Genome-Wide Identification and Characterization of RopGEF Gene Family in C4 Crops
Abstract
:1. Introduction
2. Materials and Methods
2.1. Identification of RopGEF Family Members in Foxtail Millet
2.2. Distribution of Genes on Chromosomes
2.3. Organization of Exons and Introns, Conserved Amino Acid Motif Arrangement, and Three-Dimensional (3D) Folding Structure Prediction
2.4. Phylogenetic Relationship
2.5. Plant Material, Growth Conditions, Abiotic Stresses and Hormonal Applications in Foxtail Millet
2.6. Total RNA Extraction, cDNA Reverse Transcription, and qRT-PCR Analysis
2.7. Statistical Analysis
3. Results
3.1. Identification and Annotation of RopGEF Family Members
3.2. Phylogenetic Analysis and Classification of the RopGEF Family Members
3.3. Conserved Motif Analysis and 3D Structure Prediction of the RopGEF Family Proteins
3.4. Exon–Intron Distribution of RopGEF Family Genes
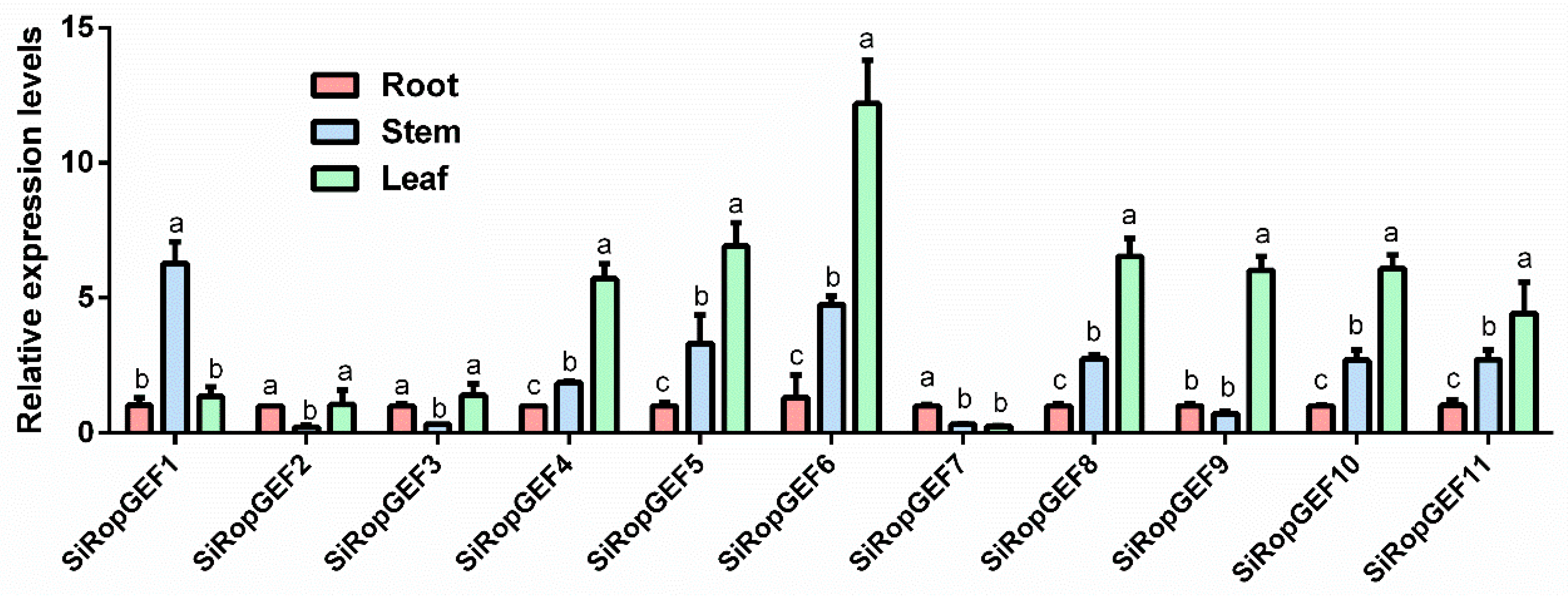
3.5. Tissue-Specific Expression Profiles of SiRopGEF Family Genes
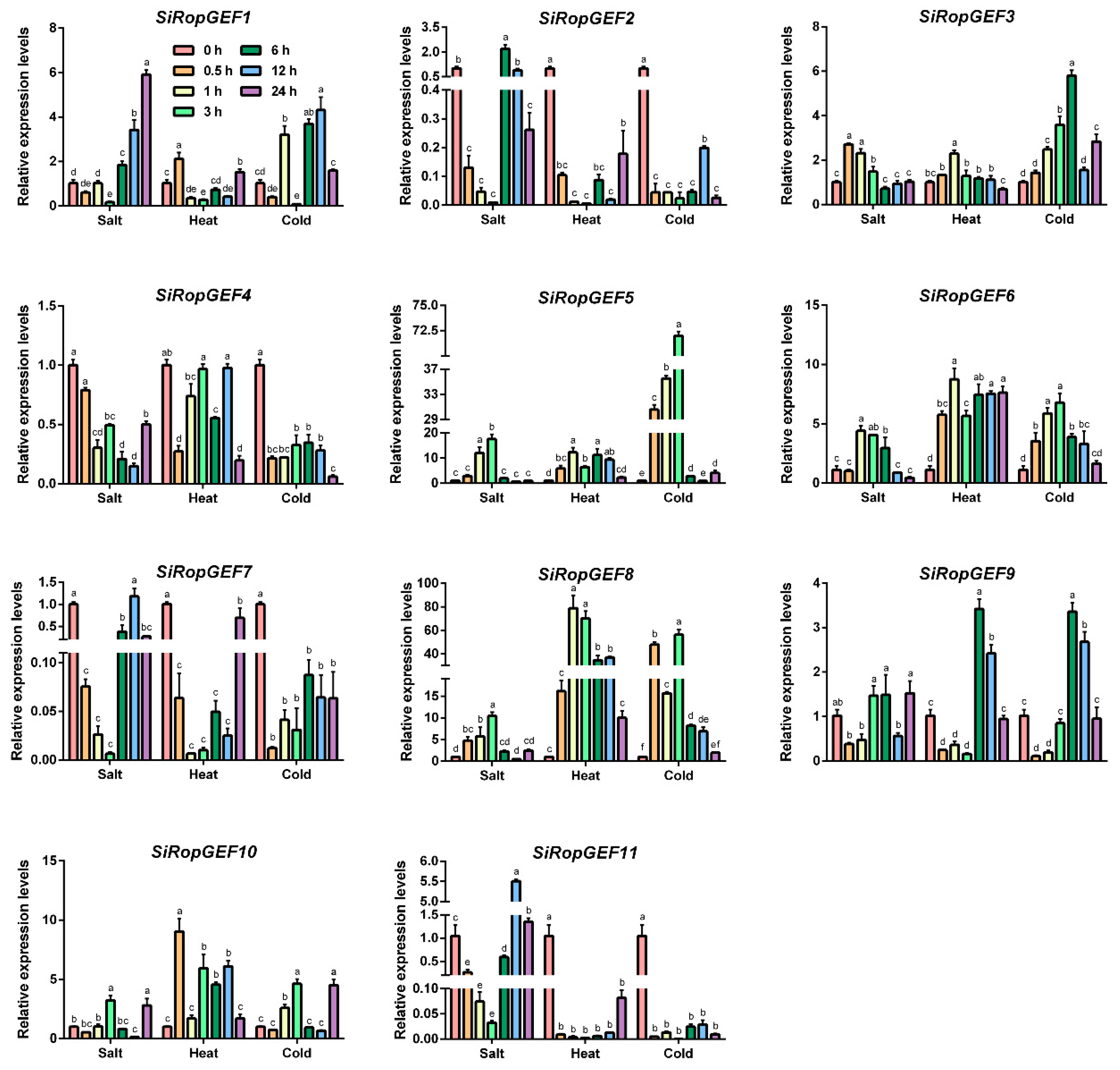
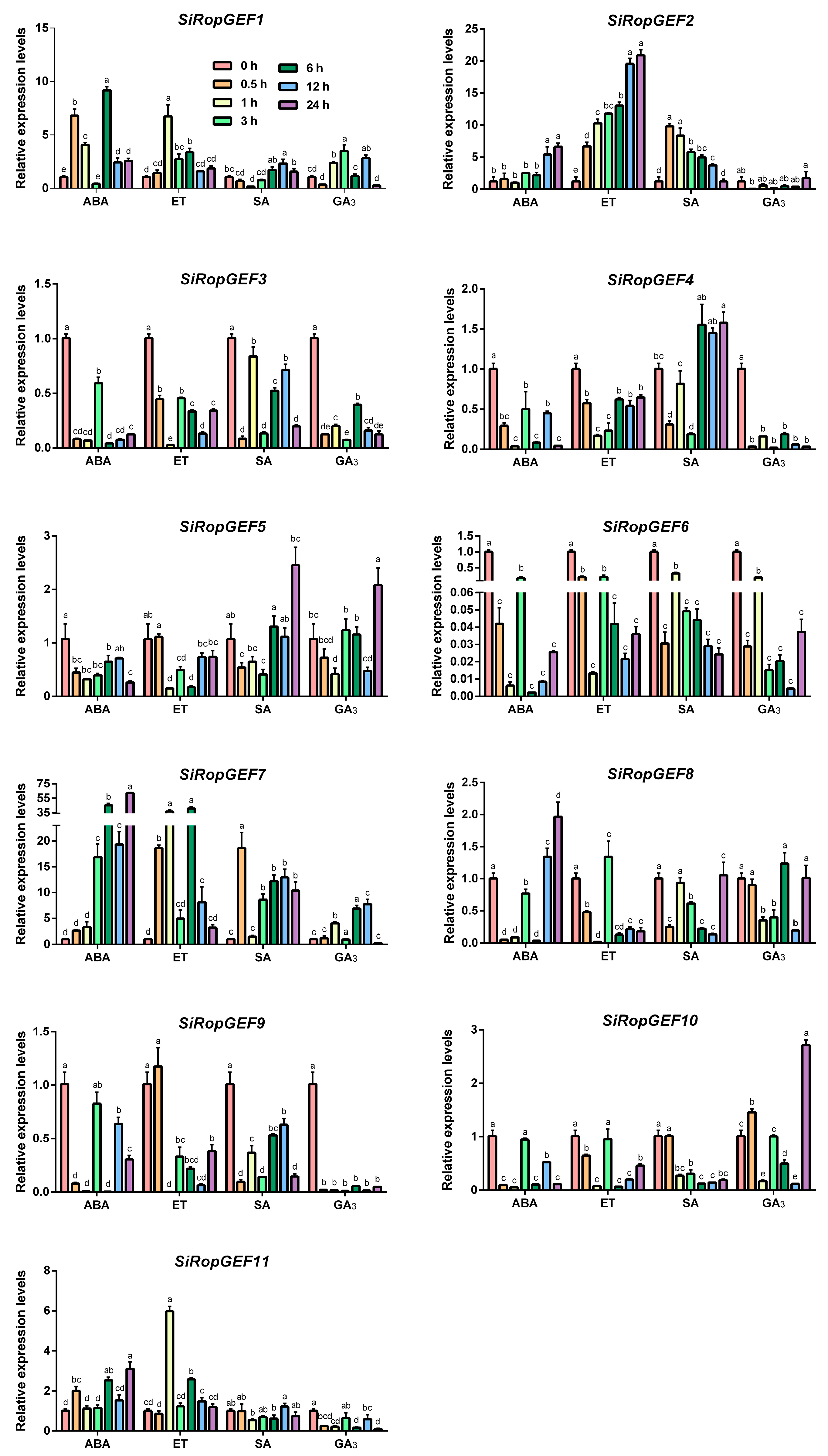
3.6. Transcriptional Profiles of SiRopGEF Family Genes under Abiotic Stresses and Phytohormone
4. Discussion
4.1. The Genetic and Protein Architectures of RopGEF Family Members Demonstrate a Significant Relationship with Evolutionary Mechanisms, Suggesting the Possibility of Functional Redundancy among the Genes within the RopGEF Family
4.2. The Tissue-Specific Expression Patterns of SiRopGEF Family Genes Have Unveiled Various Roles in the Growth and Development of Foxtail Millet
4.3. The Inducible Expression Pattern of SiRopGEF Family Genes Suggests a Crucial Role in Abiotic Stress and Hormone Signaling
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vidhyasekaran, P. G-Proteins as Molecular Switches in Signal Transduction. In PAMP Signals in Plant Innate Immunity; Springer: Berlin/Heidelberg, Germany, 2014; pp. 163–205. [Google Scholar]
- Zheng, Z.-L.; Yang, Z. The Rop GTPase: An emerging signaling switch in plants. Plant Mol. Biol. 2000, 44, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.L.; von Delft, F.; Brennan, P.E. Targeting the Small GTPase Superfamily through Their Regulatory Proteins. Angew. Chem. Int. Ed. 2020, 59, 6342–6366. [Google Scholar] [CrossRef] [PubMed]
- Fehér, A.; Lajkó, D.B. Signals fly when kinases meet Rho-of-plants (ROP) small G-proteins. Plant Sci. 2015, 237, 93–107. [Google Scholar] [CrossRef]
- Ou, H.; Yi, P. ROP GTPase-dependent polarity establishment during tip growth in plants. New Phytol. 2022, 236, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Igisch, C.P.; Miège, C.; Jaillais, Y. Cell shape: A ROP regulatory tug-of-war in pavement cell morphogenesis. Curr. Biol. 2022, 32, R116–R118. [Google Scholar] [CrossRef]
- Yang, Z.; Fu, Y. ROP/RAC GTPase signaling. Curr. Opin. Plant Biol. 2007, 10, 490–494. [Google Scholar] [CrossRef]
- Berken, A.; Thomas, C.; Wittinghofer, A. A new family of RhoGEFs activates the Rop molecular switch in plants. Nature 2005, 436, 1176–1180. [Google Scholar] [CrossRef]
- Ren, H.; Dang, X.; Yang, Y.; Huang, D.; Liu, M.; Gao, X.; Lin, D. SPIKE1 Activates ROP GTPase to Modulate Petal Growth and Shape. Plant Physiol. 2016, 172, 358–371. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Imai, K.; Akamatsu, A.; Mihashi, M.; Hayashi, N.; Shimamoto, K.; Kawasaki, T. SWAP70 functions as a Rac/Rop guanine nucleotide-exchange factor in rice. Plant J. 2012, 70, 389–397. [Google Scholar] [CrossRef]
- Shin, D.H.; Kim, T.-L.; Kwon, Y.-K.; Cho, M.-H.; Yoo, J.; Jeon, J.-S.; Hahn, T.-R.; Bhoo, S.H. Characterization of Arabidopsis RopGEF family genes in response to abiotic stresses. Plant Biotechnol. Rep. 2009, 3, 183–190. [Google Scholar] [CrossRef]
- Gu, Y.; Li, S.; Lord, E.M.; Yang, Z. Members of a Novel Class of Arabidopsis Rho Guanine Nucleotide Exchange Factors Control Rho GTPase-Dependent Polar Growth. Plant Cell 2006, 18, 366–381. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, Q.; Kita, D.; Huang, J.-b.; Liu, G.; Wu, X.; Zhu, X.; Cheung, A.Y.; Wu, H.-M.; Tao, L.-Z. RopGEF1 Plays a Critical Role in Polar Auxin Transport in Early Development. Plant Physiol. 2017, 175, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Z.; Bao, L.-J.; Zhang, S.-S.; Zhang, C.-G.; Li, X.; Li, H.-X.; Zhang, X.-L.; Bones, A.M.; Yang, Z.-B.; et al. The RopGEF2-ROP7/ROP2 Pathway Activated by phyB Suppresses Red Light-Induced Stomatal Opening. Plant Physiol. 2017, 174, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, D. ROPGEF1 and ROPGEF4 are functional regulators of ROP11 GTPase in ABA-mediated stomatal closure in Arabidopsis. FEBS Lett. 2012, 586, 1253–1258. [Google Scholar] [CrossRef]
- Chen, M.; Liu, H.; Kong, J.; Yang, Y.; Zhang, N.; Li, R.; Yue, J.; Huang, J.; Li, C.; Cheung, A.Y.; et al. RopGEF7Regulates PLETHORA-Dependent Maintenance of the Root Stem Cell Niche inArabidopsis. Plant Cell 2011, 23, 2880–2894. [Google Scholar] [CrossRef]
- Shin, D.H.; Cho, M.-H.; Kim, T.-L.; Yoo, J.; Kim, J.-I.; Han, Y.-J.; Song, P.-S.; Jeon, J.-S.; Bhoo, S.H.; Hahn, T.-R. A Small GTPase Activator Protein Interacts with Cytoplasmic Phytochromes in Regulating Root Development. J. Biol. Chem. 2010, 285, 32151–32159. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Song, J.; Tian, X.; Zhang, H.; Li, L.; Zhu, H. Arabidopsis PRK6 interacts specifically with AtRopGEF8/12 and induces depolarized growth of pollen tubes when overexpressed. Sci. China Life Sci. 2017, 61, 100–112. [Google Scholar] [CrossRef]
- Kim, E.-J.; Park, S.-W.; Hong, W.-J.; Silva, J.; Liang, W.; Zhang, D.; Jung, K.-H.; Kim, Y.-J. Genome-wide analysis of RopGEF gene family to identify genes contributing to pollen tube growth in rice (Oryza sativa). BMC Plant Biol. 2020, 20, 95. [Google Scholar] [CrossRef]
- Kim, E.-J.; Hong, W.-J.; Tun, W.; An, G.; Kim, S.-T.; Kim, Y.-J.; Jung, K.-H. Interaction of OsRopGEF3 Protein with OsRac3 to Regulate Root Hair Elongation and Reactive Oxygen Species Formation in Rice (Oryza sativa). Front. Plant Sci. 2021, 12, 661352. [Google Scholar] [CrossRef]
- Xu, Y.; Cai, W.; Chen, X.; Chen, M.; Liang, W. A small Rho GTPase OsRacB is required for pollen germination in rice. Dev. Growth Differ. 2021, 64, 88–97. [Google Scholar] [CrossRef]
- Jing, X.-Q.; Li, W.-Q.; Zhou, M.-R.; Shi, P.-T.; Zhang, R.; Shalmani, A.; Muhammad, I.; Wang, G.-F.; Liu, W.-T.; Chen, K.-M. Rice Carbohydrate-Binding Malectin-Like Protein, OsCBM1, Contributes to Drought-Stress Tolerance by Participating in NADPH Oxidase-Mediated ROS Production. Rice 2021, 14, 100. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, H.; Berberich, T.; Liu, Y.; Tao, L.-z.; Liu, T. Guanine Nucleotide Exchange Factor 7B (RopGEF7B) is involved in floral organ development in Oryza sativa. Rice 2018, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-H.; Park, J.-H.; Cho, S.-H.; Yoo, S.-C.; Li, J.; Zhang, H.; Kim, K.-S.; Koh, H.-J.; Paek, N.-C. The rice bright green leaf (bgl) locus encodes OsRopGEF10, which activates the development of small cuticular papillae on leaf surfaces. Plant Mol. Biol. 2011, 77, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Riely, B.K.; He, H.; Venkateshwaran, M.; Sarma, B.; Schraiber, J.; Ané, J.M.; Cook, D.R. Identification of legume RopGEF gene families and characterization of a Medicago truncatula RopGEF mediating polar growth of root hairs. Plant J. 2011, 65, 230–243. [Google Scholar] [CrossRef]
- Fodor-Dunai, C.; Fricke, I.; Potocký, M.; Dorjgotov, D.; Domoki, M.; Jurca, M.E.; Ötvös, K.; Žárský, V.; Berken, A.; Fehér, A. The phosphomimetic mutation of an evolutionarily conserved serine residue affects the signaling properties of Rho of plants (ROPs). Plant J. 2011, 66, 669–679. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, X.; Chen, L.; Yang, L.; Cui, X.; Cao, Y. The RopGEF Gene Family and Their Potential Roles in Responses to Abiotic Stress in Brassica rapa. Int. J. Mol. Sci. 2024, 25, 3541. [Google Scholar] [CrossRef]
- Ruan, J.; Lai, L.; Ou, H.; Yi, P. Two subtypes of GTPase-activating proteins coordinate tip growth and cell size regulation in Physcomitrium patens. Nat. Commun. 2023, 14, 7084. [Google Scholar] [CrossRef]
- Zhang, Y.; McCormick, S. A distinct mechanism regulating a pollen-specific. Proc. Natl. Acad. Sci. USA 2007, 104, 11830–11835. [Google Scholar] [CrossRef]
- Denninger, P.; Reichelt, A.; Schmidt, V.A.F.; Mehlhorn, D.G.; Asseck, L.Y.; Stanley, C.E.; Keinath, N.F.; Evers, J.-F.; Grefen, C.; Grossmann, G. Distinct RopGEFs Successively Drive Polarization and Outgrowth of Root Hairs. Curr. Biol. 2019, 29, 1854–1865.e5. [Google Scholar] [CrossRef]
- Zhao, S.; Wu, Y.; He, Y.; Wang, Y.; Xiao, J.; Li, L.; Wang, Y.; Chen, X.; Xiong, W.; Wu, Y. RopGEF2 is involved in ABA-suppression of seed germination and post-germination growth of Arabidopsis. Plant J. 2015, 84, 886–899. [Google Scholar] [CrossRef]
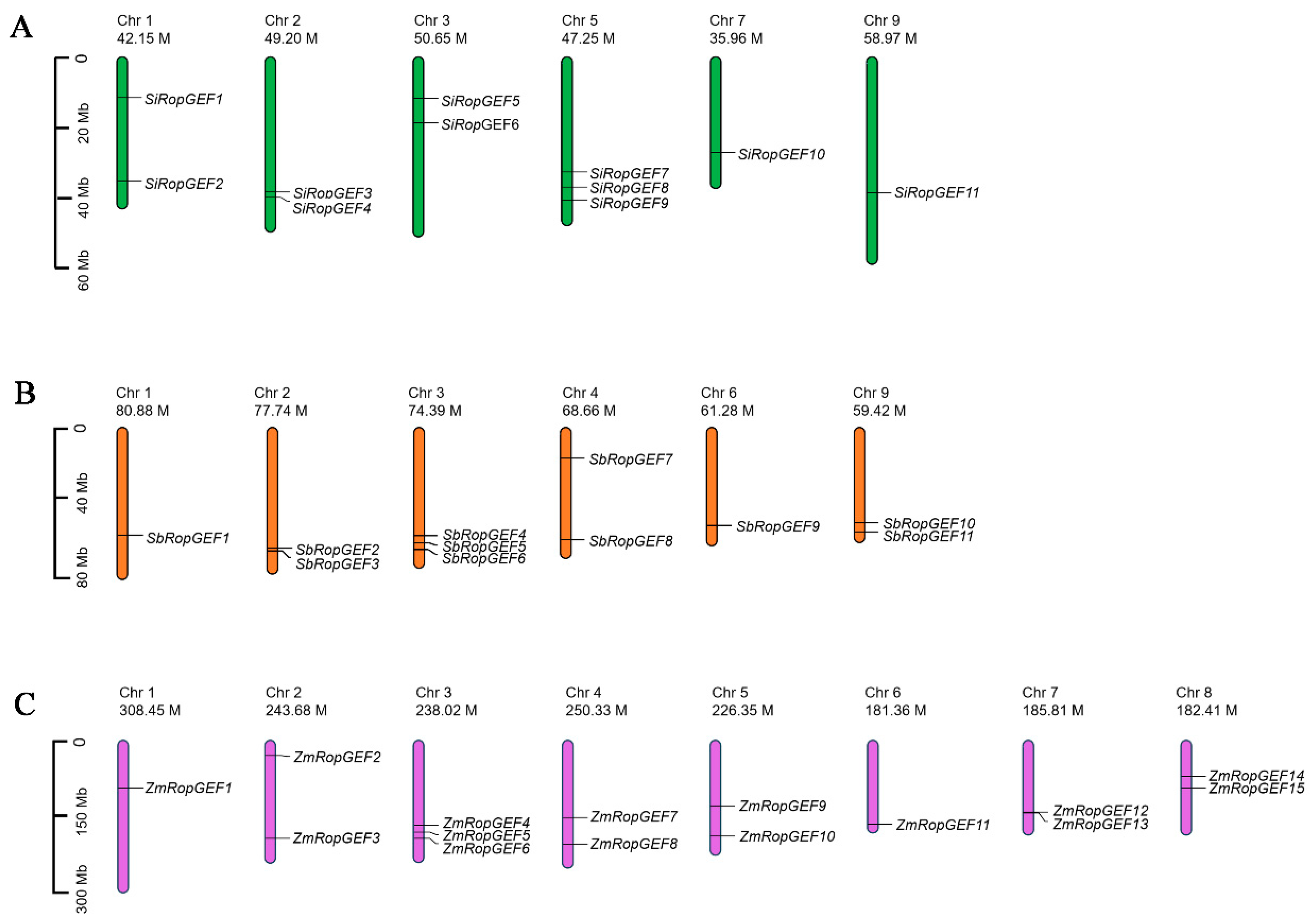
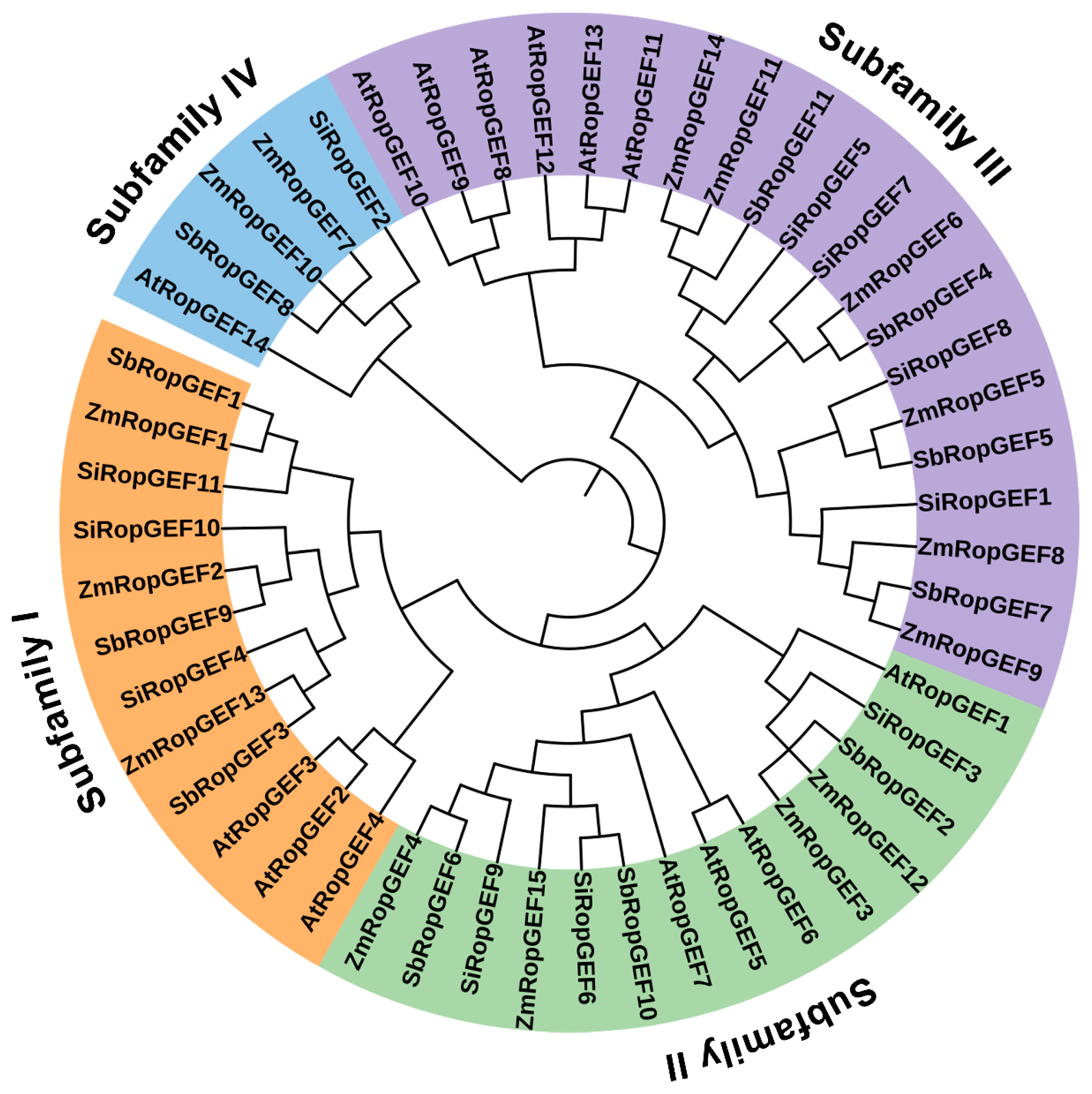
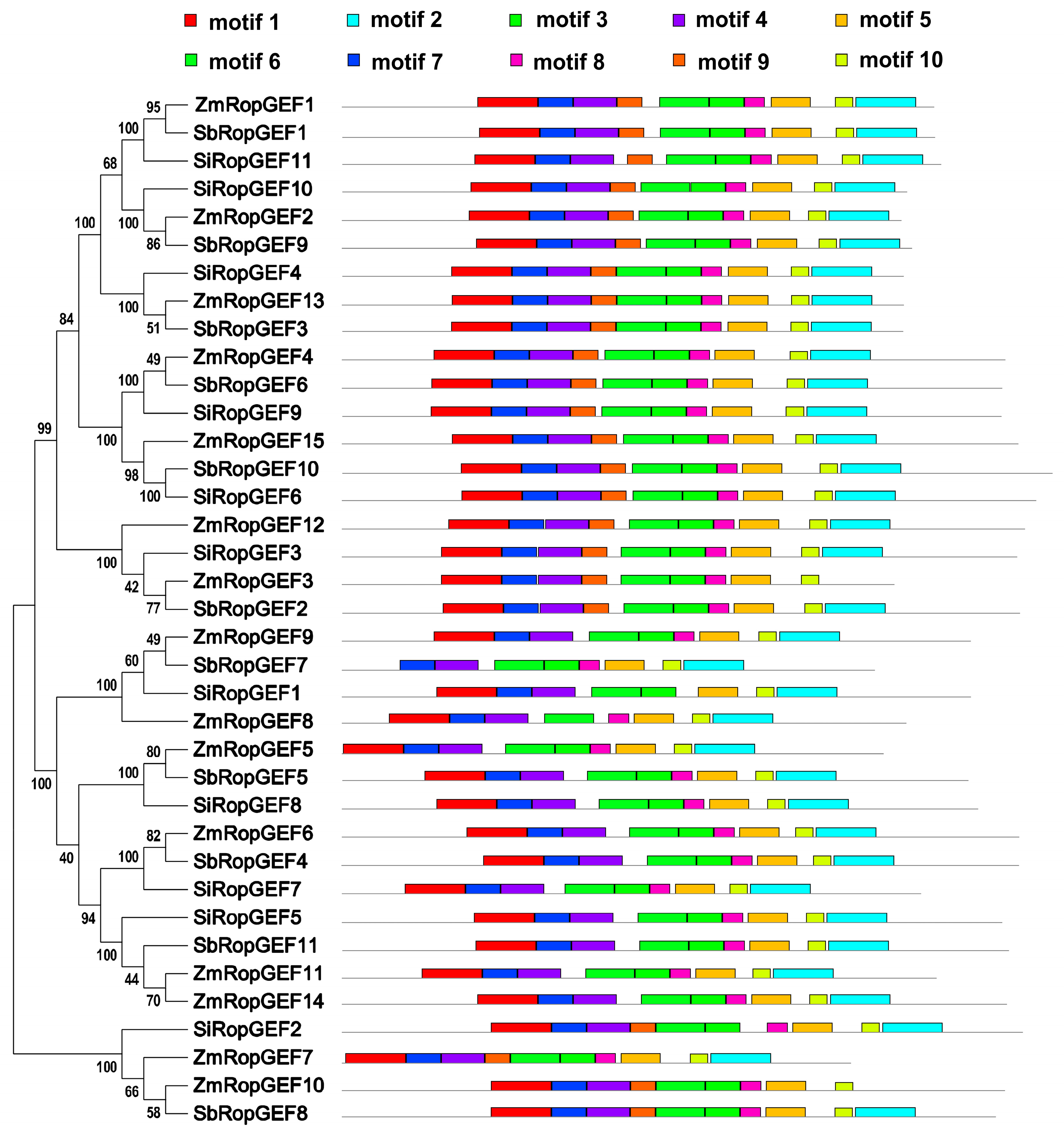
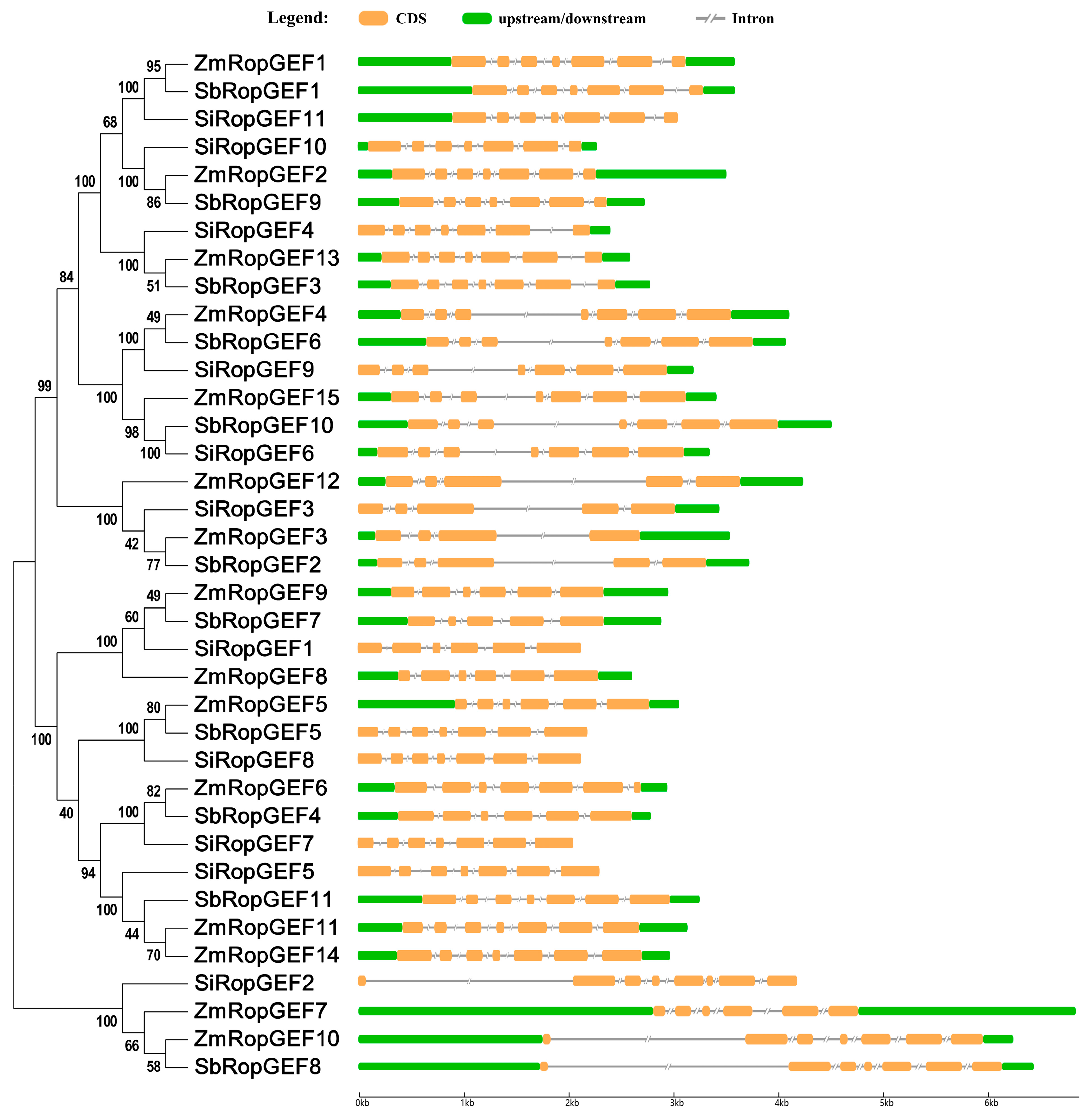
| Name | Gene ID | Genomic Location | Orientation | DNA | mRNA | PROTEIN | Exons |
|---|---|---|---|---|---|---|---|
| SiRopGEF1 | SETIT_019913mg | Chr I: 10,428,890–10,430,997 | Forward | 2108 | 1560 | 519 | 6 |
| SiRopGEF2 | SETIT_020060mg | Chr I: 35,729,463–35,733,658 | Reverse | 4196 | 1689 | 562 | 8 |
| SiRopGEF3 | SETIT_029392mg | Chr II: 38,985,249–38,988,693 | Reverse | 3445 | 2097 | 557 | 5 |
| SiRopGEF4 | SETIT_029765mg | Chr II: 40,550,390–40,552,845 | Reverse | 2456 | 1591 | 463 | 7 |
| SiRopGEF5 | SETIT_021676mg | Chr III: 10,725,845–10,728,180 | Forward | 2336 | 1638 | 545 | 7 |
| SiRopGEF6 | SETIT_021607mg | Chr III: 17,936,647–17,939,988 | Reverse | 3342 | 2154 | 573 | 7 |
| SiRopGEF7 | SETIT_001271mg | Chr V: 32,966,428–32,968,465 | Forward | 2038 | 1437 | 478 | 7 |
| SiRopGEF8 | SETIT_001004mg | Chr V: 37,519,096–37,521,213 | Forward | 2118 | 1578 | 525 | 7 |
| SiRopGEF9 | SETIT_000923mg | Chr V: 41,473,739–41,476,975 | Reverse | 3237 | 1891 | 544 | 7 |
| SiRopGEF10 | SETIT_010033mg | Chr VII: 26,959,231–26,961,508 | Forward | 2278 | 1647 | 466 | 7 |
| SiRopGEF11 | SETIT_035358mg | Chr IX: 39,178,504–39,181,535 | Reverse | 3032 | 2380 | 494 | 7 |
| SbRopGEF1 | SORBI_3001G305300 | Chr 1: 58,918,023–58,921,635 | Forward | 3613 | 2870 | 489 | 7 |
| SbRopGEF2 | SORBI_3002G284100 | Chr 2: 66,489,622–66,493,393 | Reverse | 3772 | 2281 | 559 | 5 |
| SbRopGEF3 | SORBI_3002G302800 | Chr 2: 67,888,132–67,890,903 | Reverse | 2772 | 2037 | 463 | 7 |
| SbRopGEF4 | SORBI_3003G254200 | Chr 3: 59,270,205–59,273,022 | Forward | 2818 | 2251 | 559 | 6 |
| SbRopGEF5 | SORBI_3003G303200 | Chr 3: 63,374,869–63,377,084 | Forward | 2216 | 1554 | 517 | 7 |
| SbRopGEF6 | SORBI_3003G356600 | Chr 3: 67,492,758–67,496,854 | Reverse | 4097 | 2609 | 545 | 7 |
| SbRopGEF7 | SORBI_3004G123100 | Chr 4: 13,838,770–13,841,653 | Reverse | 2884 | 2346 | 440 | 5 |
| SbRopGEF8 | SORBI_3004G269100 | Chr 4: 61,343,743–61,350,249 | Forward | 6473 | 3669 | 540 | 7 |
| SbRopGEF9 | SORBI_3006G176400 | Chr 6: 53,167,615–53,170,360 | Forward | 2746 | 2179 | 471 | 7 |
| SbRopGEF10 | SORBI_3009G159600 | Chr 9: 51,718,034–51,722,569 | Forward | 4536 | 2753 | 586 | 7 |
| SbRopGEF11 | SORBI_3009G229900 | Chr 9: 57,042,535–57,045,813 | Reverse | 3279 | 2558 | 550 | 7 |
| ZmRopGEF1 | Zm00001eb022950 | Chr 1: 89,146,259–89,149,885 | Reverse | 3627 | 2678 | 489 | 9 |
| ZmRopGEF2 | Zm00001eb074280 | Chr 2: 19,660,011–19,663,478 | Reverse | 3468 | 2940 | 462 | 7 |
| ZmRopGEF3 | Zm00001eb104060 | Chr 2: 203,405,080–203,408,547 | Reverse | 3468 | 2374 | 456 | 4 |
| ZmRopGEF4 | Zm00001eb147940 | Chr 3: 184,479,653–184,483,747 | Forward | 4095 | 2607 | 548 | 7 |
| ZmRopGEF5 | Zm00001eb152460 | Chr 3: 200,467,395–200,470,469 | Reverse | 3075 | 2394 | 447 | 8 |
| ZmRopGEF6 | Zm00001eb157000 | Chr 3: 215,085,961–215,088,881 | Reverse | 2921 | 2279 | 559 | 7 |
| ZmRopGEF7 | Zm00001eb188240 | Chr 4: 164,693,645–164,700,513 | Reverse | 6869 | 3164 | 420 | 10 |
| ZmRopGEF8 | Zm00001eb203460 | Chr 4: 228,169,534–228,172,117 | Reverse | 2584 | 2102 | 466 | 6 |
| ZmRopGEF9 | Zm00001eb237470 | Chr 5: 138,168,337–138,171,296 | Reverse | 2960 | 2495 | 519 | 6 |
| ZmRopGEF10 | Zm00001eb251920 | Chr 5: 208,547,083–208,553,330 | Reverse | 6248 | 2288 | 547 | 9 |
| ZmRopGEF11 | Zm00001eb295640 | Chr 6: 175,617,292–175,620,402 | Reverse | 3111 | 2352 | 491 | 7 |
| ZmRopGEF12 | Zm00001eb321170 | Chr 7: 153,963,165–153,967,384 | Forward | 4220 | 2554 | 564 | 5 |
| ZmRopGEF13 | Zm00001eb321410 | Chr 7: 155,027,802–155,030,401 | Reverse | 2600 | 1890 | 464 | 7 |
| ZmRopGEF14 | Zm00001eb343580 | Chr 8: 69,946,147–69,949,185 | Reverse | 3039 | 2292 | 549 | 7 |
| ZmRopGEF15 | Zm00001eb347730 | Chr 8: 97,793,292–97,796,708 | Reverse | 3417 | 2286 | 558 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, X.; Deng, N.; Cai, Y. Genome-Wide Identification and Characterization of RopGEF Gene Family in C4 Crops. Genes 2024, 15, 1112. https://doi.org/10.3390/genes15091112
Jing X, Deng N, Cai Y. Genome-Wide Identification and Characterization of RopGEF Gene Family in C4 Crops. Genes. 2024; 15(9):1112. https://doi.org/10.3390/genes15091112
Chicago/Turabian StyleJing, Xiuqing, Ning Deng, and Yongduo Cai. 2024. "Genome-Wide Identification and Characterization of RopGEF Gene Family in C4 Crops" Genes 15, no. 9: 1112. https://doi.org/10.3390/genes15091112





