Association Analysis of METTL23 Gene Polymorphisms with Reproductive Traits in Kele Pigs
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. DNA Extraction
2.3. Primer Design and Synthesis
2.4. PCR Amplification
2.5. Statistical Analysis
3. Results
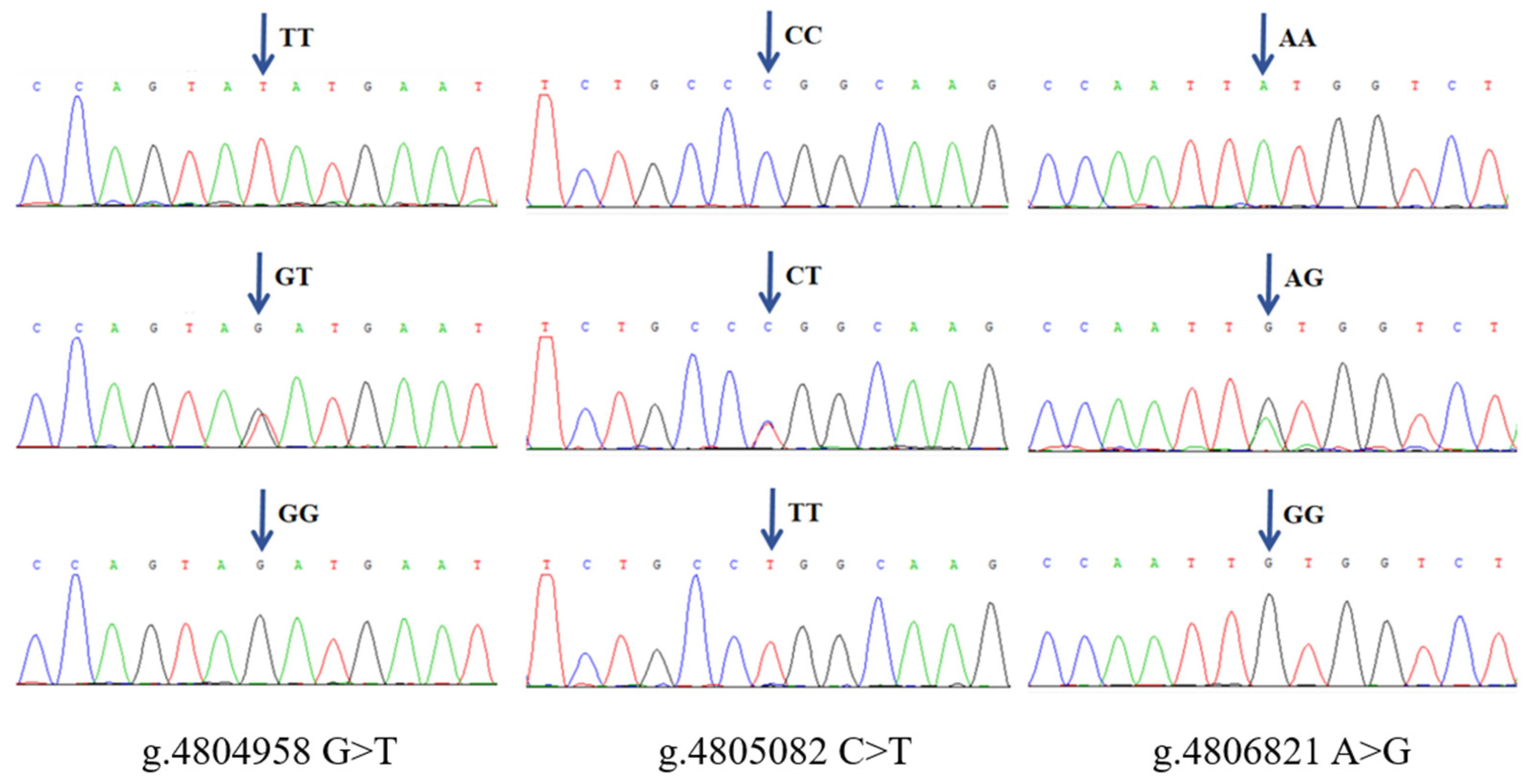
3.1. SNPs Identification
3.2. Population Genetic Analysis of SNPs
3.3. Linkage Disequilibrium Analysis of SNPs
3.4. Haplotype and Diplotype Analysis of SNPs
3.5. Association Analysis of SNPs in the METTL23 Gene with Reproductive Traits
3.6. mRNA Secondary Structure Prediction of Different Haplotypes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vigne, J.D. The origins of animal domestication and husbandry: A major change in the history of humanity and the biosphere. Comptes Rendus Biol. 2011, 334, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Zeder, M.A. The origins of agriculture in the Near East. Curr. Anthropol. 2011, 52, S221–S235. [Google Scholar] [CrossRef]
- Ramos-Onsins, S.E.; Burgos-Paz, W.; Manunza, A.; Amills, M. Mining the pig genome to investigate the domestication process. Heredity 2014, 113, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Nonneman, D.J.; Lents, C.A. Functional genomics of reproduction in pigs: Are we there yet? Mol. Reprod. Dev. 2023, 90, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, M.F.; Messer, L.A.; Vincent, A. Molecular approaches to improved pig fertility. J. Reprod. Fertil. Suppl. 1997, 52, 227–236. [Google Scholar] [CrossRef]
- Groenen, M.A.; Archibald, A.L.; Uenishi, H.; Tuggle, C.K.; Takeuchi, Y.; Rothschild, M.F.; Rogel-Gaillard, C.; Park, C.; Milan, D.; Megens, H.-J.; et al. Analyses of pig genomes provide insight into porcine demography and evolution. Nature 2012, 491, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Distl, O. Mechanisms of regulation of litter size in pigs on the genome level. Reprod. Domest. Anim. 2007, 42, 10–16. [Google Scholar] [CrossRef]
- Hui, Y.T.; Yang, Y.Q.; Liu, R.Y.; Zhang, Y.Y.; Xiang, C.J.; Liu, Z.Z.; Ding, Y.H.; Zhang, Y.L.; Wang, B.R. Significant association of APOA5 and APOC3 gene polymorphisms with meat quality traits in Kele pigs. Genet. Mol. Res. 2013, 12, 3643–3650. [Google Scholar] [CrossRef]
- Xie, J.; Li, R.; Li, S.; Ran, X.; Wang, J.; Jiang, J.; Zhao, P. Identification of Copy Number Variations in Xiang and Kele Pigs. PLoS ONE 2016, 11, e0148565. [Google Scholar] [CrossRef][Green Version]
- Liu, J.J.; Ran, X.Q.; Li, S.; Feng, Y.; Wang, J.F. Polymorphism in the first intron of follicle stimulating hormone beta gene in three Chinese pig breeds and two European pig breeds. Anim. Reprod. Sci. 2009, 111, 369–375. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, Z.; Hu, G.; Zhang, Y.; Ao, Z. Exploring characteristics of placental transcriptome and cord serum metabolome associated with low birth weight in Kele pigs. Trop. Anim. Health Prod. 2023, 55, 340. [Google Scholar] [CrossRef] [PubMed]
- Bedford, M.T. Arginine methylation at a glance. J. Cell Sci. 2007, 120, 4243–4246. [Google Scholar] [CrossRef] [PubMed]
- Blanc, R.S.; Richard, S. Arginine Methylation: The Coming of Age. Mol Cell. 2017, 65, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Guccione, E.; Richard, S. The regulation, functions and clinical relevance of arginine methylation. Nat. Rev. Mol. Cell Biol. 2019, 20, 642–657, Erratum in Nat. Rev. Mol. Cell Biol. 2019, 20, 567. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, J.; Clancy, K.W.; Thompson, P.R. Chemical biology of protein arginine modifications in epigenetic regulation. Chem. Rev. 2015, 115, 5413–5461. [Google Scholar] [CrossRef] [PubMed]
- Bedford, M.T.; Clarke, S.G. Protein arginine methylation in mammals: Who, what, and why. Mol. Cell. 2009, 33, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.H.; Fan, X.J.; Hu, Y.; Tian, X.-X.; Guo, M.; Mao, M.-W.; Fang, Z.-Y.; Wu, P.; Gao, S.-X.; Peng, C.; et al. A systematic survey of PRMT interactomes reveals the key roles of arginine methylation in the global control of RNA splicing and translation. Sci. Bull. 2021, 66, 1342–1357. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Suga, A.; Kimura, I.; Kimura, C.; Minegishi, Y.; Nakayama, M.; Yoshitake, K.; Iejima, D.; Minematsu, N.; Yamamoto, M.; et al. METTL23 mutation alters histone H3R17 methylation in normal-tension glaucoma. J. Clin. Invest. 2022, 132, e153589. [Google Scholar] [CrossRef]
- Almannai, M.; Obaid, O.; Faqeih, E.; Alasmari, A.; Samman, M.M.; Pinz, H.; Braddock, S.R.; Alkuraya, F.S. Further delineation of METTL23-associated intellectual disability. Am. J. Med. Genet. Part A 2020, 182, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Bernkopf, M.; Webersinke, G.; Tongsook, C.; Koyani, C.N.; Rafiq, M.A.; Ayaz, M.; Müller, D.; Enzinger, C.; Aslam, M.; Naeem, F.; et al. Disruption of the methyltransferase-like 23 gene METTL23 causes mild autosomal recessive intellectual disability. Hum. Mol. Genet. 2014, 23, 4015–4023. [Google Scholar] [CrossRef]
- Khan, A.; Miao, Z.; Umair, M.; Ullah, A.; Alshabeeb, M.A.; Bilal, M.; Ahmad, F.; Rappold, G.A.; Ansar, M.; Carapito, R. Two Cases of Recessive Intellectual Disability Caused by NDST1 and METTL23 Variants. Genes 2020, 11, 1021. [Google Scholar] [CrossRef] [PubMed]
- Reiff, R.E.; Ali, B.R.; Baron, B.; Yu, T.W.; Ben-Salem, S.; Coulter, M.E.; Schubert, C.R.; Hill, R.S.; Akawi, N.A.; Al-Younes, B.; et al. METTL23, a transcriptional partner of GABPA, is essential for human cognition. Hum. Mol. Genet. 2014, 23, 3456–3466. [Google Scholar] [CrossRef] [PubMed]
- Smaili, W.; Elalaoui, S.C.; Zrhidri, A.; Raymond, L.; Egéa, G.; Taoudi, M.; Mouatassim, S.E.L.; Sefiani, A.; Lyahyai, J. Exome sequencing revealed a novel homozygous METTL23 gene mutation leading to familial mild intellectual disability with dysmorphic features. Eur. J. Med. Genet. 2020, 63, 103951. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Iwata, T. Molecular genetics of inherited normal tension glaucoma. Indian J. Ophthalmol. 2024, 72, S335–S344. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.W.; Sun, Y. Epigenetics in glaucoma: A link between histone methylation and neurodegeneration. J. Clin. Invest. 2023, 133, e173784. [Google Scholar] [CrossRef] [PubMed]
- Ekure, E.N.; Adeyemo, A.; Liu, H.; Sokunbi, O.; Kalu, N.; Martinez, A.F.; Owosela, B.; Tekendo-Ngongang, C.; Addissie, Y.A.; Olusegun-Joseph, A.; et al. Exome Sequencing and Congenital Heart Disease in Sub-Saharan Africa. Circ. Genom. Precis. Med. 2021, 14, e003108. [Google Scholar] [CrossRef] [PubMed]
- Hamey, J.J.; Wienert, B.; Quinlan, K.G.R.; Wilkins, M.R. METTL21B Is a Novel Human Lysine Methyltransferase of Translation Elongation Factor 1A: Discovery by CRISPR/Cas9 Knockout. Mol. Cell. Proteom. 2017, 16, 2229–2242. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, Y.; Tsusaka, T.; Shimizu, N.; Morita, K.; Suzuki, T.; Machida, S.; Satoh, M.; Honda, A.; Hirose, M.; Kamimura, S.; et al. Histone H3 Methylated at Arginine 17 Is Essential for Reprogramming the Paternal Genome in Zygotes. Cell Rep. 2017, 20, 2756–2765. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.H.; Lee, C.Y.; Lee, T.Y.; Huang, H.D.; Hsu, J.B.; Chang, T.H. Biomarker Identification through Multiomics Data Analysis of Prostate Cancer Prognostication Using a Deep Learning Model and Similarity Network Fusion. Cancers 2021, 13, 2528. [Google Scholar] [CrossRef]
- Fabbri, M.C.; Crovetti, A.; Tinacci, L.; Bertelloni, F.; Armani, A.; Mazzei, M.; Fratini, F.; Bozzi, R.; Cecchi, F. Identification of candidate genes associated with bacterial and viral infections in wild boars hunted in Tuscany (Italy). Sci. Rep. 2022, 12, 8145. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, Y.; Yang, Z.; Ming, F.; Li, J.; Kong, D.; Wang, Y.; Chen, P.; Wang, M.; Wang, Z. Metabolic Pathway Engineering Improves Dendrobine Production in Dendrobium catenatum. Int. J. Mol. Sci. 2023, 25, 397. [Google Scholar] [CrossRef] [PubMed]
- Uenishi, H.; Eguchi, T.; Suzuki, K.; Sawazaki, T.; Toki, D.; Shinkai, H.; Okumura, N.; Hamasima, N.; Awata, T. PEDE (Pig EST Data Explorer): Construction of a database for ESTs derived from porcine full-length cDNA libraries. Nucleic Acids Res. 2004, 32, D484–D488. [Google Scholar] [CrossRef] [PubMed]
- Onishi, A.; Iwamoto, M.; Akita, T.; Mikawa, S.; Takeda, K.; Awata, T.; Hanada, H.; Perry, A.C. Pig cloning by microinjection of fetal fibroblast nuclei. Science 2000, 289, 1188–1190. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Kao, P.N.; Herschman, H.R. Protein-arginine methyltransferase I, the predominant protein-arginine methyltransferase in cells, interacts with and is regulated by interleukin enhancer-binding factor 3. J. Biol. Chem. 2000, 275, 19866–19876. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Chang, J.; Zhu, Y.; Yeldandi, A.V.; Rao, S.M.; Zhu, Y.J. Identification of protein arginine methyltransferase 2 as a coactivator for estrogen receptor alpha. J. Biol. Chem. 2002, 277, 28624–28630. [Google Scholar] [CrossRef] [PubMed]
- Tee, W.W.; Pardo, M.; Theunissen, T.W.; Yu, L.; Choudhary, J.S.; Hajkova, P.; Surani, M.A. Prmt5 is essential for early mouse development and acts in the cytoplasm to maintain ES cell pluripotency. Genes Dev. 2010, 24, 2772–2777. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Lee, J.; Kim, J.; Shen, J.; Hu, M.C.T.; Aldaz, C.M.; Bedford, M.T. Specific protein methylation defects and gene expression perturbations in coactivator-associated arginine methyltransferase 1-deficient mice. Proc. Natl. Acad. Sci. USA 2003, 100, 6464–6468. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Murata, K.; Ishida, J.; Kanou, A.; Kasuya, Y.; Fukamizu, A. Severe Hypomyelination and Developmental Defects Are Caused in Mice Lacking Protein Arginine Methyltransferase 1 (PRMT1) in the Central Nervous System. J. Biol. Chem. 2016, 291, 2237–2245. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhan, J.; Tang, X.; Li, T.; Duan, S. Characterization and identification of pork flavor compounds and their precursors in Chinese indigenous pig breeds by volatile profiling and multivariate analysis. Food Chem. 2022, 385, 132543. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Y. TET-mediated active DNA demethylation: Mechanism, function and beyond. Nat. Rev. Genet. 2017, 18, 517–534. [Google Scholar] [CrossRef]
- Alarcón, C.R.; Goodarzi, H.; Lee, H.; Liu, X.; Tavazoie, S.; Tavazoie, S.F. HNRNPA2B1 Is a Mediator of m(6)A-Dependent Nuclear RNA Processing Events. Cell 2015, 162, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, Y.; Sun, B.F.; Shi, Y.; Yang, X.; Xiao, W.; Hao, Y.-J.; Ping, X.-L.; Chen, Y.-S.; Wang, W.-J.; et al. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res. 2014, 24, 1403–1419. [Google Scholar] [CrossRef] [PubMed]
- Serrote, C.M.L.; Reiniger, L.R.S.; Silva, K.B.; Rabaiolli, S.M.D.S.; Stefanel, C.M. Determining the Polymorphism Information Content of a molecular marker. Gene 2020, 726, 144175. [Google Scholar] [CrossRef]
- Slatkin, M. Linkage disequilibrium—Understanding the evolutionary past and mapping the medical future. Nat. Rev. Genet. 2008, 9, 477–485. [Google Scholar] [CrossRef]
- Ardlie, K.G.; Kruglyak, L.; Seielstad, M. Patterns of linkage disequilibrium in the human genome. Nat. Rev. Genet. 2002, 3, 299–309, Erratum in Nat. Rev. Genet. 2002, 3, 566. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.C.B.; Viana, J.M.S.; Pereira, H.D.; Pinto, V.B.; Silva, F.F. Linkage disequilibrium and haplotype block patterns in popcorn populations. PLoS ONE 2019, 14, e0219417. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Li, Y.; Guo, H.; Lin, S.; Chen, J.; Ma, Q.; Gu, Y.; Jiang, Z.; Gui, Y. Protein Arginine Methyltransferase 6 Involved in Germ Cell Viability during Spermatogenesis and Down-Regulated by the Androgen Receptor. Int. J. Mol. Sci. 2015, 16, 29467–29481. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, T.; Hébert, J.; Li, E.; Richard, S. A mouse PRMT1 null allele defines an essential role for arginine methylation in genome maintenance and cell proliferation. Mol. Cell. Biol. 2009, 29, 2982–2996, Erratum in Mol. Cell. Biol. 2017, 37, e00298-17. [Google Scholar] [CrossRef]
- Wan, Y.; Qu, K.; Ouyang, Z.; Kertesz, M.; Li, J.; Tibshirani, R.; Makino, D.L.; Nutter, R.C.; Segal, E.; Chang, H.Y. Genome-wide measurement of RNA folding energies. Mol. Cell 2012, 48, 169–181. [Google Scholar] [CrossRef]
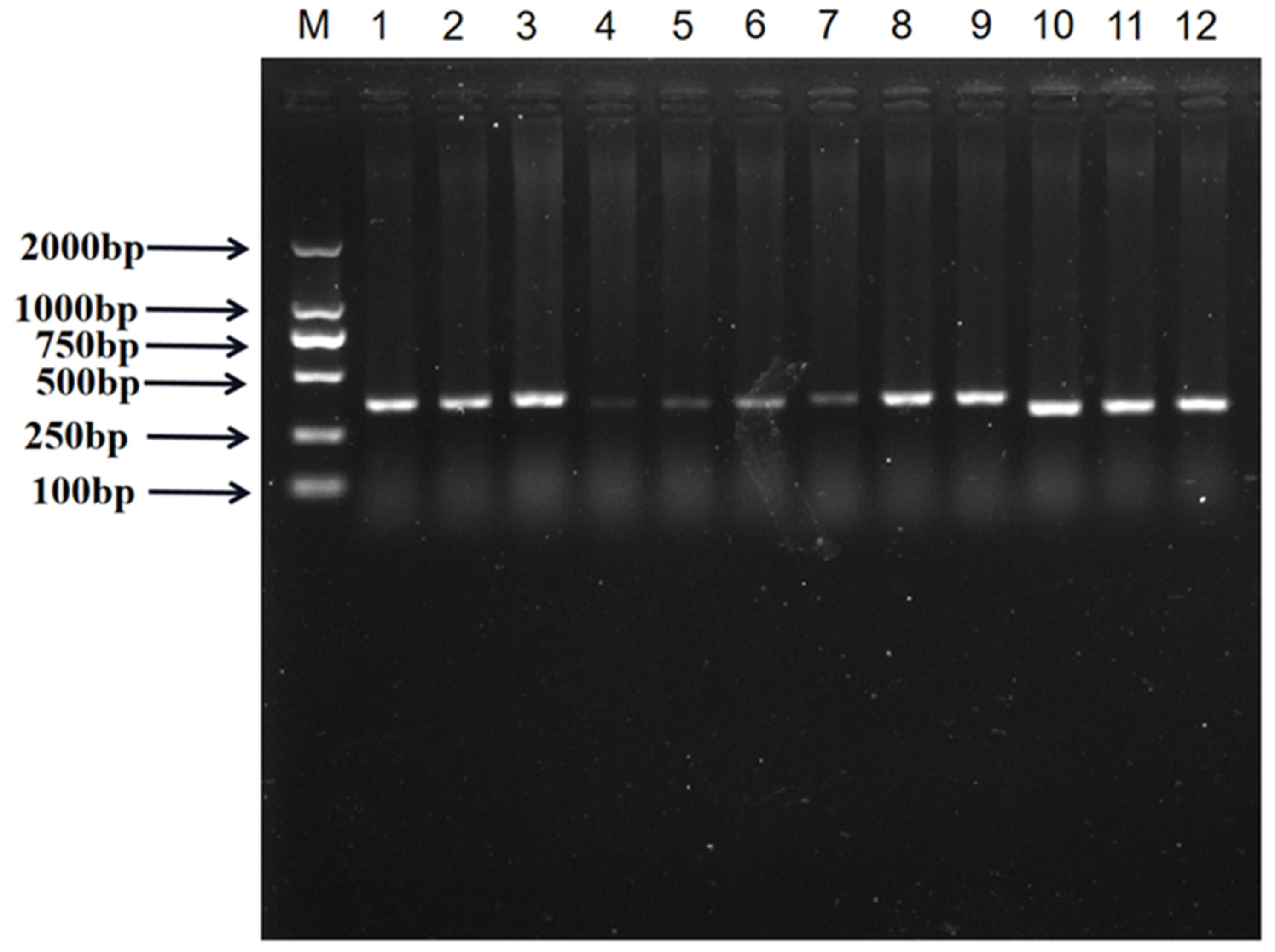

| Primers | Primer Sequences (5′→3′) | Amplified Fragment | Tm/°C | |
|---|---|---|---|---|
| P1 | F1 | TGCTGGGCACTCAGTAATTGT | g.4804918~g.4805287 369bp/Exon2 | 60 |
| R1 | AGACGCCACCAGTCCTGTTA | |||
| P2 | F2 | AAGCCATTCACCAAGACCCG | g.4806377~g.4806725 348bp/Exon3 | 60 |
| R2 | TCCATTGCATCGCTGGCTAA | |||
| P3 | F3 | TGCCTGGAAATCTGTCAGCG | g.4806523~g.4806891 368bp/Exon4 | 60 |
| R3 | ACACCAGTAAGGCTTCAGTTGAG | |||
| P4 | F4 | ACTCAACTGAAGCCTTACTGGT | g.4806869~g.4807185 316bp/Exon5 | 60 |
| R4 | AGCATTGACGCTGTCCTGAA | |||
| SNP Loci | Genotype Frequency | Allele Frequency | PIC | χ2 | |||
|---|---|---|---|---|---|---|---|
| g.4804958 G > T | GG (93) 0.408 | GT (89) 0.390 | TT (46) 0.202 | G 0.603 | T 0.397 | 0.364 | 7.774 (0.02) |
| g.4805082 C > T | CC (150) 0.658 | CT (70) 0.307 | TT (8) 0.035 | C 0.811 | T 0.189 | 0.259 | 0.002 (0.99) |
| g.4806821 A > G | AA (103) 0.452 | AG (87) 0.382 | GG (38) 0.167 | A 0.642 | G 0.357 | 0.354 | 6.537 (0.03) |
| SNP Loci | g.4804958 G > T | g.4805082 C > T | g.4806821 A > G |
|---|---|---|---|
| g.4804958 G > T | - | 1.000 | 0.513 |
| g.4805082 C > T | 0.141 | - | 1.000 |
| g.4806821 A > G | 0.128 | 0.290 | - |
| SNPs | g.4804958 G > T | g.4805082 C > T | g.4806821 A > G | Frequency | |
|---|---|---|---|---|---|
| Haplotypes | H1 (181) | T | C | A | 0.397 |
| H2 (112) | G | C | A | 0.246 | |
| H3 (86) | G | T | G | 0.189 | |
| H4 (77) | G | C | G | 0.169 | |
| Diplotypes | H1H1 (46) | TT | CC | AA | 0.202 |
| H1H2 (38) | GT | CC | AA | 0.167 | |
| H1H3 (26) | GT | CT | AG | 0.114 | |
| H1H4 (25) | GT | CC | AG | 0.110 | |
| H2H2 (19) | GG | CC | AA | 0.104 | |
| H2H3 (20) | GG | CT | AG | 0.088 | |
| H2H4 (16) | GG | CC | AG | 0.070 | |
| H3H3 (8) | GG | TT | GG | 0.035 | |
| H3H4 (24) | GG | CT | GG | 0.105 | |
| H4H4 (6) | GG | CC | GG | 0.026 | |
| SNP Loci | Genotype | Piglets Born Alive | Litter Birth Weight/kg | Average Birth Weight/kg | Number of Weaned Piglets | Weaning Litter Weight/kg | Weaning Weight/kg |
|---|---|---|---|---|---|---|---|
| g.4804958 G > T | GG (93) | 9.473 ± 2.224 a | 9.299 ± 3.154 a | 1.074 ± 0.221 | 7.484 ± 2.130 a | 39.459 ± 14.083 a | 5.257 ± 1.102 |
| GT (89) | 8.653 ± 2.667 ab | 8.277 ± 2.614 ab | 1.070 ± 0.248 | 6.776 ± 2.477 ab | 38.206 ± 13.462 a | 5.794 ± 1.310 | |
| TT (46) | 7.568 ± 2.534b | 6.403 ± 2.736 b | 1.021 ± 0.238 | 5.405 ± 2.327 b | 32.360 ± 15.524 b | 5.808 ± 1.301 | |
| g.4805082 C > T | CC (150) | 8.887 ± 2.574 | 8.375 ± 3.171 | 1.044 ± 0.243 | 6.773 ± 2.425 | 37.941 ± 15.494 | 5.590 ± 1.301 |
| CT (70) | 8.714 ± 2.583 | 8.489 ± 2.861 | 1.095 ± 0.221 | 6.986 ± 2.470 | 38.016 ± 11.852 | 5.622 ± 1.117 | |
| TT (8) | 8.250 ± 1.909 | 7.806 ± 0.895 | 1.175 ± 0.165 | 6.875 ± 1.808 | 32.363 ± 4.800 | 4.838 ± 0.605 | |
| g.4806821 A > G | AA (103) | 8.659 ± 2.302 | 7.585 ± 2.563 b | 1.002 ± 0.248 b | 6.365 ± 2.230 | 35.362 ± 16.119 | 5.475 ± 1.396 |
| AG (87) | 8.807 ± 2.417 | 8.612 ± 3.085 ab | 1.082 ± 0.220 ab | 6.952 ± 2.316 | 38.831 ± 12.402 | 5.689 ± 1.228 | |
| GG (38) | 9.1033 ± 3.047 | 9.223 ± 3.289 a | 1.125 ± 0.221 a | 7.367 ± 2.663 | 39.708 ± 13.415 | 5.567 ± 0.976 |
| Diplotypes | Piglets Born Alive | Litter Birth Weight/kg | Average Birth Weight/kg | Number of Weaned Piglets | Weaning Litter Weight/kg | Weaning Weight/kg |
|---|---|---|---|---|---|---|
| H1H1 (46) | 7.643 ± 2.792 c | 6.271 ± 2.050 d | 0.984 ± 0.235 b | 5.750 ± 2.102 c | 33.221 ± 13.936 c | 5.638 ± 0.683 ab |
| H1H2 (38) | 9.053 ± 1.930 b | 7.780 ± 2.673 cd | 0.982 ± 0.258 b | 6.605 ± 2.531 bc | 38.170 ± 17.678 bc | 5.776 ± 1.414 a |
| H1H3 (26) | 7.231 ± 2.386 c | 7.254 ± 2.491 cd | 1.233 ± 0.182 a | 5.461 ± 1.330 c | 34.508 ± 9.192 c | 6.482 ± 1.812 a |
| H1H4 (25) | 8.600 ± 2.769 b | 8.153 ± 2.424 c | 1.003 ± 0.157 ab | 7.120 ± 2.297 b | 36.588 ± 9.163 bc | 5.315 ± 0.913 b |
| H2H2 (19) | 9.368 ± 1.707 b | 9.128 ± 2.092 c | 1.071 ± 0.246 ab | 6.796 ± 1.584 bc | 32.900 ± 15.766 c | 4.632 ± 1.818 c |
| H2H3 (20) | 9.200 ± 1.056 b | 8.707 ± 2.566 c | 1.033 ± 0.270 ab | 7.450 ± 1.145 b | 40.882 ± 7.540 b | 5.497 ± 0.626 abc |
| H2H4 (16) | 10.750 ± 2.145 b | 11.325 ± 2.702 b | 1.114 ± 0.210 ab | 8.750 ± 1.949 ab | 48.431 ± 12.846 b | 5.507 ± 0.553 abc |
| H3H3 (8) | 8.250 ± 1.909 b | 7.806 ± 0.895 cd | 1.175 ± 0.165 ab | 6.875 ± 1.808 bc | 31.362 ± 4.800 c | 4.838 ± 0.605 c |
| H3H4 (24) | 8.583 ± 2.430 b | 8.265 ± 3.447 c | 1.069 ± 0.173 ab | 6.875 ± 2.863 bc | 35.231 ± 14.263 c | 5.342 ± 0.969 abc |
| H4H4 (6) | 12.500 ± 2.739 a | 12.533 ± 5.352 a | 0.997 ± 0.249 b | 9.667 ± 0.817 a | 57.933 ± 8.364 a | 5.983 ± 0.591 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Wang, C.; Wu, Y.; Xiang, J.; Zhang, Y. Association Analysis of METTL23 Gene Polymorphisms with Reproductive Traits in Kele Pigs. Genes 2024, 15, 1061. https://doi.org/10.3390/genes15081061
Sun J, Wang C, Wu Y, Xiang J, Zhang Y. Association Analysis of METTL23 Gene Polymorphisms with Reproductive Traits in Kele Pigs. Genes. 2024; 15(8):1061. https://doi.org/10.3390/genes15081061
Chicago/Turabian StyleSun, Jie, Chunyuan Wang, Yan Wu, Jin Xiang, and Yiyu Zhang. 2024. "Association Analysis of METTL23 Gene Polymorphisms with Reproductive Traits in Kele Pigs" Genes 15, no. 8: 1061. https://doi.org/10.3390/genes15081061
APA StyleSun, J., Wang, C., Wu, Y., Xiang, J., & Zhang, Y. (2024). Association Analysis of METTL23 Gene Polymorphisms with Reproductive Traits in Kele Pigs. Genes, 15(8), 1061. https://doi.org/10.3390/genes15081061





