Bioinformatic Evaluation of KLF13 Genetic Variant: Implications for Neurodevelopmental and Psychiatric Symptoms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Library Preparation and Next-Generation Sequencing (NGS)
2.2. Data Analysis
3. Results
3.1. Clinical Report
3.2. Next-Generation Sequencing (NGS)
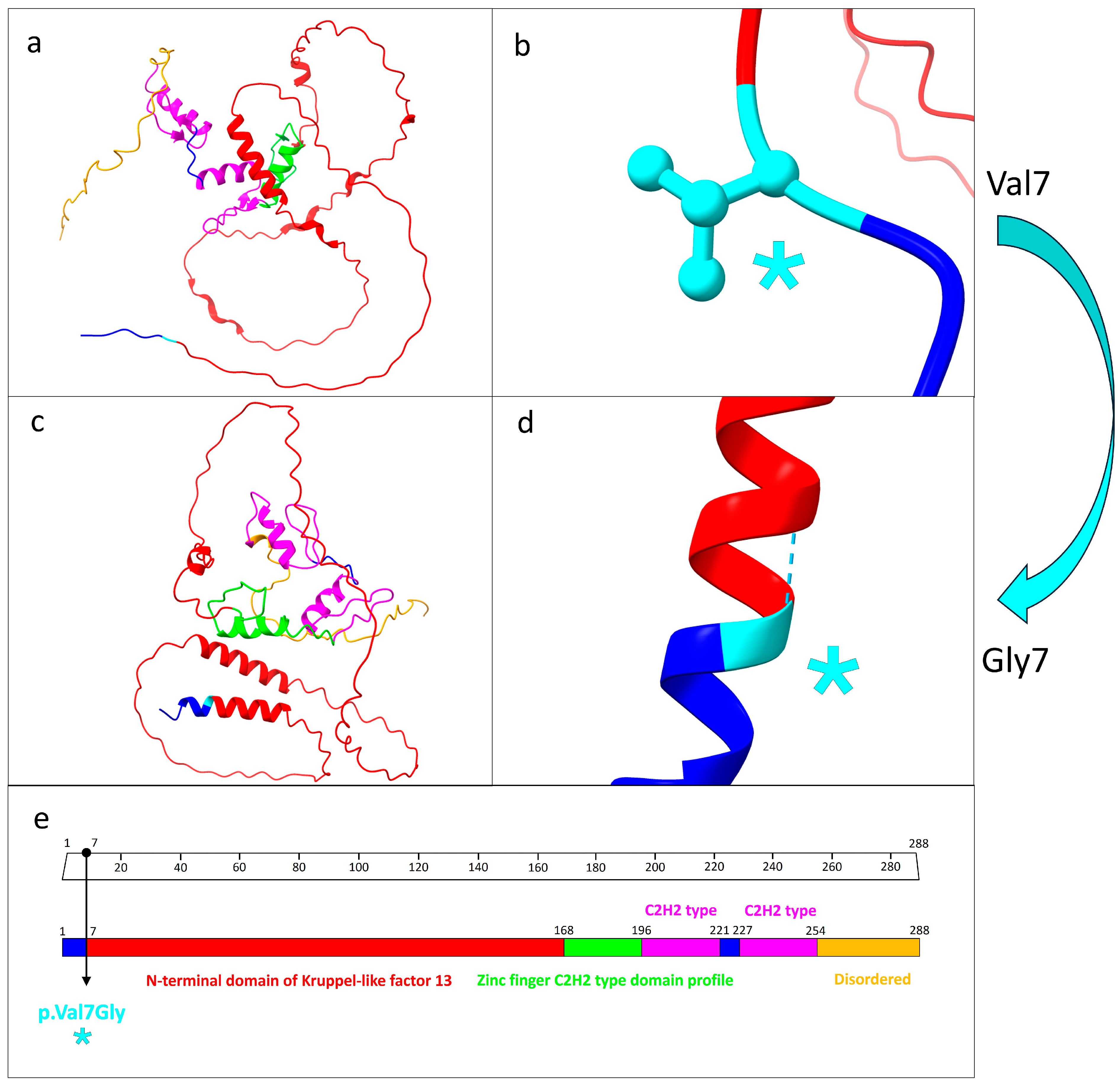
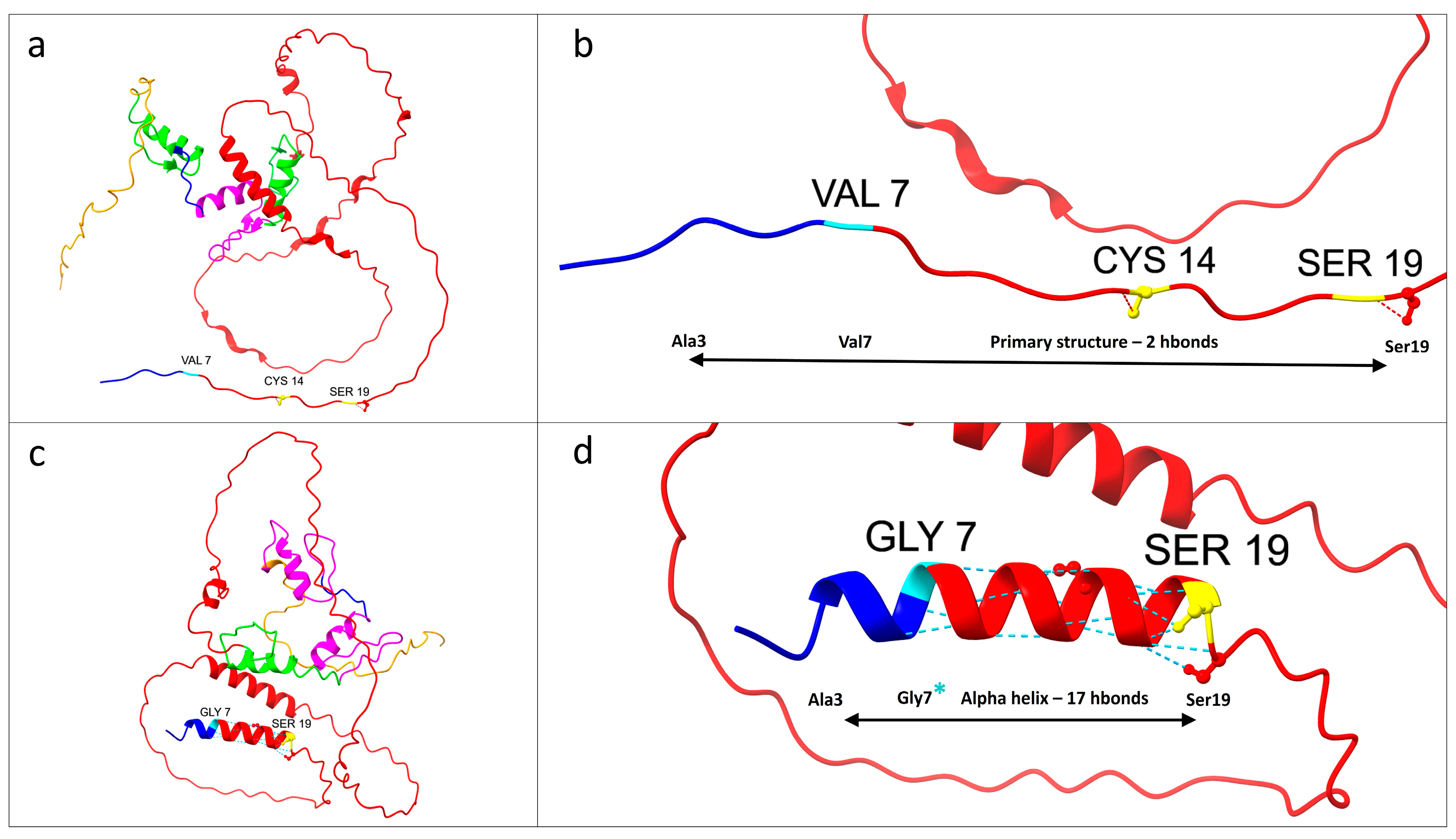
3.3. Protein Structure Prediction and Pathway Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McConnell, B.B.; Yang, V.W. Mammalian Krüppel-Like Factors in Health and Diseases. Physiol. Rev. 2010, 90, 1337–1381. [Google Scholar] [CrossRef] [PubMed]
- Presnell, J.S.; Schnitzler, C.E.; Browne, W.E. KLF/SP Transcription Factor Family Evolution: Expansion, Diversification, and Innovation in Eukaryotes. Genome Biol. Evol. 2015, 7, 2289–2309. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.; Fleetwood, J.; Eaton, S.; Crossley, M.; Bao, S. Krüppel-like Transcription Factors: A Functional Family. Int. J. Biochem. Cell Biol. 2008, 40, 1996–2001. [Google Scholar] [CrossRef] [PubMed]
- Toda, T.; Gage, F.H. Review: Adult Neurogenesis Contributes to Hippocampal Plasticity. Cell Tissue Res. 2018, 373, 693–709. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, J.; Chen, Y.; Li, Y. MiR-146a/KLF4 Axis in Epileptic Mice: A Novel Regulator of Synaptic Plasticity Involving STAT3 Signaling. Brain Res. 2022, 1790, 147988. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, C.L.M.; Price, E.M.; Mifsud, K.R.; Salatino, S.; Sharma, E.; Engledow, S.; Broxholme, J.; Goss, H.M.; Reul, J.M.H.M. Genomic Regulation of Krüppel-like-Factor Family Members by Corticosteroid Receptors in the Rat Brain. Neurobiol. Stress 2023, 23, 100532. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.; Chen, B.; Zhao, J.; Zhan, X.; Zhang, L.; Liu, X.; Dong, Q. Krüppel-like Factor 8 Ameliorates Alzheimer’s Disease by Activating β-Catenin. J. Mol. Neurosci. 2014, 52, 231–241. [Google Scholar] [CrossRef]
- Tian, H.; Jiao, Y.; Guo, M.; Wang, Y.; Wang, R.; Wang, C.; Chen, X.; Tian, W. Krüppel-like Factor 7 Deficiency Causes Autistic-like Behavior in Mice via Regulating Clock Gene. Cell Biosci. 2022, 12, 166. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Yi, X.; Wang, Y.; Liu, Q.; Ge, R. Induction of KLF4 Contributes to the Neurotoxicity of MPP + in M17 Cells: A New Implication in Parkinson’s Disease. J. Mol. Neurosci. 2013, 51, 109–117. [Google Scholar] [CrossRef]
- Zamanian, M.Y.; Golmohammadi, M.; Amin, R.S.; Bustani, G.S.; Romero-Parra, R.M.; Zabibah, R.S.; Oz, T.; Jalil, A.T.; Soltani, A.; Kujawska, M. Therapeutic Targeting of Krüppel-Like Factor 4 and Its Pharmacological Potential in Parkinson’s Disease: A Comprehensive Review. Mol. Neurobiol. 2024, 61, 3596–3606. [Google Scholar] [CrossRef]
- El-Deeb, A.M.; Mohamed, A.F.; EL-Yamany, M.F.; El-Tanbouly, D.M. Novel Trajectories of the NK1R Antagonist Aprepitant in Rotenone-Induced Parkinsonism-like Symptoms in Rats: Involvement of ERK5/KLF4/P62/Nrf2 Signaling Axis. Chem. Biol. Interact. 2023, 380, 110562. [Google Scholar] [CrossRef]
- Hong, W.; Gong, P.; Pan, X.; Liu, Y.; Qi, G.; Qi, C.; Qin, S. Krüppel-like Factor 7 Deficiency Disrupts Corpus Callosum Development and Neuronal Migration in the Developing Mouse Cerebral Cortex. Brain Pathol. 2023, 33, e13186. [Google Scholar] [CrossRef]
- Scobie, K.N.; Hall, B.J.; Wilke, S.A.; Klemenhagen, K.C.; Fujii-Kuriyama, Y.; Ghosh, A.; Hen, R.; Sahay, A. Krüppel-Like Factor 9 Is Necessary for Late-Phase Neuronal Maturation in the Developing Dentate Gyrus and during Adult Hippocampal Neurogenesis. J. Neurosci. 2009, 29, 9875–9887. [Google Scholar] [CrossRef]
- Powis, Z.; Petrik, I.; Cohen, J.S.; Escolar, D.; Burton, J.; van Ravenswaaij-Arts, C.M.A.; Sival, D.A.; Stegmann, A.P.A.; Kleefstra, T.; Pfundt, R.; et al. De Novo Variants in KLF7 Are a Potential Novel Cause of Developmental Delay/Intellectual Disability, Neuromuscular and Psychiatric Symptoms. Clin. Genet. 2018, 93, 1030–1038. [Google Scholar] [CrossRef]
- Mao, Y.; Chen, Y.; Zhang, Z. Molecular Function of Krüppel-like Factor 7 in Biology. Acta Biochim. Biophys. Sin. 2023, 55, 713–725. [Google Scholar] [CrossRef]
- Roberts, J.L.; Hovanes, K.; Dasouki, M.; Manzardo, A.M.; Butler, M.G. Chromosomal Microarray Analysis of Consecutive Individuals with Autism Spectrum Disorders or Learning Disability Presenting for Genetic Services. Gene 2014, 535, 70–78. [Google Scholar] [CrossRef]
- Yin, K.-J.; Hamblin, M.; Fan, Y.; Zhang, J.; Chen, Y.E. Krüpple-like Factors in the Central Nervous System: Novel Mediators in Stroke. Metab. Brain Dis. 2015, 30, 401–410. [Google Scholar] [CrossRef]
- Ray, S.K. The Transcription Regulator Kruppel-Like Factor 4 and Its Dual Roles of Oncogene in Glioblastoma and Tumor Suppressor in Neuroblastoma. Immunopathol. Dis. Ther. 2016, 7, 127–139. [Google Scholar] [CrossRef]
- Spielmann, M.; Reichelt, G.; Hertzberg, C.; Trimborn, M.; Mundlos, S.; Horn, D.; Klopocki, E. Homozygous Deletion of Chromosome 15q13.3 Including CHRNA7 Causes Severe Mental Retardation, Seizures, Muscular Hypotonia, and the Loss of KLF13 and TRPM1 Potentially Cause Macrocytosis and Congenital Retinal Dysfunction in Siblings. Eur. J. Med. Genet. 2011, 54, e441–e445. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Purmann, C.; Ma, S.; Shrestha, A.; Davis, K.N.; Ho, M.; Huang, Y.; Pattni, R.; Wong, W.H.; et al. Network Effects of the 15q13.3 Microdeletion on the Transcriptome and Epigenome in Human-Induced Neurons. Biol. Psychiatry 2021, 89, 497–509. [Google Scholar] [CrossRef]
- Körner, M.B.; Velluva, A.; Bundalian, L.; Radtke, M.; Lin, C.-C.; Zacher, P.; Bartolomaeus, T.; Kirstein, A.S.; Mrestani, A.; Scholz, N.; et al. Altered Gene Expression Profiles Impair the Nervous System Development in Individuals with 15q13.3 Microdeletion. Sci. Rep. 2022, 12, 13507. [Google Scholar] [CrossRef]
- Malwade, S.; Gasthaus, J.; Bellardita, C.; Andelic, M.; Moric, B.; Korshunova, I.; Kiehn, O.; Vasistha, N.A.; Khodosevich, K. Identification of Vulnerable Interneuron Subtypes in 15q13.3 Microdeletion Syndrome Using Single-Cell Transcriptomics. Biol. Psychiatry 2022, 91, 727–739. [Google Scholar] [CrossRef]
- Wiemerslage, L.; Islam, R.; van der Kamp, C.; Cao, H.; Olivo, G.; Ence-Eriksson, F.; Castillo, S.; Larsen, A.L.; Bandstein, M.; Dahlberg, L.S.; et al. A DNA Methylation Site within the KLF13 Gene Is Associated with Orexigenic Processes Based on Neural Responses and Ghrelin Levels. Int. J. Obes. 2017, 41, 990–994. [Google Scholar] [CrossRef]
- Ávila-Mendoza, J.; Subramani, A.; Sifuentes, C.J.; Denver, R.J. Molecular Mechanisms for Krüppel-Like Factor 13 Actions in Hippocampal Neurons. Mol. Neurobiol. 2020, 57, 3785–3802. [Google Scholar] [CrossRef]
- Bernhardt, C.; Sock, E.; Fröb, F.; Hillgärtner, S.; Nemer, M.; Wegner, M. KLF9 and KLF13 Transcription Factors Boost Myelin Gene Expression in Oligodendrocytes as Partners of SOX10 and MYRF. Nucleic Acids Res. 2022, 50, 11509–11528. [Google Scholar] [CrossRef]
- Lavallée, G.; Andelfinger, G.; Nadeau, M.; Lefebvre, C.; Nemer, G.; Horb, M.E.; Nemer, M. The Kruppel-like Transcription Factor KLF13 Is a Novel Regulator of Heart Development. EMBO J. 2006, 25, 5201–5213. [Google Scholar] [CrossRef]
- Li, W.; Li, B.; Li, T.; Zhang, E.; Wang, Q.; Chen, S.; Sun, K. Identification and Analysis of KLF13 Variants in Patients with Congenital Heart Disease. BMC Med. Genet. 2020, 21, 78. [Google Scholar] [CrossRef]
- Wang, S.-S.; Wang, T.-M.; Qiao, X.-H.; Huang, R.-T.; Xue, S.; Dong, B.-B.; Xu, Y.-J.; Liu, X.-Y.; Yang, Y.-Q. KLF13 Loss-of-Function Variation Contributes to Familial Congenital Heart Defects. Eur. Rev. Med. Pharm. Sci. 2020, 24, 11273–11285. [Google Scholar] [CrossRef]
- Zeng, N.; Jian, Z.; Zhu, W.; Xu, J.; Fan, Y.; Xiao, F. KLF13 Overexpression Protects Sepsis-induced Myocardial Injury and LPS-induced Inflammation and Apoptosis. Int. J. Exp. Pathol. 2023, 104, 23–32. [Google Scholar] [CrossRef]
- Henson, B.J.; Gollin, S.M. Overexpression of KLF13 and FGFR3 in Oral Cancer Cells. Cytogenet. Genome Res. 2010, 128, 192–198. [Google Scholar] [CrossRef]
- Wang, Q.; Peng, R.; Wang, B.; Wang, J.; Yu, W.; Liu, Y.; Shi, G. Transcription Factor KLF13 Inhibits AKT Activation and Suppresses the Growth of Prostate Carcinoma Cells. Cancer Biomark. 2018, 22, 533–541. [Google Scholar] [CrossRef]
- Li, B.; Pang, S.; Dou, J.; Zhou, C.; Shen, B.; Zhou, Y. The Inhibitory Effect of LINC00261 Upregulation on the Pancreatic Cancer EMT Process Is Mediated by KLF13 via the MTOR Signaling Pathway. Clin. Transl. Oncol. 2022, 24, 1059–1072. [Google Scholar] [CrossRef]
- Ding, Y.; Xu, Y.; Fu, Y.; Zhang, H.; Zhao, L.; Fan, X. Kruppel-like Factor 13 Inhibits Cell Proliferation of Gastric Cancer by Inducing Autophagic Degradation of β-Catenin. Discov. Oncol. 2022, 13, 121. [Google Scholar] [CrossRef]
- Khan, K.; Gulzar, A.; Badshah, Y.; Ashraf, N.M.; Raq, M.; Hamid, A.; Shabbir, M.; Afsar, T.; Almajwal, A.; Arshad, M.; et al. Determining KLF13 Structure, Uniqueness, and Possible Molecular Crosstalk in Prostate Cancer. 2022; preprint. [Google Scholar] [CrossRef]
- Vinci, M.; Costanza, C.; Galati Rando, R.; Treccarichi, S.; Saccone, S.; Carotenuto, M.; Roccella, M.; Calì, F.; Elia, M.; Vetri, L. STXBP6 Gene Mutation: A New Form of SNAREopathy Leads to Developmental Epileptic Encephalopathy. Int. J. Mol. Sci. 2023, 24, 16436. [Google Scholar] [CrossRef]
- Calì, F.; Forster, P.; Kersting, C.; Mirisola, M.G.; D’Anna, R.; De Leo, G.; Romano, V. DXYS156: A Multi-Purpose Short Tandem Repeat Locus for Determination of Sex, Paternal and Maternal Geographic Origins and DNA Fingerprinting. Int. J. Leg. Med. 2002, 116, 133–138. [Google Scholar] [CrossRef]
- Desvignes, J.-P.; Bartoli, M.; Delague, V.; Krahn, M.; Miltgen, M.; Béroud, C.; Salgado, D. VarAFT: A Variant Annotation and Filtration System for Human next Generation Sequencing Data. Nucleic Acids Res. 2018, 46, W545–W553. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Kopanos, C.; Tsiolkas, V.; Kouris, A.; Chapple, C.E.; Albarca Aguilera, M.; Meyer, R.; Massouras, A. VarSome: The Human Genomic Variant Search Engine. Bioinformatics 2019, 35, 1978–1980. [Google Scholar] [CrossRef]
- Quinodoz, M.; Royer-Bertrand, B.; Cisarova, K.; Di Gioia, S.A.; Superti-Furga, A.; Rivolta, C. DOMINO: Using Machine Learning to Predict Genes Associated with Dominant Disorders. Am. J. Hum. Genet. 2017, 101, 623–629. [Google Scholar] [CrossRef]
- Treccarichi, S.; Calì, F.; Vinci, M.; Ragalmuto, A.; Musumeci, A.; Federico, C.; Costanza, C.; Bottitta, M.; Greco, D.; Saccone, S.; et al. Implications of a De Novo Variant in the SOX12 Gene in a Patient with Generalized Epilepsy, Intellectual Disability, and Childhood Emotional Behavioral Disorders. Curr. Issues Mol. Biol. 2024, 46, 6407–6422. [Google Scholar] [CrossRef]
- Tessarech, M.; Friocourt, G.; Marguet, F.; Lecointre, M.; Le Mao, M.; Díaz, R.M.; Mignot, C.; Keren, B.; Héron, B.; De Bie, C.; et al. De Novo Variants in SP9 Cause a Novel Form of Interneuronopathy Characterized by Intellectual Disability, Autism Spectrum Disorder, and Epilepsy with Variable Expressivity. Genet. Med. 2024, 26, 101087. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Mendoza, J.; Delgado-Rueda, K.; Urban-Sosa, V.A.; Carranza, M.; Luna, M.; Martínez-Moreno, C.G.; Arámburo, C. KLF13 Regulates the Activity of the GH-Induced JAK/STAT Signaling by Targeting Genes Involved in the Pathway. Int. J. Mol. Sci. 2023, 24, 11187. [Google Scholar] [CrossRef] [PubMed]
- Koh, I.-U.; Lee, H.-J.; Hwang, J.-Y.; Choi, N.-H.; Lee, S. Obesity-Related CpG Methylation (Cg07814318) of Kruppel-like Factor-13 (KLF13) Gene with Childhood Obesity and Its Cis-Methylation Quantitative Loci. Sci. Rep. 2017, 7, 45368. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; He, P.; Bialkowska, A.B.; Yang, V.W. SP and KLF transcription factors in digestive physiology and diseases. Gastroenterology 2017, 152, 1845–1875. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, T.; Ohashi, K.; Shibata, R.; Kambara, T.; Uemura, Y.; Yuasa, D.; Kataoka, Y.; Miyabe, M.; Matsuo, K.; Joki, Y.; et al. Transcriptional Regulation of an Insulin-Sensitizing Adipokine Adipolin/CTRP12 in Adipocytes by Krüppel-Like Factor 15. PLoS ONE 2013, 8, e83183. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wei, H.; Song, T.; Yang, Y.; Zhang, F.; Zhou, Y.; Peng, J.; Jiang, S. KLF13 Promotes Porcine Adipocyte Differentiation through PPARγ Activation. Cell Biosci. 2015, 5, 28. [Google Scholar] [CrossRef]
- Scohy, S.; Gabant, P.; Van Reeth, T.; Hertveldt, V.; Drèze, P.-L.; Van Vooren, P.; Rivière, M.; Szpirer, J.; Szpirer, C. Identification of KLF13 and KLF14 (SP6), Novel Members of the SP/XKLF Transcription Factor Family. Genomics 2000, 70, 93–101. [Google Scholar] [CrossRef]
- Swamynathan, S.K. Krüppel-like Factors: Three Fingers in Control. Hum. Genom. 2010, 4, 263. [Google Scholar] [CrossRef]
- Pearson, R.C.M.; Funnell, A.P.W.; Crossley, M. The Mammalian Zinc Finger Transcription Factor Krüppel-like Factor 3 (KLF3/BKLF). IUBMB Life 2011, 63, 86–93. [Google Scholar] [CrossRef]
- Camacho-Vanegas, O.; Till, J.; Miranda-Lorenzo, I.; Ozturk, B.; Camacho, S.C.; Martignetti, J.A. Shaking the Family Tree: Identification of Novel and Biologically Active Alternatively Spliced Isoforms across the KLF Family of Transcription Factors. FASEB J. 2013, 27, 432–436. [Google Scholar] [CrossRef]
- Quach, T.T.; Stratton, H.J.; Khanna, R.; Kolattukudy, P.E.; Honnorat, J.; Meyer, K.; Duchemin, A.-M. Intellectual Disability: Dendritic Anomalies and Emerging Genetic Perspectives. Acta Neuropathol. 2021, 141, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Alemany-González, M.; Gener, T.; Nebot, P.; Vilademunt, M.; Dierssen, M.; Puig, M.V. Prefrontal–Hippocampal Functional Connectivity Encodes Recognition Memory and Is Impaired in Intellectual Disability. Proc. Natl. Acad. Sci. USA 2020, 117, 11788–11798. [Google Scholar] [CrossRef] [PubMed]
- Xian, W.; Cao, J.; Yuan, X.; Wang, G.; Jin, Q.; Zhang, H.; Zhou, G.; You, L. Deficiency of Intellectual Disability-Related Gene Brpf1 Attenuated Hippocampal Excitatory Synaptic Transmission and Impaired Spatial Learning and Memory Ability. Front. Cell Dev. Biol. 2021, 9, 711792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hong, S.; Maniar, K.P.; Cheng, S.; Jie, C.; Rademaker, A.W.; Krensky, A.M.; Clayberger, C. KLF13 Regulates the Differentiation-Dependent Human Papillomavirus Life Cycle in Keratinocytes through STAT5 and IL-8. Oncogene 2016, 35, 5565–5575. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.L.; Macdonald, A. Manipulation of JAK/STAT Signalling by High-Risk HPVs: Potential Therapeutic Targets for HPV-Associated Malignancies. Viruses 2020, 12, 977. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Moreno, C.G.; Ticante-Carrizales, A.G.P.; González-Gallardo, A.; Carranza, M.; Luna, M.; Arámburo, C.; Ávila-Mendoza, J. Designing Strategies To Enhance Gh-Dependent Axon Regeneration In Klf13 Deficient Retinal Ganglion Cells. Investig. Ophthalmol. Vis. Sci. 2024, 65, 3441. [Google Scholar] [CrossRef]
- Nicolas, C.S.; Peineau, S.; Amici, M.; Csaba, Z.; Fafouri, A.; Javalet, C.; Collett, V.J.; Hildebrandt, L.; Seaton, G.; Choi, S.-L.; et al. The JAK/STAT Pathway Is Involved in Synaptic Plasticity. Neuron 2012, 73, 374–390. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yuan, W.; Liu, Y.; Zhang, Y.; Wang, Z.; Zhou, X.; Ning, G.; Zhang, L.; Yao, L.; Feng, S.; et al. The Role of the JAK-STAT Pathway in Neural Stem Cells, Neural Progenitor Cells and Reactive Astrocytes after Spinal Cord Injury. Biomed. Rep. 2015, 3, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, C.S.; Amici, M.; Bortolotto, Z.A.; Doherty, A.; Csaba, Z.; Fafouri, A.; Dournaud, P.; Gressens, P.; Collingridge, G.L.; Peineau, S. The Role of JAK-STAT Signaling within the CNS. Jak-Stat 2013, 2, e22925. [Google Scholar] [CrossRef]
- Qin, H.; Buckley, J.A.; Li, X.; Liu, Y.; Fox, T.H.; Meares, G.P.; Yu, H.; Yan, Z.; Harms, A.S.; Li, Y.; et al. Inhibition of the JAK/STAT Pathway Protects Against α-Synuclein-Induced Neuroinflammation and Dopaminergic Neurodegeneration. J. Neurosci. 2016, 36, 5144–5159. [Google Scholar] [CrossRef]
- Lashgari, N.-A.; Roudsari, N.M.; Momtaz, S.; Sathyapalan, T.; Abdolghaffari, A.H.; Sahebkar, A. The Involvement of JAK/STAT Signaling Pathway in the Treatment of Parkinson’s Disease. J. Neuroimmunol. 2021, 361, 577758. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Singh, M.K.; Shyam, H.; Mishra, A.; Kumar, S.; Kumar, A.; Kushwaha, J. Role of JAK/STAT in the Neuroinflammation and Its Association with Neurological Disorders. Ann. Neurosci. 2021, 28, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-J. Signal Transducer and Activator of Transcription 6 (STAT6) and Attention-Deficit Hyperactivity Disorder: A Speculative Hypothesis. Med. Hypotheses 2006, 67, 1342–1344. [Google Scholar] [CrossRef] [PubMed]
- Shariq, A.S.; Brietzke, E.; Rosenblat, J.D.; Pan, Z.; Rong, C.; Ragguett, R.-M.; Park, C.; McIntyre, R.S. Therapeutic Potential of JAK/STAT Pathway Modulation in Mood Disorders. Rev. Neurosci. 2018, 30, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Yun, Q.; Zhang, J.; Bao, J. Downregulation of KLF13 through DNMT1-Mediated Hypermethylation Promotes Glioma Cell Proliferation and Invasion. Onco Targets 2019, 12, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, M.; Tian, N.; Li, D.; Wu, F.; Hu, P.; Wang, Z.; Wang, L.; Hao, W.; Kang, J.; et al. The Antibiotic Clofoctol Suppresses Glioma Stem Cell Proliferation by Activating KLF13. J. Clin. Investig. 2019, 129, 3072–3085. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Mendoza, J.; Subramani, A.; Denver, R.J. Krüppel-Like Factors 9 and 13 Block Axon Growth by Transcriptional Repression of Key Components of the CAMP Signaling Pathway. Front. Mol. Neurosci. 2020, 13, 602638. [Google Scholar] [CrossRef] [PubMed]
- Willcutt, E.G.; Nigg, J.T.; Pennington, B.F.; Solanto, M.V.; Rohde, L.A.; Tannock, R.; Loo, S.K.; Carlson, C.L.; McBurnett, K.; Lahey, B.B. Validity of DSM-IV Attention Deficit/Hyperactivity Disorder Symptom Dimensions and Subtypes. J. Abnorm. Psychol. 2012, 121, 991–1010. [Google Scholar] [CrossRef] [PubMed]
- Al-Mubarak, B.R.; Omar, A.; Baz, B.; Al-Abdulaziz, B.; Magrashi, A.I.; Al-Yemni, E.; Jabaan, A.; Monies, D.; Abouelhoda, M.; Abebe, D.; et al. Whole Exome Sequencing in ADHD Trios from Single and Multi-Incident Families Implicates New Candidate Genes and Highlights Polygenic Transmission. Eur. J. Hum. Genet. 2020, 28, 1098–1110. [Google Scholar] [CrossRef]
- Brikell, I.; Kuja-Halkola, R.; Larsson, H. Heritability of Attention-deficit Hyperactivity Disorder in Adults. Am. J. Med. Genet. Part. B Neuropsychiatr. Genet. 2015, 168, 406–413. [Google Scholar] [CrossRef]
- Faraone, S.V.; Larsson, H. Genetics of Attention Deficit Hyperactivity Disorder. Mol. Psychiatry 2019, 24, 562–575. [Google Scholar] [CrossRef] [PubMed]
- Arpawong, T.E.; Klopack, E.T.; Kim, J.K.; Crimmins, E.M. ADHD Genetic Burden Associates with Older Epigenetic Age: Mediating Roles of Education, Behavioral and Sociodemographic Factors among Older Adults. Clin. Epigenetics 2023, 15, 67. [Google Scholar] [CrossRef] [PubMed]
- Kleefstra, T.; Kramer, J.M.; Neveling, K.; Willemsen, M.H.; Koemans, T.S.; Vissers, L.E.L.M.; Wissink-Lindhout, W.; Fenckova, M.; van den Akker, W.M.R.; Kasri, N.N.; et al. Disruption of an EHMT1-Associated Chromatin-Modification Module Causes Intellectual Disability. Am. J. Hum. Genet. 2012, 91, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Vogel-Ciernia, A.; Wood, M.A. Neuron-Specific Chromatin Remodeling: A Missing Link in Epigenetic Mechanisms Underlying Synaptic Plasticity, Memory, and Intellectual Disability Disorders. Neuropharmacology 2014, 80, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Larizza, L.; Finelli, P. Developmental Disorders with Intellectual Disability Driven by Chromatin Dysregulation: Clinical Overlaps and Molecular Mechanisms. Clin. Genet. 2019, 95, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Song, C.-Z.; Keller, K.; Chen, Y.; Stamatoyannopoulos, G. Functional Interplay between CBP and PCAF in Acetylation and Regulation of Transcription Factor KLF13 Activity. J. Mol. Biol. 2003, 329, 207–215. [Google Scholar] [CrossRef]
- Ahn, Y.-T.; Huang, B.; McPherson, L.; Clayberger, C.; Krensky, A.M. Dynamic Interplay of Transcriptional Machinery and Chromatin Regulates “Late” Expression of the Chemokine RANTES in T Lymphocytes. Mol. Cell. Biol. 2007, 27, 253–266. [Google Scholar] [CrossRef]
- Krensky, A.M.; Ahn, Y.-T. Mechanisms of Disease: Regulation of RANTES (CCL5) in Renal Disease. Nat. Clin. Pract. Nephrol. 2007, 3, 164–170. [Google Scholar] [CrossRef]
- Walton, E.; Pingault, J.-B.; Cecil, C.A.M.; Gaunt, T.R.; Relton, C.L.; Mill, J.; Barker, E.D. Epigenetic Profiling of ADHD Symptoms Trajectories: A Prospective, Methylome-Wide Study. Mol. Psychiatry 2017, 22, 250–256. [Google Scholar] [CrossRef]
- Hamza, M.; Halayem, S.; Bourgou, S.; Daoud, M.; Charfi, F.; Belhadj, A. Epigenetics and ADHD: Toward an Integrative Approach of the Disorder Pathogenesis. J. Atten. Disord. 2019, 23, 655–664. [Google Scholar] [CrossRef]
- Song, C.-Z.; Keller, K.; Murata, K.; Asano, H.; Stamatoyannopoulos, G. Functional Interaction between Coactivators CBP/P300, PCAF, and Transcription Factor FKLF2. J. Biol. Chem. 2002, 277, 7029–7036. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Ahn, Y.-T.; McPherson, L.; Clayberger, C.; Krensky, A.M. Interaction of PRP4 with Krüppel-Like Factor 13 Regulates CCL5 Transcription. J. Immunol. 2007, 178, 7081–7087. [Google Scholar] [CrossRef] [PubMed]
- Huttlin, E.L.; Bruckner, R.J.; Navarrete-Perea, J.; Cannon, J.R.; Baltier, K.; Gebreab, F.; Gygi, M.P.; Thornock, A.; Zarraga, G.; Tam, S.; et al. Dual Proteome-Scale Networks Reveal Cell-Specific Remodeling of the Human Interactome. Cell 2021, 184, 3022–3040.e28. [Google Scholar] [CrossRef] [PubMed]
- Bannwarth, S.; Ait-El-Mkadem, S.; Chaussenot, A.; Genin, E.C.; Lacas-Gervais, S.; Fragaki, K.; Berg-Alonso, L.; Kageyama, Y.; Serre, V.; Moore, D.G.; et al. A Mitochondrial Origin for Frontotemporal Dementia and Amyotrophic Lateral Sclerosis through CHCHD10 Involvement. Brain 2014, 137, 2329–2345. [Google Scholar] [CrossRef]
- Stilling, R.M.; Rönicke, R.; Benito, E.; Urbanke, H.; Capece, V.; Burkhardt, S.; Bahari-Javan, S.; Barth, J.; Sananbenesi, F.; Schütz, A.L.; et al. K-Lysine Acetyltransferase 2a Regulates a Hippocampal Gene Expression Network Linked to Memory Formation. EMBO J. 2014, 33, 1912–1927. [Google Scholar] [CrossRef] [PubMed]
- Panahzadeh, F.; Mirnasuri, R.; Rahmati, M. The Effect of Endurance Training on the Expression of PRDX6 and KAT2B Genes in Hippocampus of β Amyloid-Induced Rat Model of Alzheimer’s Disease: An Experimental Study. J. Rafsanjan Univ. Med. Sci. 2020, 19, 485–498. [Google Scholar] [CrossRef]
- Smigiel, K.S.; Parks, W.C. Matrix Metalloproteinases and Leukocyte Activation. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2017; pp. 167–195. [Google Scholar]
- Beroun, A.; Mitra, S.; Michaluk, P.; Pijet, B.; Stefaniuk, M.; Kaczmarek, L. MMPs in Learning and Memory and Neuropsychiatric Disorders. Cell. Mol. Life Sci. 2019, 76, 3207–3228. [Google Scholar] [CrossRef]


| Criteria for Classification of Variants | Category Code | Description |
|---|---|---|
| Strong | PS2 | De novo (both maternity and paternity confirmed) in a patient with the disease and no family history. |
| Moderate | PM2 | Absent from controls (or at extremely low frequency if recessive) in Exome Sequencing Project, 1000 Genomes Project, or Exome Aggregation Consortium. |
| Supporting | PP4 | Patient’s phenotype or family history is highly specific for a disease with a single genetic etiology. |
| Benign supporting | BP4 | Multiple lines of computational evidence suggest no impact on gene or gene product (conservation, evolutionary, splicing impact) |
| ACMG variant classification | Likely pathogenic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinci, M.; Greco, D.; Treccarichi, S.; Chiavetta, V.; Figura, M.G.; Musumeci, A.; Greco, V.; Federico, C.; Calì, F.; Saccone, S. Bioinformatic Evaluation of KLF13 Genetic Variant: Implications for Neurodevelopmental and Psychiatric Symptoms. Genes 2024, 15, 1056. https://doi.org/10.3390/genes15081056
Vinci M, Greco D, Treccarichi S, Chiavetta V, Figura MG, Musumeci A, Greco V, Federico C, Calì F, Saccone S. Bioinformatic Evaluation of KLF13 Genetic Variant: Implications for Neurodevelopmental and Psychiatric Symptoms. Genes. 2024; 15(8):1056. https://doi.org/10.3390/genes15081056
Chicago/Turabian StyleVinci, Mirella, Donatella Greco, Simone Treccarichi, Valeria Chiavetta, Maria Grazia Figura, Antonino Musumeci, Vittoria Greco, Concetta Federico, Francesco Calì, and Salvatore Saccone. 2024. "Bioinformatic Evaluation of KLF13 Genetic Variant: Implications for Neurodevelopmental and Psychiatric Symptoms" Genes 15, no. 8: 1056. https://doi.org/10.3390/genes15081056







