Abstract
Takenouchi–Kosaki syndrome (TKS) is a rare congenital disease caused by a de novo mutation in the Cell Division Cycle 42 (CDC42) gene. Patients with TKS present facial and body dysmorphisms, hematologic and immune dysregulation, intellectual disability, neurodevelopmental delay and hearing loss. The aim of this study is to review the literature, focusing on hearing and language abilities in children with TKS. A systematic search on PubMed, Scopus and Web of Science databases was performed, including twelve studies for a total of 13 patients. Hearing loss (HL) occurs in a great percentage of patients (84.6%); nonetheless, auditory threshold, severity of HL and language abilities were reported in a few cases. In two studies, auditory rehabilitation strategies were described. Although several studies have investigated the hematological features of TKS, still only a few authors have focused on the audiological and language abilities of these children. Given the fact that HL has a significant impact on behaviors, communications skills, and quality of life, it is important to adequately assess and rehabilitate patients early with this syndrome. Further studies are needed to improve the knowledge about this topic and improve the quality of life of patients with TKS.
1. Introduction
The Takenouchi–Kosaki syndrome (TKS) is rare a congenital disease caused by a de novo heterozygous mutation in the CDC42 (Cell Division Cycle 42) gene. In the majority of cases, patients present a p.Tyr64Cys mutation [1]; this gene variant is associated with the most severe form of the disease. CDC42 encodes a member of the RAS superfamily of low-molecular-weight GTP/GDP-binding proteins. In particular, it is involved in the intracellular signaling and biological processes of hematologic cells [2] and plays a major role in the control of a broad spectrum of activities, including hematopoiesis, neurodevelopment and hearing function [3].
In 2015, Takenouchi reported the first patient with TKS [4]; up to now, only a few other cases have been described. Patients with TKS present facial and body dysmorphisms and immunohematological dysregulations, including macrothrombocytopenia, lymphopenia, hypoimmunoglobulin, immunodeficiency, anemia and leukopenia. These conditions often require the administration of long-term corticosteroid therapy. Moreover, intellectual disability, neurodevelopmental delay and hearing loss have been reported—with a different degree of disease severity—in these patients [1,2,3,4,5,6,7,8,9,10,11,12,13,14].
Several studies have investigated the association between CDC42 mutations and hematologic disorders, focusing on pharmacological treatment approaches. Conversely, auditory function and language development in children with TKS has not been extensively analyzed so far. Consequently, there is not only a lack of knowledge on this topic but also on the possible rehabilitative strategies, on the communications outcomes and on the quality-of-life level of children with TKS [1,2,3,4,5,6,7,8,9,10,11,12,13,14].
The purpose of this study is to review the literature to identify the most common clinical features of patients with TKS, focusing on auditory and language abilities. The secondary aim is to present the most common therapeutic and rehabilitative strategies and the communication outcomes of these patients.
2. Materials and Methods
A literature search was performed through PubMed, Scopus and Web of Science databases according to the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) 2020 Statement guidelines [15].
The selection of articles was performed by 3 authors. After obtaining records from the databases, one author reviewed abstracts and titles and excluded duplicated records and articles not directly related to TKS. After this first screening procedure, all the full texts of the remaining studies were read by all the authors independently, filling a database with important notes and/or reasons for exclusion. Finally, each author reviewed the two other databases, and all the relevant data were included in a single table (Table 1).

Table 1.
Descriptive data of patients.
The search was performed without any filter, including range, time and using the term “Takenouchi Kosaki syndrome”. Last search was performed on 14 February 2024. Eligibility of the articles was assessed using two main inclusion criteria: (1) studies including patients with genetically confirmed TKS; (2) studies with clinical description of cases. All texts examined were written in English or French—languages spoken by the authors of this review. Articles without a report of clinical phenotype or without genetic analysis confirming TKS were excluded. Patients’ data, extracted from the selected studies, included gender, age, genetic analysis, clinical features, laboratory data, imaging, audiology and language skills, therapy and rehabilitation approaches. Figure 1 summarizes the identification process of the selected articles.
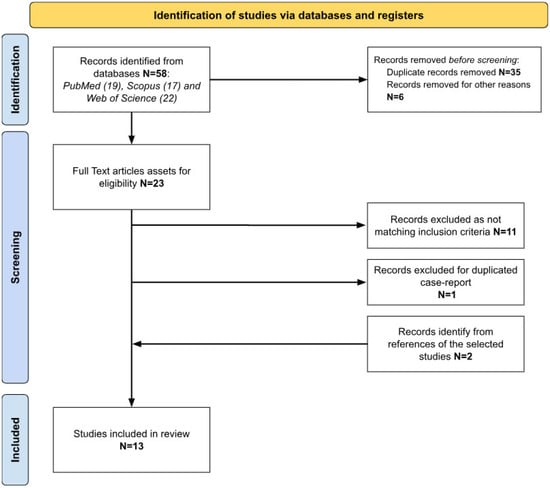
Figure 1.
Identification process of the selected studies according to PRISMA criteria.
Data were analyzed in accordance with the Helsinki Declaration, the Italian privacy and sensitive data laws, and the in-house regulations of our hospital.
3. Results
A total of 58 studies were retrieved, 13 of which were included in the present study (Figure 1). Table 1 summarizes the data of the included reports.
3.1. Population
Patients’ population is composed of 13 subjects; of these, 69.2% were female and 30.8% were male. The mean age was 13.5 years (0.75–26).
Studies were conducted in different countries (Table 1).
3.2. Genetic Analysis
Genetic analysis identified a de novo heterozygous mutation in the CDC42 gene in all patients. The most common variation was a de novo c.191A>G p.Tyr64Cys missense mutation. It was carried by nine patients (69.2% of the cases). In the other percentage of cases, patients presented a different variation of the mutation, such as p.Y64C (7.7%), c.203G>A p. Arg68Gln (7.7%), c.68A>G p.Tyr23Cys (7.7%) and c.242G>A p.Cys81Tyr (7.7%).
3.3. Clinical and Laboratory Features
Physical and facial dysmorphisms were the most common features reported and described in these patients (100%). These conditions were almost always associated with thrombocytopenia (92.3%), a developmental delay (84.6%) and intellectual disabilities (IDs) (46.1%). The disease was comorbid in 76.9% of cases, with immunological alterations such as hypogammaglobulinemia (38.4%), leukocytopenia (30.8%), anemia (23%) and lymphopenia (15.3%).
3.4. Imaging
Neuroimaging was performed with MRI (69.2%) or CT scans (7.7%). Ventriculomegaly was present in 84.6% of patients who underwent MRI, corpus callosum hypoplasia in the 15.4% of cases and cerebellar atrophy in the 38.5% of cases. Two different radiological variants, the Dandy–Walker variant [5] and hypoplastic cerebellar vermis, were reported [6]. Normal findings were reported in another case [7].
3.5. Audiological Evaluation
The audiological evaluation was reported in 11 out of 13 studies. In the study where it was not reported, a hearing test was recommended. In 10 (76.9%) patients, sensorineural hearing loss (SNHL) was diagnosed. The extent of hearing loss severity ranged from mild to profound [2,6,8]. One patient was diagnosed with “deafness” [1], and no further data or information were available. Two cases reported on an audiological assessment through an auditory brainstem response [2,8].
3.6. Therapy and Rehabilitation
Auditory rehabilitation with hearing aids (HAs) was proposed in a subject. In this case, the patient with the aided threshold was able to understand simple requests and developed language skills; however, they were limited due to his severe ID [4]. In the presence of mild hearing loss and ID, specific programs at schools and individualized follow-up cares were proposed [9].
One patient, who was not able to read nor write, used an iPad with a Pro Logic II program for general communication, primarily at school and at home, due to anxiety in other settings [2].
For recurrent ear infections, two studies described the use of myringotomy and tympanostomy tubes [3,12].
None of the identified subjects were treated by other means, such as a cochlear implant.
4. Discussion
Takenouchi–Kosaki syndrome is a rare genetic disease caused by a mutation in the CDC42 gene. The syndrome is characterized by the presence of hematologic disorders and body abnormalities, which are often in comorbidity with neurophysiopathological impairments and hearing loss of a different entity [7,9]. Although several variants were described, the p.Tyr64Cys mutation was detected in the vast majority of cases (69.2%). The derived mutant allele determined a hypomorphic effect of the gene and caused a reduction in GTPase activity. GTPase is a RHO family protein that regulates a wide range of functions, such as cell morphology, cell migration, cell cycle and actin dynamics [16,17]. The modified functioning of GTPase activity explains the multiple symptoms of TKS. In particular, the presence of the p.Tyr64Cys mutation seems to be responsible for the most prototypic and severe forms of the disease [2,5,7,9,10,11,18,19,20].
As proposed by Hamada et al. (2020), the p.Tyr64Cys mutation affects Cdc42-dependent cell polarity organization, and it is critical for axon elongation, dendritic arbor formation and migration during the corticogenesis; these processes could underlie the pathophysiology of ID [16,17,21] and, eventually, sensorineural hearing loss.
Despite there being a significant number and a high prevalence of patients with TKS and audiological impairment (84.6%), only a few studies have focused on the description of the hearing threshold and levels of language development over the recent years, as well as on the different possible auditory rehabilitation strategies [1,2,3,4,5,6,7,8,9,10,11,12,13,14]. Consequently, there are no significant data to discuss and compare on this topic. According to the results of the present review, HL may occur at different degrees of severity, ranging from mild to severe. According to the findings within the literature, HL severity seems to be strongly related to the variant type of the mutated gene. In particular, the presence of the p.Tyr64Cys mutation could be related to a more severe degree of HL.
It is well known that HL has a negative impact on the development of language and speech abilities, on behavioral disorders and on the quality of life of people with it [22]. Consequently, it is very important to assess patients’ hearing threshold early and to adequately rehabilitate their auditory functions. Thus, common rehabilitation strategies include hearing aids, alone or in combination with augmentative alternative communication (AAC) tools [9,11]. Nonetheless, good results may be obtained by also using hearing aids, cochlear implants and tactile vibrators devices. Children with TKS may also benefit from speech and language therapy in order to improve both perceptive and receptive abilities.
Interestingly, five studies were from Japan, two from Poland, two from USA, one from Italy, one from South Africa, one from Canada and one from Belgium.
Due to the complexity of the TKS condition, a multidisciplinary assessment of these patients is recommended. Therefore, an audiological and phoniatric evaluation is mandatory to detect the level of these patients’ auditory and language skills and propose adequate rehabilitation strategies to improve their communication skills and quality of life. The auditory strategies available, at present, may not be fully effective in rehabilitating these subjects; hopefully, further approaches will be eventually identified and proposed in the future.
It is likely that a valid solution for patients to fully recover from TKS will be gene therapy [23]. In this regard, recent studies suggested that genetic-based nanotechnological strategies are effective for treating different diseases, including HL [24]. However, up to now, the limited number of pathological samples as well as the inaccuracy of immortalized cell lines and mouse models used hamper the establishment of ideal models for rare diseases. These obstacles may be overcome by using induced pluripotent stem cell (iPSC)-based models. Disease-specific iPSCs have been used to understand cell functions. To date, studies on TKS patient-derived iPSCs (TKSiPSCs) demonstrated to be the more effective cellular model for investigating macrothrombocytopenia in a stepwise procedure throughout the differentiation process from pluripotent stem cells (PSCs) to platelets [10].
It is possible to speculate that future studies using iPSCs will also eventually be able explain the occurrence of hearing impairment in TKS and may potentially be used to recover hearing loss in these patients [10]. Nonetheless, all these approaches should be reconsidered and eventually tailored according to patients’ global medical conditions and their specific needs. As children with TKS may improve auditory and develop language skills, even if at a lower rate compared to their counterparts without a hearing impairment, adequate follow-ups should always be performed.
Drawbacks: the main limitation of this study is the limited number of studies on TKS that are available, with there being insufficient data on auditory function and language levels regarding the described cases.
5. Conclusions
The present review shows that hearing loss is a common feature of patients with TKS, a rare congenital syndrome presenting body and facial dysmorphism and hematological alterations due to a mutation of the CDC42 gene, which encodes an RHO family GTPase.
Different variants of the mutation [the main one is c.1449T > C/p. (Tyr64Cys)] describe different phenotypes and severity of comorbidities, which can include intellectual disability, development delay, lymphedema, hypothyroidism, recurrent infections and sensorineural hearing loss.
Audiological testing and eventual related rehabilitative interventions should always be performed in the case of a TKS diagnosis. In fact, assessing auditory thresholds and language abilities is crucial to provide these children with the opportunity of developing appropriate communication skills and achieving an adequate quality of life.
There is still insufficient information available on this topic, and further studies are necessary, particularly involving the molecular pathophysiology of this disorder. Hopefully, in the future, genetic therapeutic approaches will be developed for this condition, as per other similar conditions.
Author Contributions
Conceptualization, V.C. and A.C.; methodology, V.C.; software, V.C., S.P. and M.P.; validation, V.C., A.C. and E.G.; formal analysis, V.C., S.P. and M.P.; investigation, V.C., S.P. and M.P.; resources, V.C., E.G. and A.C.; data curation V.C. and A.C.; writing—original draft preparation, V.C., S.P. and M.P.; writing—review and editing, V.C., S.P., M.P. and A.C.; visualization, V.C., S.P., M.P. and A.C.; supervision, V.C., E.G., A.C.; project administration, V.C., E.G., A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yamano, S.; Iguchi, A.; Ishikawa, K.; Sakamoto, A.; Uchiyama, T.; Yanagi, K.; Kaname, T.; Kunishima, S.; Ishiguro, A. Splenectomy as an Effective Treatment for Macrothrombocytopenia in Takenouchi-Kosaki Syndrome. Int. J. Hematol. 2023, 117, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, S.; Krumbach, O.H.F.; Pantaleoni, F.; Coppola, S.; Amin, E.; Pannone, L.; Nouri, K.; Farina, L.; Dvorsky, R.; Lepri, F.; et al. Functional Dysregulation of CDC42 Causes Diverse Developmental Phenotypes. Am. J. Hum. Genet. 2018, 102, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Szczawinska-Poplonyk, A.; Ploski, R.; Bernatowska, E.; Pac, M. A Novel CDC42 Mutation in an 11-Year Old Child Manifesting as Syndromic Immunodeficiency, Autoinflammation, Hemophagocytic Lymphohistiocytosis, and Malignancy: A Case Report. Front. Immunol. 2020, 11, 318. [Google Scholar] [CrossRef] [PubMed]
- Takenouchi, T.; Kosaki, R.; Niizuma, T.; Hata, K.; Kosaki, K. Macrothrombocytopenia and Developmental Delay with a de Novo CDC42 Mutation: Yet Another Locus for Thrombocytopenia and Developmental Delay. Am. J. Med. Genet. A 2015, 167, 2822–2825. [Google Scholar] [CrossRef] [PubMed]
- Bucciol, G.; Pillay, B.; Casas-Martin, J.; Delafontaine, S.; Proesmans, M.; Lorent, N.; Coolen, J.; Tousseyn, T.; Bossuyt, X.; Ma, C.S.; et al. Systemic Inflammation and Myelofibrosis in a Patient withTakenouchi-Kosaki Syndrome Due to CDC42 Tyr64CysMutation. J. Clin. Immunol. 2020, 40, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Szczawińska-Popłonyk, A.; Popłonyk, N.; Badura-Stronka, M.; Juengling, J.; Huhn, K.; Biskup, S.; Bancerz, B.; Walkowiak, J. The Clinical Phenotype with Gastrostomy and Abdominal Wall Infection in a Pediatric Patient with Takenouchi-Kosaki Syndrome Due to a Heterozygous c.191A > G (p.Tyr64Cys) Variant in CDC42: A Case Report. Front. Genet. 2023, 14, 1108852. [Google Scholar] [CrossRef] [PubMed]
- Flynn, K.; Feben, C.; Lamola, L.; Carstens, N.; Krause, A.; Lombard, Z.; for DDD-Africa as members of the H3Africa Consortium. Ending a Diagnostic Odyssey—The First Case of Takenouchi–Kosaki Syndrome in an African Patient. Clin. Case Rep. 2021, 9, 2144–2148. [Google Scholar] [CrossRef] [PubMed]
- Santoro, C.; Gaudino, G.; Torella, A.; Piluso, G.; Perrotta, S.; Miraglia Del Giudice, E.; Nigro, V.; Grandone, A. Intermittent Macrothrombocytopenia in a Novel Patient with Takenouchi-Kosaki Syndrome and Review of Literature. Eur. J. Med. Genet. 2021, 64, 104358. [Google Scholar] [CrossRef]
- Motokawa, M.; Watanabe, S.; Nakatomi, A.; Kondoh, T.; Matsumoto, T.; Morifuji, K.; Sawada, H.; Nishimura, T.; Nunoi, H.; Yoshiura, K.; et al. A Hot-Spot Mutation in CDC42 (p.Tyr64Cys) and Novel Phenotypes in the Third Patient with Takenouchi-Kosaki Syndrome. J. Hum. Genet. 2018, 63, 387–390. [Google Scholar] [CrossRef]
- Uehara, T.; Suzuki, H.; Okamoto, N.; Kondoh, T.; Ahmad, A.; O’Connor, B.C.; Yoshina, S.; Mitani, S.; Kosaki, K.; Takenouchi, T. Pathogenetic Basis of Takenouchi-Kosaki Syndrome: Electron Microscopy Study Using Platelets in Patients and Functional Studies in a Caenorhabditis Elegans Model. Sci. Rep. 2019, 9, 4418. [Google Scholar] [CrossRef]
- Takenouchi, T.; Okamoto, N.; Ida, S.; Uehara, T.; Kosaki, K. Further Evidence of a Mutation in CDC42 as a Cause of a Recognizable Syndromic Form of Thrombocytopenia. Am. J. Med. Genet. A 2016, 170, 852–855. [Google Scholar] [CrossRef] [PubMed]
- McAuley, J.; Gunderman, L.; Khojah, A. Thrombocytopenia, hypogammaglobulinemia, and poor vaccine response in a patient with takenouchi-kosaki syndrome. Ann. Allergy. Asthma Immunol. 2022, 129, S144. [Google Scholar] [CrossRef]
- Stubbs, L.; Grimes, A.; Forbes, L.; Burrage, L.; Vogel, T.; Pereira, M. Immune-Mediated Chronic Macrothrombocytopenia, Musculoskeletal Abnormalities, and Dysmorphic Features in a Patient with Takenouchi-Kosaki Syndrome. J. Clin. Immunol. 2021, 41 (Suppl. S1), S135–S141. [Google Scholar]
- Ishikawa, K.; Uchiyama, T.; Kaname, T.; Kawai, T.; Ishiguro, A. Autoimmune Hemolytic Anemia Associated with Takenouchi–Kosaki Syndrome. Pediatr. Int. 2021, 63, 1528–1530. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Mosaddeghzadeh, N.; Ahmadian, M.R. The RHO Family GTPases: Mechanisms of Regulation and Signaling. Cells 2021, 10, 1831. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Liu, B.; Zhang, H.; Fu, F.; Yang, X.; Fan, L.; Zheng, M.; Zhang, S. The Role of Cell Division Control Protein 42 in Tumor and Non-Tumor Diseases: A Systematic Review. J. Cancer 2022, 13, 800–814. [Google Scholar] [CrossRef]
- Lam, M.T.; Coppola, S.; Krumbach, O.H.F.; Prencipe, G.; Insalaco, A.; Cifaldi, C.; Brigida, I.; Zara, E.; Scala, S.; Di Cesare, S.; et al. A Novel Disorder Involving Dyshematopoiesis, Inflammation, and HLH Due to Aberrant CDC42 Function. J. Exp. Med. 2019, 216, 2778–2799. [Google Scholar] [CrossRef] [PubMed]
- Coppola, S.; Insalaco, A.; Zara, E.; Di Rocco, M.; Marafon, D.P.; Spadaro, F.; Pannone, L.; Farina, L.; Pasquini, L.; Martinelli, S.; et al. Mutations at the C-Terminus of CDC42 Cause Distinct Hematopoietic and Autoinflammatory Disorders. J. Allergy Clin. Immunol. 2022, 150, 223–228. [Google Scholar] [CrossRef]
- El Masri, R.; Delon, J. Une palmitoylation de CDC42 causée par une mutation déclenche un syndrome auto-inflammatoire sévère. Med. Sci. 2020, 36, 987–990. [Google Scholar] [CrossRef]
- Hamada, N.; Ito, H.; Shibukawa, Y.; Morishita, R.; Iwamoto, I.; Okamoto, N.; Nagata, K. Neuropathophysiological Significance of the c.1449T>C/p.(Tyr64Cys) Mutation in the CDC42 Gene Responsible for Takenouchi-Kosaki Syndrome. Biochem. Biophys. Res. Commun. 2020, 529, 1033–1037. [Google Scholar] [CrossRef]
- Carew, P.; Shepherd, D.A.; Smith, L.; Soh, Q.R.; Sung, V. Frontiers | Language and Health-Related Quality of Life Outcomes of Children Early-Detected with Unilateral and Mild Bilateral Hearing Loss. Front. Pediatr. 2023, 11, 1210282. [Google Scholar] [CrossRef]
- Rivolta, M.N. Developing a stem cell-based therapy for the treatment of hearing loss. Hear. Balance Commun. 2015, 13, 148–152. [Google Scholar] [CrossRef]
- Valentini, C.; Szeto, B.; Kysar, J.W.; Lalwani, A.K. Inner ear gene delivery: Vectors and routes. Hear. Balance Commun. 2020, 18, 278–285. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).