High Prevalence of ESBL Genes in Commensal Escherichia coli of the Urinary Tract: Implications for Antibiotic Stewardship among Residents of Ghanaian Elderly Nursing Care Homes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Sample Collection
2.2. Bacterial Isolation and Identification
2.3. Antimicrobial Susceptibility Testing
2.4. DNA Extraction and Polymerase Chain Reaction (PCR)
2.4.1. Screening for ESBL Genes
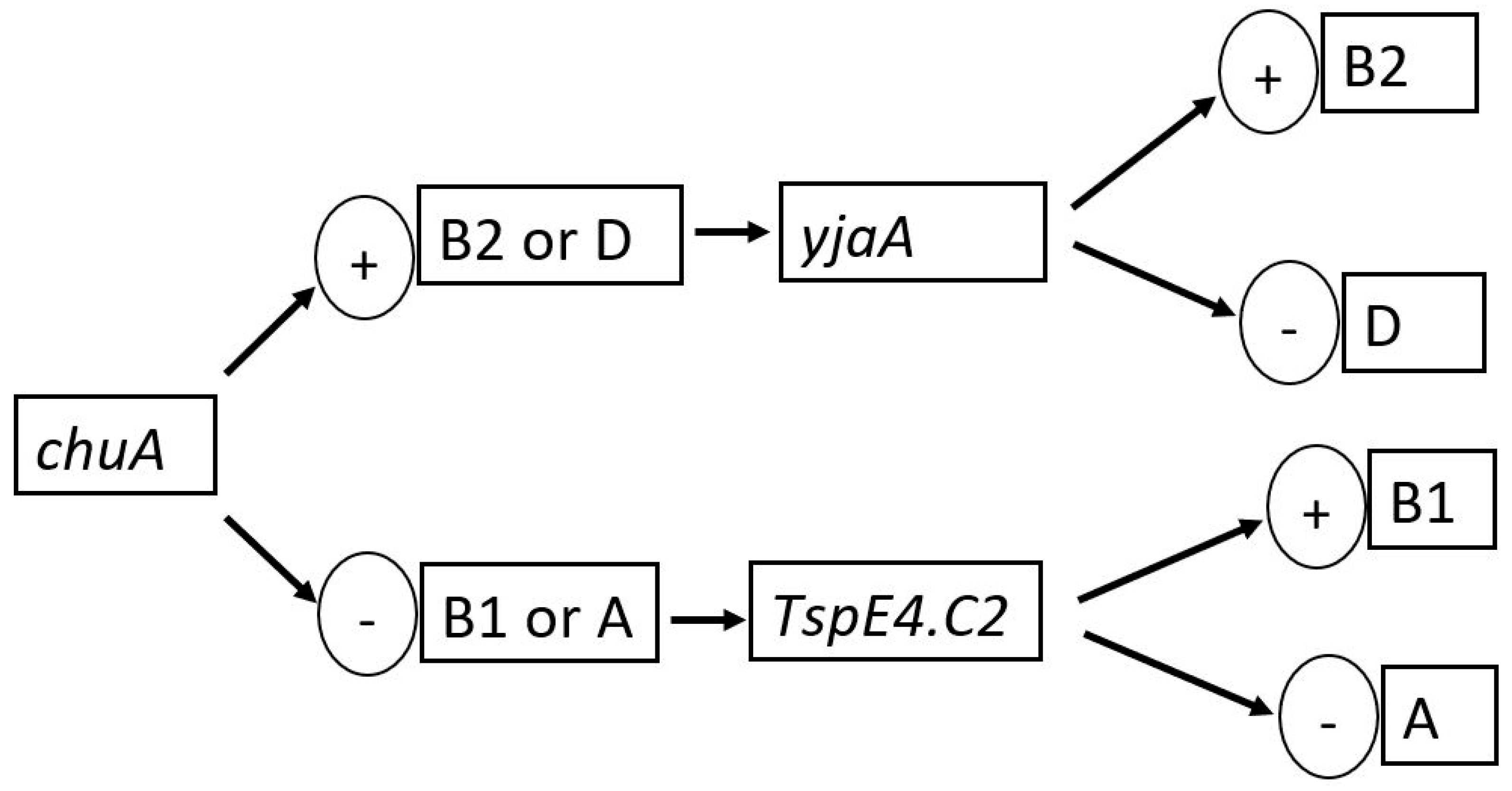
2.4.2. Phylogenetic Grouping
2.5. Statistical Analysis
2.6. Ethical Considerations
3. Results
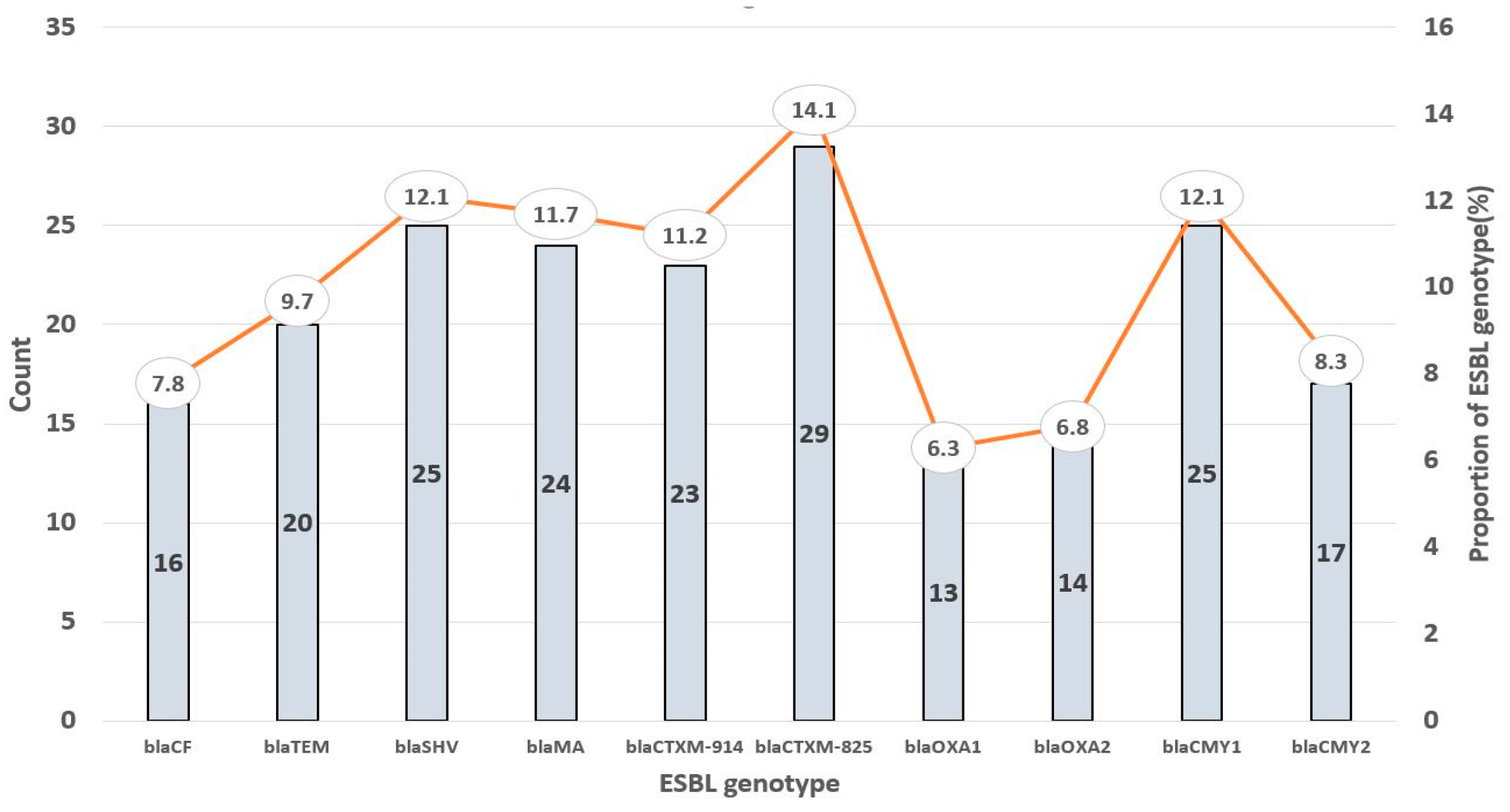
3.1. Demographics of the Participants and the Prevalence of E. coli and ESBL Genes
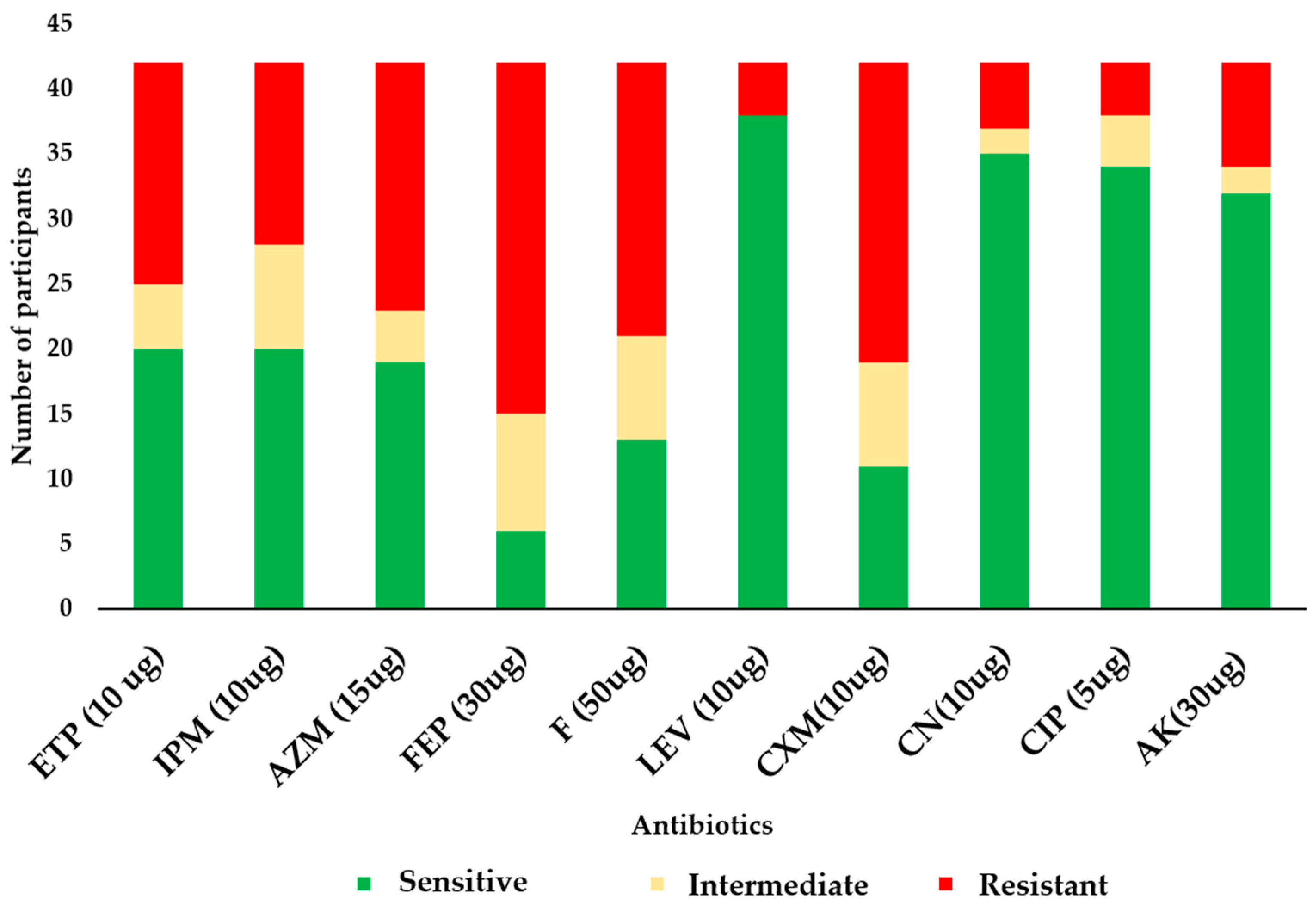
3.2. Antibiogram of the E. coli Isolates
3.3. Phylogroups of the E. coli Isolates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abayneh, M.; Tesfaw, G.; Abdissa, A. Isolation of Extended-Spectrum β—Lactamase- (ESBL-) Producing Escherichia coli and Klebsiella pneumoniae from Patients with Community-Onset Urinary Tract Infections in Jimma University Specialized Hospital, Southwest Ethiopia. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 4846159. [Google Scholar] [CrossRef] [PubMed]
- Kiratisin, P.; Apisarnthanarak, A.; Laesripa, C.; Saifon, P. Molecular Characterization and Epidemiology of Extended-Spectrum- β-Lactamase-Producing Escherichia coli and Klebsiella pneumoniae Isolates Causing Health Care-Associated Infection in Thailand, Where the CTX-M Family Is Endemic. Antimicrob. Agents Chemother. 2008, 52, 2818–2824. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Deeny, S.R.; Van Kleef, E.; Bou-Antoun, S.; Hope, R.J.; Robotham, J.V. Seasonal changes in the incidence of Escherichia coli bloodstream infection: Variation with region and place of onset. Clin. Microbiol. Infect. 2015, 21, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Asafo-Adjei, K.; Mensah, J.E.; Labi, A.K.; Dayie, N.T.K.D.; Donkor, E.S. Urinary tract infections among bladder outlet obstruction patients in Accra, Ghana: Aetiology, antibiotic resistance, and risk factors. Diseases. 2018, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Donkor, E.S.; Horlutu, P.; Dayie, N.T.K.D.; Labi, A.K. Community acquired urinary tract infections among adults in Accra, Ghana. Infect. Drug Resist. 2019, 12, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Asare, M.; Acquah, S.E.; Agyare, C.; Ayeh-Kumi, P.F. Prevalence of extended-spectrum β- lactamase-producing Escherichia coli among patients attending a tertiary hospital in Ghana. BMC Res. Notes 2021, 14, 1–8. [Google Scholar]
- Dwomoh, F.P.; Kotey, F.C.N.; Dayie, N.T.K.D.; Osei, M.M.; Amoa-Owusu, F.; Bannah, V.; Alzahrani, F.M.; Halawani, I.F.; Alzahrani, K.J.; Egyir, B.; et al. Phenotypic and genotypic detection of carbapenemase-producing Escherichia coli and Klebsiella pneumoniae in Accra, Ghana. PLoS ONE 2022, 17, e0279715. [Google Scholar] [CrossRef]
- Owusu, F.A.; Obeng-Nkrumah, N.; Gyinae, E.; Kodom, S.; Tagoe, R.; Tabi, B.K.A.; Dayie, N.T.K.D.; Opintan, J.A.; Egyir, B. Occurrence of carbapenemases, extended-spectrum β-lactamases and AmpCs among β-lactamase-producing Gram-negative bacteria from clinical sources in Accra, Ghana. Antibiotics 2023, 12, 1016. [Google Scholar] [CrossRef]
- Dayie, N.T.K.D.; Egyir, B.; Amoa-Owusu, F.; Owusu-Nyantakyi, C.; Adu, B.; Kotey, F.C.N.; Donkor, E.S.; Stabler, R.A. Genome sequences of extended-spectrum β-lactamase producing-Escherichia coli recovered from mid-stream urine samples in Accra, Ghana. Microorganisms 2024, 12, 1139. [Google Scholar] [CrossRef]
- Dzowela, T.; Mwenya, D. Prevalence and patterns of extended-spectrum β-lactamase-producing Escherichia coli isolated from clinical specimens in a tertiary hospital, Lusaka, Zambia. BMC Microbiol. 2021, 21, 1–9. [Google Scholar]
- Afriyie, D.K.; Asare, M.; Sule, A.A. Antimicrobial susceptibility patterns and plasmid profiles of extended-spectrum β-lactamase producing Escherichia coli isolates from hospitals in Ghana. Int. J. Microbiol. 2010, 8, 1–8. [Google Scholar]
- Ko, M.-C.; Liu, C.-K.; Woung, L.-C.; Lee, W.-K.; Jeng, H.-S.; Lu, S.-H.; Chiang, H.-S.; Li, C.-Y. Species and Antimicrobial Resistance of Uropathogens Isolated from Patients with Urinary Catheter. Tohoku J. Exp. Med. 2008, 214, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, P.; Cercenado, E.; Rodríguez-Créixems, M.; Díaz, M.D.; Vicente, T.; Bouza, E. The CLED agar option in urine culture routine. Diagn. Microbiol. Infect. Dis. 1992, 15, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 33rd ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2023. [Google Scholar]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Kiiru, J.; Kiriuki, S.; Goddeeris, B.M. Analysis of β-lactamase phenotype and carriage of selected β-lactamase genes among Escherichia coli obtained from Kenyan patients during an 18-year peroid. BiodMed Cent. Microbiol. 2012, 12, 155. [Google Scholar] [CrossRef]
- Clermont, O.; Bonacorsi, S.; Bingen, E. Rapid and Simple Determination of the Escherichia coli Phylogenetic Group. Appl. Environ. Microbiol. 2000, 66, 4555–4558. [Google Scholar] [CrossRef] [PubMed]
- Donkor, E.S.; Osei, J.A.; Anim-Baidoo, I.; Darkwah, S. Risk of asymptomatic bacteriuria among people with sickle cell disease in Accra, Ghana. Diseases 2023, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Pitout, J.D.; Laupland, K.B. Extended-spectrum β-lactamase-producing Enterobacteriaceae: An emerging public-health concern. Lancet Infect. Dis. 2008, 8, 159–166. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Z.; Zhang, Y.; Zhang, Z.; Lei, L.; Xia, Z. Increasing Prevalence of ESBL-Producing Multidrug Resistance Escherichia coli From Diseased Pets in Beijing, China From 2012 to 2017. Front. Microbiol. 2019, 10, 2852. [Google Scholar] [CrossRef]
- Abujnah, A.A.; Zorgani, A.; Sabri MA, M.; El-Mohammady, H.; Khalek, R.A.; Ghenghesh, K.S. Multidrug resistance and extended-spectrum β-lactamases genes among Escherichia coli from patients with urinary tract infections in Northwestern Libya. Libyan J. Med. 2015, 10, 26412. [Google Scholar] [CrossRef]
- Ibrahim, D.R.; Dodd, C.E.R.; Stekel, D.J.; Meshioye, R.T.; Diggle, M.; Lister, M.; Hobman, J.L. Multidrug-Resistant ESBL-Producing, E. coli in Clinical Samples from the UK. Antibiotics 2023, 12, 169. [Google Scholar] [CrossRef]
- Tumbarello, M.; Sanguinetti, M.; Montuori, E.; Trecarichi, E.M.; Posteraro, B.; Fiori, B.; Citton, R.; D’Inzeo, T.; Fadda, G.; Cauda, R.; et al. Predictors of Mortality in Patients with Bloodstream Infections Caused by Extended-Spectrum-β-Lactamase-Producing Enterobacteriaceae: Importance of Inadequate Initial Antimicrobial Treatment. Antimicrob. Agents Chemother. 2007, 51, 3469. [Google Scholar] [CrossRef]
- Ligon, M.M.; Joshi, C.S.; Fashemi, B.E.; Salazar, A.M.; Mysorekar, I.U. Effects of aging on urinary tract epithelial homeostasis and immunity. Dev. Biol. 2023, 493, 29–39. [Google Scholar] [CrossRef]
- Ouedraogo, A.-S.; Sanou, M.; Kissou, A.; Sanou, S.; Solaré, H.; Kaboré, F.; Poda, A.; Aberkane, S.; Bouzinbi, N.; Sano, I.; et al. High prevalence of extended-spectrum ß-lactamase producing enterobacteriaceae among clinical isolates in Burkina Faso. BMC Infect. Dis. 2016, 16, 326. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, M.L.; Furuno, J.P.; Harris, A.D.; Johnson, J.K.; Shardell, M.D.; McGregor, J.C.; Thom, K.A.; Cosgrove, S.E.; Sakoulas, G.; Perencevich, E.N. Comparative effectiveness of nafcillin or cefazolin versus vancomycin in methicillin-susceptible Staphylococcus aureus bacteremia. BMC Infect. Dis. 2011, 11, 279. [Google Scholar] [CrossRef]
- Advisory Group on Integrated Surveillance of Antimicrobial Resistance (AGISAR). Critically Important Antimicrobials for Human Medicine; World Health Organisation: Geneva, Switzerland, 2011; 38p. [Google Scholar]
- Zurfluh, K.; Hächler, H.; Nüesch-Inderbinen, M.; Stephan, R. Characteristics of extended-spectrum β-lactamase- and carbapenemase-producing Enterobacteriaceae isolates from rivers and lakes in Switzerland. Appl. Environ. Microbiol. 2013, 79, 3021–3026. [Google Scholar] [CrossRef]
- Russo, T.A.; Johnson, J.R. Proposal for a new inclusive designation for extraintestinal pathogenic isolates of Escherichia coli: ExPEC. J. Infect. Dis. 2000, 181, 1753–1754. [Google Scholar] [CrossRef] [PubMed]
- Manges, A.R.; Johnson, J.R.; Foxman, B.; O’Bryan, T.T.; Fullerton, K.E.; Riley, L.W. Widespread Distribution of Urinary Tract Infections Caused by a Multidrug-Resistant Escherichia coli Clonal Group. N. Engl. J. Med. 2001, 345, 1007–1013. [Google Scholar] [CrossRef]
- Picard, B.; Garcia, J.S.; Gouriou, S.; Duriez, P.; Brahimi, N.; Bingen, E.; Elion, J.; Denamur, E. The link between phylogeny and virulence in Escherichia coli extraintestinal infection. Infect. Immun. 1999, 67, 546–553. [Google Scholar] [CrossRef]
- Ludden, C.; Raven, K.E.; Jamrozy, D.; Gouliouris, T.; Blane, B.; Coll, F.; De Goffau, M.; Naydenova, P.; Horner, C.; Hernandez-Garcia, J.; et al. One Health Genomic Surveillance of Escherichia coli Demonstrates Distinct Lineages and Mobile Genetic Elements in Isolates from Humans versus Livestock. mBio 2019, 10, e02693-18. [Google Scholar] [CrossRef] [PubMed]
| Target Gene | 5′–3′ Sequence | Size (bp) | Annealing Temperature (°C) |
|---|---|---|---|
| blaTEM | ATGAGTATTCAACAT TTC CG | 840 | 55.1 |
| CCAATGCTTAATCAG TGA GG | |||
| blaSHV | TTCGCCTGTGTATTATCTCCCTG | 854 | 51.2 |
| TTAGCGTTGCCAGTGYTCG | |||
| blaMA | ATGTGCAGYACCAGTAARGTKATGGC | 593 | 51.2 |
| TGGGTRAARTARGTSACCAGAAYCAGCGG | |||
| blaCTX-M 825 | CGC TTT GCC ATG TGC AGC ACC | 307 | 57.7 |
| GCT CAG TAC GAT CGA GCC | |||
| blaCTX-M 914 | GCT CAG TAC GAT CGA GCC | 474 | 56.3 |
| GTA AGC TGA CGC AAC GTC TG | |||
| blaCMY-1 | GTGGTGGATGCCAGCATCC | 915 | 56.1 |
| GGTCGAGCCGGTCTTGTTGAA | |||
| blaCMY-2 | GCACTTAGCCACCTATACGGCAG | 758 | 52.5 |
| GCTTTTCAAGAATGCGCCAGG | |||
| blaOXA-1 | ATGAAAAACACAATACATATCAACTTCGC | 820 | 51.2 |
| GTGTGTTTAGAATGGTGATCGCATT | |||
| blaOXA-2 | ACGATAGTTGTGGCAGACGAAC | 602 | 56.1 |
| ATYCTGTTTGGCGTATCRATATTC | |||
| CF | ATGATGAAAAAATCGTTATGC | 1200 | 56.1 |
| TTGCAGCTTTTCAAGAATGCGC |
| Target Gene | 5′–3′ Sequence | Size (bp) |
|---|---|---|
| TspE4.C2 | GAGTAATGTCGGGGCATTCA | 840 |
| CGCGCCAACAAAGTATTACG | ||
| chuA | GACGAACCAACGGTCAGGAT | 854 |
| TGCCGCCAGTACCAAAGACA | ||
| yjaA | TGAAGTGTCAGGAGACGCTG | 593 |
| ATGGAGAATGCGTTCCTCAAC |
| E. coli ESBL Gene Carriage | No. of Isolates (%) | p Value |
|---|---|---|
| Single gene | 2 (4.9%) | 1.0 |
| Double genes | 7 (17.1%) | |
| Triple genes | 3 (7.3%) | |
| Multiple gene (≥4) | 29 (70.1%) |
| Phylogenetic Groups | No. (%) of Isolates Positive for Amplification | ||
|---|---|---|---|
| ChuA | vjaA | Tspe4.C2 | |
| A (n = 28) | 0 | 8 (28.6) | 0 |
| B1 (n = 11) | 0 | 4 (36.4) | 11 (100) |
| B2 (n = 0) | 0 | 0 | 0 |
| D (n = 2) | 2 (100) | 0 | 2 (100) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armah, E.; Osae-Nyarko, L.; Idun, B.; Ahiabu, M.K.; Agyapong, I.; Kwarteng, F.B.; Oppong, M.; Mohammed, N.; Kotey, F.C.N.; Osei-Atweneboana, M.Y.; et al. High Prevalence of ESBL Genes in Commensal Escherichia coli of the Urinary Tract: Implications for Antibiotic Stewardship among Residents of Ghanaian Elderly Nursing Care Homes. Genes 2024, 15, 985. https://doi.org/10.3390/genes15080985
Armah E, Osae-Nyarko L, Idun B, Ahiabu MK, Agyapong I, Kwarteng FB, Oppong M, Mohammed N, Kotey FCN, Osei-Atweneboana MY, et al. High Prevalence of ESBL Genes in Commensal Escherichia coli of the Urinary Tract: Implications for Antibiotic Stewardship among Residents of Ghanaian Elderly Nursing Care Homes. Genes. 2024; 15(8):985. https://doi.org/10.3390/genes15080985
Chicago/Turabian StyleArmah, Emmanuel, Lawrencia Osae-Nyarko, Bright Idun, Mawutor Kwame Ahiabu, Isaac Agyapong, Freda Boampong Kwarteng, Mercy Oppong, Naael Mohammed, Fleischer C. N. Kotey, Mike Yaw Osei-Atweneboana, and et al. 2024. "High Prevalence of ESBL Genes in Commensal Escherichia coli of the Urinary Tract: Implications for Antibiotic Stewardship among Residents of Ghanaian Elderly Nursing Care Homes" Genes 15, no. 8: 985. https://doi.org/10.3390/genes15080985





