Radiation-Tolerant Fibrivirga spp. from Rhizosphere Soil: Genome Insights and Potential in Agriculture
Abstract
1. Introduction
2. Results
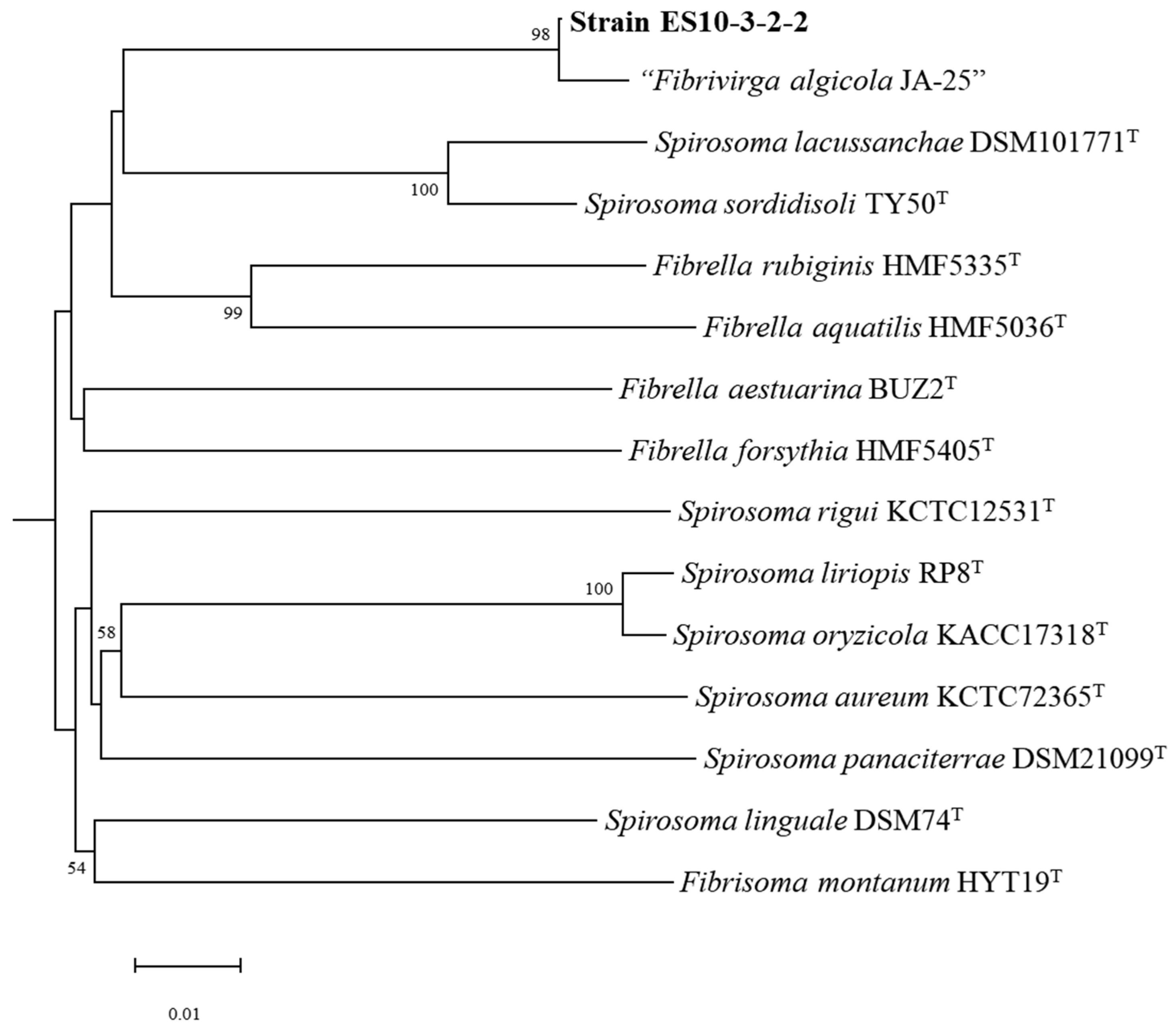
2.1. Strain Isolation and Identification
2.2. Genome Sequencing and Analysis
2.2.1. Genome Properties
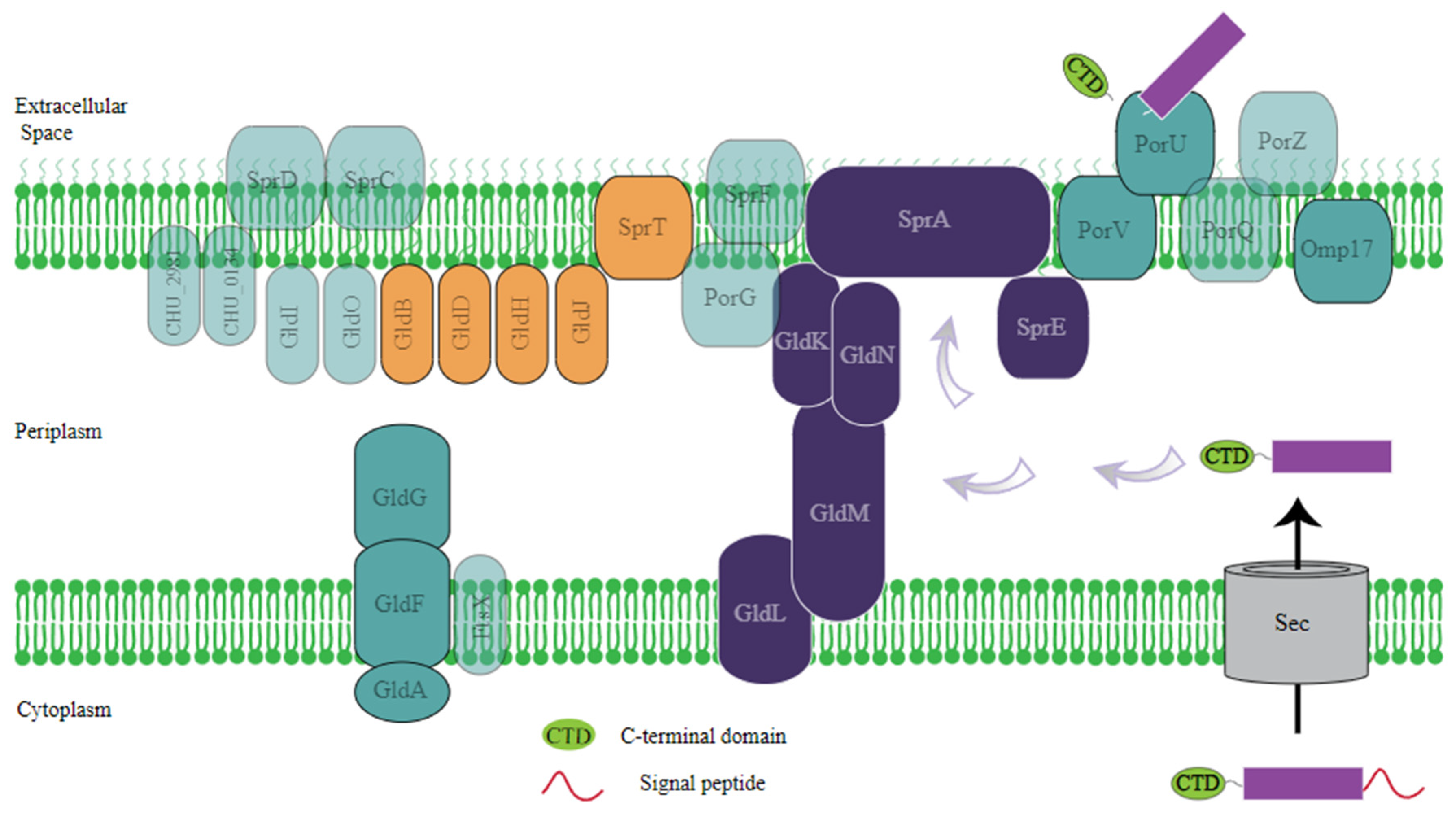
2.2.2. Radiation Resistance Genes and Pathways
2.2.3. Genes Involved in Plant Growth
2.3. Radiation-Resistance Analysis
2.4. Plant-Growth Analysis
3. Discussion
4. Materials and Methods
4.1. Isolation and Growth
4.2. Genome Sequencing and Phylogenomic Analysis
4.3. Genome Annotation
4.4. Radiation Resistant Analysis
4.4.1. γ Irradiation Analysis
4.4.2. UVC Irradiation Analysis
4.5. Plant Growth Analysis
Plant Cultivation and Treatments
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, O.S.; Babalola, O.O. The rhizosphere microbial complex in plant health: A review of interaction dynamics. J. Integr. Agric. 2022, 21, 2168–2182. [Google Scholar] [CrossRef]
- Adomako, M.O.; Roiloa, S.; Yu, F.-H. Potential roles of soil microorganisms in regulating the effect of soil nutrient heterogeneity on plant performance. Microorganisms 2022, 10, 2399. [Google Scholar] [CrossRef]
- Kabato, W.S.; Janda, T.; Molnár, Z. Unveiling the significance of rhizosphere: Implications for plant growth, stress response, and sustainable agriculture. Plant Physiol. Biochem. 2023, 206, 108290. [Google Scholar]
- Omae, N.; Tsuda, K. Plant-Microbiota Interactions in Abiotic Stress Environments. Mol. Plant-Microbe Interact. 2022, 35, 511–526. [Google Scholar] [CrossRef]
- L’Annunziata, M.F. Introduction: Radioactivity and our well-being. In Radioactivity; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–45. [Google Scholar]
- Daly, M.J. A new perspective on radiation resistance based on Deinococcus radiodurans. Nat. Rev. Microbiol. 2009, 7, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, A.; Garrido-Chamorro, S.; Barreiro, C. Microorganisms and Climate Change: A Not So Invisible Effect. Microbiol. Res. 2023, 14, 918–947. [Google Scholar] [CrossRef]
- Filloux, A. Bacterial protein secretion systems: Game of types. Microbiology 2022, 168. [Google Scholar] [CrossRef]
- Lasica, A.M.; Ksiazek, M.; Madej, M.; Potempa, J. The type IX secretion system (T9SS): Highlights and recent insights into its structure and function. Front. Cell. Infect. Microbiol. 2017, 7, 215. [Google Scholar] [CrossRef]
- Krisko, A.; Radman, M. Biology of extreme radiation resistance: The way of Deinococcus radiodurans. Cold Spring Harb. Perspect. Biol. 2013, 5, a012765. [Google Scholar] [CrossRef]
- Megali, L.; Glauser, G.; Rasmann, S. Fertilization with beneficial microorganisms decreases tomato defenses against insect pests. Agron. Sustain. Dev. 2014, 34, 649–656. [Google Scholar] [CrossRef]
- Nazir, N.; Kamili, A.; Shah, D. Mechanism of Plant Growth Promoting Rhizobacteria (PGPR) in enhancing plant growth—A Review. Int. J. Manag. Technol. Eng. 2019, 8, 709–721. [Google Scholar]
- Neilands, J.B. Microbial iron compounds. Annu. Rev. Biochem. 1981, 50, 715–731. [Google Scholar] [CrossRef] [PubMed]
- García-López, M.; Meier-Kolthoff, J.P.; Tindall, B.J.; Gronow, S.; Woyke, T.; Kyrpides, N.C.; Hahnke, R.L.; Göker, M. Analysis of 1,000 type-strain genomes improves taxonomic classification of Bacteroidetes. Front. Microbiol. 2019, 10, 2083. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Cho, J.Y.; Jung, D.-H.; Jang, S.W.; Eom, J.H.; Nam, S.W.; Kwon, D.R.; Ryu, J.; Kim, K.T. Fibrivirga algicola gen. nov., sp. nov., an algicidal bacterium isolated from a freshwater river. Antonie Van Leeuwenhoek 2022, 115, 899–909. [Google Scholar] [CrossRef]
- Lefort, V.; Desper, R. FastME OG. 2.0: A comprehensive, accurate, and fast distance-based phylogeny inference program. Mol. Biol. Evol. 2015, 32, 2798–2800. [Google Scholar] [CrossRef] [PubMed]
- Farris, J.S. Estimating phylogenetic trees from distance matrices. Am. Nat. 1972, 106, 645–668. [Google Scholar] [CrossRef]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen 2017, 58, 235–263. [Google Scholar] [CrossRef]
- Petit, C.; Sancar, A. Nucleotide excision repair: From E. coli to man. Biochimie 1999, 81, 15–25. [Google Scholar] [CrossRef]
- Earl, A.M.; Rankin, S.K.; Kim, K.-P.; Lamendola, O.N.; Battista, J.R. Genetic evidence that the uvsE gene product of Deinococcus radiodurans R1 is a UV damage endonuclease. J. Bacteriol. 2002, 184, 1003–1009. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Braun, V. Membrane topology of the Escherichia coli ExbD protein. J. Bacteriol. 1992, 174, 5485–5487. [Google Scholar] [CrossRef] [PubMed]
- Krewulak, K.D.; Vogel, H.J. Structural biology of bacterial iron uptake. Biochim. Biophys. Acta (BBA)-Biomembr. 2008, 1778, 1781–1804. [Google Scholar] [CrossRef] [PubMed]
- Ho, B.T.; Dong, T.G.; Mekalanos, J.J. A view to a kill: The bacterial type VI secretion system. Cell Host Microbe 2014, 15, 9–21. [Google Scholar] [PubMed]
- Spiewak, H.L.; Shastri, S.; Zhang, L.; Schwager, S.; Eberl, L.; Vergunst, A.C.; Thomas, M.S. Burkholderia cenocepacia utilizes a type VI secretion system for bacterial competition. Microbiologyopen 2019, 8, e00774. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, A.K.; Vivek-Ananth, R.P.; Chivukula, N.; Rajaram, S.V.; Mohanraj, K.; Khare, D.; Acharya, C.; Samal, A. T9GPred: A Comprehensive Computational Tool for the Prediction of Type 9 Secretion System, Gliding Motility, and the Associated Secreted Proteins. ACS Omega 2023, 8, 34091–34102. [Google Scholar] [CrossRef]
- Kämpfer, P.; Lodders, N.; Huber, B.; Falsen, E.; Busse, H.-J. Deinococcus aquatilis sp. nov., isolated from water. Int. J. Syst. Evol. Microbiol. 2008, 58, 2803–2806. [Google Scholar] [CrossRef] [PubMed]
- Ogwu, M.C.; Srinivasan, S.; Dong, K.; Ramasamy, D.; Waldman, B.; Adams, J.M. Community Ecology of Deinococcus in Irradiated Soil. Microb. Ecol. 2019, 78, 855–872. [Google Scholar] [CrossRef] [PubMed]
- Ogwu, M.C.; Kerfahi, D.; Song, H.; Dong, K.; Seo, H.; Lim, S.; Srinivasan, S.; Kim, M.K.; Waldman, B.; Adams, J.M. Changes in soil taxonomic and functional diversity resulting fromgamma irradiation. Sci. Rep. 2019, 9, 7894. [Google Scholar] [CrossRef] [PubMed]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef]
- Hernández-Plaza, A.; Szklarczyk, D.; Botas, J.; Cantalapiedra, C.P.; Giner-Lamia, J.; Mende, D.R.; Kirsch, R.; Rattei, T.; Letunic, I.; Jensen, L.J.; et al. eggNOG 6.0: Enabling comparative genomics across 12 535 organisms. Nucleic Acids Res. 2023, 51, D389–D394. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023, 51, D587–D592. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, K.J.; Simmons, L.A. Bacterial DNA excision repair pathways. Nat. Rev. Microbiol. 2022, 20, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.R.; Tanaka, M.; Saveliev, S.V.; Jolivet, E.; Earl, A.M.; Cox, M.M.; Battista, J.R. Preserving genome integrity: The DdrA protein of Deinococcus radiodurans R1. PLoS Biol. 2004, 2, e304. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Lu, H.; Wang, L.; Chen, H.; Xu, Z.; Hu, Y.; Tian, B.; Hua, Y. DdrB stimulates single-stranded DNA annealing and facilitates RecA-independent DNA repair in Deinococcus radiodurans. DNA Repair 2010, 9, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Basu, B.; Apte, S.K. Gamma radiation-induced proteome of Deinococcus radiodurans primarily targets DNA repair and oxidative stress alleviation. Mol. Cell. Proteom. 2012, 11. [Google Scholar] [CrossRef]
- Lim, S.; Jung, J.-H.; Blanchard, L.; de Groot, A. Conservation and diversity of radiation and oxidative stress resistance mechanisms in Deinococcus species. FEMS Microbiol. Rev. 2019, 43, 19–52. [Google Scholar] [CrossRef]
- Erill, I.; Campoy, S.; Barbé, J. Aeons of distress: An evolutionary perspective on the bacterial SOS response. FEMS Microbiol. Rev. 2007, 31, 637–656. [Google Scholar] [CrossRef]
- Yin, R.; Cheng, J.; Lin, J. The role of the type VI secretion system in the stress resistance of plant-associated bacteria. Stress Biol. 2024, 4, 16. [Google Scholar] [CrossRef]
- Bladergroen, M.R.; Badelt, K.; Spaink, H. Infection-blocking genes of a symbiotic Rhizobium leguminosarum strain that are involved in temperature-dependent protein secretion. Mol. Plant-Microbe Interact. 2003, 16, 53–64. [Google Scholar] [CrossRef]
- Lin, J.; Xu, L.; Yang, J.; Wang, Z.; Shen, X. Beyond dueling: Roles of the type VI secretion system in microbiome modulation, pathogenesis and stress resistance. Stress Biol. 2021, 1, 11. [Google Scholar] [CrossRef]
- Han, Y.; Wang, T.; Chen, G.; Pu, Q.; Liu, Q.; Zhang, Y.; Xu, L.; Wu, M.; Liang, H. A Pseudomonas aeruginosa type VI secretion system regulated by CueR facilitates copper acquisition. PLoS Pathog. 2019, 15, e1008198. [Google Scholar]
- Trunk, K.; Peltier, J.; Liu, Y.-C.; Dill, B.D.; Walker, L.; Gow, N.A.; Stark, M.J.; Quinn, J.; Strahl, H.; Trost, M. The type VI secretion system deploys antifungal effectors against microbial competitors. Nat. Microbiol. 2018, 3, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Coulthurst, S. The Type VI secretion system: A versatile bacterial weapon. Microbiology 2019, 165, 503–515. [Google Scholar] [CrossRef]
- Wan, B.; Zhang, Q.; Ni, J.; Li, S.; Wen, D.; Li, J.; Xiao, H.; He, P.; Ou, H.-Y.; Tao, J. Type VI secretion system contributes to Enterohemorrhagic Escherichia coli virulence by secreting catalase against host reactive oxygen species (ROS). PLoS Pathog. 2017, 13, e1006246. [Google Scholar] [CrossRef]
- Bendor, L.; Weyrich, L.S.; Linz, B.; Rolin, O.Y.; Taylor, D.L.; Goodfield, L.L.; Smallridge, W.E.; Kennett, M.J.; Harvill, E.T. Type six secretion system of Bordetella bronchiseptica and adaptive immune components limit intracellular survival during infection. PLoS ONE 2015, 10, e0140743. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Cheng, J.; Chen, K.; Guo, C.; Zhang, W.; Yang, X.; Ding, W.; Ma, L.; Wang, Y.; Shen, X. The icmF3 locus is involved in multiple adaptation-and virulence-related characteristics in Pseudomonas aeruginosa PAO1. Front. Cell. Infect. Microbiol. 2015, 5, 70. [Google Scholar] [CrossRef]
- Chen, L.; Zou, Y.; Kronfl, A.A.; Wu, Y. Type VI secretion system of Pseudomonas aeruginosa is associated with biofilm formation but not environmental adaptation. Microbiologyopen 2020, 9, e991. [Google Scholar] [CrossRef]
- Wang, J.; Yin, R.; Yang, J.; Cheng, J.; Lin, J. Research progress in the function of type VI secretion system in plant-associated bacteria. Acta Microbiol. Sin. 2023, 63, 2573–2594. [Google Scholar]
- Persello-Cartieaux, F.; David, P.; Sarrobert, C.; Thibaud, M.-C.; Achouak, W.; Robaglia, C.; Nussaume, L. Utilization of mutants to analyze the interaction between Arabidopsis thaliana and its naturally root-associated Pseudomonas. Planta 2001, 212, 190–198. [Google Scholar] [CrossRef]
- Ryu, C.-M.; Hu, C.-H.; Locy, R.D.; Kloepper, J.W. Study of mechanisms for plant growth promotion elicited by rhizobacteria in Arabidopsis thaliana. Plant Soil. 2005, 268, 285–292. [Google Scholar] [CrossRef]
- Scavino, A.F.; Pedraza, R.O. The role of siderophores in plant growth-promoting bacteria. In Bacteria in Agrobiology: Crop Productivity; Springer: Berlin/Heidelberg, Germany, 2013; pp. 265–285. [Google Scholar]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.-K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Khasheii, B.; Mahmoodi, P.; Mohammadzadeh, A. Siderophores: Importance in bacterial pathogenesis and applications in medicine and industry. Microbiol. Res. 2021, 250, 126790. [Google Scholar] [CrossRef] [PubMed]
- Guillon, L.; Altenburger, S.; Graumann, P.L.; Schalk, I.J. Deciphering protein dynamics of the siderophore pyoverdine pathway in Pseudomonas aeruginosa. PLoS ONE 2013, 8, e79111. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Herrero, A.; Peacock, R.S.; Howard, S.P.; Vogel, H.J. The solution structure of the periplasmic domain of the TonB system ExbD protein reveals an unexpected structural homology with siderophore-binding proteins. Mol. Microbiol. 2007, 66, 872–889. [Google Scholar] [CrossRef] [PubMed]
- Alam, A. Soil degradation: A challenge to sustainable agriculture. Int. J. Sci. Res. Agric. Sci. 2014, 1, 50–55. [Google Scholar] [CrossRef]
- Sultana, S.; Alam, S.; Karim, M.M. Screening of siderophore-producing salt-tolerant rhizobacteria suitable for supporting plant growth in saline soils with iron limitation. J. Agric. Food Res. 2021, 4, 100150. [Google Scholar] [CrossRef]
- De Serrano, L.O. Biotechnology of siderophores in high-impact scientific fields. Biomol. Concepts 2017, 8, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Pahari, A.; Mishra, B. Characterization of siderophore producing rhizobacteria and its effect on growth performance of different vegetables. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1398–1405. [Google Scholar] [CrossRef]
- Loper, J.E.; Henkels, M.D. Utilization of heterologous siderophores enhances levels of iron available to Pseudomonas putida in the rhizosphere. Appl. Environ. Microbiol. 1999, 65, 5357–5363. [Google Scholar] [CrossRef]
- Zhang, Q.; Gong, M.; Xu, X.; Li, H.; Deng, W. Roles of auxin in the growth, development, and stress tolerance of horticultural plants. Cells 2022, 11, 2761. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J. Auxin and plant-microbe interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a001438. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Han, L.; Xia, S.; Zhu, R.; Kang, E.; Shang, Z. ATANN3 is involved in extracellular ATP-regulated auxin distribution in Arabidopsis thaliana seedlings. Plants 2023, 12, 330. [Google Scholar] [CrossRef] [PubMed]
- Kazan, K. Auxin and the integration of environmental signals into plant root development. Ann. Bot. 2013, 112, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D. Alleviation of salt stress by plant growth regulators and IAA producing bacteria in wheat. Acta Physiol. Plant. 2009, 31, 861–864. [Google Scholar] [CrossRef]
- Tsavkelova, E.; Klimova, S.Y.; Cherdyntseva, T.; Netrusov, A. Hormones and hormone-like substances of microorganisms: A review. Appl. Biochem. Microbiol. 2006, 42, 229–235. [Google Scholar] [CrossRef]
- Zepeda, M.A.; Ruiz, V.V.; Cota, F.I.P.; Chinchilla-Soto, C.; de la Cruz Torres, E.; Ibba, M.I.; Alvarado, M.I.E.; de los Santos Villalobos, S. Genomic insights of a native bacterial consortium for wheat production sustainability. Curr. Res. Microb. Sci. 2024, 6, 100230. [Google Scholar]
- Malhotra, H.; Vandana Sharma, S.; Pandey, R. Phosphorus nutrition: Plant growth in response to deficiency and excess. In Plant Nutrients and Abiotic Stress Tolerance; Springer: Berlin/Heidelberg, Germany, 2018; pp. 171–190. [Google Scholar]
- Wang, Y.; Li, W.; Du, B.; Li, H. Effect of biochar applied with plant growth-promoting rhizobacteria (PGPR) on soil microbial community composition and nitrogen utilization in tomato. Pedosphere 2021, 31, 872–881. [Google Scholar] [CrossRef]
- Cho, C.; Lee, D.; Jeong, D.; Kim, S.; Kim, M.K.; Srinivasan, S. Characterization of radiation-resistance mechanism in Spirosoma montaniterrae DY10T in terms of transcriptional regulatory system. Sci. Rep. 2023, 13, 4739. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-J.; Srinivasan, S.; Lim, S.; Joe, M.; Lee, S.H.; Kwon, S.A.; Kwon, Y.J.; Lee, J.; Choi, J.J.; Lee, H.M.; et al. Hymenobacter swuensis sp. nov., a Gamma-Radiation-Resistant Bacteria Isolated from Mountain Soil. Curr. Microbiol. 2014, 68, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Chalita, M.; Ha, S.-M.; Na, S.-I.; Yoon, S.-H.; Chun, J. ContEst16S: An algorithm that identifies contaminated prokaryotic genomes using 16S RNA gene sequences. Int. J. Syst. Evol. Microbiol. 2017, 67, 2053–2057. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Göker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Hyatt, D.; Chen, G.-L.; LoCascio, P.F.; Land, M.L.; Larimer, F.W.; Hauser, L.J. Prodigal: Prokaryotic gene recognition and translation initiation site identification. BMC Bioinform. 2010, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Overbeek, R.; Olson, R.; Pusch, G.D.; Olsen, G.J.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Parrello, B.; Shukla, M. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 2014, 42, D206–D214. [Google Scholar] [CrossRef] [PubMed]
- Brettin, T.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Olsen, G.J.; Olson, R.; Overbeek, R.; Parrello, B.; Pusch, G.D. RASTtk: A modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci. Rep. 2015, 5, 8365. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Kim, D.-U.; Kang, M.-S.; Jang, J.H.; Kim, S.J.; Kim, M.J.; Lee, J.Y.; Lee, Y.S.; Zhang, J.; Lim, S. Roseomonas radiodurans sp. nov., a gamma-radiation-resistant bacterium isolated from gamma ray-irradiated soil. Int. J. Syst. Evol. Microbiol. 2018, 68, 2443–2447. [Google Scholar] [CrossRef]
- Im, S.; Song, D.; Joe, M.; Kim, D.; Park, D.-H.; Lim, S. Comparative survival analysis of 12 histidine kinase mutants of Deinococcus radiodurans after exposure to DNA-damaging agents. Bioprocess. Biosyst. Eng. 2013, 36, 781–789. [Google Scholar] [CrossRef]
- Selvam, K.; Duncan, J.R.; Tanaka, M.; Battista, J.R. DdrA, DdrD, and PprA: Components of UV and mitomycin C resistance in Deinococcus radiodurans R1. PLoS ONE 2013, 8, e69007. [Google Scholar] [CrossRef]
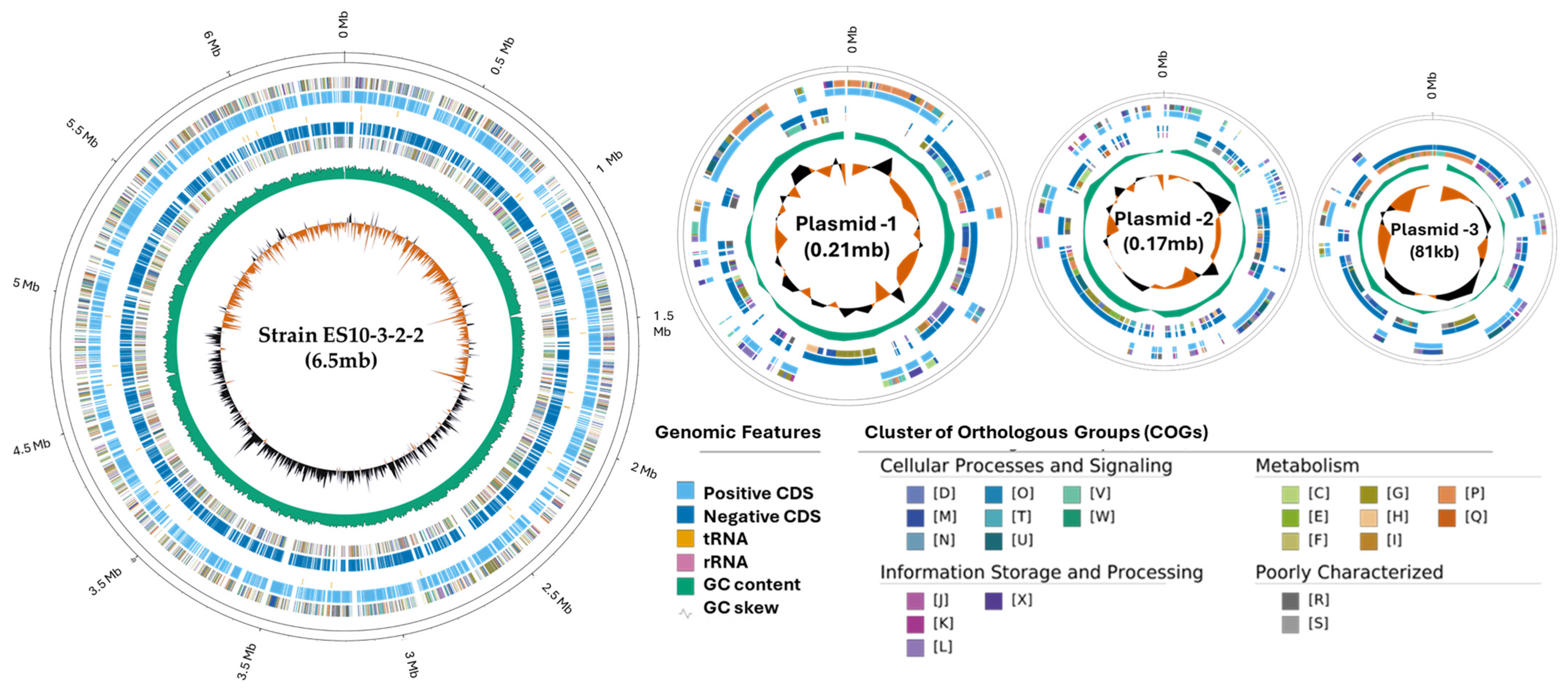

| Features | Chromosome | Plasmid 1 | Plasmid 2 | Plasmid 3 |
|---|---|---|---|---|
| Strain | ES10-3-2-2 | ES10-3-2-2 | ES10-3-2-2 | ES10-3-2-2 |
| Number of contigs | 1 | 1 | 1 | 1 |
| GenBank Accession No. | CP015317 | CP155472 | CP155473 | CP155474 |
| Chromosome size (pb) | 6,408,035 | 212,574 | 175,683 | 81,564 |
| GC content | 52.8 | 52.4 | 49.0 | 52.9 |
| Sequencing coverage | 71 | 71 | 71 | 71 |
| Genes | 5252 | 189 | 159 | 91 |
| rRNA | 9 | 0 | 0 | 0 |
| 5s rRNAs | 3 | 0 | 0 | 0 |
| 16S rRNAs | 3 | 0 | 0 | 0 |
| 23S rRNA | 3 | 0 | 0 | 0 |
| tRNAs | 43 | 0 | 0 | 0 |
| ncRNAs | 2 | 0 | 0 | 0 |
| Pseudo genes | 45 | 0 | 0 | 0 |
| S. No. | Length | Function | Sequence Similarity (BLAST) | |
|---|---|---|---|---|
| D. radiodurans R1 | E. coli K12 | |||
| 1 | 999 | Excinuclease ABC subunit A paralog | 39.2 | 44.2 |
| 2 | 977 | Excinuclease ABC subunit A | 51.8 | 51.9 |
| 3 | 767 | ATP-dependent DNA helicase UvrD/PcrA | 37.5 | 37.2 |
| 4 | 674 | Excinuclease ABC subunit B | 50.0 | 54.8 |
| 5 | 605 | Excinuclease ABC subunit C | 31.1 | 33.4 |
| 6 | 845 | Excinuclease ABC subunit A paralog | 71.3 | 40.3 |
| 7 | 690 | DNA ligase (NAD (+)) | 34.4 | 40.6 |
| 8 | 886 | Excinuclease ABC subunit A paralog of unknown function | 63.5 | 38.0 |
| S. No. | Length | Function | Sequence Similarity (BLAST) | |
|---|---|---|---|---|
| D. radiodurans R1 | E. coli K12 | |||
| 1 | 703 | ATP-dependent DNA helicase RecG | 41.1 | 40.0 |
| 2 | 193 | Crossover junction endodeoxyribonuclease RuvC | 38.6 | 36.8 |
| 3 | 365 | DNA recombination and repair protein RecF | 26.3 | 26.4 |
| 4 | 231 | DNA recombination and repair protein RecO | 0.0 | 0.0 |
| 5 | 459 | DNA repair protein RadA | 42.9 | 46.0 |
| 6 | 555 | DNA repair protein RecN | 30.7 | 29.8 |
| 7 | 1029 | Exonuclease SbcC | 34.8 | 50.0 |
| 8 | 418 | Exonuclease SbcD | 32.3 | 32.5 |
| 9 | 836 | Helicase PriA essential for oriC/DnaA | 36.6 | 37.6 |
| 10 | 196 | Holliday junction ATP-dependent DNA helicase RuvA | 33.5 | 39.7 |
| 11 | 343 | Holliday junction ATP-dependent DNA helicase RuvB | 54.4 | 59.3 |
| 12 | 363 | RecA protein | 59.2 | 59.8 |
| 13 | 206 | Recombination protein RecR | 45.8 | 40.4 |
| 14 | 590 | Single-stranded, DNA-specific exonuclease RecJ | 32.2 | 31.6 |
| KEGG Map | Distinct ECs | Strain ES10-3-2-2 | “F. algicola JA-25” | Fibrella aestuarina BUZ 2T | Spirosoma linguale SM 74T |
|---|---|---|---|---|---|
| Biosynthesis of plant hormones | 131 | 66 (50.4%) | 66 (50.4%) | 76 (58.0%) | 75 (57.3%) |
| Brassinosteroid biosynthesis | 3 | 1 (33.3%) | 1 (33.3%) | 2 (66.7%) | 2 (66.7%) |
| Fatty acid biosynthesis | 21 | 10 (47.6%) | 10 (47.6%) | 10 (47.6%) | 12 (57.1%) |
| Terpenoid backbone biosynthesis | 27 | 10 (37.0%) | 10 (37.0%) | 15 (55.6%) | 14 (51.9%) |
| Zeatin biosynthesis | 9 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srinivasan, S. Radiation-Tolerant Fibrivirga spp. from Rhizosphere Soil: Genome Insights and Potential in Agriculture. Genes 2024, 15, 1048. https://doi.org/10.3390/genes15081048
Srinivasan S. Radiation-Tolerant Fibrivirga spp. from Rhizosphere Soil: Genome Insights and Potential in Agriculture. Genes. 2024; 15(8):1048. https://doi.org/10.3390/genes15081048
Chicago/Turabian StyleSrinivasan, Sathiyaraj. 2024. "Radiation-Tolerant Fibrivirga spp. from Rhizosphere Soil: Genome Insights and Potential in Agriculture" Genes 15, no. 8: 1048. https://doi.org/10.3390/genes15081048
APA StyleSrinivasan, S. (2024). Radiation-Tolerant Fibrivirga spp. from Rhizosphere Soil: Genome Insights and Potential in Agriculture. Genes, 15(8), 1048. https://doi.org/10.3390/genes15081048






