Whole Genome Analysis in Consanguineous Families Reveals New Loci for Speech Sound Disorder (SSD)
Abstract
:1. Introduction
2. Materials and Methods
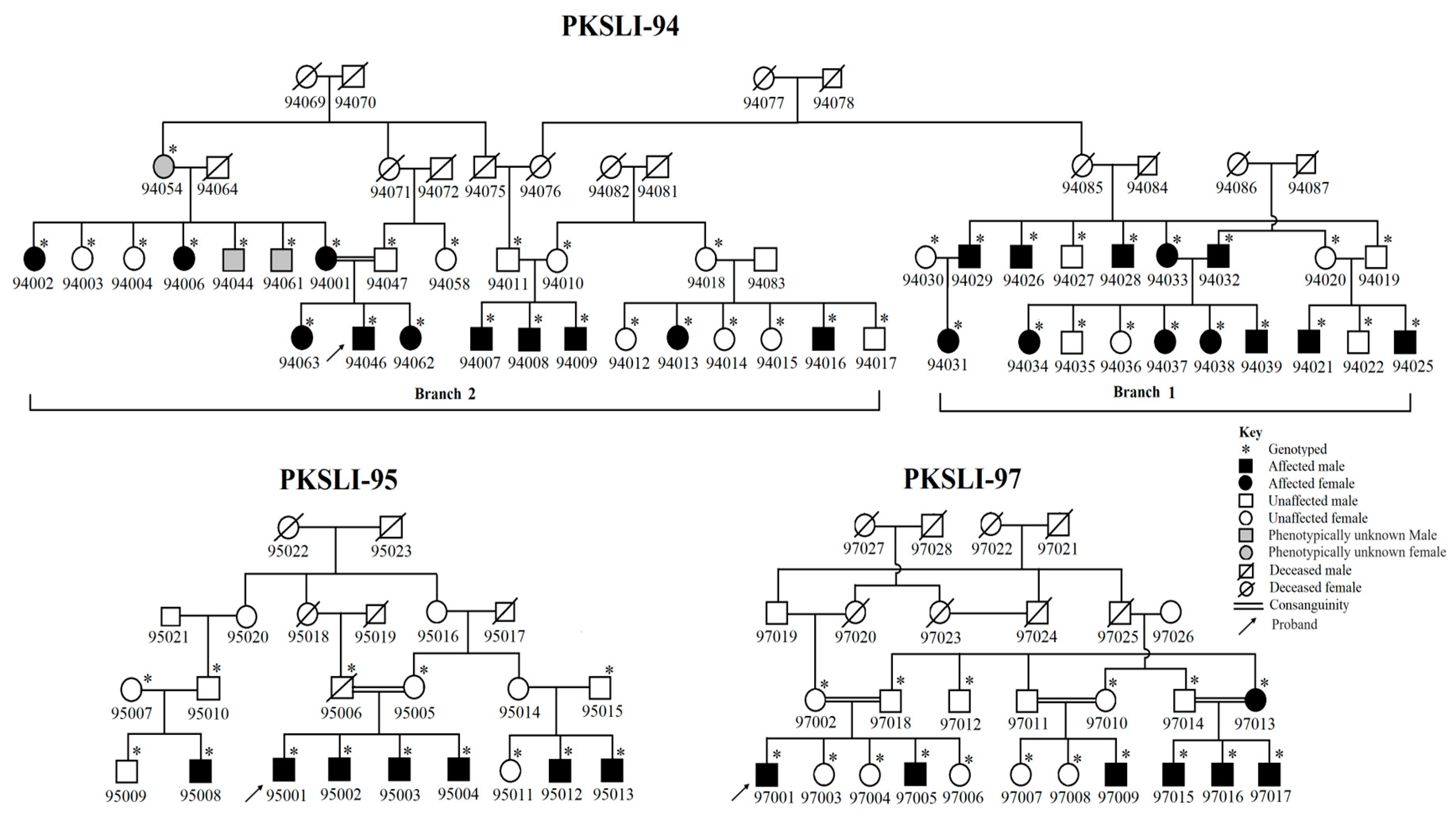
2.1. Identification and Enrollment of Families
2.2. Collection and Evaluation of Speech Data
2.3. DNA Extraction and Whole-Genome SNP Genotyping
2.4. Linkage Analysis
2.5. Homozygosity Mapping
2.6. Haplotyping
3. Results
3.1. Speech Articulation Analysis
3.2. Linkage Analysis
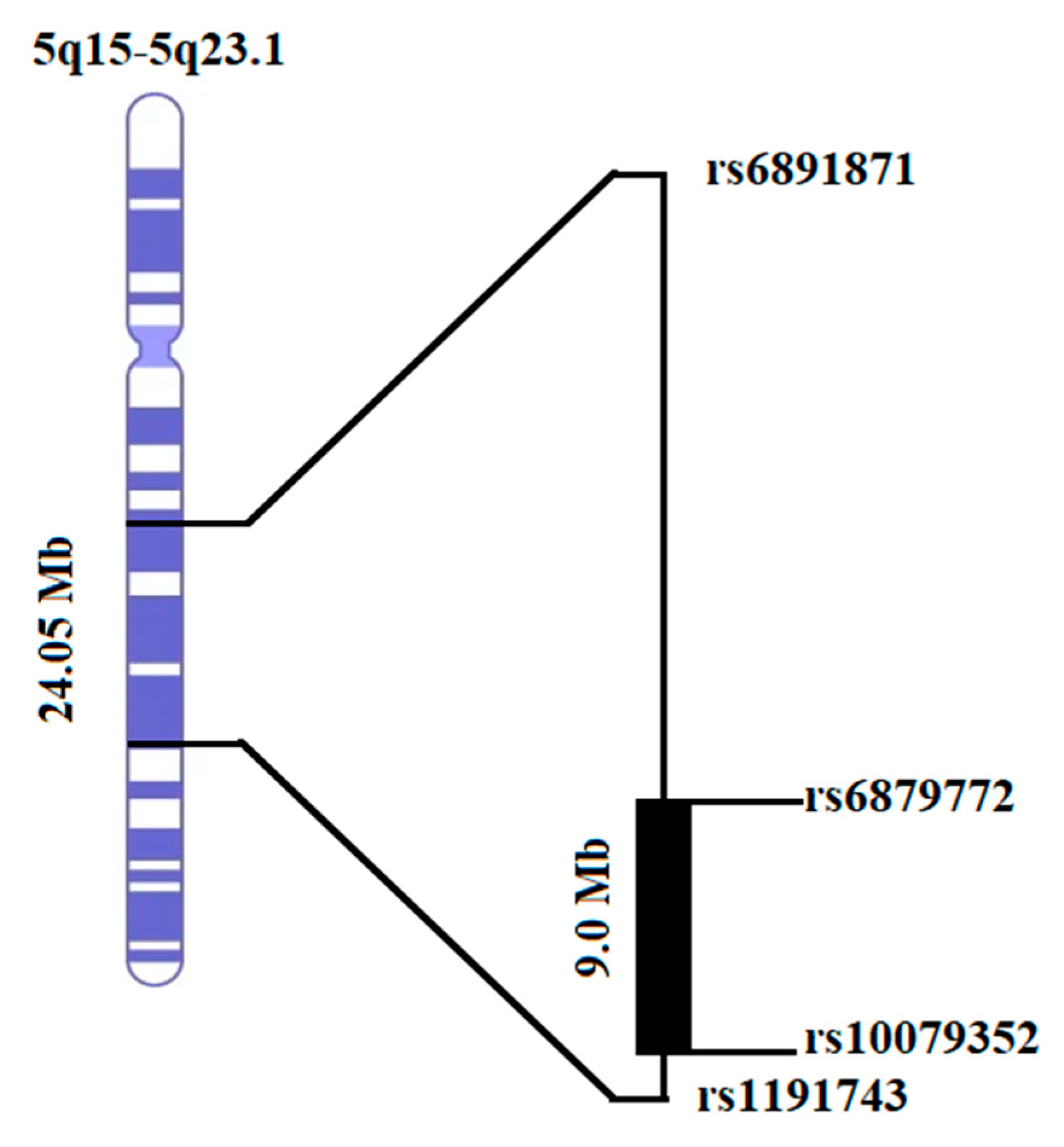
3.3. Homozygosity Mapping and Haplotype Analysis
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stein, C.M.; Millard, C.; Kluge, A.; Miscimarra, L.E.; Cartier, K.C.; Freebairn, L.A.; Hansen, A.J.; Shriberg, L.D.; Taylor, H.G.; Lewis, B.A. Speech sound disorder influenced by a locus in 15q14 region. Behav. Genet. 2006, 36, 858–868. [Google Scholar] [CrossRef] [PubMed]
- American Speech-Language-Hearing Association. Speech Sound Disorders-Articulation and Phonology. 2021. Available online: https://www.asha.org/practice-portal/clinical-topics/articulation-and-phonology/#collapse_1 (accessed on 15 October 2022).
- Feldman, H.M. Evaluation and management of language and speech disorders in preschool children. Pediatr. Rev. 2005, 26, 40. [Google Scholar] [CrossRef] [PubMed]
- Munson, B.; Edwards, J.; Beckman, M.E. Phonological knowledge in typical and atypical speech–sound development. Top. Lang. Disord. 2005, 25, 190–206. [Google Scholar] [CrossRef]
- McLeod, S.; Verdon, S. International Expert Panel on Multilingual Children’s Speech. Tutorial: Speech assessment for multilingual children who do not speak the same language(s) as the speech-language pathologist. Am. J. Speech-Lang. Pathol. 2017, 26, 691–708. [Google Scholar] [CrossRef]
- McCormack, J.; McLeod, S.; McAllister, L.; Harrison, L.J. A systematic review of the association between childhood speech impairment and participation across the lifespan. Int. J. Speech-Lang. Pathol. 2009, 11, 155–170. [Google Scholar] [CrossRef]
- Lewis, B.A.; Freebairn, L.; Tag, J.; Ciesla, A.A.; Iyengar, S.K.; Stein, C.M.; Taylor, H.G. Adolescent outcomes of children with early speech sound disorders with and without language impairment. Am. J. Speech-Lang. Pathol. 2015, 24, 150–163. [Google Scholar] [CrossRef]
- Fisher, S.E.; Lai, C.S.; Monaco, A.P. Deciphering the genetic basis of speech and language disorders. Annu. Rev. Neurosci. 2003, 26, 57–80. [Google Scholar] [PubMed]
- Kang, C.; Drayna, D. Genetics of speech and language disorders. Annu. Rev. Genom. Hum. Genet. 2011, 12, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Okanoya, K.; Koike, T.; Sasaki, E.; Okano, H.; Watanabe, S.; Iriki, A. Human speech-and reading-related genes display partially overlapping expression patterns in the marmoset brain. Brain Lang. 2014, 133, 26–38. [Google Scholar] [CrossRef]
- Aslam, I.; Mumtaz, N.; Saqulain, G. Prevalence of speech sound disorders among primary school children. J. Islamabad Med. Dent. Coll. 2020, 9, 195–200. [Google Scholar]
- Campbell, T.F.; Dollaghan, C.A.; Rockette, H.E.; Paradise, J.L.; Feldman, H.M.; Shriberg, L.D.; Sabo, D.L.; Kurs-Lasky, M. Risk factors for speech delay of unknown origin in 3-year-old children. Child Dev. 2003, 74, 346–357. [Google Scholar] [CrossRef]
- Shriberg, L.D.; Tomblin, J.B.; McSweeny, J.L. Prevalence of speech delay in 6-year-old children and comorbidity with language impairment. J. Speech Lang. Hear. Res. 1999, 42, 1461–1481. [Google Scholar] [CrossRef]
- McKinnon, D.H.; McLeod, S.; Reilly, S. The prevalence of stuttering, voice, and speech-sound disorders in primary school students in Australia. Lang. Speech Hear. Serv. Sch. 2007, 38, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Sedehi, M.; Rahimi-Madiseh, M.; Mohamadi, O. The prevalence of stuttering, voice disorder and speech sound disorders in preschoolers in Shahrkord, Iran. Int. J. Child Youth Fam. Stud. 2016, 7, 456–471. [Google Scholar]
- Al-Sabi, Y. The JISH Speech, Language, and Hearing School Readiness Screening in Jeddah, Saudi Arabia. J. Otolaryngol. ENT Res. 2017, 7, 00221. [Google Scholar] [CrossRef]
- Flipsen, P., Jr. Emergence and prevalence of persistent and residual speech errors. In Seminars in Speech and Language; Thieme Medical Publishers: New York, NY, USA, 2015; pp. 217–223. [Google Scholar]
- Pennington, B.F.; Bishop, D.V. Relations among speech, language, and reading disorders. Annu. Rev. Psychol. 2009, 60, 283–306. [Google Scholar] [CrossRef] [PubMed]
- Shriberg, L.; Austin, D. Comorbidity of speech-language disorder: Implications for a phenotype marker for speech delay. Speech-Lang. Connect. 1998, 73–117. [Google Scholar]
- Eadie, P.; Morgan, A.; Ukoumunne, O.C.; Ttofari Eecen, K.; Wake, M.; Reilly, S. Speech sound disorder at 4 years: Prevalence, comorbidities, and predictors in a community cohort of children. Dev. Med. Child Neurol. 2015, 57, 578–584. [Google Scholar] [CrossRef]
- Hafeez, H.; Yasmin, T.; Raza, M.H.; Mubarak, L.; Ashraf, K.; Samra, M.M.; Basra, M.A.R. Receptive vocabulary, memory span, and speech articulation in Pakistani children with developmental language disorders. Child Neuropsychol. 2022, 1–22. [Google Scholar] [CrossRef]
- Lewis, B.A.; Avrich, M.A.A.; Freebairn, M.L.A.; Taylor, H.G.; Iyengar, S.K.; Stein, C.M. Subtyping children with speech sound disorders by endophenotypes. Top. Lang. Disord. 2011, 31, 112. [Google Scholar] [CrossRef]
- Goldman, R.; Fristoe, M. Goldman Fristoe Test of Articulation: GFTA; American Guidance Service: New York, NY, USA, 1969. [Google Scholar]
- Noveen, S.; Butt, A.K.; Alam, M.B. Development of a test for articulation and phonological disorders in Urdu speaking children. J. Riphah Coll. Rehabil. Sci. 2017, 5, 89–93. [Google Scholar]
- Bishop, D.V. Motor immaturity and specific speech and language impairment: Evidence for a common genetic basis. Am. J. Med. Genet. 2002, 114, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Bishop, D.V.; Adams, C.V.; Norbury, C.F. Distinct genetic influences on grammar and phonological short-term memory deficits: Evidence from 6-year-old twins. Genes Brain Behav. 2006, 5, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.A.; Short, E.J.; Iyengar, S.K.; Taylor, H.G.; Freebairn, M.L.; Tag, M.J.; Avrich, M.A.A.; Stein, C.M. Speech-sound disorders and attention-deficit/hyperactivity disorder symptoms. Top. Lang. Disord. 2012, 32, 247. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.A.; Thompson, L.A. A study of developmental speech and language disorders in twins. J. Speech Lang. Hear. Res. 1992, 35, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Felsenfeld, S.; McGue, M.; Broen, P.A. Familial aggregation of phonological disorders: Results from a 28-year follow-up. J. Speech Lang. Hear. Res. 1995, 38, 1091–1107. [Google Scholar] [CrossRef]
- Lewis, B.A. Pedigree analysis of children with phonology disorders. J. Learn. Disabil. 1992, 25, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Gamsiz, E.D.; Viscidi, E.W.; Frederick, A.M.; Nagpal, S.; Sanders, S.J.; Murtha, M.T.; Schmidt, M.; Triche, E.W.; Geschwind, D.H.; State, M.W. Intellectual disability is associated with increased runs of homozygosity in simplex autism. Am. J. Hum. Genet. 2013, 93, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Andres, E.M.; Hafeez, H.; Yousaf, A.; Riazuddin, S.; Rice, M.L.; Basra, M.A.R.; Raza, M.H. A genome-wide analysis in consanguineous families reveals new chromosomal loci in specific language impairment (SLI). Eur. J. Hum. Genet. 2019, 27, 1274–1285. [Google Scholar] [CrossRef]
- Lin, P.-I.; Kuo, P.-H.; Chen, C.-H.; Wu, J.-Y.; Gau, S.S.; Wu, Y.-Y.; Liu, S.-K. Runs of homozygosity associated with speech delay in autism in a taiwanese han population: Evidence for the recessive model. PLoS ONE 2013, 8, e72056. [Google Scholar] [CrossRef]
- Gandin, I.; Faletra, F.; Faletra, F.; Carella, M.; Pecile, V.; Ferrero, G.B.; Biamino, E.; Palumbo, P.; Palumbo, O.; Bosco, P. Excess of runs of homozygosity is associated with severe cognitive impairment in intellectual disability. Genet. Med. 2015, 17, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, P.; Newbury, D.F.; Jara, L.; De Barbieri, Z.; Mirza, G.; Palomino, H.M.; Fernández, M.A.; Cazier, J.-B.; Monaco, A.P.; Palomino, H. Genome-wide analysis of genetic susceptibility to language impairment in an isolated Chilean population. Eur. J. Hum. Genet. 2011, 19, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, P.; Nudel, R.; Hoischen, A.; Fernández, M.A.; Simpson, N.H.; Gilissen, C.; Reader, R.H.; Jara, L.; Magdalena Echeverry, M.; Francks, C. Correction: Exome Sequencing in an Admixed Isolated Population IndicatesNFXL1 Variants Confer a Risk for Specific Language Impairment. PLoS Genet. 2015, 11, e1005336. [Google Scholar] [CrossRef] [PubMed]
- Andres, E.M.; Earnest, K.K.; Smith, S.D.; Rice, M.L.; Raza, M.H. Pedigree-based gene mapping supports previous loci and reveals novel suggestive loci in specific language impairment. J. Speech Lang. Hear. Res. 2020, 63, 4046–4061. [Google Scholar] [CrossRef]
- Yasmin, T.; Hafeez, H.; Nadee, A.; Lubna, M.; Tarar, S.A.; Raza, M.H.; Basra, M.A.R. Working memory span and receptive vocabulary assessment in Urdu speaking children with speech sound disorder. Acta Psychol. 2022, 231, 103777. [Google Scholar] [CrossRef]
- Gildersleeve-Neumann, C.E.; Kester, E.S.; Davis, B.L.; Peña, E.D. English speech sound development in preschool-aged children from bilingual English–Spanish environments. Lang. Speech Hear. Serv. Sch. 2008, 39, 314–328. [Google Scholar] [CrossRef]
- Holm, A.; Dodd, B.; Stow, C.; Pert, S. Identification and differential diagnosis of phonological disorder in bilingual children. Lang. Test. 1999, 16, 271–292. [Google Scholar] [CrossRef]
- Waring, R.; Eadie, P.; Rickard Liow, S.; Dodd, B. Do children with phonological delay have phonological short-term and phonological working memory deficits? Child Lang. Teach. Ther. 2017, 33, 33–46. [Google Scholar] [CrossRef]
- DNA Collection Kits for Research. Available online: https://www.dnagenotek.com/us/products/collection-human/oragene-discover/500-series/OGR-500.html (accessed on 20 April 2020).
- Genotyping. Available online: https://grcf.jhmi.edu/genotyping/ (accessed on 1 February 2022).
- Ponomarenko, P.; Ryutov, A.; Maglinte, D.T.; Baranova, A.; Tatarinova, T.V.; Gai, X. Clinical utility of the low-density Infinium QC genotyping Array in a genomics-based diagnostics laboratory. BMC Med. Genom. 2017, 10, 57. [Google Scholar] [CrossRef]
- Pinese, M.; Lacaze, P.; Rath, E.M.; Stone, A.; Brion, M.-J.; Ameur, A.; Nagpal, S.; Puttick, C.; Husson, S.; Degrave, D. The Medical Genome Reference Bank contains whole genome and phenotype data of 2570 healthy elderly. Nat. Commun. 2020, 11, 435. [Google Scholar] [CrossRef]
- Zhao, S.; Jing, W.; Samuels, D.C.; Sheng, Q.; Shyr, Y.; Guo, Y. Strategies for processing and quality control of Illumina genotyping arrays. Brief. Bioinform. 2018, 19, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.; Elston, R.; Sellers, T.; Bailey-Wilson, J.; Gersting, J.; Deen, D.; Sorant, A.; Tran, L.; Amos, C.; Siervogel, R. Stepwise oligogenic segregation and linkage analysis illustrated with dopamine-β-hydroxylase activity. Am. J. Med. Genet. 1990, 35, 425–432. [Google Scholar] [CrossRef]
- Morton, N.E. Sequential tests for the detection of linkage. Am. J. Hum. Genet. 1955, 7, 277. [Google Scholar]
- Gibson, J.; Morton, N.E.; Collins, A. Extended tracts of homozygosity in outbred human populations. Hum. Mol. Genet. 2006, 15, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Seelow, D.; Schuelke, M.; Hildebrandt, F.; Nürnberg, P. Homozygosity Mapper—An interactive approach to homozygosity mapping. Nucleic Acids Res. 2009, 37, W593–W599. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, T.; Andres, E.M.; Ashraf, K.; Basra, M.A.R.; Raza, M.H. Genome-wide analysis of runs of homozygosity in Pakistani controls with no history of speech or language-related developmental phenotypes. Ann. Hum. Biol. 2023, 50, 101–108. [Google Scholar] [CrossRef] [PubMed]
- McGrath, L.M.; Hutaff-Lee, C.; Scott, A.; Boada, R.; Shriberg, L.D.; Pennington, B.F. Children with comorbid speech sound disorder and specific language impairment are at increased risk for attention-deficit/hyperactivity disorder. J. Abnorm. Child Psychol. 2008, 36, 151–163. [Google Scholar] [CrossRef]
- Stein, C.M.; Schick, J.H.; Taylor, H.G.; Shriberg, L.D.; Millard, C.; Kundtz-Kluge, A.; Russo, K.; Minich, N.; Hansen, A.; Freebairn, L.A. Pleiotropic effects of a chromosome 3 locus on speech-sound disorder and reading. Am. J. Hum. Genet. 2004, 74, 283–297. [Google Scholar] [CrossRef]
- Nopola-Hemmi, J.; Myllyluoma, B.; Haltia, T.; Taipale, M.; Ollikainen, V.; Ahonen, T.; Voutilainen, A.; Kere, J.; Widén, E. A dominant gene for developmental dyslexia on chromosome 3. J. Med. Genet. 2001, 38, 658–664. [Google Scholar] [CrossRef]
- Smith, S.D.; Pennington, B.F.; Boada, R.; Shriberg, L.D. Linkage of speech sound disorder to reading disability loci. J. Child Psychol. Psychiatry 2005, 46, 1057–1066. [Google Scholar] [CrossRef]
- Pennington, B.F. From single to multiple deficit models of developmental disorders. Cognition 2006, 101, 385–413. [Google Scholar] [CrossRef] [PubMed]
- Shriberg, L.D.; Paul, R.; Flipsen, P. Chapter 3: From postbehaviorism to the postgenomic era. In Childhood Speech Sound Disorders, 2nd ed.; Plural Publishing: Oxfordshire, UK, 2009; pp. 1–33. [Google Scholar]
- Kang, C.; Riazuddin, S.; Mundorff, J.; Krasnewich, D.; Friedman, P.; Mullikin, J.C.; Drayna, D. Mutations in the lysosomal enzyme–targeting pathway and persistent stuttering. N. Engl. J. Med. 2010, 362, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.H.; Mattera, R.; Morell, R.; Sainz, E.; Rahn, R.; Gutierrez, J.; Paris, E.; Root, J.; Solomon, B.; Brewer, C. Association between rare variants in AP4E1, a component of intracellular trafficking, and persistent stuttering. Am. J. Hum. Genet. 2015, 97, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Schuettpelz, J.; Janer, A.; Antonicka, H.; Shoubridge, E.A. The role of the mitochondrial outer membrane protein SLC25A46 in mitochondrial fission and fusion. Life Sci. Alliance 2023, 6, e202301914. [Google Scholar] [CrossRef] [PubMed]
- Janer, A.; Prudent, J.; Paupe, V.; Fahiminiya, S.; Majewski, J.; Sgarioto, N.; Des Rosiers, C.; Forest, A.; Lin, Z.Y.; Gingras, A.C. SLC 25A46 is required for mitochondrial lipid homeostasis and cristae maintenance and is responsible for Leigh syndrome. EMBO Mol. Med. 2016, 8, 1019–1038. [Google Scholar] [CrossRef] [PubMed]
- Hammer, M.B.; Ding, J.; Mochel, F.; Eleuch-Fayache, G.; Charles, P.; Coutelier, M.; Gibbs, J.R.; Arepalli, S.K.; Chong, S.B.; Hernandez, D.G. SLC25A46 mutations associated with autosomal recessive cerebellar ataxia in North African families. Neurodegener. Dis. 2017, 17, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Mochel, F.; Rastetter, A.; Ceulemans, B.; Platzer, K.; Yang, S.; Shinde, D.N.; Helbig, K.L.; Lopergolo, D.; Mari, F.; Renieri, A. Variants in the SK2 channel gene (KCNN2) lead to dominant neurodevelopmental movement disorders. Brain 2020, 143, 3564–3573. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Sun, L.D.; Atkins, C.M.; Soderling, T.R.; Wilson, M.A.; Tonegawa, S. An important role of neural activity-dependent CaMKIV signaling in the consolidation of long-term memory. Cell 2001, 106, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.L.; Smith, S.D.; Gayán, J. Convergent genetic linkage and associations to language, speech and reading measures in families of probands with specific language impairment. J. Neurodev. Disord. 2009, 1, 264–282. [Google Scholar] [CrossRef] [PubMed]
- Gabel, L.A.; Gibson, C.J.; Gruen, J.R.; LoTurco, J.J. Progress towards a cellular neurobiology of reading disability. Neurobiol. Dis. 2010, 38, 173–180. [Google Scholar] [CrossRef]
- Londin, E.R.; Meng, H.; Gruen, J.R. A transcription map of the 6p22. 3 reading disability locus identifying candidate genes. BMC Genom. 2003, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Moores, C.A.; Perderiset, M.; Francis, F.; Chelly, J.; Houdusse, A.; Milligan, R.A. Mechanism of microtubule stabilization by doublecortin. Mol. Cell 2004, 14, 833–839. [Google Scholar] [CrossRef] [PubMed]

| Individual IDs | Addition Errors | Omission Errors | Substitution Errors | Total Errors | Correct Consonants | PCC |
|---|---|---|---|---|---|---|
| 94001 | 0 | 0 | 10 | 10 | 74 | 88 |
| 94002 | 3 | 11 | 44 | 58 | 26 | 31 |
| 94003 | 1 | 0 | 2 | 3 | 81 | 96 |
| 94004 | 2 | 0 | 7 | 9 | 75 | 89 |
| 94006 | 0 | 0 | 14 | 14 | 70 | 83 |
| 94007 | 1 | 0 | 19 | 20 | 64 | 76 |
| 94008 | 1 | 2 | 33 | 36 | 48 | 57 |
| 94009 | 18 | 4 | 56 | 78 | 6 | 7 |
| 94010 | 0 | 0 | 0 | 0 | 84 | 100 |
| 94011 | 0 | 1 | 3 | 4 | 80 | 95 |
| 94012 | 0 | 0 | 4 | 4 | 80 | 95 |
| 94013 | 1 | 2 | 19 | 22 | 62 | 74 |
| 94014 | 0 | 2 | 2 | 4 | 80 | 95 |
| 94015 | 1 | 1 | 5 | 7 | 77 | 92 |
| 94016 | 2 | 0 | 22 | 24 | 60 | 71 |
| 94017 | 0 | 0 | 2 | 2 | 82 | 98 |
| 94018 | 0 | 0 | 1 | 1 | 83 | 99 |
| 94019 | 0 | 1 | 5 | 6 | 78 | 93 |
| 94020 | 0 | 1 | 6 | 7 | 77 | 92 |
| 94021 | 0 | 2 | 13 | 15 | 69 | 82 |
| 94022 | 1 | 2 | 3 | 6 | 78 | 93 |
| 94025 | 1 | 2 | 23 | 26 | 58 | 69 |
| 94026 | 2 | 3 | 25 | 30 | 54 | 64 |
| 94027 | 0 | 0 | 0 | 0 | 84 | 100 |
| 94028 | 0 | 2 | 9 | 11 | 73 | 87 |
| 94029 | 0 | 2 | 16 | 18 | 66 | 79 |
| 94030 | 0 | 0 | 1 | 1 | 83 | 99 |
| 94031 | 3 | 9 | 18 | 30 | 54 | 64 |
| 94032 | 0 | 2 | 8 | 10 | 74 | 88 |
| 94033 | 0 | 0 | 28 | 28 | 56 | 67 |
| 94034 | 1 | 3 | 15 | 19 | 65 | 77 |
| 94035 | 0 | 3 | 2 | 5 | 79 | 94 |
| 94036 | 0 | 0 | 4 | 4 | 80 | 95 |
| 94037 | 1 | 4 | 4 | 9 | 75 | 89 |
| 94038 | 1 | 12 | 41 | 54 | 30 | 36 |
| 94039 | 1 | 10 | 37 | 48 | 36 | 43 |
| 94046 | 3 | 3 | 32 | 38 | 46 | 55 |
| 94047 | 0 | 1 | 2 | 3 | 81 | 96 |
| 94058 | 1 | 1 | 7 | 9 | 75 | 89 |
| 94062 | 1 | 1 | 10 | 12 | 72 | 86 |
| 94063 | 5 | 0 | 25 | 30 | 54 | 64 |
| Individual IDs | Addition Errors | Omission Errors | Substitution Errors | Total Errors | Correct Consonants | PCC |
|---|---|---|---|---|---|---|
| 95001 | 3 | 3 | 25 | 31 | 53 | 63 |
| 95002 | 0 | 1 | 12 | 13 | 71 | 85 |
| 95003 | 0 | 1 | 9 | 10 | 74 | 88 |
| 95004 | 4 | 4 | 40 | 48 | 36 | 43 |
| 95005 | 0 | 1 | 7 | 8 | 76 | 90 |
| 95006 | 0 | 0 | 1 | 1 | 83 | 99 |
| 95007 | 0 | 0 | 1 | 1 | 83 | 99 |
| 95008 | 0 | 1 | 16 | 17 | 67 | 80 |
| 95009 | 0 | 0 | 6 | 6 | 78 | 93 |
| 95010 | 0 | 0 | 3 | 3 | 81 | 96 |
| 95011 | 0 | 1 | 5 | 6 | 78 | 93 |
| 95012 | 1 | 1 | 8 | 10 | 74 | 88 |
| 95013 | 4 | 5 | 18 | 27 | 57 | 68 |
| 95014 | 1 | 0 | 5 | 6 | 78 | 93 |
| 95015 | 0 | 0 | 0 | 0 | 84 | 100 |
| Individual IDs | Addition Errors | Omission Errors | Substitution Errors | Total Errors | Correct Consonants | PCC |
|---|---|---|---|---|---|---|
| 97001 | 0 | 2 | 21 | 23 | 61 | 73 |
| 97002 | 0 | 0 | 1 | 1 | 83 | 99 |
| 97003 | 0 | 1 | 7 | 8 | 76 | 90 |
| 97004 | 0 | 0 | 1 | 1 | 83 | 99 |
| 97005 | 0 | 0 | 14 | 14 | 70 | 83 |
| 97006 | 0 | 2 | 3 | 5 | 79 | 94 |
| 97007 | 3 | 0 | 6 | 9 | 75 | 89 |
| 97008 | 1 | 0 | 6 | 7 | 77 | 92 |
| 97009 | 0 | 0 | 12 | 12 | 72 | 86 |
| 97010 | 0 | 0 | 0 | 0 | 84 | 100 |
| 97011 | 0 | 0 | 0 | 0 | 84 | 100 |
| 97012 | 0 | 0 | 8 | 8 | 76 | 90 |
| 97013 | 0 | 1 | 9 | 10 | 74 | 88 |
| 97014 | 0 | 0 | 1 | 1 | 83 | 99 |
| 97015 | 0 | 1 | 14 | 15 | 69 | 82 |
| 97016 | 5 | 5 | 20 | 30 | 54 | 64 |
| 97017 | 0 | 1 | 11 | 12 | 72 | 86 |
| 97018 | 0 | 0 | 4 | 4 | 80 | 95 |
| Chromosome | SNP ID | hg 19 Position (Mb) | Single-Point LOD Score |
|---|---|---|---|
| 5 | rs1045706 | 108.714 | −2.157 |
| 5 | rs6879772 | 109.613 | 0.471 |
| 5 | rs381661 | 110.085 | 0.471 |
| 5 | rs456236 | 110.087 | 3.131 |
| 5 | rs6893213 | 110.198 | 0.471 |
| 5 | rs1837253 | 110.401 | 2.689 |
| 5 | rs10455025 | 110.404 | 3.131 |
| 5 | rs2162893 | 110.558 | 0.471 |
| 5 | rs115192 | 110.919 | 2.689 |
| 5 | rs27342 | 111.465 | 2.689 |
| 5 | rs1850407 | 112.721 | 3.131 |
| 5 | rs26959 | 112.736 | 3.131 |
| 5 | rs1496390 | 113.725 | 2.689 |
| 5 | rs28019 | 115.733 | 3.131 |
| 5 | rs1021516 | 116.544 | 0.471 |
| 5 | rs6891356 | 116.549 | 0.471 |
| 5 | rs13187808 | 117.223 | 3.131 |
| 5 | rs10079352 | 117.494 | 3.131 |
| 5 | rs13157174 | 117.912 | −1.912 |
| Linkage Loci (hg 19) | Single-Point LOD Score | Mode of Inheritance | Affection Status | Family/ Branch | Homozygosity |
|---|---|---|---|---|---|
| 16q12.1-q12.2 | 2.13 | Recessive | Phenotype | PKSLI-94 (Branch 1) | No |
| 6p22.1-p22.1 | 2.13 | Recessive | Phenotype | PKSLI-95 | No |
| 14q12-q21.1 | 2.80 | Recessive | Phenotype | PKSLI-95 | No |
| 5q21.3-5q23.1 | 3.13 | Recessive | Phenotype | PKSLI-97 | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasmin, T.; Sadia, A.; Nadeem, L.; Basra, M.A.R.; Rice, M.L.; Raza, M.H. Whole Genome Analysis in Consanguineous Families Reveals New Loci for Speech Sound Disorder (SSD). Genes 2024, 15, 1069. https://doi.org/10.3390/genes15081069
Yasmin T, Sadia A, Nadeem L, Basra MAR, Rice ML, Raza MH. Whole Genome Analysis in Consanguineous Families Reveals New Loci for Speech Sound Disorder (SSD). Genes. 2024; 15(8):1069. https://doi.org/10.3390/genes15081069
Chicago/Turabian StyleYasmin, Tahira, Aatika Sadia, Laraib Nadeem, Muhammad Asim Raza Basra, Mabel L. Rice, and Muhammad Hashim Raza. 2024. "Whole Genome Analysis in Consanguineous Families Reveals New Loci for Speech Sound Disorder (SSD)" Genes 15, no. 8: 1069. https://doi.org/10.3390/genes15081069





