Comparison of Mutations Induced by Different Doses of Fast-Neutron Irradiation in the M1 Generation of Sorghum (Sorghum bicolor)
Abstract
1. Introduction
2. Results
2.1. Effect of FN Irradiation on Seed Germination
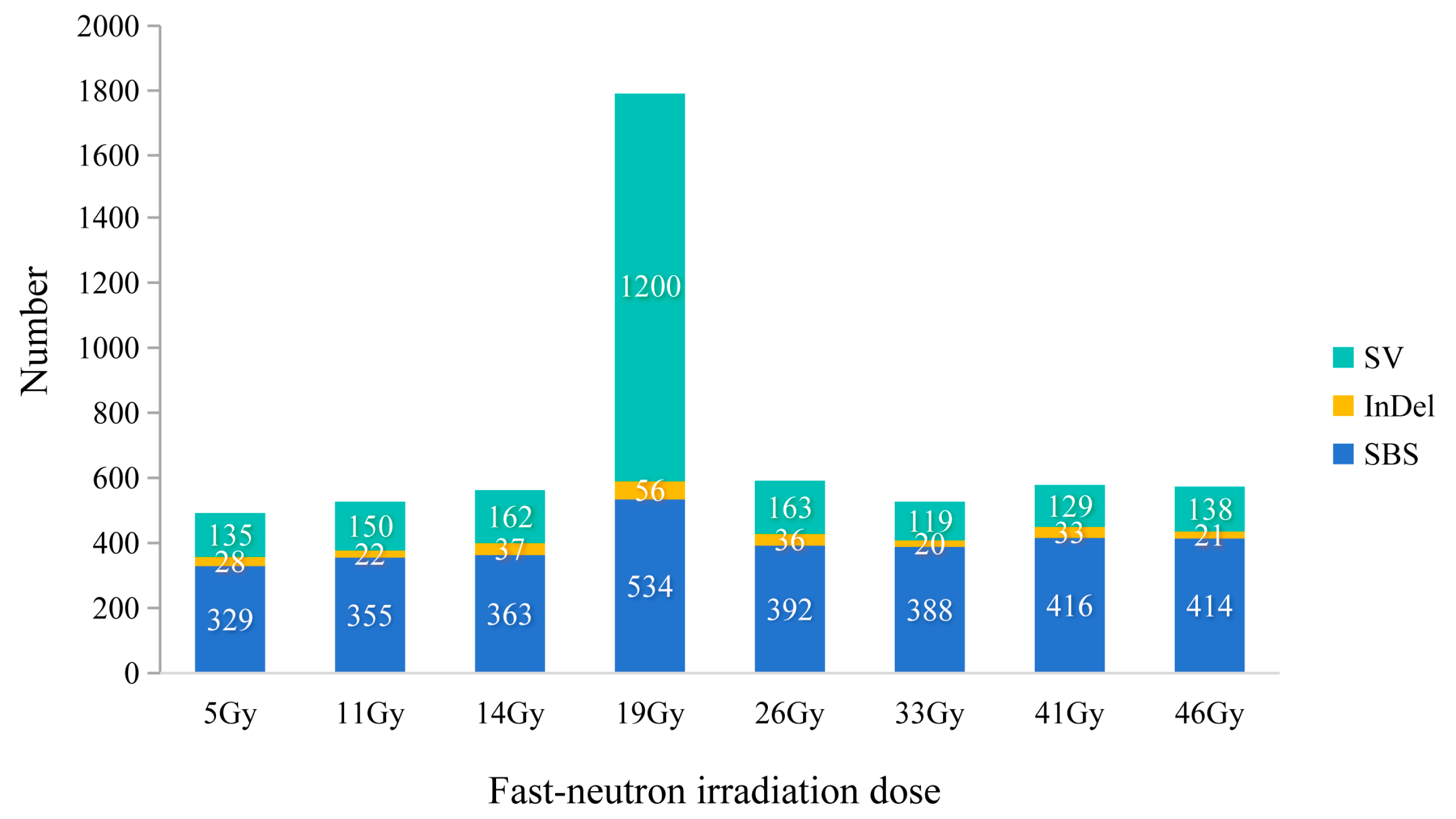
2.2. Identification of Genome-Wide Mutations in FN Lines
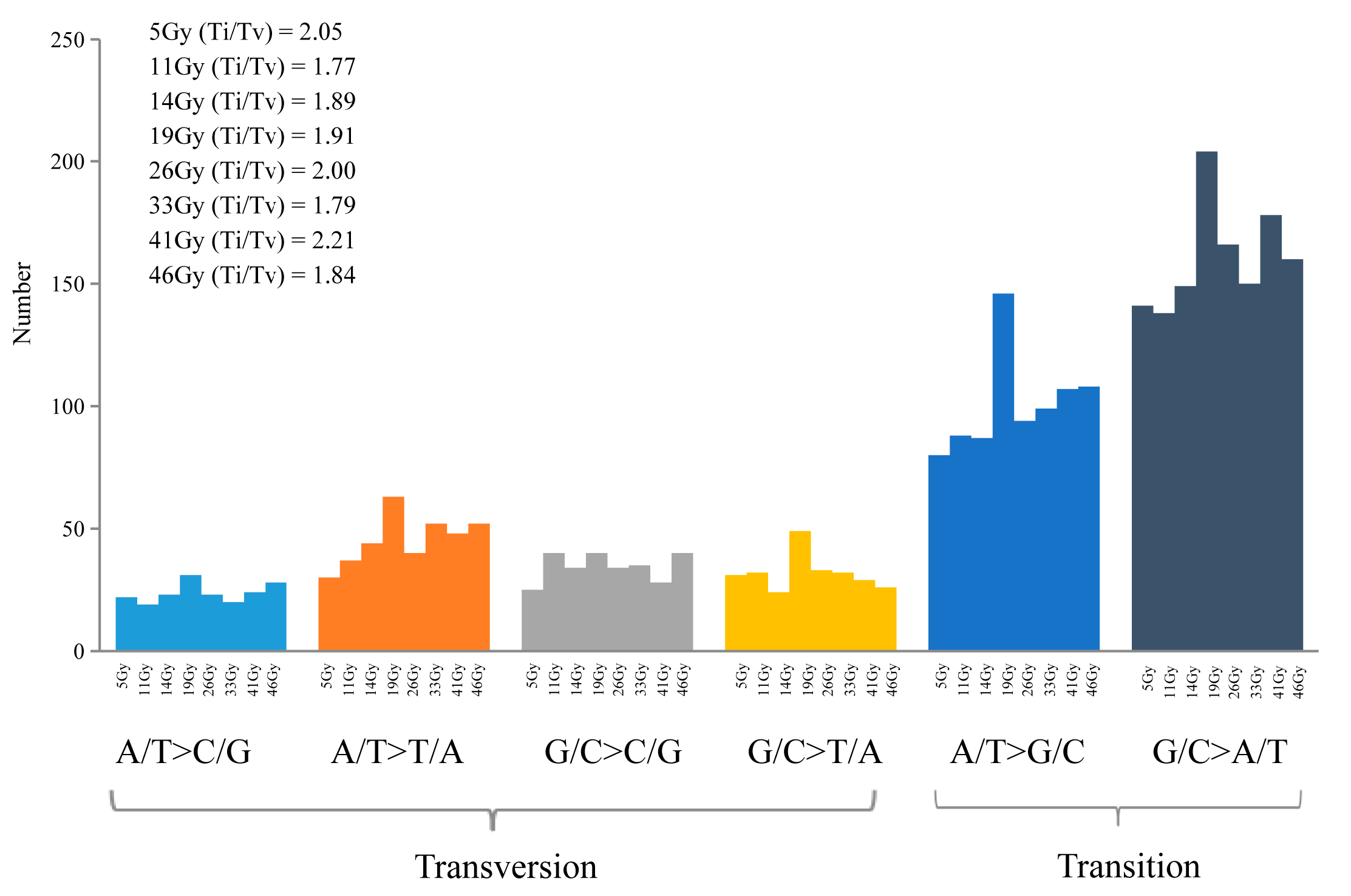
2.3. Characteristics of the SBSs Induced by FN Irradiation
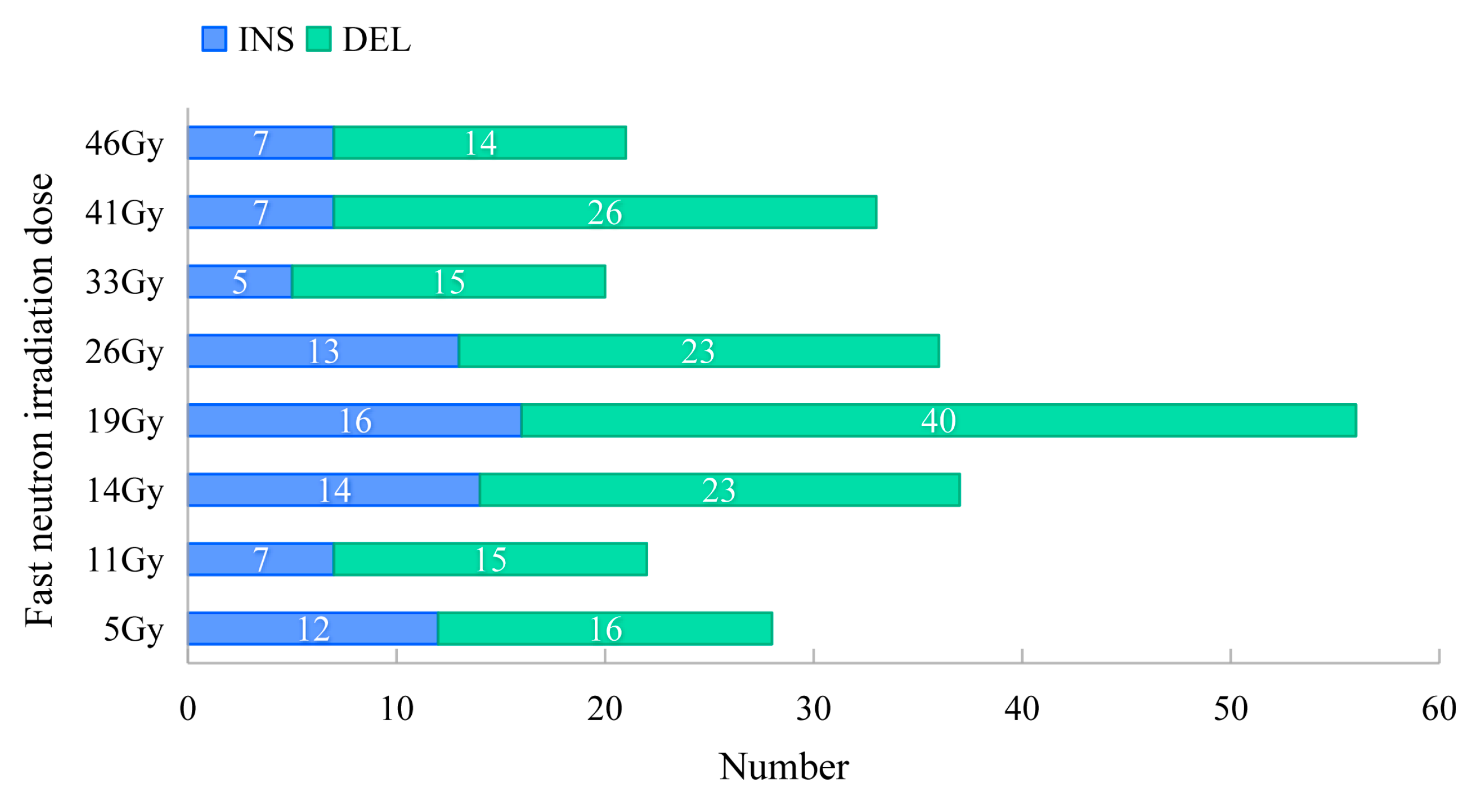
2.4. Characteristics of InDels and SVs Induced by FN Irradiation
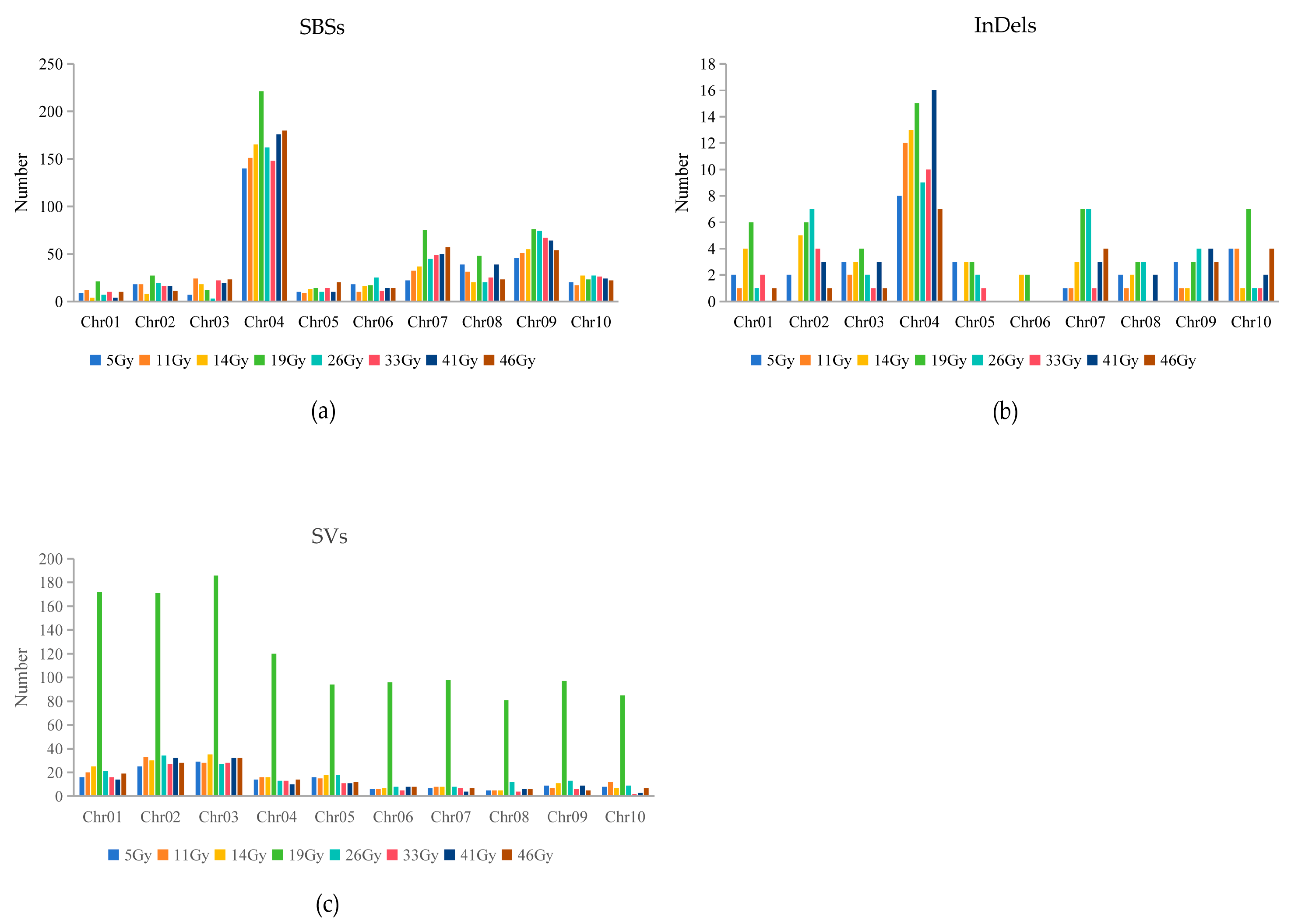
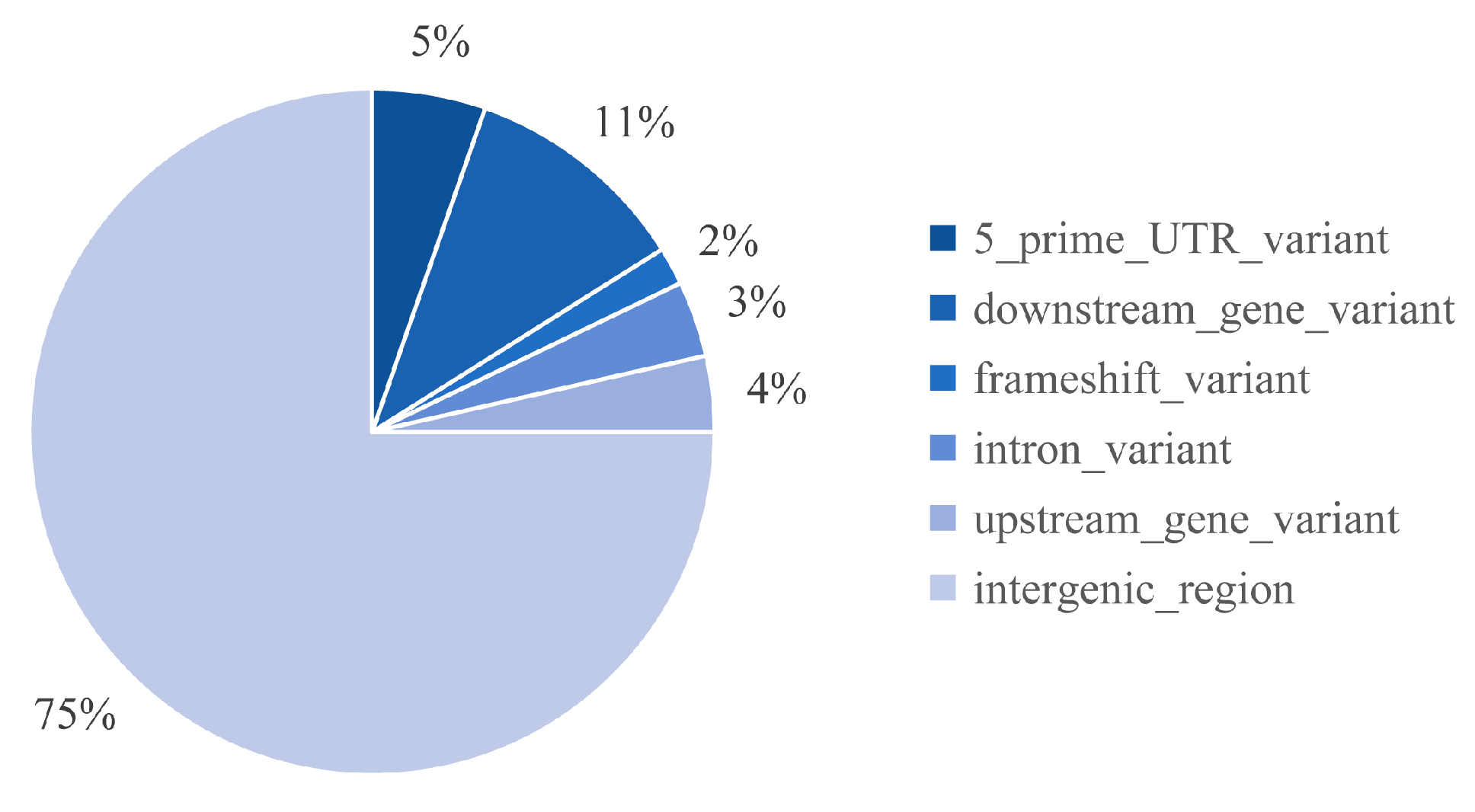
2.5. Distribution of Mutations Induced by FN Irradiation
2.6. Genes Affected by FN Irradiation
3. Discussion
4. Materials and Methods
4.1. Plant Material and Generation of the Mutant Library
4.2. Germination Test
4.3. Whole-Genome Re-Sequencing
4.4. Genomic Variant Detection and Annotation
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paterson, A.H.; Bowers, J.E.; Feltus, F.A. Genomics of sorghum, a semi-arid cereal and emerging model for tropical grass genomics. In Genomics of Tropical Crop Plants; Moore, P.H., Mingeds, R., Eds.; Springer: New York, NY, USA, 2008; pp. 469–482. [Google Scholar]
- Bollam, S.; Romana, K.K.; Rayaprolu, L.; Vemula, A.; Das, R.R.; Rathore, A.; Gandham, P.; Chander, G.; Deshpande, S.P.; Gupta, R. Nitrogen use efficiency in sorghum: Exploring native variability for traits under variable n-regimes. Front. Plant Sci. 2021, 12, 643192. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, M.; Liu, F.; Zou, J.; Lu, X.; Diao, X. Current status and future prospective of sorghum production and seed industry in China. Sci. Agric. Sin. 2021, 54, 471–482. [Google Scholar]
- Tollenaar, D. Untersuchungen euber Mutation bei Tabak. II. Einige kuenstlich erzeugte Chromosom-Mutanten. Genetica 1938, 20, 285–294. [Google Scholar] [CrossRef]
- Pathirana, R. Plant mutation breeding in agriculture. CABI Rev. 2011, 6, 1–20. [Google Scholar] [CrossRef]
- Sikora, P.; Chawade, A.; Larsson, M.; Olsson, J.; Olsson, O. Mutagenesis as a tool in plant genetics, functional genomics, and breeding. Int. J. Plant Genom. 2011, 2011, 314829. [Google Scholar] [CrossRef] [PubMed]
- Gillmor, C.S.; Lukowitz, W. EMS Mutagenesis of Arabidopsis Seeds. In Plant Embryogenesis: Methods in Molecular Biology; Bayer, M., Ed.; Humana Press: New York, NY, USA, 2020. [Google Scholar]
- Ma, L.; Kong, F.; Sun, K.; Wang, T.; Guo, T. From classical radiation to modern radiation: Past, present, and future of radiation mutation breeding. Front. Public Health 2021, 9, 68071. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, R.; Nozawa, S.; Hase, Y.; Narumi, I.; Hidema, J.; Sakamoto, A.N. Mutational effects of γ-rays and carbon ion beams on Arabidopsis seedlings. J. Radiat. Res. 2013, 54, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, Y.; Oike, T.; Niimi, A.; Yamauchi, M.; Sato, H.; Limsirichaikul, S.; Held, K.D.; Nakano, T.; Shibata, A. Clustered DNA double-strand break formation and the repair pathway following heavy-ion irradiation. J. Radiat. Res. 2019, 60, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, S.; Rana, N.; Bansal, R.; Vishwakarma, G.; Mehetre, S.T.; Das, B.K.; Kumar, M.; Yadav, S.K.; Sonah, H.; Sharma, T.R.; et al. Expanding avenue of fast neutron mediated mutagenesis for crop improvement. Plants 2019, 8, 164. [Google Scholar] [CrossRef]
- Hendry, J.H. The slower cellular recovery after higher-LET irradiations, including neutrons, focuses on the quality of DNA breaks. Radiat. Res. 1991, 128, S111–S113. [Google Scholar] [CrossRef]
- Belfield, E.J.; Gan, X.; Mithani, A.; Brown, C.; Jiang, C.; Franklin, K.; Alvey, E.; Wibowo, A.; Jung, M.; Bailey, K.; et al. Genome-wide analysis of mutations in mutant lineages selected following fast-neutron irradiation mutagenesis of Arabidopsis thaliana. Genome Res. 2012, 22, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chern, M.; Jain, R.; Martin, J.A.; Schackwitz, W.S.; Jiang, L.; Vega-Sánchez, M.E.; Lipzen, A.M.; Barry, K.W.; Schmutz, J.; et al. Genome-wide sequencing of 41 rice (Oryza sativa L.) mutated lines reveals diverse mutations induced by fast-neutron irradiation. Mol. Plant 2016, 9, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- Bolon, Y.T.; Haun, W.J.; Xu, W.W.; Grant, D.; Stacey, M.G.; Nelson, R.T.; Gerhardt, D.J.; Jeddeloh, J.A.; Stacey, G.; Muehlbauer, G.J.; et al. Phenotypic and genomic analyses of a fast neutron mutant population resource in soybean. Plant Physiol. 2011, 156, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Wyant, S.R.; Rodriguez, M.F.; Carter, C.K.; Parrott, W.A.; Jackson, S.A.; Stupar, R.M.; Morrell, P.L. Fast neutron mutagenesis in soybean enriches for small indels and creates frameshift mutations. G3 2022, 12, jkab431. [Google Scholar] [CrossRef] [PubMed]
- Domoney, C.; Knox, M.; Moreau, C.; Ambrose, M.; Palmer, S.; Smith, P.; Christodoulou, V.; Isaac, P.G.; Hegarty, M.; Blackmore, T.; et al. Exploiting a fast neutron mutant genetic resource in Pisum sativum (pea) for functional genomics. Funct. Plant Biol. 2013, 40, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, R. Physical mutagenesis in medicago truncatula using fast neutron bombardment (FNB) for symbiosis and developmental biology studies. In Functional Genomics in Medicago truncatula: Methods in Molecular Biology; Cañas, L., Beltrán, J., Eds.; Humana Press: New York, NY, USA, 2018. [Google Scholar]
- Wang, J.; Sui, J.; Xie, Y.; Guo, H.; Qiao, L.; Zhao, L.; Yu, S.; Liu, L. Generation of peanut mutants by fast neutron irradiation combined with in vitro culture. J. Radiat. Res. 2015, 56, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, I.; Rafii, M.Y.; Nazli, M.H.; Ramlee, S.I.; Harun, A.R.; Oladosu, Y. Determination of lethal (LD) and growth reduction (GR) doses on acute and chronic gamma-irradiated Bambara groundnut [Vigna subterranea (L.) Verdc.]varieties. J. Radiat. Res. Appl. Sci. 2021, 14, 133–145. [Google Scholar] [CrossRef]
- Guha Mallick, R.; Pramanik, S.; Pandit, M.K.; Gupta, A.K.; Roy, S.; Jambhulkar, S.; Sarker, A.; Nath, R.; Bhattacharyya, S. Radiosensitivity of seedling traits to varying gamma doses, optimum dose determination and variation in determined doses due to different time of sowings after irradiation and methods of irradiation in faba bean genotypes. Int. J. Radiat. Biol. 2023, 99, 534–550. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Yao, Z.; Feng, H.; Yin, Y. Effects of different dosages of neutron radiation on seed germination and seedling growth of needle leaf pea. Chin. Agric. Sci. Bull. 2015, 31, 200–204. [Google Scholar]
- Zhang, L.; Zhao, B.; Wan, P.; Luo, W.; Li, Y.; Yang, K.; Cao, J.; Sun, W.; Zhao, C.; Zhang, H. The screening of mutants from jingnong 6 induced by fast neutron in Azuki bean (Vigna angularisi). Chin. Agric. Sci. Bull. 2012, 28, 53–58. [Google Scholar]
- Jain, S.M. Mutagenesis in crop improvement under the climate change. Rom. Biotechnol. Lett. 2010, 15, 88–106. [Google Scholar]
- Wang, C.Y.; Zhu, Z.X.; Li, D.; Cong, L.; Zhang, L. EMS mutagenesis, mutant screening and identification of sorghum. Biotechnol. Bull. 2014, 9, 78–83. [Google Scholar]
- Jiao, Y.; Burke, J.; Chopra, R.; Burow, G.; Chen, J.; Wang, B.; Hayes, C.; Emendack, Y.; Ware, D.; Xin, Z. A sorghum mutant resource as an efficient platform for gene discovery in grasses. Plant Cell 2016, 28, 1551–1562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, W.; Bai, W.; Zhang, Y.; Wang, G.; Shao, R.; Xue, D. Screening and identification of ems mutants from sweet sorghum. Chin. Agric. Sci. Bull. 2020, 3, 22–25. [Google Scholar]
- Simons, J.M.; Herbert, T.C.; Kauffman, C.; Batete, M.Y.; Simpson, A.T.; Katsuki, Y.; Le, D.; Amundson, D.; Buescher, E.M.; Weil, C.; et al. Systematic prediction of EMS-induced mutations in a sorghum mutant population. Plant Direct. 2022, 6, e404. [Google Scholar] [CrossRef]
- Fan, X.; Wang, H.; Nie, M.; Zhao, X.; Zhang, Y.; Yang, H.; Zhang, X.; Liang, D.; Duan, Y.; Liu, Q. Effects of EMS mutagenesis on emergence and agronomic traits in sorghum. Crops 2020, 36, 47–54. [Google Scholar]
- Paterson, A.H.; Bowers, J.E.; Bruggmann, R.; Dubchak, I.; Grimwood, J.; Gundlach, H.; Haberer, G.; Hellsten, U.; Mitros, T.; Poliakov, A.; et al. The Sorghum bicolor genome and the diversification of grasses. Nature 2009, 457, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Maluszynski, M.; Szarejko, I.; Bhatia, C.R.; Nichterlein, K.; Lagoda, P.J. Methodologies for generating variability Part 4, Mutation techniques. In Plant Breeders and Farmer Participation; Ceccarelli, S., Guimaraes, E.P., Weltzien, E., Eds.; FAO: Rome, Italy, 2009; pp. 159–194. [Google Scholar]
- Bolon, Y.T.; Stec, A.O.; Michno, J.M.; Roessler, J.; Bhaskar, P.B.; Ries, L.; Dobbels, A.A.; Campbell, B.W.; Young, N.P.; Anderson, J.E.; et al. Genome resilience and prevalence of segmental duplications following fast neutron irradiation of soybean. Genetics 2014, 198, 967–981. [Google Scholar] [CrossRef]
- Naito, K.; Kusaba, M.; Shikazono, N.; Takano, T.; Tanaka, A.; Tanisaka, T.; Nishimura, M. Transmissible and nontransmissible mutations induced by irradiating Arabidopsis thaliana pollen with γ-rays and carbon ions. Genetics 2005, 169, 881–889. [Google Scholar] [CrossRef]
- Xin, Z.; Jiao, Y.; Burow, G.; Hayes, C.M.; Chen, J.; Burke, J.J.; Pugh, N.A.; Ware, D.H. Registration of 252 sequenced sorghum mutants as a community reverse genetic resource. J. Plant Regist. 2023, 17, 599. [Google Scholar] [CrossRef]
- Jiao, Y.; Nigam, D.; Barry, K.; Daum, C.; Yoshinaga, Y.; Lipzen, A.; Khan, A.; Parasa, S.; Wei, S.; Lu, Z.; et al. A large sequenced mutant library-valuable reverse genetic resource that covers 98% of sorghum genes. Plant J. 2024, 117, 1543–1557. [Google Scholar] [CrossRef] [PubMed]
- Sega, G.A. A review of the genetic effects of ethyl methanesulfonate. Mutat. Res./Rev. Genet. Toxicol. 1984, 134, 113–142. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Guo, H.; Fu, M.; Xie, Y.; Zhao, L.; Gu, J.; Zhao, S.; Ding, Y.; Du, Q.; Zhang, J.; et al. A large-scale whole-exome sequencing mutant resource for functional genomics in wheat. Plant Biotechnol. J. 2023, 21, 2047–2056. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, Z.; Shu, Q. Molecular nature of chemically and physically induced mutants in plants: A review. Plant Genet. Resour. 2014, 12, S74–S78. [Google Scholar] [CrossRef]
- Thompson, O.; Edgley, M.; Strasbourger, P.; Flibotte, S.; Ewing, B.; Adair, R.; Au, V.; Chaudhry, I.; Fernando, L.; Hutter, H.; et al. The million mutation project: A new approach to genetics in Caenorhabditis elegans. Genome Res. 2013, 23, 1749–1762. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, G.; Geng, J.; Geng, Z.; Dou, H.; Liu, X.; An, Z.; Zhang, H.; Wang, Y. Genome-wide analysis of mutations induced by carbon ion beam irradiation in cotton. Front. Plant Sci. 2023, 14, 1056662. [Google Scholar] [CrossRef] [PubMed]
- Monroe, J.G.; Srikant, T.; Carbonell-Bejerano, P.; Becker, C.; Lensink, M.; Exposito-Alonso, M.; Klein, M.; Hildebrandt, J.; Neumann, M.; Kliebenstein, D.; et al. Mutation bias reflects natural selection in Arabidopsis thaliana. Nature 2022, 302, 101–105. [Google Scholar] [CrossRef]
- Weng, M.L.; Becker, C.; Hildebrandt, J.; Neumann, M.; Rutter, M.T.; Shaw, R.G.; Weigel, D.; Fenster, C.B. Fine-grained analysis of spontaneous mutation spectrum and frequency in Arabidopsis thaliana. Genetics 2019, 211, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.J.; Ward, J.F. Constraints on energy deposition and target size of multiply damaged sites associated with DNA double-strand breaks. Int. J. Radiat. Biol. 2009, 61, 737–748. [Google Scholar] [CrossRef]
- Nikjoo, H.; O’Neill, P.; Wilson, W.E.; Goodhead, D.T. Computational approach for determining the spectrum of DNA damage induced by ionizing radiation. Radiat. Res. 2001, 156, 577–583. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Layer, R.M.; Chiang, C.; Quinlan, A.R.; Hall, I.M. LUMPY: A probabilistic framework for structural variant discovery. Genome Biol. 2014, 15, R84. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Liu, Y.; Yan, H.; You, Q.; Yi, X.; Du, Z.; Xu, W.; Su, Z. agriGO v2.0: A GO analysis toolkit for the agricultural community, 2017 update. Nucleic Acids Res. 2017, 45, W122–W129. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
| Mutant ID | Number of Raw Reads | Number of Clean Reads | Number of Clean Bases (Gb) | Mapping Rate (%) | Depth of Coverage ≥ 20× (%) | Depth of Coverage ≥ 10× (%) | Depth of Coverage ≥ 5× (%) | Q20% | Q30% | GC% |
|---|---|---|---|---|---|---|---|---|---|---|
| Bs5_1 | 105691374 | 103875740 | 15.51 | 98.19 | 43.79 | 92.33 | 98.91 | 97.02 | 92.32 | 43.52 |
| Bs5_2 | 108522048 | 106884932 | 15.96 | 98.71 | 47.20 | 93.62 | 99.08 | 97.17 | 92.65 | 43.34 |
| Bs5_3 | 105939082 | 104319734 | 15.57 | 97.51 | 42.28 | 92.51 | 98.98 | 97.19 | 92.66 | 43.54 |
| Bs5_4 | 98457400 | 97053746 | 14.49 | 97.22 | 34.18 | 89.73 | 98.69 | 97.21 | 92.76 | 43.69 |
| Bs5_5 | 107326540 | 105233474 | 15.72 | 97.97 | 44.45 | 93.04 | 99.03 | 96.89 | 92.12 | 43.57 |
| Bs11_1 | 107332754 | 105249272 | 15.71 | 97.79 | 46.33 | 89.45 | 97.36 | 96.91 | 92.23 | 44.78 |
| Bs11_2 | 105666644 | 103826436 | 15.51 | 98.03 | 43.64 | 92.58 | 98.99 | 96.98 | 92.24 | 43.52 |
| Bs11_3 | 106434116 | 104599640 | 15.62 | 98.47 | 44.95 | 92.93 | 98.98 | 96.99 | 92.26 | 43.46 |
| Bs11_4 | 107693462 | 105623622 | 15.77 | 98.18 | 44.03 | 92.91 | 99.02 | 96.89 | 92.13 | 43.42 |
| Bs11_5 | 105326402 | 103602506 | 15.46 | 98.51 | 43.76 | 92.80 | 98.99 | 97.04 | 92.33 | 43.44 |
| Bs14_1 | 108693522 | 106686736 | 15.93 | 98.38 | 45.76 | 93.19 | 99.04 | 96.95 | 92.29 | 43.38 |
| Bs14_2 | 109430936 | 107883362 | 16.1 | 98.64 | 48.44 | 93.89 | 99.12 | 97.21 | 92.87 | 43.37 |
| Bs14_3 | 107017144 | 105162890 | 15.7 | 98.37 | 45.48 | 92.85 | 99.01 | 97 | 92.3 | 43.41 |
| Bs14_4 | 107153690 | 105324132 | 15.73 | 98.03 | 44.67 | 92.73 | 98.99 | 97.01 | 92.32 | 43.46 |
| Bs14_5 | 104585328 | 103151406 | 15.39 | 98.65 | 42.51 | 92.33 | 98.96 | 97.25 | 92.98 | 43.29 |
| Bs19_1 | 108469448 | 106800680 | 15.94 | 97.45 | 45.40 | 93.48 | 99.11 | 97.17 | 92.89 | 43.59 |
| Bs19_2 | 108122626 | 106404360 | 15.89 | 98.34 | 45.61 | 93.56 | 99.12 | 97.1 | 92.49 | 43.39 |
| Bs19_3 | 108469448 | 106800680 | 15.94 | 97.45 | 45.40 | 93.48 | 99.11 | 97.17 | 92.89 | 43.59 |
| Bs19_4 | 107796868 | 105588026 | 15.77 | 98.4 | 45.48 | 93.44 | 99.10 | 96.85 | 92.04 | 43.43 |
| Bs19_5 | 108507344 | 106160820 | 15.85 | 98.52 | 46.64 | 93.43 | 99.08 | 96.69 | 91.78 | 43.41 |
| Bs26_1 | 109657890 | 108202156 | 16.14 | 98.61 | 47.79 | 94.23 | 99.13 | 97.52 | 93.47 | 43.22 |
| Bs26_2 | 108054944 | 105624632 | 15.78 | 98.28 | 46.02 | 93.43 | 99.08 | 96.7 | 91.75 | 43.51 |
| Bs26_3 | 109460390 | 107190882 | 16.01 | 98.29 | 47.89 | 94.05 | 99.15 | 96.76 | 91.92 | 43.45 |
| Bs26_4 | 108326044 | 106128352 | 15.84 | 97.8 | 45.16 | 93.36 | 99.09 | 96.81 | 91.93 | 43.49 |
| Bs26_5 | 108149020 | 106462900 | 15.89 | 98.85 | 48.22 | 94.08 | 99.17 | 97.16 | 92.79 | 43.35 |
| Bs33_1 | 108844444 | 107644812 | 16.05 | 97.03 | 45.41 | 89.30 | 97.11 | 97.6 | 93.74 | 44.87 |
| Bs33_2 | 109326180 | 107551264 | 16.04 | 98.18 | 46.63 | 90.42 | 97.59 | 97.25 | 92.95 | 44.43 |
| Bs33_3 | 109850900 | 108089326 | 16.12 | 98.48 | 49.80 | 91.11 | 97.66 | 97.25 | 92.92 | 44.57 |
| Bs33_4 | 108885036 | 107371884 | 16.02 | 98.65 | 48.37 | 91.61 | 97.97 | 97.41 | 93.24 | 44.24 |
| Bs33_5 | 108389176 | 106722290 | 15.93 | 98.62 | 46.88 | 94.00 | 99.15 | 97.36 | 93.07 | 43.22 |
| Bs41_1 | 108968604 | 107382338 | 16 | 98.18 | 48.31 | 90.02 | 97.24 | 97.34 | 93.1 | 44.75 |
| Bs41_2 | 108959848 | 107167034 | 15.98 | 94.38 | 43.29 | 87.89 | 96.80 | 97.18 | 92.77 | 45.56 |
| Bs41_3 | 107782964 | 106085224 | 15.83 | 98.29 | 44.79 | 88.96 | 97.21 | 97.24 | 92.82 | 44.56 |
| Bs41_4 | 109794748 | 107912634 | 16.1 | 98.38 | 47.03 | 90.68 | 97.71 | 97.13 | 92.67 | 44.38 |
| Bs41_5 | 107973556 | 105970066 | 15.81 | 97.55 | 45.69 | 89.84 | 97.45 | 97.03 | 92.47 | 44.64 |
| Bs46_1 | 107332754 | 105249272 | 15.71 | 97.79 | 46.33 | 89.45 | 97.36 | 96.91 | 92.23 | 44.78 |
| Bs46_2 | 108282810 | 106441380 | 15.88 | 98.71 | 46.43 | 88.89 | 97.03 | 97.09 | 92.54 | 44.64 |
| Bs46_3 | 107892514 | 106163432 | 15.83 | 98.19 | 46.04 | 88.70 | 96.96 | 97.17 | 92.69 | 44.79 |
| Bs46_4 | 105055526 | 103510568 | 15.44 | 98 | 44.71 | 88.34 | 96.98 | 97.27 | 93.09 | 44.8 |
| Bs46_5 | 108503148 | 106871610 | 15.94 | 98.18 | 45.50 | 88.57 | 96.84 | 97.29 | 92.95 | 44.67 |
| BTx623 | 108293796 | 106194916 | 15.86 | 99.01 | 48.73 | 93.69 | 99.11 | 97.12 | 92.56 | 43.42 |
| Mean | 107668792 | 105857785 | 15.80 | 98.10 | 45.59 | 91.83 | 98.40 | 97.10 | 92.59 | 43.90 |
| Line | ID | Chr | Pos | Ref | Alt | GeneID |
|---|---|---|---|---|---|---|
| 5 Gy | Chr01_62221922 | Chr01 | 62221922 | G | GGC | Sobic.001G299900.v5.1 |
| Chr04_57358919 | Chr04 | 57358919 | TCGA | T | Sobic.004G197700.v5.1 | |
| Chr04_57358941 | Chr04 | 57358941 | C | T | Sobic.004G197700.v5.1 | |
| Chr04_57358981 | Chr04 | 57358981 | A | T | Sobic.004G197700.v5.1 | |
| Chr09_28770284 | Chr09 | 28770284 | T | G | Sobic.009G095560.v5.1 | |
| 11 Gy | Chr03_6409060 | Chr03 | 6409060 | CA | CAA,C | Sobic.003G074700.v5.1 |
| 14 Gy | Chr01_62221922 | Chr01 | 62221922 | G | GGC | Sobic.001G299900.v5.1 |
| Chr01_10357147 | Chr01 | 10357147 | T | A | Sobic.001G130400.v5.1 | |
| Chr02_48202647 | Chr02 | 48202647 | TCA | T | Sobic.002G155500.v5.1 | |
| 19 Gy | Chr02_48202647 | Chr02 | 48202647 | TCA | T | Sobic.002G155500.v5.1 |
| Chr03_70852413 | Chr03 | 70852413 | CAG | C | Sobic.003G317500.v5.1 | |
| Chr03_79824423 | Chr03 | 79824423 | AG | A | Sobic.003G431800.v5.1 | |
| Chr04_28733502 | Chr04 | 28733502 | G | A | Sobic.004G135383.v5.1 | |
| Chr04_28733574 | Chr04 | 28733574 | A | T | Sobic.004G135383.v5.1 | |
| Chr05_6918726 | Chr05 | 6918726 | C | T,G | Sobic.005G062200.v5.1 | |
| Chr05_10737537 | Chr05 | 10737537 | TC | T | Sobic.005G080062.v5.1 | |
| Chr06_58090348 | Chr06 | 58090348 | ACTCT | A,ACT | Sobic.006G219600.v5.1 | |
| Chr07_97864 | Chr07 | 97864 | CGAGA | CGA,C | Sobic.007G000900.v5.1 | |
| 26 Gy | Chr01_30720439 | Chr01 | 30720439 | T | C | Sobic.001G257400.v5.1 |
| Chr01_62221922 | Chr01 | 62221922 | G | GGC | Sobic.001G299900.v5.1 | |
| Chr02_48202647 | Chr02 | 48202647 | TCA | T | Sobic.002G155500.v5.1 | |
| Chr02_68565702 | Chr02 | 68565702 | TC | T | Sobic.002G295100.v5.1 | |
| Chr08_62900559 | Chr08 | 62900559 | T | A | Sobic.008G164526.v5.1 | |
| Chr09_24008650 | Chr09 | 24008650 | T | C | Sobic.009G092900.v5.1 | |
| 33 Gy | Chr01_62221922 | Chr01 | 62221922 | G | GGC | Sobic.001G299900.v5.1 |
| 41 Gy | Chr04_57358919 | Chr04 | 57358919 | TCGA | T | Sobic.004G197700.v5.1 |
| Chr04_57358988 | Chr04 | 57358988 | CGGG | C | Sobic.004G197700.v5.1 | |
| Chr04_57358993 | Chr04 | 57358993 | ACGG | A | Sobic.004G197700.v5.1 | |
| Chr04_57359003 | Chr04 | 57359003 | CGAT | C | Sobic.004G197700.v5.1 | |
| Chr04_57358941 | Chr04 | 57358941 | C | T | Sobic.004G197700.v5.1 | |
| Chr04_57358981 | Chr04 | 57358981 | A | T | Sobic.004G197700.v5.1 | |
| Chr05_6918726 | Chr05 | 6918726 | C | T,G | Sobic.005G062200.v5.1 | |
| Chr08_62900559 | Chr08 | 62900559 | T | A | Sobic.008G164526.v5.1 | |
| Chr08_62900574 | Chr08 | 62900574 | A | G | Sobic.008G164526.v5.1 | |
| 46 Gy | Chr01_62221922 | Chr01 | 62221922 | G | GGC | Sobic.001G299900.v5.1 |
| Chr01_73368414 | Chr01 | 73368414 | A | G | Sobic.001G408300.v5.1 | |
| Chr04_57358833 | Chr04 | 57358833 | G | A | Sobic.004G197700.v5.1 | |
| Chr04_57358850 | Chr04 | 57358850 | TG | T | Sobic.004G197700.v5.1 | |
| Chr10_60900949 | Chr10 | 60900949 | CAG | C | Sobic.010G254300.v5.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, N.; Liang, S.; Zhou, L.; Yuan, X.; Li, C.; Chen, X.; Zhao, H. Comparison of Mutations Induced by Different Doses of Fast-Neutron Irradiation in the M1 Generation of Sorghum (Sorghum bicolor). Genes 2024, 15, 976. https://doi.org/10.3390/genes15080976
Yuan N, Liang S, Zhou L, Yuan X, Li C, Chen X, Zhao H. Comparison of Mutations Induced by Different Doses of Fast-Neutron Irradiation in the M1 Generation of Sorghum (Sorghum bicolor). Genes. 2024; 15(8):976. https://doi.org/10.3390/genes15080976
Chicago/Turabian StyleYuan, Na, Shuaiqiang Liang, Ling Zhou, Xingxing Yuan, Chunhong Li, Xin Chen, and Han Zhao. 2024. "Comparison of Mutations Induced by Different Doses of Fast-Neutron Irradiation in the M1 Generation of Sorghum (Sorghum bicolor)" Genes 15, no. 8: 976. https://doi.org/10.3390/genes15080976
APA StyleYuan, N., Liang, S., Zhou, L., Yuan, X., Li, C., Chen, X., & Zhao, H. (2024). Comparison of Mutations Induced by Different Doses of Fast-Neutron Irradiation in the M1 Generation of Sorghum (Sorghum bicolor). Genes, 15(8), 976. https://doi.org/10.3390/genes15080976






