RETRACTED: Analysis of the Molecular Mechanism of Energy Metabolism in the Sex Differentiation of Chickens Based on Transcriptome Sequencing
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Experimental Animals
2.2. Chicken Embryo Hatching and Gonad Collection
2.3. Isolation and Culture of ESCs
2.4. Genetic Sex Identification
2.5. Paraffin Sections and Hematoxylin–Eosin (H&E) Staining
2.6. RNA Isolation and Library Preparation
2.7. RNA Sequencing and Differentially Expressed Genes Analysis
2.8. Quantitative Real-Time PCR
2.9. Determination of Injection Drug Concentration
2.10. Embryo Injection Experiment
2.11. PAS Staining
2.12. Hormone Level Detection
2.13. Statistical Analysis of Data
3. Results
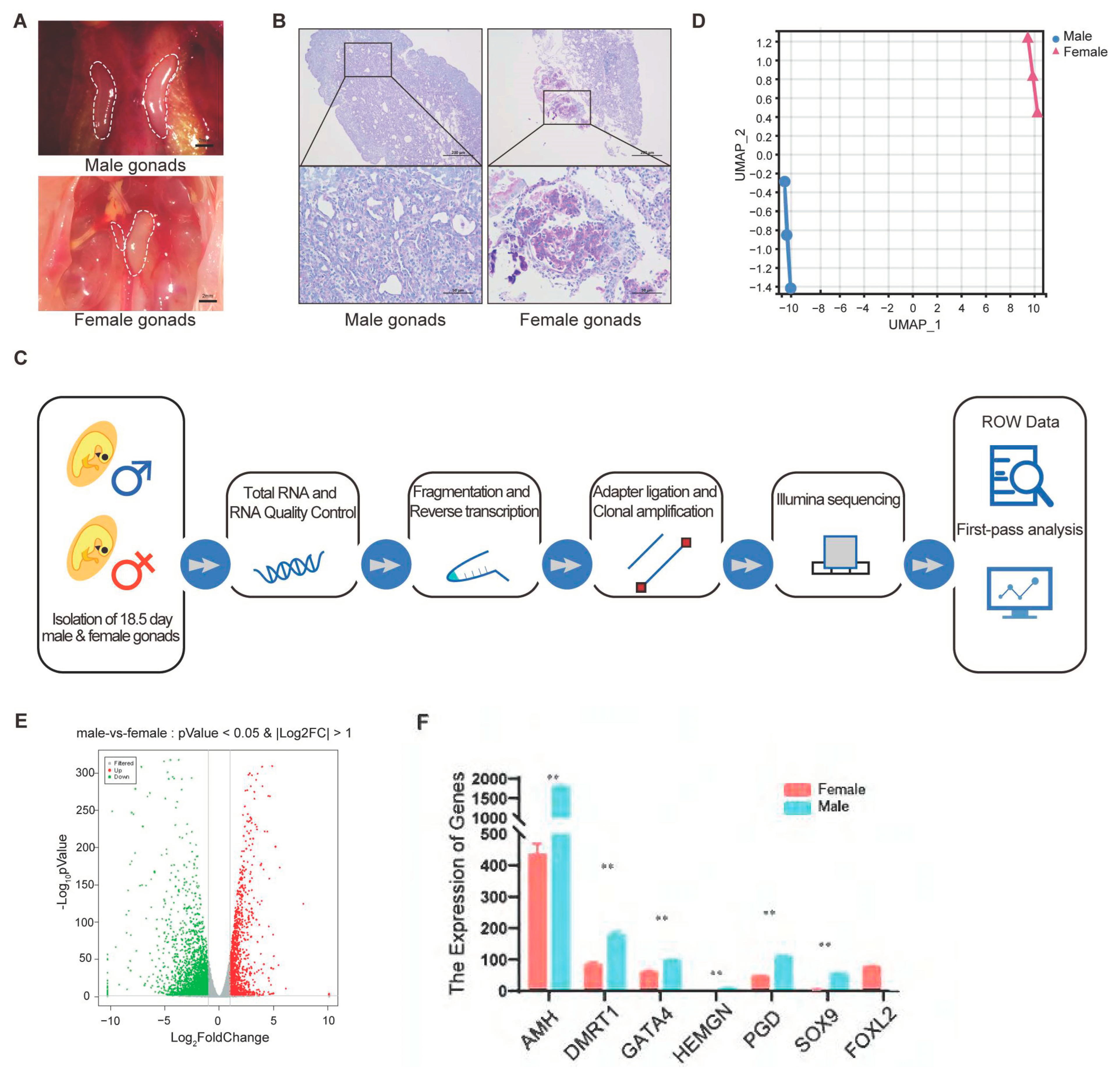
3.1. Exploring Sex Differentiation in Chickens: Initial Findings and Gene Expression Patterns
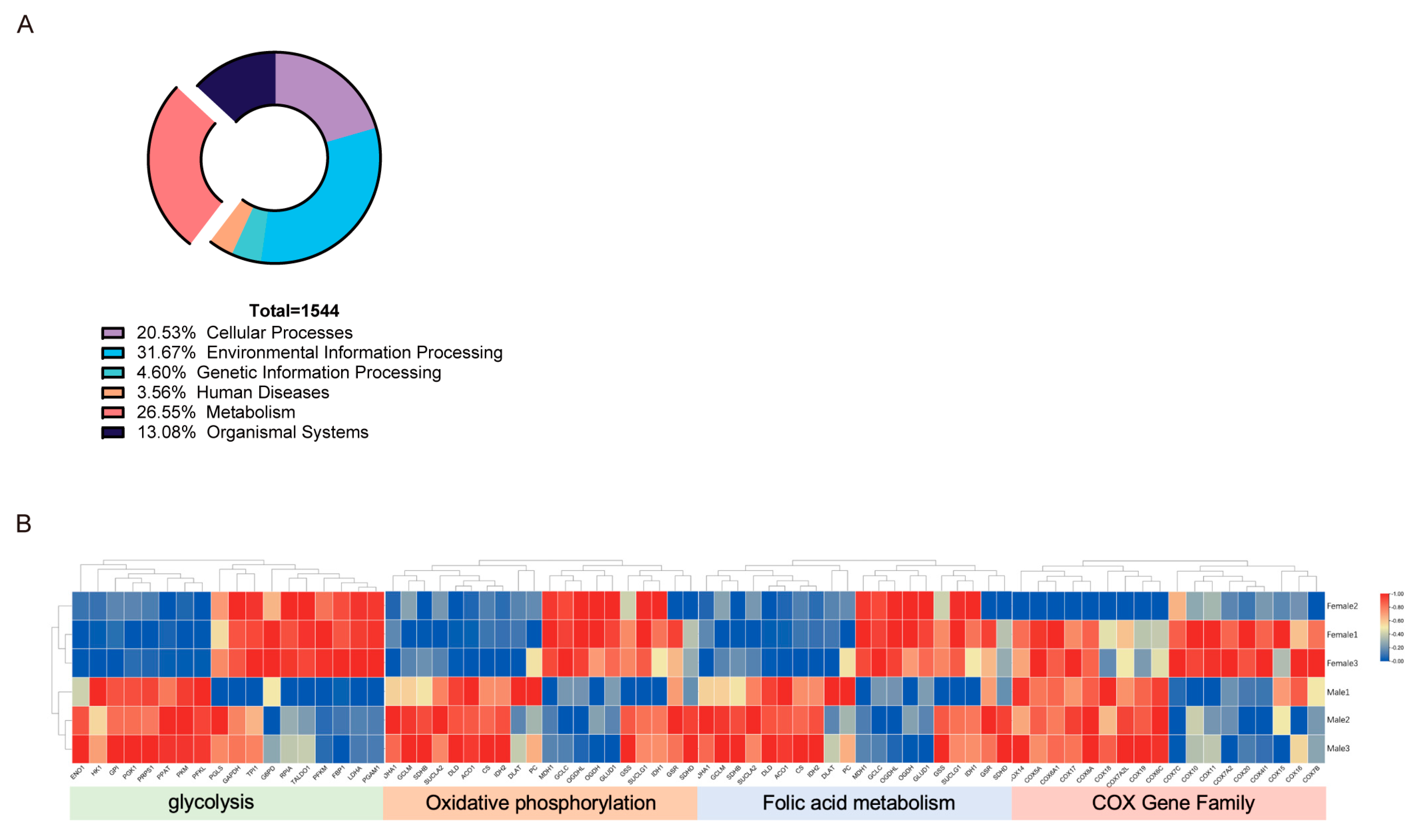
3.2. Metabolic Processes in Gonad Development and Sex Determination
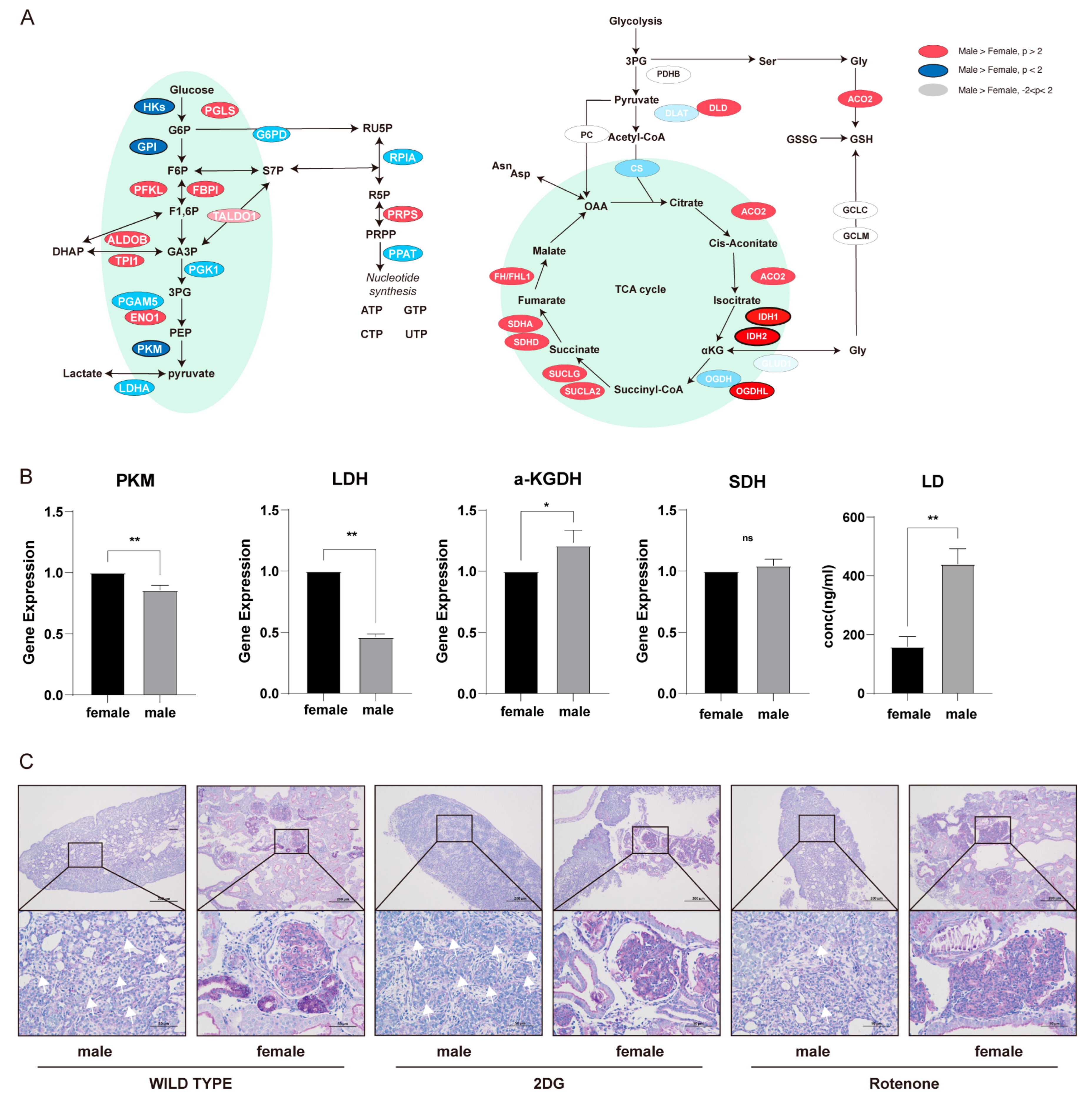
3.3. Role of Energy Metabolism and Glycolysis in Sex Determination and Differentiation
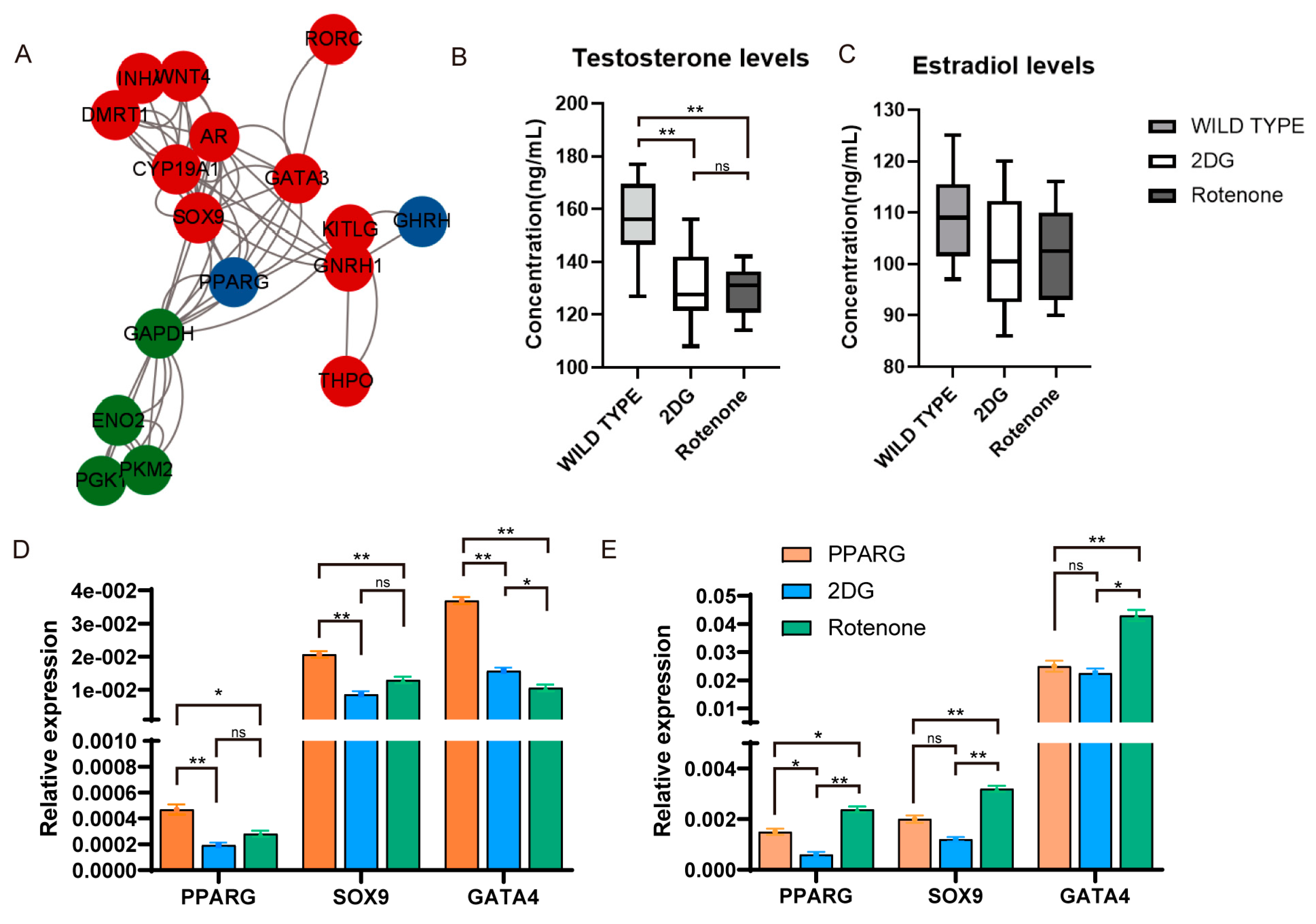
3.4. Construction of a Regulatory Network for Energy Metabolism to Regulate Sex Determination in Chickens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hawkins, A.; Burdine, K.H.; Amaral-Phillips, D.M.; Costa, J.H.C. Effects of Housing System on Dairy Heifer Replacement Cost From Birth to Calving: Evaluating Costs of Confinement, Dry-Lot, and Pasture-Based Systems and Their Impact on Total Rearing Investment. Front. Vet. Sci. 2020, 7, 625. [Google Scholar] [CrossRef] [PubMed]
- van der Heide, E.M.M.; Lourenco, D.A.L.; Chen, C.Y.; Herring, W.O.; Sapp, R.L.; Moser, D.W.; Tsuruta, S.; Masuda, Y.; Ducro, B.J.; Misztal, I. Sexual dimorphism in livestock species selected for economically important traits. J. Anim. Sci. vol. 2016, 94, 3684–3692. [Google Scholar] [CrossRef] [PubMed]
- Gautron, J.; Réhault-Godbert, S.; Van de Braak, T.; Dunn, I. Review: What are the challenges facing the table egg industry in the next decades and what can be done to address them? Anim. Int. J. Anim. Biosci. 2021, 15 (Suppl 1), 100282. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.G.; Li, D.; Xue, Z.D.; Zhang, R.Q.; Shi, H.Z.; Xue, J.; Quan, F.S. Research progress of the applications of sex control technologies in livestock production. J. Shanxi Agric. Sci. 2022, 50, 433–438. [Google Scholar]
- Boersma, P.D.; Davies, E.M. Sexing monomorphic birds by vent measurements. Ornithology 1987, 104, 779–783. [Google Scholar] [CrossRef]
- Dubiec, A.; Magdalena, Z.-N. Molecular techniques for sex identification in birds. Biol. Lett. 2006, 43, 3–12. [Google Scholar]
- Garner, J.L.; Niles, K.M.; McGraw, S.; Yeh, J.R.; Cushnie, D.W.; Hermo, L.; Nagano, M.C.; Trasler, J.M. Stability of DNA Methylation Patterns in Mouse Spermatogonia Under Conditions of MTHFR Deficiency and Methionine Supplementation1. Biol. Reprod. 2013, 89, 125. [Google Scholar] [CrossRef]
- Hirst, C.E.; Major, A.T.; Smith, C.A. Sex determination and gonadal sex differentiation in the chicken model. Int. J. Dev. Biol. 2018, 62, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Riel, J.M.; Ruthig, V.A.; Ortega, E.A.; Mitchell, M.J.; Ward, M.A. Two genes substitute for the mouse Y chromosome for spermatogenesis and reproduction. Science 2016, 351, 514–516. [Google Scholar] [CrossRef]
- Nakamura, M. Sex determination in amphibians. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2009; Volume 20. [Google Scholar]
- Augstenová, B.; Ma, W.J. Decoding Dmrt1: Insights into vertebrate sex determination and gonadal sex differentiation. arXiv 2024, arXiv:2407.12060. [Google Scholar]
- Bullejos, M.; Adrián, R.-G.; Álvaro, S.R. Sex determination and gonadal differentiation in amphibians. Horm. Reprod. Vertebr. 2024, 2, 1–31. [Google Scholar]
- Griffiths, R. Sex identification using DNA markers. In Molecular Methods in Ecology; John Wiley & Sons: Hoboken, NJ, USA, 2000; pp. 295–321. [Google Scholar]
- Lee, H.J.; Seo, M.; Choi, H.J.; Rengaraj, D.; Jung, K.M.; Park, J.S.; Han, J.Y. DMRT1 gene disruption alone induces incomplete gonad feminization in chicken. FASEB J. 2021, 35, e21876. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, X.; Wang, X.; Sun, C.; Zheng, J.; Li, J.; Yang, N. The m6A methylation regulates gonadal sex differentiation in chicken embryo. J. Anim. Sci. Biotechnol. 2022, 13, 52. [Google Scholar] [CrossRef]
- Zhu, Z.-X.; Matsubara, K.; Shams, F.; Dobry, J.; Wapstra, E.; Gamble, T.; Sarre, S.D.; Georges, A.; Graves, J.A.M.; Zhou, Q.; et al. Diversity of reptile sex chromosome evolution revealed by cytogenetic and linked-read sequencing. Zool. Res. 2022, 43, 719–733. [Google Scholar] [CrossRef]
- Estermann, M.A.; Major, A.T.; Smith, C.A. Genetic Regulation of Avian Testis Development. Genes 2021, 12, 1459. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Sezaki, M.; Bertocchini, F.; Fukuda, K.; Sheng, G. HINTW, a W-chromosome HINT gene in chick, is expressed ubiquitously and is a robust female cell marker applicable in intraspecific chimera studies: HINTW, A Ubiquitous Female Cell Marker in Chick. Genesis 2014, 52, 424–430. [Google Scholar] [CrossRef]
- Ma, X.; Liu, F.; Chen, Q.; Sun, W.; Shen, J.; Wu, K.; Ge, C. Foxl2 is required for the initiation of the female pathway in a temperature-dependent sex determination system in Trachemys scripta. Development 2022, 149, dev200863. [Google Scholar] [CrossRef]
- Wang, P.; Li, W.; Liu, Z.; He, X.; Hong, Q.; Lan, R.; Liu, Y.; Chu, M. Identification of WNT4 alternative splicing patterns and effects on proliferation of granulosa cells in goat. Int. J. Biol. Macromol. 2022, 223, 1230–1242. [Google Scholar] [CrossRef]
- Jin, K.; Zhou, J.; Zuo, Q.-S.; Li, J.-C.; Jiuzhou, S.; Zhang, Y.-N.; Chang, G.-B.; Chen, G.-H.; Li, B.-C. UBE2I stimulates female gonadal differentiation in chicken (Gallus gallus) embryos. J. Integr. Agric. 2021, 20, 2986–2994. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, P.; Sun, X.; Zhang, C.; Shi, X.; Li, J.; Jiang, J.; Chen, C.; Zhang, Y.; Chen, G.; et al. JUN promotes chicken female differentiation by inhibiting Smad2. Cytotechnology 2021, 73, 101–113. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, C.; Yuan, X.; Li, J.; Zhang, M.; Shi, X.; Jin, K.; Zhang, Y.; Zuo, Q.; Chen, G.; et al. Spin1z induces the male pathway in the chicken by down-regulating Tcf4. Gene 2021, 780, 145521. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Sun, X.; Xu, P.; Zhang, C.; Zhang, M.; Yuan, X.; Jiang, J.; Jin, K.; Chen, C.; Zuo, Q.; et al. HMGCS1 Promotes male differentiation of chicken embryos by regulating the generate of cholesterol. All Life 2021, 14, 577–587. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, S.; Lian, Z.; Jiang, J.; Gao, X.; Hu, C.; Zuo, Q.; Zhang, Y.; Chen, G.; Jin, K.; et al. Tle4z1 facilitate the male sexual differentiation of chicken embryos. Front. Physiol. 2022, 13, 856980. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Zhou, J.; Zuo, Q.; Song, J.; Zhang, Y.; Chang, G.; Chen, G.; Li, B. Transcriptome Sequencing and Comparative Analysis of Amphoteric ESCs and PGCs in Chicken (Gallus gallus). Animals 2020, 10, 2228. [Google Scholar] [CrossRef] [PubMed]
- Guioli, S.; Nandi, S.; Zhao, D.; Burgess-Shannon, J.; Lovell-Badge, R.; Clinton, M. Gonadal Asymmetry and Sex Determination in Birds. Sex Dev. 2014, 8, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, R.; Korn, R.M. A CHD1 gene is Z chromosome linked in the chicken Gallus domesticus. Gene 1997, 197, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhao, J.; Xu, X.; Zuo, Q.; Zhang, Y.; Jin, K.; Han, W.; Li, B. Inhibition of Autophagy Maintains ESC Pluripotency and Inhibits Primordial Germ Cell Formation in Chickens. Stem Cells Int. 2023, 2023, e4956871. [Google Scholar] [CrossRef]
- Sakae, Y.; Tanaka, M. Metabolism and sex differentiation in animals from a starvation perspective. Sex. Dev. 2021, 15, 168–178. [Google Scholar] [CrossRef]
- Tramunt, B.; Smati, S.; Grandgeorge, N.; Lenfant, F.; Arnal, J.F.; Montagner, A.; Gourdy, P. Sex and gender differences in developmental programming of metabolism. Mol. Metab. 2018, 15, 8–19. [Google Scholar]
- Suresh, M.; Singh, K.K. Singh. Sex-specific differences in mitochondrial function and its role in health disparities. In Principles of Gender-Specific Medicine; Academic Press: Cambridge, MA, USA, 2023; pp. 129–144. [Google Scholar]
- Valencak, T.G.; Osterrieder, A.; Schulz, T.J. Sex matters: The effects of biological sex on adipose tissue biology and energy metabolism. Redox Biol. 2017, 12, 806–813. [Google Scholar] [CrossRef]
- Smith, C.A.; Major, A.T.; Estermann, M.A. The role of SRY in sex determination and the genetic pathways involved in gonadal development. The Curious Case of Avian Sex Determination. Trends Genet. 2021, 37, 496–497. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S. Evolution of Genetic and Gene Regulatory Sex Differences in Mammals. Massachusetts Institute of Technology: Cambridge, MA, USA, 2019. [Google Scholar]
- Almansa-Ordonez, A.; Bellido, R.; Vassena, R.; Barragan, M.; Zambelli, F. Oxidative stress in reproduction: A mitochondrial perspective. Biology 2020, 9, 269. [Google Scholar] [CrossRef] [PubMed]
- Rinn, J.L.; Michael, S. Sexual dimorphism in mammalian gene expression. Trends Genet. 2005, 21, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Ishikawa, M.; Sugai, H.; Funaki, A.; Kimura, Y.; Sumitomo, M.; Ueno, K. Sex hormones influence expression and function of peroxisome proliferator-activated receptor γ in adipocytes: Pathophysiological aspects. Horm. Mol. Biol. Clin. Investig. 2014, 20, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Cheng, Z. Hormonal regulation of metabolism—Recent lessons learned from insulin and estrogen. Clin. Sci. 2023, 137, 415–434. [Google Scholar] [CrossRef]
- Santos, D.; Luzio, A.; Coimbra, A.M. Zebrafish sex differentiation and gonad development: A review on the impact of environmental factors. Aquat. Toxicol. 2017, 191, 141–163. [Google Scholar] [CrossRef]
- Bucher, M.; Kadam, L.; Ahuna, K.; Myatt, L. Differences in glycolysis and mitochondrial respiration between cytotrophoblast and syncytiotrophoblast in-vitro: Evidence for sexual dimorphism. Int. J. Mol. Sci. 2021, 22, 10875. [Google Scholar] [CrossRef]
- Guiguen, Y.; Fostier, A.; Herpin, A. Sex determination and differentiation in fish: Genetic, genomic, and endocrine aspects. In Sex Control in Aquaculture; Wiley: Hoboken, NJ, USA, 2018; pp. 35–63. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Zhao, Z.; Cheng, F.; Wang, Z.; Geng, Q.; Wang, Y.; Niu, Y.; Zuo, Q.; Zhang, Y. RETRACTED: Analysis of the Molecular Mechanism of Energy Metabolism in the Sex Differentiation of Chickens Based on Transcriptome Sequencing. Genes 2024, 15, 1035. https://doi.org/10.3390/genes15081035
Zhao Z, Zhao Z, Cheng F, Wang Z, Geng Q, Wang Y, Niu Y, Zuo Q, Zhang Y. RETRACTED: Analysis of the Molecular Mechanism of Energy Metabolism in the Sex Differentiation of Chickens Based on Transcriptome Sequencing. Genes. 2024; 15(8):1035. https://doi.org/10.3390/genes15081035
Chicago/Turabian StyleZhao, Ziduo, Zongyi Zhao, Fufu Cheng, Zhe Wang, Qingqing Geng, Yingjie Wang, Yingjie Niu, Qisheng Zuo, and Yani Zhang. 2024. "RETRACTED: Analysis of the Molecular Mechanism of Energy Metabolism in the Sex Differentiation of Chickens Based on Transcriptome Sequencing" Genes 15, no. 8: 1035. https://doi.org/10.3390/genes15081035
APA StyleZhao, Z., Zhao, Z., Cheng, F., Wang, Z., Geng, Q., Wang, Y., Niu, Y., Zuo, Q., & Zhang, Y. (2024). RETRACTED: Analysis of the Molecular Mechanism of Energy Metabolism in the Sex Differentiation of Chickens Based on Transcriptome Sequencing. Genes, 15(8), 1035. https://doi.org/10.3390/genes15081035





