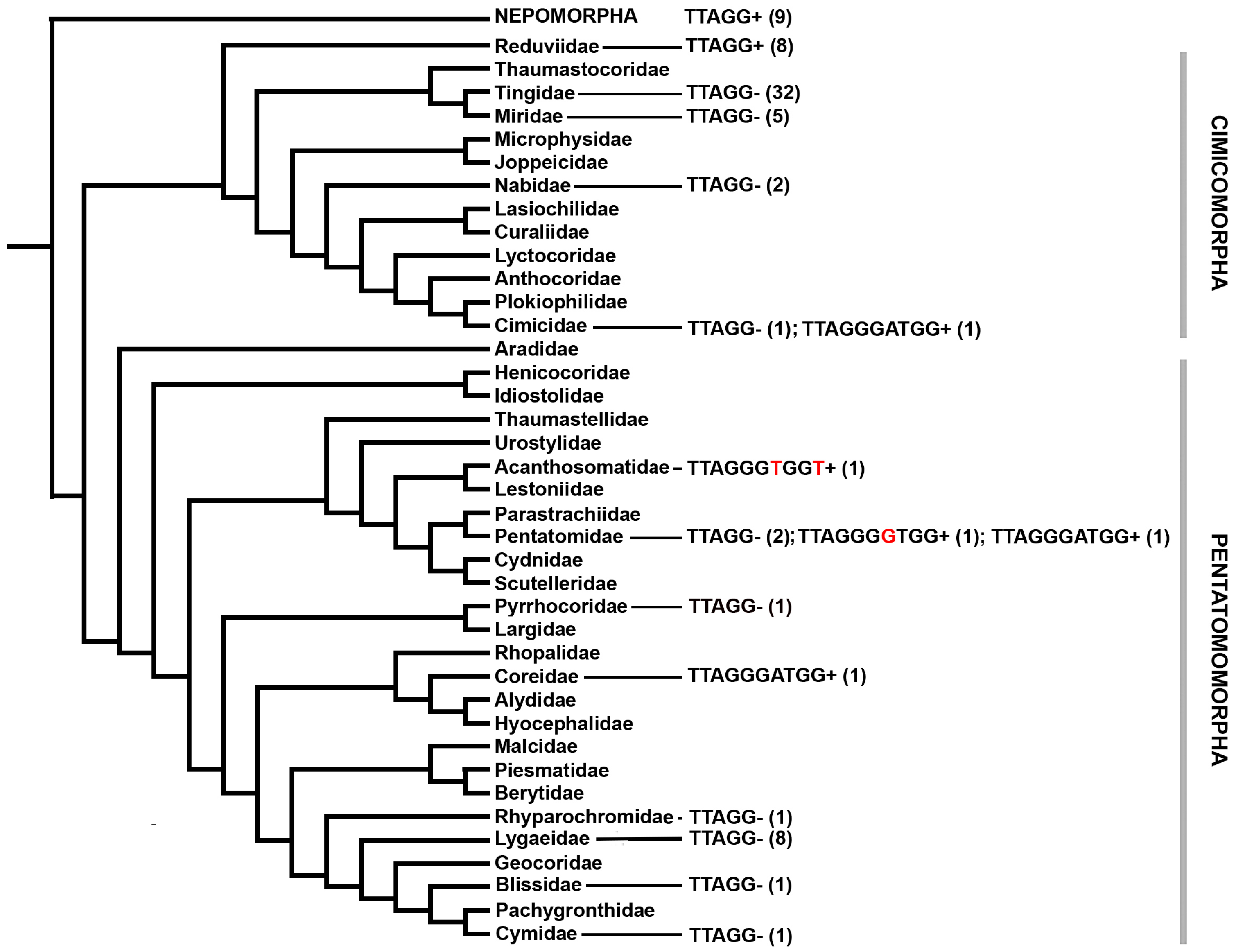
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muller, H.J. The remaking of chromosomes. Collect. Net 1938, 8, 182–195. [Google Scholar]
- Blackburn, E.H. Structure and Function of Telomeres. Nature 1991, 350, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Zakian, V.A. Telomeres: Beginning to understand the end. Science 1995, 270, 1601–1607. [Google Scholar] [CrossRef] [PubMed]
- Bochman, M.; Paeschke, K.; Zakian, V.A. DNA secondary structures: Stability and function of G-quadruplex structures. Nat. Rev. Genet. 2012, 13, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Čapková-Frydrychová, R.; Mason, J.M.; Peška, V. Telomere flexibility and versatility: A role of telomeres in adaptive potential. Front. Genet. 2021, 12, 771938. [Google Scholar] [CrossRef] [PubMed]
- Greider, C.W.; Blackburn, E.H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature 1989, 337, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Traut, W.; Szczepanowski, M.; Vítková, M.; Opitz, C.; Marec, F.; Zrzavý, J. The telomere repeat motif of basal Metazoa. Chromosome Res. 2007, 15, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Lukhtanov, V.A.; Kuznetsova, V.G. What genes and chromosomes say about the origin and evolution of insects and other arthropods. Russ. J. Genet. 2010, 46, 1115–1122. [Google Scholar] [CrossRef]
- Mason, J.M.; Randall, T.A.; Frydrychová, R.C. Telomerase lost? Chromosoma 2016, 125, 65–73. [Google Scholar] [CrossRef]
- Kuznetsova, V.; Grozeva, S.; Gokhman, V. Telomere structure in insects: A review. J. Zool. Syst. Evol. Res. 2020, 58, 127–158. [Google Scholar] [CrossRef]
- Peška, V.; Garcia, S. Origin Diversity and Evolution of Telomere Sequences in Plants. Front. Plant Sci. 2020, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Lukhtanov, V.A. Diversity and evolution of telomere and subtelomere DNA sequences in insects. BioRxiv 2022. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Xiong, X.; Appel, A.G.; Zhang, C.; Wang, X. Profiles of telomeric repeats in Insecta reveal diverse forms of telomeric motifs in Hymenopterans. Life Sci. Alliance 2022, 5, e202101163. [Google Scholar] [CrossRef] [PubMed]
- Lukhtanov, V.A.; Pazhenkova, E.A. Diversity and evolution of telomeric motifs and telomere DNA organization in insects. Biol. J. Linn. Soc. 2023, 140, 536–555. [Google Scholar] [CrossRef]
- Frydrychová, R.; Grossmann, P.; Trubač, P.; Vítková, M.; Marec, F. Phylogenetic distribution of TTAGG telomeric repeats in insects. Genome 2004, 47, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Frydrychová, R.; Marec, F. Repeated losses of TTAGG telomere repeats in evolution of beetles (Coleoptera). Genetica 2002, 115, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Gibbs, R.A.; Weinstock, G.M.; Gibbs, R.; Richards, S.; Muzny, D.; Gibbs, R.A.; Weinstock, G.M.; Attaway, T.; Bell, S.; et al. The genome of the model beetle and pest Tribolium castaneum. Nature 2008, 452, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Mravinac, B.; Meštrović, N.; Cavrak, V.V.; Plohl, M. TCAGG an alternative telomeric sequence in insects. Chromosoma 2011, 120, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Prušáková, D.; Peška, V.; Pekár, S.; Bubeník, M.; Čížek, L.; Bezděk, A.; Frydrychová, R.C. Telomeric DNA sequences in beetle taxa vary with species richness. Sci. Rep. 2021, 11, 1331. [Google Scholar] [CrossRef]
- Meyne, J.; Hirai, H.; Imai, H.T. FISH analysis of the telomere sequences of bulldog ants (Myrmecia: Formicidae). Chromosoma 1995, 104, 14–18. [Google Scholar] [CrossRef]
- Lyčka, M.; Bubeník, M.; Závodník, M.; Peška, V.; Fajkus, P.; Demko, M.; Fajkus, J.; Fojtová, M. TeloBase: A community-curated database of telomere sequences across the tree of life. Nucleic Acids Res. 2024, 52, D311–D321. [Google Scholar] [CrossRef]
- Sahara, K.; Marec, F.; Traut, W. TTAGG telomeric repeats in chromosomes of some insects and other arthropods. Chromosome Res. 1999, 7, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Grozeva, S.; Kuznetsova, V.; Anokhin, B. Karyotypes male meiosis and comparative FISH mapping of 18S ribosomal DNA and telomeric (TTAGG)n repeat in eight species of true bug (Hemiptera Heteroptera). Comp. Cytogenet. 2011, 5, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, V.G.; Grozeva, S.M.; Anokhin, B.A. The first finding of (TTAGG)n telomeric repeat in chromosomes of true bugs (Heteroptera, Belostomatidae). Comp. Cytogenet. 2012, 6, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Golub, N.V.; Golub, V.B.; Kuznetsova, V.G. Distribution of the major rDNA loci among four hemipteran species of the family Tingidae (Heteroptera, Cimicomorpha). Folia Biol. 2017, 65, 155–158. [Google Scholar] [CrossRef]
- Golub, N.V.; Golub, V.B.; Anokhin, B.A.; Kuznetsova, V.G. Comparative cytogenetics of lace bugs (Tingidae, Heteroptera): New data and a brief overview. Insects 2022, 13, 608. [Google Scholar] [CrossRef] [PubMed]
- Golub, N.V.; Maryańska-Nadachowska, A.; Anokhin, B.A.; Kuznetsova, V.G. Expanding the chromosomal evolution understanding of lygaeioid true bugs (Lygaeoidea, Pentatomomorpha, Heteroptera) by classical and molecular cytogenetic analysis. Genes 2023, 14, 725. [Google Scholar] [CrossRef] [PubMed]
- Pita, S.; Panzera, F.; Mora, P.; Vela, J.; Palomeque, T.; Lorite, P. The presence of the ancestral insect telomeric motif in kissing bugs (Triatominae) rules out the hypothesis of its loss in evolutionarily advanced Heteroptera (Cimicomorpha). Comp. Cytogenet. 2016, 10, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Angus, R.B.; Jeangirard, C.; Stoianova, D.; Grozeva, S.; Kuznetsova, V.G. A chromosomal analysis of Nepa cinerea Linnaeus 1758 and Ranatra linearis (Linnaeus, 1758) (Heteroptera, Nepidae). Comp. Cytogenet. 2017, 11, 641–657. [Google Scholar] [CrossRef]
- Chirino, M.G.; Dalíková, M.; Marec, F.; Bressa, M.J. Chromosomal distribution of interstitial telomeric sequences as signs of evolution through chromosome fusion in six species of the giant water bugs (Hemiptera, Belostoma). Ecol. Evol. 2017, 7, 5227–5235. [Google Scholar] [CrossRef]
- Grozeva, S.; Anokhin, B.A.; Simov, N.; Kuznetsova, V.G. New evidence for the presence of the telomeric motif (TTAGG)n in the family Reduviidae and its absence in the families Nabidae and Miridae (Hemiptera, Cimicomorpha). Comp. Cytogenet. 2019, 13, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Gapon, D.A.; Kuznetsova, V.G.; Maryańska-Nadachowska, A. A new species of the genus Rhaphidosoma Amyot et Serville 1843 (Heteroptera, Reduviidae) with data on its chromosome complement. Comp. Cytogenet. 2021, 15, 467. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, K.B.; Visser, S.; Dalíková, M.; Provazníková, I.; Urbaneja, A.; Pérez-Hedo, M.; Marec, F.; Werren, J.H.; Zwaan, B.J.; Pannebakker, B.A.; et al. Jekyll or Hyde? The genome (and more) of Nesidiocoris tenuis a zoophytophagous predatory bug that is both a biological control agent and a pest. Insect Mol. Biol. 2021, 30, 188–209. [Google Scholar] [CrossRef] [PubMed]
- Cabral-de-Mello, D.C.; Mora, P.; Rico-Porras, J.M.; Ferretti, A.B.S.M.; Palomeque, T.; Lorite, P. The spread of satellite DNAs in euchromatin and insights into the multiple sex chromosome evolution in Hemiptera revealed by repeatome analysis of the bug Oxycarenus hyalinipennis. Insect Mol. Biol. 2023, 32, 725–737. [Google Scholar] [CrossRef]
- Schaefer, C.W.; Panizzi, A.R. Heteroptera of Economic Importance; CRC Press: Boca Raton, FL, USA, 2000; pp. 3–8. [Google Scholar] [CrossRef]
- Reinhardt, K.; Siva-Jothy, M.T. Biology of the bed bugs (Cimicidae). Annu. Rev. Entomol. 2007, 52, 351–374. [Google Scholar] [CrossRef] [PubMed]
- Zorrilla-Vaca, A.; Silva-Medina, M.M.; Escandón-Vargas, K. Bedbugs Cimex spp.: Their current world resurgence and healthcare impact. Asian Pac. J. Trop. Dis. 2015, 5, 342–352. [Google Scholar] [CrossRef]
- Akhoundi, M.; Sereno, D.; Durand, R.; Mirzaei, A.; Bruel, C.; Delaunay, P.; Marty, P.; Izri, A. Bed Bugs (Hemiptera, Cimicidae): Overview of Classification Evolution and Dispersion. Int. J. Environ. Res. Public Health 2020, 17, 24576. [Google Scholar] [CrossRef]
- Koganemaru, R.; Miller, D.M. The bed bug problem: Past present and future control methods. Pestic. Biochem. Physiol. 2013, 106, 177–189. [Google Scholar] [CrossRef]
- Grozeva, S.; Kuznetsova, V.; Anokhin, B. Bed bug cytogenetics: Karyotype sex chromosome system FISH mapping of 18S rDNA and male meiosis in Cimex lectularius Linnaeus 1758 (Heteroptera: Cimicidae). Comp. Cytogenet. 2010, 4, 151–160. [Google Scholar] [CrossRef]
- Sadílek, D.; Urfus, T.; Vilímová, J. Genome size and sex chromosome variability of bed bugs feeding on animal hosts compared to Cimex lectularius parasitizing human (Heteroptera: Cimicidae). Cytom. A 2019, 95, 1158–1166. [Google Scholar] [CrossRef]
- Law, S.T.S.; Nong, W.; Li, C.; Chong, T.K.; Yip, H.Y.; Swale, T.; Chiu, S.W.; Chung, R.Y.N.; Lam, H.M.; Wong, S.Y.; et al. Genome of tropical bed bug Cimex hemipterus (Cimicidae, Hemiptera) reveals tetraspanin expanded in bed bug ancestor. Insect Sci. 2024. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Weirauch, C.; Schuh, R.T.; Cassis, G.; Wheeler, W.C. Revisiting habitat and lifestyle transitions in Heteroptera (Insecta: Hemiptera): Insights from a combined morphological and molecular phylogeny. Cladistics 2019, 35, 67–105. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoianova, D.; Grozeva, S.; Golub, N.V.; Anokhin, B.A.; Kuznetsova, V.G. The First FISH-Confirmed Non-Canonical Telomeric Motif in Heteroptera: Cimex lectularius Linnaeus, 1758 and C. hemipterus (Fabricius, 1803) (Hemiptera, Cimicidae) Have a 10 bp Motif (TTAGGGATGG)n. Genes 2024, 15, 1026. https://doi.org/10.3390/genes15081026
Stoianova D, Grozeva S, Golub NV, Anokhin BA, Kuznetsova VG. The First FISH-Confirmed Non-Canonical Telomeric Motif in Heteroptera: Cimex lectularius Linnaeus, 1758 and C. hemipterus (Fabricius, 1803) (Hemiptera, Cimicidae) Have a 10 bp Motif (TTAGGGATGG)n. Genes. 2024; 15(8):1026. https://doi.org/10.3390/genes15081026
Chicago/Turabian StyleStoianova, Desislava, Snejana Grozeva, Natalia V. Golub, Boris A. Anokhin, and Valentina G. Kuznetsova. 2024. "The First FISH-Confirmed Non-Canonical Telomeric Motif in Heteroptera: Cimex lectularius Linnaeus, 1758 and C. hemipterus (Fabricius, 1803) (Hemiptera, Cimicidae) Have a 10 bp Motif (TTAGGGATGG)n" Genes 15, no. 8: 1026. https://doi.org/10.3390/genes15081026




