Screening Wheat Genotypes for Specific Genes Linked to Drought Tolerance
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Layout
2.3. Traits Measured
2.4. Selection of Genotypes Tolerant to Drought Stress
2.5. Statistical Analyses of the Phenotypic Data
2.6. Molecular Screening for the Best Wheat Genotypes under Drought Stress
2.6.1. DNA Extraction
2.6.2. Drought-Specific Markers
2.6.3. Gel Electrophoresis
3. Results
3.1. Effects of Drought Stress on the Studied Traits
3.2. Genetic Variation in Yield Traits under Drought Stress
3.3. Gene Screening for the Drought-Tolerant Wheat Genotypes
4. Discussion
4.1. Phenotypic and Genetic Variation in Drought Tolerance
4.2. Molecular Screening for the Tested Wheat Genotypes in This Study
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| N | Normal conditions |
| D | Drought conditions |
| SPL | Spike length |
| GNPS | Grain number per spike |
| NSPS | Number of spikelets per spike |
| GYPS | Grain yield per spike |
| RDD | Reduction due to drought effect |
| TFs | Transcription factors |
| RCBD | Randomized complete block design |
| ASR | Average of sum ranks |
References
- Medhat, A.; Al-Naggar, M.; Abd, M.; Abd El-Shafi, E.-M.; Helmy El-Shal, M.; Anany, A.H. Evaluation of Egyptian Wheat Landraces (Triticum aestivum L.) For Drought Tolerance, Agronomic, Grain Yield and Quality Traits. Plant Arch. 2020, 20, 3487–3504. [Google Scholar]
- Challinor, A.J.; Watson, J.; Lobell, D.B.; Howden, S.M.; Smith, D.R.; Chhetri, N. A Meta-Analysis of Crop Yield under Climate Change and Adaptation. Nat. Clim. Change 2014, 4, 287–291. [Google Scholar] [CrossRef]
- World Food and Agriculture—Statistical Yearbook 2022; FAO: Rome, Italy, 2022.
- Kulkarni, M.; Soolanayakanahally, R.; Ogawa, S.; Uga, Y.; Selvaraj, M.G.; Kagale, S. Drought Response in Wheat: Key Genes and Regulatory Mechanisms Controlling Root System Architecture and Transpiration Efficiency. Front. Chem. 2017, 5, 106. [Google Scholar] [CrossRef]
- Eltaher, S.; Sallam, A.; Emara, H.A.; Nower, A.A.; Salem, K.F.M.; Börner, A.; Baenziger, P.S.; Mourad, A.M.I. Genome-Wide Association Mapping Revealed SNP Alleles Associated with Spike Traits in Wheat. Agronomy 2022, 12, 1469. [Google Scholar] [CrossRef]
- Sallam, A.; Alqudah, A.M.; Dawood, M.F.A.; Baenziger, P.S.; Börner, A. Drought Stress Tolerance in Wheat and Barley: Advances in Physiology, Breeding and Genetics Research. Int. J. Mol. Sci. 2019, 20, 3137. [Google Scholar] [CrossRef]
- Flexas, J.; Barón, M.; Bota, J.; Ducruet, J.M.; Gallé, A.; Galmés, J.; Jiménez, M.; Pou, A.; Ribas-Carbó, M.; Sajnani, C.; et al. Photosynthesis Limitations during Water Stress Acclimation and Recovery in the Drought-Adapted Vitis Hybrid Richter-110 (V. berlandieri × V. rupestris). J. Exp. Bot. 2009, 60, 2361–2377. [Google Scholar] [CrossRef]
- Fischer, R.A. Wheat Physiology: A Review of Recent Developments. Crop Pasture Sci. 2010, 62, 95–114. [Google Scholar] [CrossRef]
- Aprile, A.; Havlickova, L.; Panna, R.; Marè, C.; Borrelli, G.M.; Marone, D.; Perrotta, C.; Rampino, P.; De Bellis, L.; Curn, V.; et al. Different Stress Responsive Strategies to Drought and Heat in Two Durum Wheat Cultivars with Contrasting Water Use Efficiency. BMC Genom. 2013, 14, 821. [Google Scholar] [CrossRef]
- Thabet, S.G.; Moursi, Y.S.; Karam, M.A.; Graner, A.; Alqudah, A.M. Genetic Basis of Drought Tolerance during Seed Germination in Barley. PLoS ONE 2018, 13, e0206682. [Google Scholar] [CrossRef]
- Rasheed, A.; Wen, W.; Gao, F.; Zhai, S.; Jin, H.; Liu, J.; Guo, Q.; Zhang, Y.; Dreisigacker, S.; Xia, X.; et al. Development and Validation of KASP Assays for Genes Underpinning Key Economic Traits in Bread Wheat. Theor. Appl. Genet. 2016, 129, 1843–1860. [Google Scholar] [CrossRef]
- Agarwal, P.K.; Agarwal, P.; Reddy, M.K.; Sopory, S.K. Role of DREB Transcription Factors in Abiotic and Biotic Stress Tolerance in Plants. Plant Cell Rep. 2006, 25, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Egawa, C.; Kobayashi, F.; Ishibashi, M.; Nakamura, T.; Nakamura, C.; Takumi, S. Differential Regulation of Transcript Accumulation and Alternative Splicing of a DREB2 Homolog under Abiotic Stress Conditions in Common Wheat. Genes Genet. Syst. 2006, 81, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Abdalrahman, M.; EL-defrawy, M.M.; Dawood, M.; Börner, A. Genetic Variation in Peduncle Traits and Their Association with Spike Traits under Normal and Drought Conditions in Spring Wheat. In Proceedings of the Cereal Molecular Breeding and Genetics—International Symposium, Assuit, Egypt, 9 September 2022. [Google Scholar]
- Pour-Aboughadareh, A.; Yousefian, M.; Moradkhani, H.; Moghaddam Vahed, M.; Poczai, P.; Siddique, K.H.M. IPASTIC: An Online Toolkit to Estimate Plant Abiotic Stress Indices. Appl. Plant Sci. 2019, 7, e11278. [Google Scholar] [CrossRef]
- Utz, H.F. PLABSTAT A Computer Program for Statistical Analysis of Plant Breeding Experiments Version 3A-Pre of 2005-08-16; Institute of Plant Breeding, Seed Science and Population Genetics, University of Hohenheim: Stuttgart, Germany, 1986. [Google Scholar]
- Thermo Scientific. GeneJET Plant Genomic DNA Purification Mini Kit #K0791, #K0792; Thermo Scientific: Waltham, MA, USA, 2016. [Google Scholar]
- Ahmed, A.A.M.; Dawood, M.F.A.; Elfarash, A.; Mohamed, E.A.; Hussein, M.Y.; Börner, A.; Sallam, A. Genetic and Morpho-Physiological Analyses of the Tolerance and Recovery Mechanisms in Seedling Stage Spring Wheat under Drought Stress. Front. Genet. 2022, 13, 1010272. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, Z.; Xiao, H.; Yang, Y. Characterization of TaDREB1 in Wheat Genotypes with Different Seed Germination under Osmotic Stress. Hereditas 2018, 155, 26. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Iqbal, I.; Mehmood Rana, R.; Ur Rehman, S.; Haidery, Q.; Ahmad, F.; Ijaz, A.; Muhammad Imran Umar, H. A Comprehensive Review of Effects of Water Stress and Tolerance in Wheat (Triticuma estivum L.). Trop. Plant Res. 2015, 3, 271–275. [Google Scholar]
- Qaseem, M.F.; Qureshi, R.; Shaheen, H. Effects of Pre-Anthesis Drought, Heat and Their Combination on the Growth, Yield and Physiology of Diverse Wheat (Triticum aestivum L.) Genotypes Varying in Sensitivity to Heat and Drought Stress. Sci. Rep. 2019, 9, 6955. [Google Scholar] [CrossRef]
- Liu, H.; Searle, I.R.; Mather, D.E.; Able, A.J.; Able, J.A. Morphological, Physiological and Yield Responses of Durum Wheat to Pre-Anthesis Water-Deficit Stress Are Genotype Dependent. Crop Pasture Sci. 2015, 66, 1024–1038. [Google Scholar] [CrossRef]
- Barnabás, B.; Jäger, K.; Fehér, A. The Effect of Drought and Heat Stress on Reproductive Processes in Cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef]
- Kiliç, H.; Yağbasanlar, T. The Effect of Drought Stress on Grain Yield, Yield Components and Some Quality Traits of Durum Wheat (Triticum turgidum ssp. durum) Cultivars. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 164–170. [Google Scholar] [CrossRef]
- Paknejad, F.; Nasri, M.; Moghadam, H.T.; Zahedi, H.; Alahmadi, M.J. Effects of Drought Stress on Chlorophyll Fluorescence Parameters, Chlorophyll Content and Grain Yield of Wheat Cultivars. J. Biol. Sci. 2007, 7, 841–847. [Google Scholar] [CrossRef]
- Sallam, A.; Mourad, A.M.I.; Hussain, W.; Stephen Baenziger, P. Genetic Variation in Drought Tolerance at Seedling Stage and Grain Yield in Low Rainfall Environments in Wheat (Triticum aestivum L.). Euphytica 2018, 214, 169. [Google Scholar] [CrossRef]
- Reynolds, M.P.; Pierre, C.S.; Saad, A.S.I.; Vargas, M.; Condon, A.G. Evaluating Potential Genetic Gains in Wheat Associated with Stress-Adaptive Trait Expression in Elite Genetic Resources under Drought and Heat Stress. Crop Sci. 2007, 47, S-172–S-189. [Google Scholar] [CrossRef]
- Mourad, A.M.I.; Amin, A.E.-E.A.Z.; Dawood, M.F.A. Genetic Variation in Kernel Traits under Lead and Tin Stresses in Spring Wheat Diverse Collection. Environ. Exp. Bot. 2021, 192, 104646. [Google Scholar] [CrossRef]
- Hasseb, N.M.; Sallam, A.; Karam, M.A.; Gao, L.; Wang, R.R.C.; Moursi, Y.S. High-LD SNP Markers Exhibiting Pleiotropic Effects on Salt Tolerance at Germination and Seedlings Stages in Spring Wheat. Plant Mol. Biol. 2022, 108, 585–603. [Google Scholar] [CrossRef]
- Sallam, A.; Awadalla, R.A.; Elshamy, M.M.; Börner, A.; Heikal, Y.M. Genome-Wide Analysis for Root and Leaf Architecture Traits Associated with Drought Tolerance at the Seedling Stage in a Highly Ecologically Diverse Wheat Population. Comput. Struct. Biotechnol. J. 2024, 23, 870–882. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Luo, T.; Zhao, H.; Su, Y.; Ji, W.; Li, H. Identification of Wheat DREB Genes and Functional Characterization of TaDREB3 in Response to Abiotic Stresses. Gene 2020, 740, 144514. [Google Scholar] [CrossRef]
- Huang, X.; Song, X.; Chen, R.; Zhang, B.; Li, C.; Liang, Y.; Qiu, L.; Fan, Y.; Zhou, Z.; Zhou, H.; et al. Genome-Wide Analysis of the DREB Subfamily in Saccharum spontaneum Reveals Their Functional Divergence During Cold and Drought Stresses. Front. Genet. 2020, 10, 1326. [Google Scholar] [CrossRef]
- Eltaher, S.; Hashem, M.; Ahmed, A.A.M.; Baenziger, P.S.; Börner, A.; Sallam, A. Effectiveness of TaDreb-B1 and 1-FEH W3 KASP Markers in Spring and Winter Wheat Populations for Marker-Assisted Selection to Improve Drought Tolerance. Int. J. Mol. Sci. 2023, 24, 8986. [Google Scholar] [CrossRef]
- Zhao, J.-Y.; Lu, Z.-W.; Sun, Y.; Fang, Z.-W.; Chen, J.; Zhou, Y.-B.; Chen, M.; Ma, Y.-Z.; Xu, Z.-S.; Min, D.-H. The Ankyrin-Repeat Gene GmANK114 Confers Drought and Salt Tolerance in Arabidopsis and Soybean. Front. Plant Sci. 2020, 11, 584167. [Google Scholar] [CrossRef]
- Kaur, A.; Madhu; Upadhyay, S.K. EF-Hand Domain-Containing Proteins: Diversity and Role in Plants. In Cation Transporters in Plants; Elsevier: Amsterdam, The Netherlands, 2022; pp. 185–203. [Google Scholar]
- Anwar, A.; Wang, K.; Wang, J.; Shi, L.; Du, L.; Ye, X. Expression of Arabidopsis Ornithine Aminotransferase (AtOAT) Encoded Gene Enhances Multiple Abiotic Stress Tolerances in Wheat. Plant Cell Rep. 2021, 40, 1155–1170. [Google Scholar] [CrossRef] [PubMed]
- Sornaraj, P.; Luang, S.; Lopato, S.; Hrmova, M. Basic Leucine Zipper (BZIP) Transcription Factors Involved in Abiotic Stresses: A Molecular Model of a Wheat BZIP Factor and Implications of Its Structure in Function. Biochim. Biophys. Acta (BBA)—General. Subj. 2016, 1860, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xin, M.; Qin, J.; Peng, H.; Ni, Z.; Yao, Y.; Sun, Q. Temporal Transcriptome Profiling Reveals Expression Partitioning of Homeologous Genes Contributing to Heat and Drought Acclimation in Wheat (Triticum aestivum L.). BMC Plant Biol. 2015, 15, 152. [Google Scholar] [CrossRef] [PubMed]
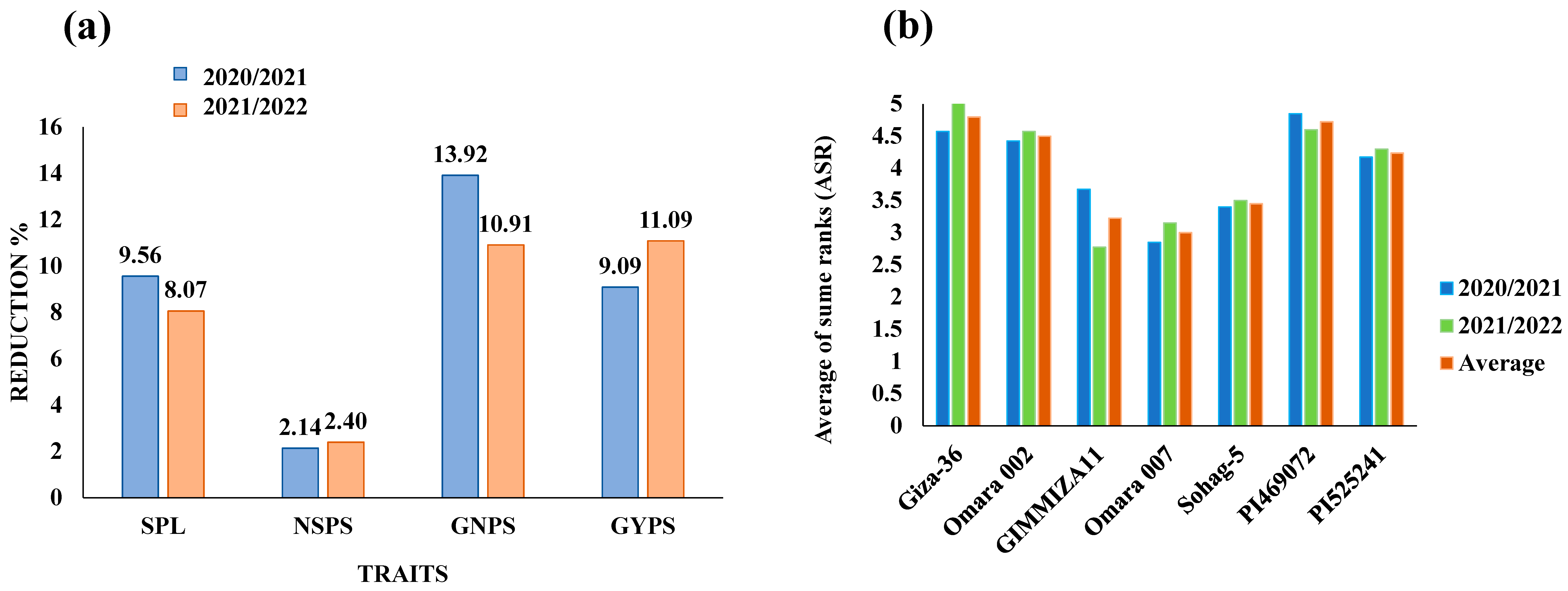
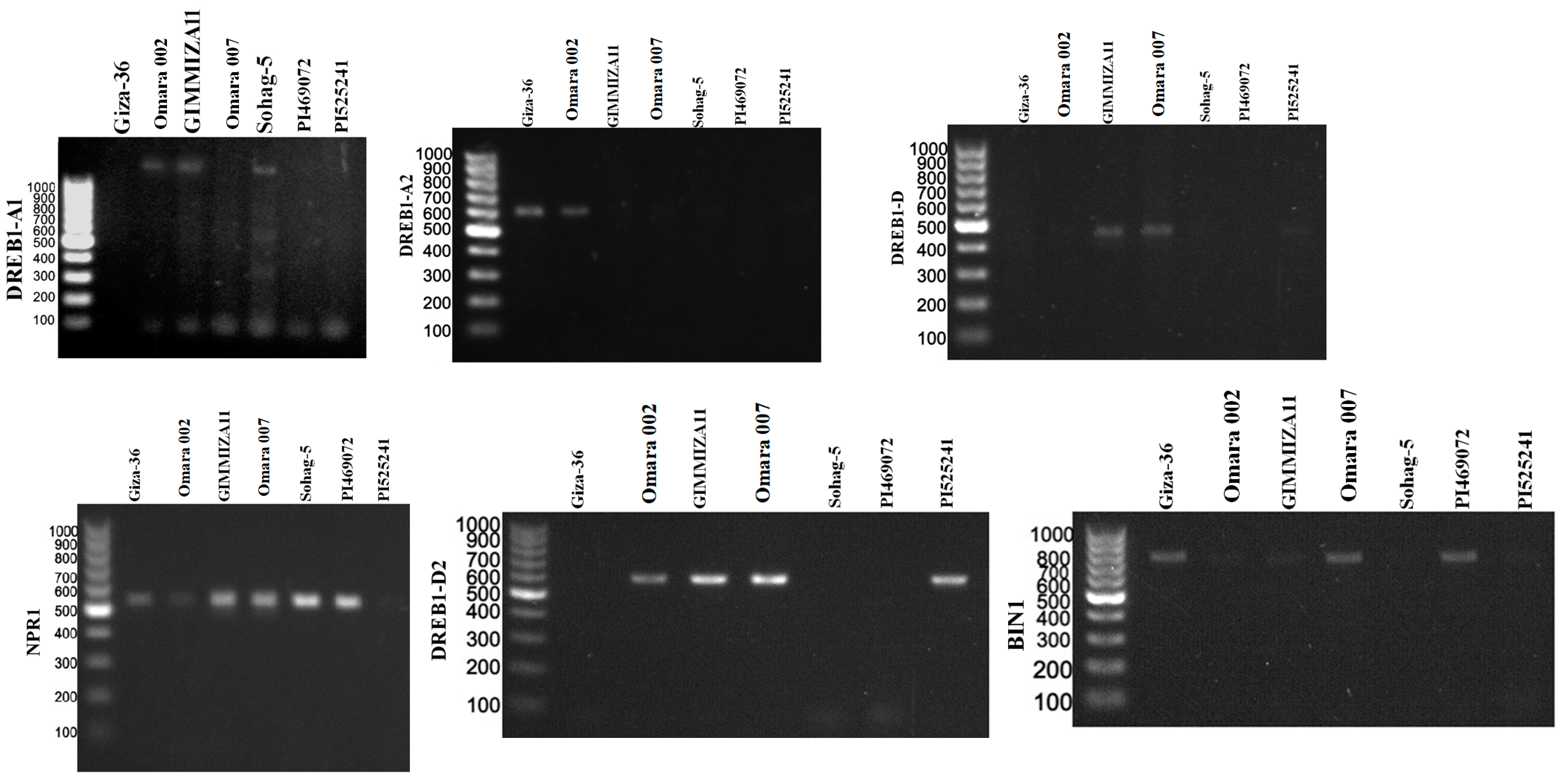
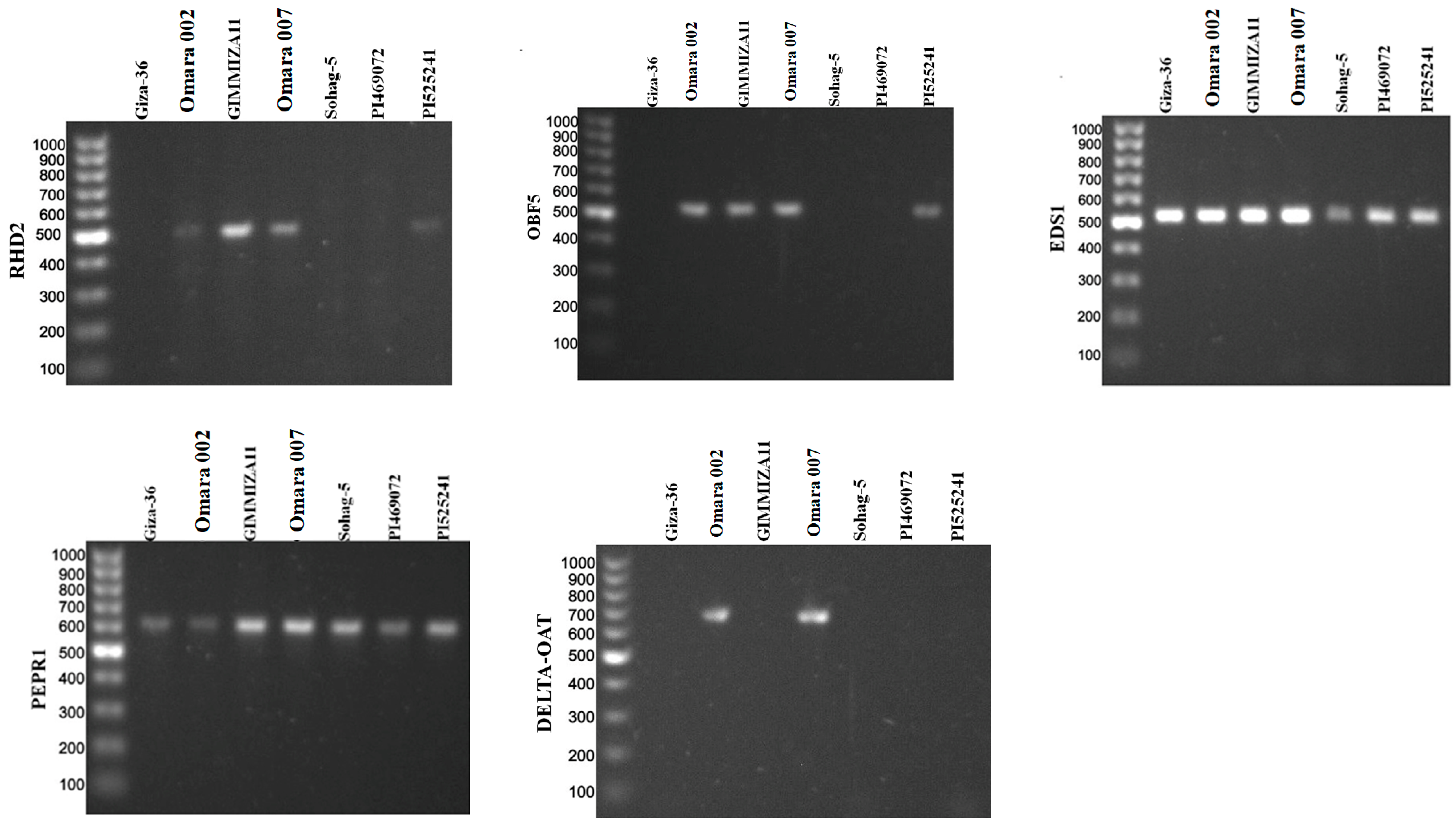

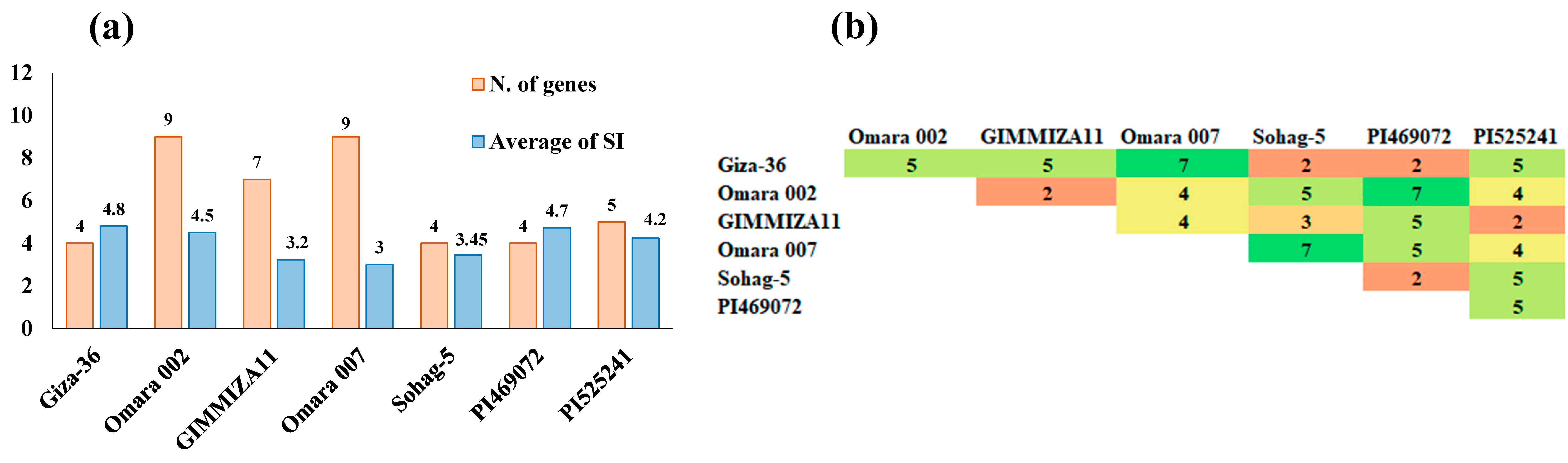
| Source | DF | SPL | NSPS | GNPS | GYPS |
|---|---|---|---|---|---|
| Y | 1 | 21.875 ** | 5.59 * | 1739.85 ** | 3.21 * |
| T | 1 | 0.096 | 0.0182 | 51.07 | 0.23 |
| R | 1 | 12.99 * | 2.16 | 2420.13 ** | 2.11 * |
| G | 6 | 243.93 ** | 45.64 ** | 1570.55 ** | 3.14 ** |
| GY | 6 | 7.04 ** | 2.52 * | 145.8 | 0.182 |
| GT | 6 | 1.11 | 1.98 | 120.25 | 0.143 |
| GRTY | 34 | 0.48 | 102.21 | 1.11 | 1.77 |
| Item | Year | Normal | Drought | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SPL | NSPS | GNPS | GYPS | SPL | NSPS | GNPS | GYPS | ||
| Minimum | 2020/2021 | 7.67 | 23 | 69 | 3.07 | 7.67 | 23 | 56.33 | 3.37 |
| 2021/2022 | 7.67 | 21.67 | 60 | 3.45 | 8 | 25 | 58 | 2.99 | |
| Maximum | 2020/2021 | 29 | 31.67 | 119.33 | 6.56 | 20.67 | 30.33 | 100.67 | 5.46 |
| 2021/2022 | 26.67 | 33.67 | 149 | 7.77 | 20.67 | 31 | 110.67 | 5.48 | |
| Mean | 2020/2021 | 14.17 | 27.79 | 87.83 | 4.7 | 13.02 | 27.12 | 78.25 | 4.18 |
| 2021/2022 | 14.19 | 27.79 | 91.31 | 4.78 | 12.83 | 27.19 | 78.6 | 4.35 | |
| Control | Drought | ||||||
|---|---|---|---|---|---|---|---|
| SPL | NSPS | NSPS | GYPS | SPL | NSPS | NSPS | GYPS |
| 0.98 ** | 0.92 ** | 0.85 ** | 0.72 ** | 0.99 ** | 0.89 ** | 0.83 ** | 0.98 ** |
| Season | 2020/2021 | 2021/2022 | Average | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Genotype | SPL | NSPS | GNPS | GYPS | SPL | NSPS | GNPS | GYPS | |
| Giza-36 | 4 | 3.8 | 6.5 | 4 | 4.4 | 5.8 | 6.1 | 3.8 | 4.8 |
| Omara 002 | 3.9 | 2.7 | 5.7 | 5.4 | 4.2 | 2.8 | 5.4 | 5.9 | 4.5 |
| GIMMIZA11 | 2 | 4.7 | 3.8 | 4.2 | 2.3 | 4.2 | 3 | 1.6 | 3.225 |
| Omara 007 | 3.4 | 4.2 | 2.4 | 1.4 | 3.4 | 3.4 | 2.7 | 3.1 | 3 |
| Sohag-5 | 3.8 | 6.4 | 1.4 | 2 | 5.1 | 4.4 | 1.6 | 2.9 | 3.45 |
| PI469072 | 5.5 | 4 | 4.5 | 5.4 | 3.8 | 4.4 | 4.8 | 5.4 | 4.725 |
| PI525241 | 5.4 | 2 | 3.7 | 5.6 | 4.8 | 2.7 | 4.4 | 5.3 | 4.2375 |
| No. of Genotypes | Genotype | Country | BIN1 | NIM1 | RHD2 | OAT | OBF5 | PEPR1 | EDS1 | DREB1-A1 | DREB1-A2 | DREB1-D | DREB1-D2 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Giza-36 | Egypt | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 4 |
| 2 | Omara 002 | Egypt | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 9 |
| 3 | GIMMIZA11 | Egypt | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 7 |
| 4 | Omara 007 | Egypt | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 9 |
| 5 | Sohag-5 | Egypt | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 4 |
| 6 | PI469072 | Greece | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 4 |
| 7 | PI525241 | Morocco | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 5 |
| Total | 2 | 6 | 4 | 2 | 4 | 7 | 7 | 3 | 2 | 1 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sallam, A.; El-Defrawy, M.M.H.; Dawood, M.F.A.; Hashem, M. Screening Wheat Genotypes for Specific Genes Linked to Drought Tolerance. Genes 2024, 15, 1119. https://doi.org/10.3390/genes15091119
Sallam A, El-Defrawy MMH, Dawood MFA, Hashem M. Screening Wheat Genotypes for Specific Genes Linked to Drought Tolerance. Genes. 2024; 15(9):1119. https://doi.org/10.3390/genes15091119
Chicago/Turabian StyleSallam, Ahmed, Mohamed M. H. El-Defrawy, Mona F. A. Dawood, and Mostafa Hashem. 2024. "Screening Wheat Genotypes for Specific Genes Linked to Drought Tolerance" Genes 15, no. 9: 1119. https://doi.org/10.3390/genes15091119
APA StyleSallam, A., El-Defrawy, M. M. H., Dawood, M. F. A., & Hashem, M. (2024). Screening Wheat Genotypes for Specific Genes Linked to Drought Tolerance. Genes, 15(9), 1119. https://doi.org/10.3390/genes15091119






