Multiple Localization Analysis of the Major QTL—sfw 2.2 for Controlling Single Fruit Weight Traits in Melon Based on SLAF Sequencing
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Phenotyping
2.2. Phenotype Collection
2.3. SLAF-Seq and Indel Molecular Marker Screening
2.4. Candidate Gene Screening
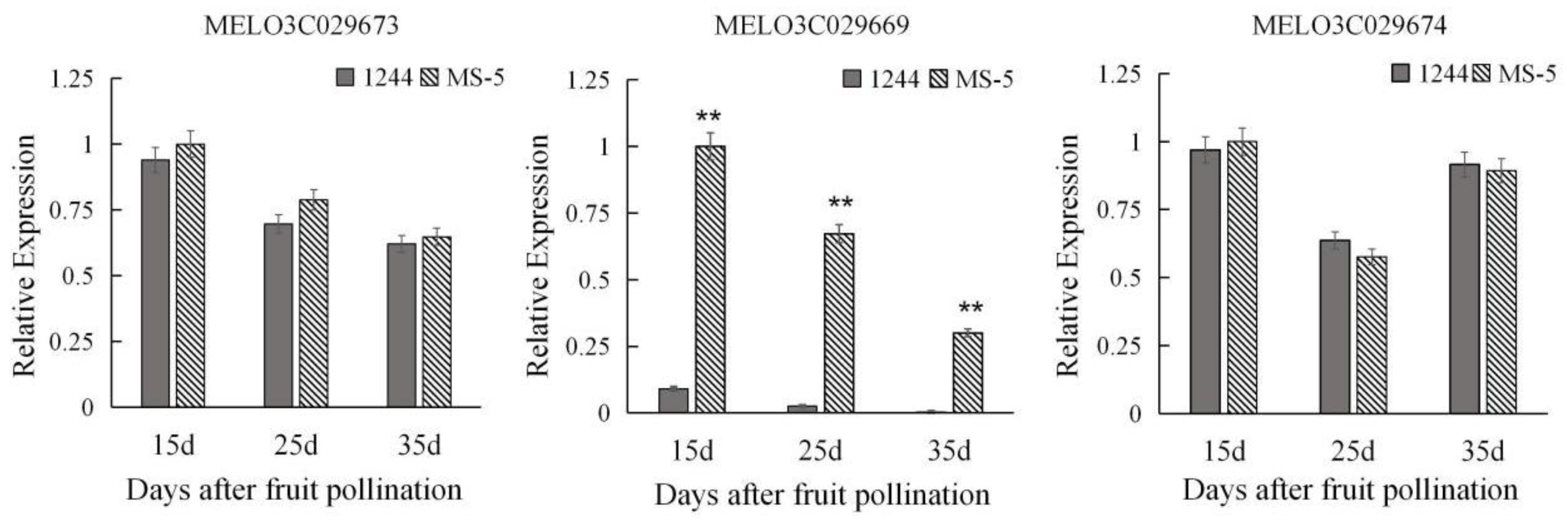
2.5. Candidate Gene qRT—PCR Verification
2.6. Data Analysis
2.6.1. Field Experiment
2.6.2. Linkage Map Construction and QTL Analysis
2.6.3. qRT-PCR Data Analysis
3. Results
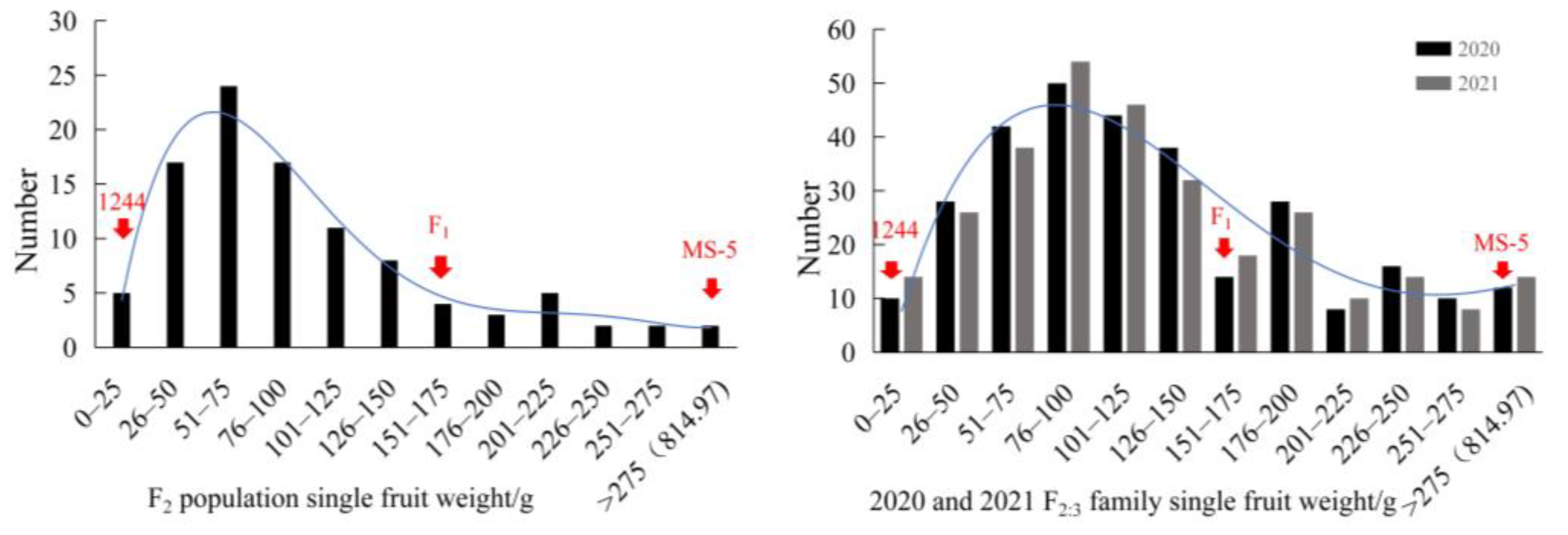
3.1. Analysis of Single Fruit Weight
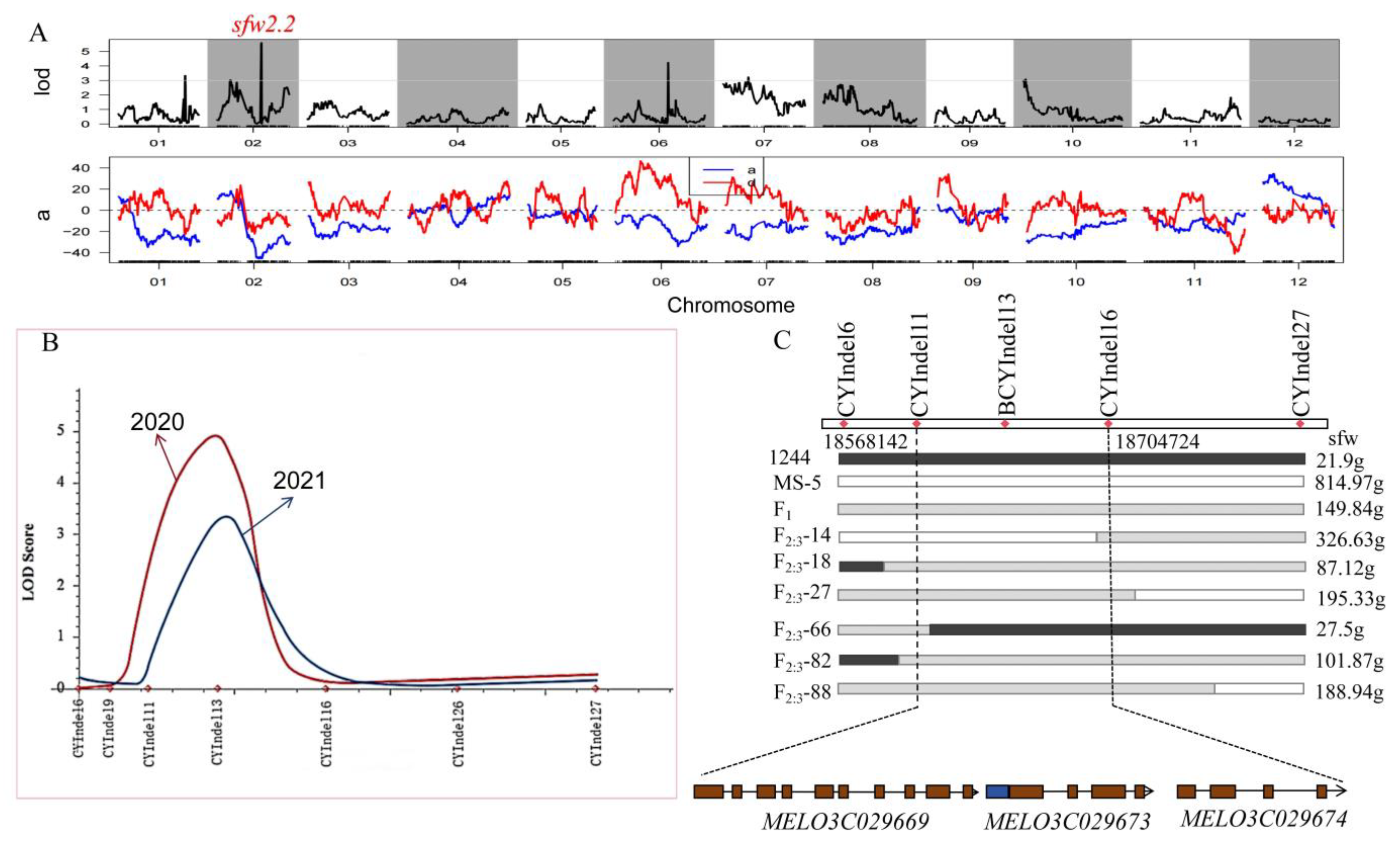
3.2. SLAF Sequencing and QTL Analysis of Melon Single Fruit Weight in the F2 Population
3.3. Further Localization of the sfw2.2 Locus
3.4. Candidate Genes and Preliminary Functional Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quero-García, J.; Campoy, J.; Barreneche, T.; Le Dantec, L.; Wenden, B.; Fouché, M.; Dirlewanger, E.; Silva, H.; Cai, L.; Iezzoni, A. Present and future of marker-assisted breeding in sweet and sour cherry. Acta Hortic. 2019, 2019, 1235. [Google Scholar] [CrossRef]
- Shi, P.; Xu, Z.; Zhang, S.; Wang, X.; Ma, X.; Zheng, J.; Xing, L.; Zhang, D.; Ma, J.; Han, M.; et al. Construction of a high-density SNP-based genetic map and identification of fruit-related QTLs and candidate genes in peach [Prunus persica (L.) Batsch]. BMC Plant Biol. 2020, 20, 438. [Google Scholar] [CrossRef]
- Illa-Berenguer, E.; Van Houten, J.; Huang, Z.; van der Knaap, E. Rapid and reliable identification of tomato fruit weight and locule number loci by QTL-seq. Theor. Appl. Genet. 2015, 128, 1329–1342. [Google Scholar] [CrossRef] [PubMed]
- Curtolo, M.; Cristofani-Yaly, M.; Gazaffi, R.; Takita, M.A.; Figueira, A.; Machado, M.A. QTL mapping for fruit quality in Citrus using DArTseq markers. BMC Genom. 2017, 18, 289. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yan, A.; Sun, L.; Zhang, G.; Wang, X.; Ren, J.; Xu, H. Novel stable QTLs identification for berry quality traits based on high-density genetic linkage map construction in table grape. BMC Plant Biol. 2020, 20, 411. [Google Scholar] [CrossRef]
- Natarajan, S.; Hossain, M.R.; Kim, H.-T.; Denison, M.I.J.; Ferdous, M.J.; Jung, H.-J.; Park, J.-I.; Nou, I.-S. ddRAD-seq derived genome-wide SNPs, high density linkage map and QTLs for fruit quality traits in strawberry (Fragaria x ananassa). 3 Biotech 2020, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Mengist, M.F.; Bostan, H.; Young, E.; Kay, K.L.; Gillitt, N.; Ballington, J.; Kay, C.D.; Ferruzzi, M.G.; Ashrafi, H.; Lila, M.A.; et al. High-density linkage map construction and identification of loci regulating fruit quality traits in blueberry. Hortic. Res. 2021, 8, 169. [Google Scholar] [CrossRef]
- Nantawan, U.; Kanchana-Udomkan, C.; Bar, I.; Ford, R. Linkage mapping and quantitative trait loci analysis of sweetness and other fruit quality traits in papaya. BMC Plant Biol. 2019, 19, 499. [Google Scholar] [CrossRef]
- Fresnedo-Ramírez, J.; Frett, T.J.; Sandefur, P.J.; Salgado-Rojas, A.; Clark, J.R.; Gasic, K.; Peace, C.P.; Anderson, N.; Hartmann, T.P.; Byrne, D.H.; et al. QTL mapping and breeding value estimation through pedigree-based analysis of fruit size and weight in four diverse peach breeding programs. Tree Genet. Genomes 2016, 12, 25. [Google Scholar] [CrossRef]
- Fresnedo-Ramírez, J.; Bink, M.C.A.M.; van de Weg, E.; Famula, T.R.; Crisosto, C.H.; Frett, T.J.; Gasic, K.; Peace, C.P.; Gradziel, T.M. QTL mapping of pomological traits in peach and related species breeding germplasm. Mol. Breed. 2015, 35, 166. [Google Scholar] [CrossRef]
- Desnoues, E.; Baldazzi, V.; Génard, M.; Mauroux, J.-B.; Lambert, P.; Confolent, C.; Quilot-Turion, B. Dynamic QTLs for sugars and enzyme activities provide an overview of genetic control of sugar metabolism during peach fruit development. J. Exp. Bot. 2016, 67, 3419–3431. [Google Scholar] [CrossRef]
- Da Silva Linge, C.; Bassi, D.; Bianco, L.; Pacheco, I.; Pirona, R.; Rossini, L. Genetic dissection of fruit weight and size in an F2 peach (Prunus persica (L.) Batsch) progeny. Mol. Breed. 2015, 35, 71. [Google Scholar] [CrossRef]
- Kim, M.; Nguyen, T.T.P.; Ahn, J.-H.; Kim, G.-J.; Sim, S.-C. Genome-wide association study identifies QTL for eight fruit traits in cultivated tomato (Solanum lycopersicum L.). Hortic. Res. 2021, 8, 203. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Tian, M.; Du, H.; Liu, S.; Yu, R.; Shen, H. Quantitative Trait Loci Mapping and Comparative Transcriptome Analysis of Fruit Weight (FW) in Watermelon (Citrullus lanatus L.). Genes 2024, 15, 933. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Amanullah, S.; Pan, J.; Chen, G.; Liu, S.; Ma, S.; Wang, J.; Gao, P.; Wang, X. Identification of putative genetic regions for watermelon rind hardness and related traits by BSA-seq and QTL mapping. Euphytica 2021, 217, 19. [Google Scholar] [CrossRef]
- Li, N.; Kong, S.; Zhou, D.; Li, N.; Shang, J.; Wang, J.; Ma, S. Mapping and validation of a new quantitative trait locus (QTL) for fruit size in watermelon (Citrullus lanatus). Sci. Hortic. 2023, 318, 112054. [Google Scholar] [CrossRef]
- Han, X.; Min, Z.; Wei, M.; Li, Y.; Wang, D.; Zhang, Z.; Hu, X.; Kong, Q. QTL mapping for pumpkin fruit traits using a GBS-based high-density genetic map. Euphytica 2022, 218, 106. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, B.; Peng, Q.; He, X.; Lin, Y.; Wang, M.; Liang, Z.; Xie, D.; Hu, K. Genetic analysis and QTL mapping of fruit-related traits in wax gourd (Benincasa hispida). Euphytica 2018, 214, 136. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Li, M.; Yang, Y.; Li, Z.; Xu, Y.; Wang, H.; Wang, D.; Zhang, Y.; Wang, H.; et al. Molecular mapping for fruit-related traits, and joint identification of candidate genes and selective sweeps for seed size in melon. Genomics 2022, 114, 110306. [Google Scholar] [CrossRef]
- Amanullah, S.; Gao, P.; Osae, B.A.; Saroj, A.; Yang, T.; Liu, S.; Weng, Y.; Luan, F. Genetic linkage mapping and QTLs identification for morphology and fruit quality related traits of melon by SNP based CAPS markers. Sci. Hortic. 2021, 278, 109849. [Google Scholar] [CrossRef]
- Monforte, A.J.; Diaz, A.; Caño-Delgado, A.; van der Knaap, E. The genetic basis of fruit morphology in horticultural crops: Lessons from tomato and melon. J. Exp. Bot. 2013, 65, 4625–4637. [Google Scholar] [CrossRef] [PubMed]
- Monforte, A.J.; Eduardo, I.; Abad, S.; Arus, P. Inheritance mode of fruit traits in melon: Heterosis for fruit shape and its correlation with genetic distance. Euphytica 2005, 144, 31–38. [Google Scholar] [CrossRef]
- Díaz, A.; Martín-Hernández, A.M.; Dolcet-Sanjuan, R.; Garcés-Claver, A.; Álvarez, J.M.; Garcia-Mas, J.; Picó, B.; Monforte, A.J. Quantitative trait loci analysis of melon (Cucumis melo L.) domestication-related traits. Theor. Appl. Genet. 2017, 130, 1837–1856. [Google Scholar] [CrossRef] [PubMed]
- Díaz, A.; Zarouri, B.; Fergany, M.; Eduardo, I.; Álvarez, J.M.; Picó, B.; Monforte, A.J. Mapping and Introgression of QTL Involved in Fruit Shape Transgressive Segregation into ‘Piel de Sapo’ Melon (Cucucumis melo L.). PLoS ONE 2014, 9, e104188. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Lian, Q.; Zhang, Z.; Fu, Q.; He, Y.; Ma, S.; Ruggieri, V.; Monforte, A.J.; Wang, P.; Julca, I.; et al. A comprehensive genome variation map of melon identifies multiple domestication events and loci influencing agronomic traits. Nat. Genet. 2019, 51, 1607–1615. [Google Scholar] [CrossRef]
- Campos, M.; Gonzalo, M.J.; Díaz, A.; Picó, B.; Gómez-Guillamón, M.L.; Monforte, A.J.; Esteras, C. A Novel Introgression Line Library Derived from a Wild Melon Gives Insights into the Genetics of Melon Domestication, Uncovering New Genetic Variability Useful for Breeding. Int. J. Mol. Sci. 2023, 24, 10099. [Google Scholar] [CrossRef]
- Pereira, L.; Ruggieri, V.; Pérez, S.; Alexiou, K.G.; Fernández, M.; Jahrmann, T.; Pujol, M.; Garcia-Mas, J. QTL mapping of melon fruit quality traits using a high-density GBS-based genetic map. BMC Plant Biol. 2018, 18, 324. [Google Scholar] [CrossRef]
- Ramamurthy, R.K.; Waters, B.M. Identification of fruit quality and morphology QTLs in melon (Cucumis melo) using a population derived from flexuosus and cantalupensis botanical groups. Euphytica 2015, 204, 163–177. [Google Scholar] [CrossRef]
- Lian, Q.; Fu, Q.; Xu, Y.; Hu, Z.; Zheng, J.; Zhang, A.; He, Y.; Wang, C.; Xu, C.; Chen, B.; et al. QTLs and candidate genes analyses for fruit size under domestication and differentiation in melon (Cucumis melo L.) based on high resolution maps. BMC Plant Biol. 2021, 21, 126. [Google Scholar] [CrossRef]
- Pereira, L.; Santo Domingo, M.; Argyris, J.; Mayobre, C.; Valverde, L.; Martín-Hernández, A.M.; Pujol, M.; Garcia-Mas, J. A novel introgression line collection to unravel the genetics of climacteric ripening and fruit quality in melon. Sci. Rep. 2021, 11, 11364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, C.; Xie, M.; Liu, J.; Kong, Z.; Su, H. Actin Bundles in The Pollen Tube. Int. J. Mol. Sci. 2018, 19, 3710. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, S.-R.; Shu, S.; Ren, Y.; Sun, J. Construction of a genetic linkage map of rootstock-used pumpkin using SSR markers and QTL analysis for cold tolerance. Sci. Hortic. 2017, 220, 107–113. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, P.; Bhadauria, V.; Chen, X.; Ma, B. An efficient CAPS marker for bacterial-blight resistance gene xa25 in rice. Can. J. Plant Pathol. 2017, 39, 117–120. [Google Scholar] [CrossRef]
- Seo, J.-H.; Dhungana, S.K.; Kang, B.-K.; Baek, I.-Y.; Sung, J.-S.; Ko, J.-Y.; Jung, C.-S.; Kim, K.-S.; Jun, T.-H. Development and Validation of SNP and InDel Markers for Pod-Shattering Tolerance in Soybean. Int. J. Mol. Sci. 2022, 23, 2382. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Dai, D.; Wang, L.; Sheng, Y.; Wang, D.; Li, D.; Tian, L.; Luan, F. QTL analysis of flowering-related traits by specific length amplified fragment sequencing in melon. Crop Sci. 2021, 62, 203–215. [Google Scholar] [CrossRef]
- Niedz, R.P.; Song, Z.; Miao, H.; Zhang, S.; Wang, Y.; Zhang, S.P.; Gu, X.F. Genetic Analysis and QTL Mapping of Fruit Peduncle Length in Cucumber (Cucumis sativus L.). PLoS ONE 2016, 11, e0167845. [Google Scholar] [CrossRef]
- He, Y.; Li, L.; Yao, Y.; Li, Y.; Zhang, H.; Fan, M. Transcriptome-wide N6-methyladenosine (m6A) methylation in watermelon under CGMMV infection. BMC Plant Biol. 2021, 21, 516. [Google Scholar] [CrossRef]
- Wang, X.; Ando, K.; Wu, S.; Reddy, U.K.; Tamang, P.; Bao, K.; Hammar, S.A.; Grumet, R.; McCreight, J.D.; Fei, Z. Genetic characterization of melon accessions in the U.S. National Plant Germplasm System and construction of a melon core collection. Mol. Hortic. 2021, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Hazman, M.; Kabil, F.; Elhamid, S.A.; Nick, P. Double lysis: An integrative time-saving method yielding high-quality RNA from strawberry. J. Genet. Eng. Biotechnol. 2020, 18, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Sun, W.; Yue, Z.; Gong, B.; Yang, X.; Wu, K.; Liu, C.; Xu, Y. Phenotypic Diversity and Relationships of Fruit Traits in Persimmon (Diospyros kaki Thunb.) Germplasm Resources. Agriculture 2023, 13, 1804. [Google Scholar] [CrossRef]
- Gong, C.; Zhao, S.; Yang, D.; Lu, X.; Anees, M.; He, N.; Zhu, H.; Zhao, Y.; Liu, W. Genome-wide association analysis provides molecular insights into natural variation in watermelon seed size. Hortic. Res. 2022, 9, uhab074. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-X.; Dai, D.-Y.; Wang, L.; Yang, L.-M.; Li, D.-D.; Wang, C.; Ji, P.; Sheng, Y.-Y. SLAF marker based QTL mapping of fruit-related traits reveals a major-effect candidate locus ff2.1 for flesh firmness in melon. J. Integr. Agric. 2023, 22, 3331–3345. [Google Scholar] [CrossRef]
- Rehman, F.; Gong, H.; Li, Z.; Zeng, S.; Yang, T.; Ai, P.; Pan, L.; Huang, H.; Wang, Y. Identification of fruit size associated quantitative trait loci featuring SLAF based high-density linkage map of goji berry (Lycium spp.). BMC Plant Biol. 2020, 20, 474. [Google Scholar] [CrossRef] [PubMed]
- Harel-Beja, R.; Tzuri, G.; Portnoy, V.; Lotan-Pompan, M.; Lev, S.; Cohen, S.; Dai, N.; Yeselson, L.; Meir, A.; Libhaber, S.E.; et al. A genetic map of melon highly enriched with fruit quality QTLs and EST markers, including sugar and carotenoid metabolism genes. Theor. Appl. Genet. 2010, 121, 511–533. [Google Scholar] [CrossRef] [PubMed]
- Zalapa, J.E.; Staub, J.E.; McCreight, J.D.; Chung, S.M.; Cuevas, H. Detection of QTL for yield-related traits using recombinant inbred lines derived from exotic and elite US Western Shipping melon germplasm. Theor. Appl. Genet. 2007, 114, 1185–1201. [Google Scholar] [CrossRef] [PubMed]
- Monforte, A.J.; Oliver, M.; Gonzalo, M.J.; Alvarez, J.M.; Dolcet-Sanjuan, R.; Arús, P. Identification of quantitative trait loci involved in fruit quality traits in melon (Cucumis melo L.). Theor. Appl. Genet. 2004, 108, 750–758. [Google Scholar] [CrossRef]
- Zhang, T.; Ding, Z.; Liu, J.; Qiu, B.; Gao, P. QTL mapping of pericarp and fruit-related traits in melon (Cucumis melo L.) using SNP-derived CAPS markers. Sci. Hortic. 2020, 265, 109243. [Google Scholar] [CrossRef]
- Zhu, W.-Y.; Huang, L.; Chen, L.; Yang, J.-T.; Wu, J.-N.; Qu, M.-L.; Yao, D.-Q.; Guo, C.-L.; Lian, H.-L.; He, H.-L.; et al. A High-Density Genetic Linkage Map for Cucumber (Cucumis sativus L.): Based on Specific Length Amplified Fragment (SLAF) Sequencing and QTL Analysis of Fruit Traits in Cucumber. Front. Plant Sci. 2016, 7, 437. [Google Scholar] [CrossRef]
- Sheng, Y.; Pan, Y.; Li, Y.; Yang, L.; Weng, Y.; Flachowsky, H. Quantitative trait loci for fruit size and flowering time-related traits under domestication and diversifying selection in cucumber (Cucumis sativus). Plant Breed. 2020, 139, 176–191. [Google Scholar] [CrossRef]
- Hernández-Bautista, A.; Lobato-Ortiz, R.; García-Zavala, J.J.; López-Fortoso, F.; Cruz-Izquierdo, S.; Chávez-Servia, J.L.; Cadeza-Espinosa, M. Quantitative trait locus mapping associated with earliness and fruit weight in tomato. Sci. Agric. 2016, 73, 478–486. [Google Scholar] [CrossRef]
- Lopez-Moreno, H.; Basurto-Garduño, A.C.; Torres-Meraz, M.A.; Diaz-Valenzuela, E.; Arellano-Arciniega, S.; Zalapa, J.; Sawers, R.J.H.; Cibrián-Jaramillo, A.; Diaz-Garcia, L. Genetic analysis and QTL mapping of domestication-related traits in chili pepper (Capsicum annuum L.). Front. Genet. 2023, 14, 1101401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, J.; Liu, W.; Liu, N.; Zhang, Y.; Xu, M.; Liu, S.; Ma, X.; Zhang, Y. Construction of a high-density genetic map and identification of quantitative trait loci linked to fruit quality traits in apricots using specific-locus amplified fragment sequencing. Front. Plant Sci. 2022, 13, 798700. [Google Scholar] [CrossRef] [PubMed]
- Marco, C.; Irina, B.; Remo, C.; Chiozzotto, R.; Silvestri, C.; Rossini, L.; Bassi, D. Genetic and phenotypic analyses reveal major quantitative loci associated to fruit size and shape traits in a non-flat peach collection (P. persica L. Batsch). Hortic. Res. 2021, 8, 232. [Google Scholar] [CrossRef]
- Pan, L.; Wang, M.; Yang, Y.; Chen, C.; Dai, H.; Zhang, Z.; Hua, B.; Miao, M. Whole-genome resequencing identified QTLs, candidate genes and Kompetitive Allele-Specific PCR markers associated with the large fruit of Atlantic Giant (Cucurbita maxima). Front. Plant Sci. 2022, 13, 942004. [Google Scholar] [CrossRef]
- Chang, Y.; Zheng, W.; Wang, S.; He, X.; He, P.; Li, H.; Wang, H.; Li, L. QTL mapping combined RNA-seq technology identified potential genes involved in regulation of apple size. Sci. Hortic. 2023, 319, 112150. [Google Scholar] [CrossRef]
- Ning, Y.; Wei, K.; Li, S.; Zhang, L.; Chen, Z.; Lu, F.; Yang, P.; Yang, M.; Liu, X.; Liu, X.; et al. Fine Mapping of fw6.3, a Major-Effect Quantitative Trait Locus That Controls Fruit Weight in Tomato. Plants 2023, 12, 2065. [Google Scholar] [CrossRef] [PubMed]
- Kaźmińska, K.; Hallmann, E.; Korzeniewska, A.; Niemirowicz-Szczytt, K.; Bartoszewski, G. Identification of Fruit-Associated QTLs in Winter Squash (Cucurbita maxima Duchesne) Using Recombinant Inbred Lines. Genes 2020, 11, 419. [Google Scholar] [CrossRef]
- Osae, B.A.; Amanullah, S.; Liu, H.; Liu, S.; Saroj, A.; Zhang, C.; Liu, T.; Gao, P.; Luan, F. CAPS marker-base genetic linkage mapping and QTL analysis for watermelon ovary, fruit and seed-related traits. Euphytica 2022, 218, 39. [Google Scholar] [CrossRef]
- Santo Domingo, M.; Mayobre, C.; Pereira, L.; Argyris, J.; Valverde, L.; Martín-Hernández, A.M.; Garcia-Mas, J.; Pujol, M. Fruit morphology and ripening-related QTLs in a newly developed introgression line collection of the elite varieties ‘Védrantais’ and ‘Piel de Sapo’. Plants 2022, 11, 3120. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhang, S.; Liu, J.; Liu, S.; Su, H. Arabidopsis FIM4 and FIM5 regulates the growth of root hairs in an auxin-insensitive way. Plant Signal. Behav. 2018, 13, e1473667. [Google Scholar] [CrossRef] [PubMed]


| Number | Primer Name | Genomic Location | Forward Sequence | Reverse Sequence |
|---|---|---|---|---|
| 1 | CYIndel6 | 18,524,772–18,524,791 | CCTAAGGCTAAAGGATATCG | AAACATTTTGGAAGGGTAAG |
| 2 | CYIndel9 | 18,560,853–18,561,288 | AGTAAAGATGGGGTGAAAAC | TGTGAAAAATGAGGATGGAA |
| 3 | CYIndel11 | 18,568,142–18,568,533 | GCTGTAGAACACATTAAGGACGA | TAAAGACCTTGGAAAATCGAATA |
| 4 | CYIndel13 | 18,602,885–18,603,339 | CTCCTGCTTGTGTCCTCATG | TCAGTCTTCTCAAATCCTCCC |
| 5 | CYIndel16 | 18,704,309–18,704,724 | TCTTTCGGCTTCACTTGCTT | GAAGAGCGTTGTGCTGTGG |
| 6 | CYIndel26 | 18,906,922–18,907,357 | TTTGTGAGAGACAAGTTTGAGT | ATACAGGAAGATTTTCTGCTAC |
| 7 | CYIndel27 | 18,920,443–18,920,801 | TCCTCGAAGTCGGTGGTAAC | TGGTCCTATCAATGAGGCAAAT |
| Generation | Mean ± SD | CV | Maximum | Minimum | Range | H2 of Trait | |
|---|---|---|---|---|---|---|---|
| 1244 | 22.59 ± 11.74 a | 51.97% | 43.83 | 3.83 | 40.00 | 52.51% | |
| MS-5 | 814.97 ± 70.12 b | 8.60% | 931.84 | 682.72 | 249.12 | ||
| F1 | 160.17 ± 30.06 | 18.77% | 201.40 | 99.15 | 102.25 | ||
| F2 | 100.89 ± 71.97 | Max: 170.99 ± 72.60 a | 71.34% | 406.80 | 17.40 | 389.40 | |
| Min: 59.72 ± 23.57 b | |||||||
| F2:3-2020 | 106.37 ± 63.14 | Max: 163.42 ± 67.33 a | 59.36% | 395.70 | 18.69 | 377.01 | |
| Min: 65.32 ± 25.81 b | |||||||
| F2:3-2021 | 112.29 ± 58.39 | Max: 151.60 ± 55.24 a | 52.00% | 326.63 | 19.46 | 307.17 | |
| Min: 70.52 ± 20.13 b | |||||||
| Trait | QTLsite | Linkage Group | Genome Start Position | Genome End Position | LOD | ADD | PVE (%) | Gene Number |
|---|---|---|---|---|---|---|---|---|
| Single fruit weight | sfw1.1 | 1 | 34,295,745 | 34,435,727 | 3.30 | −28.38 | 8.30 | 17 |
| sfw2.1 | 2 | 1,626,517 | 1,635,504 | 3.00 | 17.81 | 3.10 | 1 | |
| sfw2.2 | 2 | 18,344,184 | 18,881,371 | 5.60 | −45.10 | 17.00 | 15 | |
| sfw6.1 | 6 | 30,841,187 | 38,291,645 | 3.20 | −21.93 | 8.60 | 354 | |
| sfw7.1 | 7 | 4,758,046 | 7,547,788 | 3.20 | −25.56 | 5.90 | 113 | |
| sfw10.1 | 10 | 485,913 | 504,601 | 3.10 | −30.64 | 8.30 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Y.; Wang, D.; Che, Y.; Wang, L.; Zhang, F.; Liu, T.; Sheng, Y. Multiple Localization Analysis of the Major QTL—sfw 2.2 for Controlling Single Fruit Weight Traits in Melon Based on SLAF Sequencing. Genes 2024, 15, 1138. https://doi.org/10.3390/genes15091138
Cai Y, Wang D, Che Y, Wang L, Zhang F, Liu T, Sheng Y. Multiple Localization Analysis of the Major QTL—sfw 2.2 for Controlling Single Fruit Weight Traits in Melon Based on SLAF Sequencing. Genes. 2024; 15(9):1138. https://doi.org/10.3390/genes15091138
Chicago/Turabian StyleCai, Yi, Di Wang, Ye Che, Ling Wang, Fan Zhang, Tai Liu, and Yunyan Sheng. 2024. "Multiple Localization Analysis of the Major QTL—sfw 2.2 for Controlling Single Fruit Weight Traits in Melon Based on SLAF Sequencing" Genes 15, no. 9: 1138. https://doi.org/10.3390/genes15091138
APA StyleCai, Y., Wang, D., Che, Y., Wang, L., Zhang, F., Liu, T., & Sheng, Y. (2024). Multiple Localization Analysis of the Major QTL—sfw 2.2 for Controlling Single Fruit Weight Traits in Melon Based on SLAF Sequencing. Genes, 15(9), 1138. https://doi.org/10.3390/genes15091138





