Expression, Purification, and Anti-UV Irradiation Effect of RsSOD on HCE-T Human Corneal Epithelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain and Cell Line
2.2. Bioinformatics Analysis
2.3. Expression and Purification of RsSOD Protein
2.4. Stability Determination of RsSOD Protein
2.5. Effect of RsSOD Protein on the Proliferation of HCE-T Cells
2.6. Effect of Long-Term or Short-Term UV Irradiation on the Proliferation of HCE-T Cells
2.7. Effect of RsSOD on the Proliferation of HCE-T Cells under UV Irradiation
2.8. Statistical Analysis
3. Results
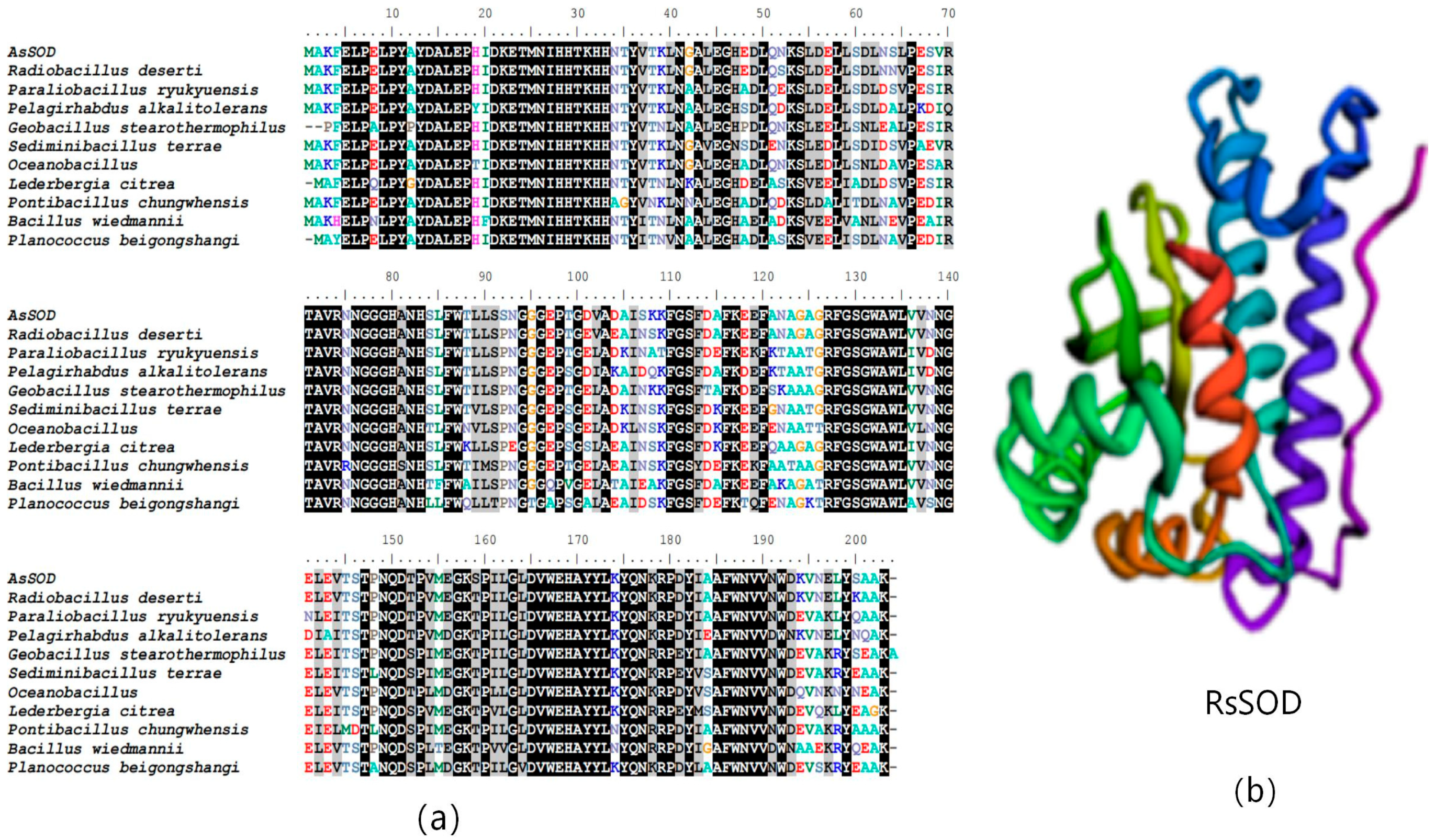
3.1. Bioinformatics Analysis
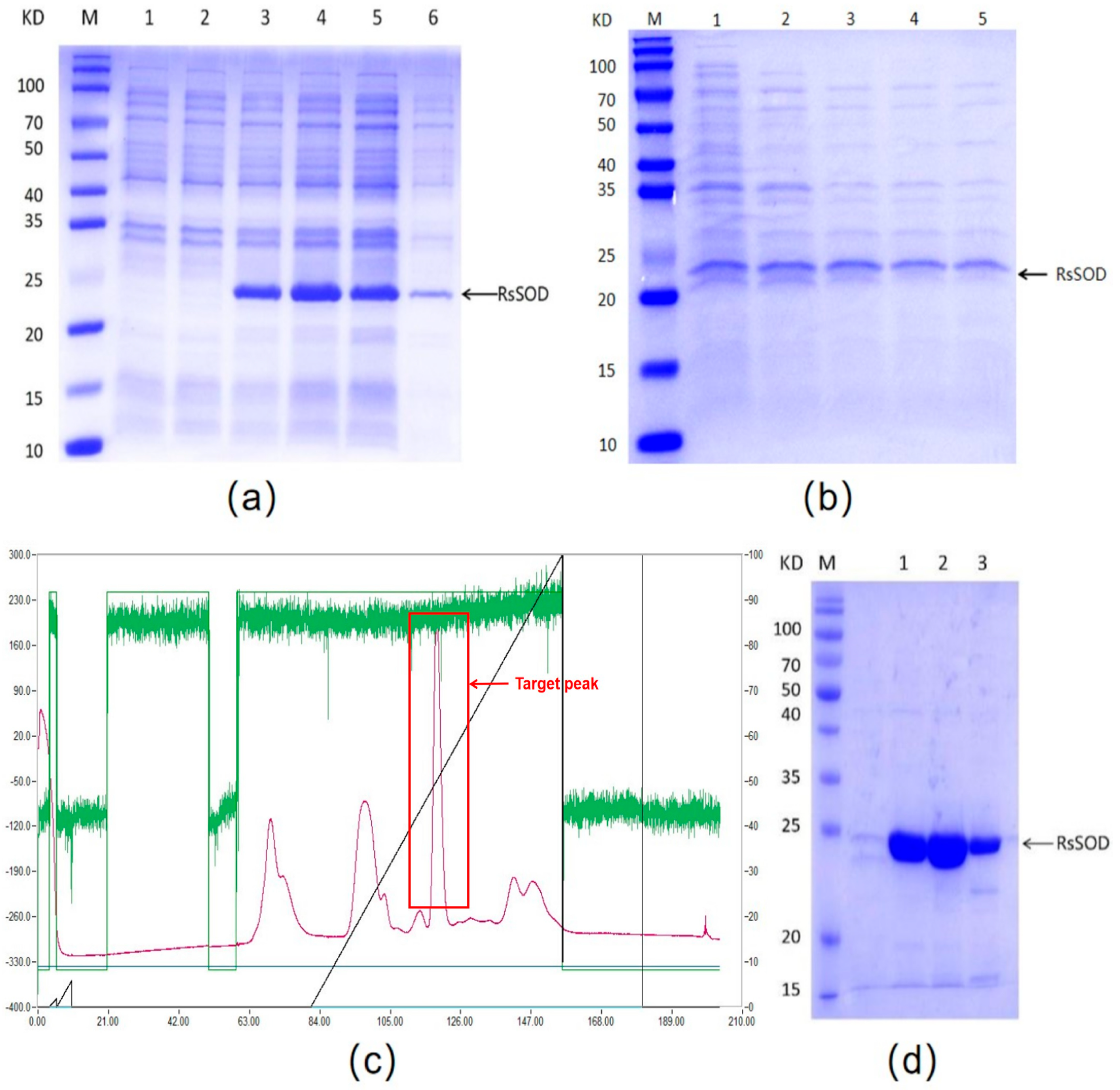
3.2. Expression and Purification of RsSOD Protein
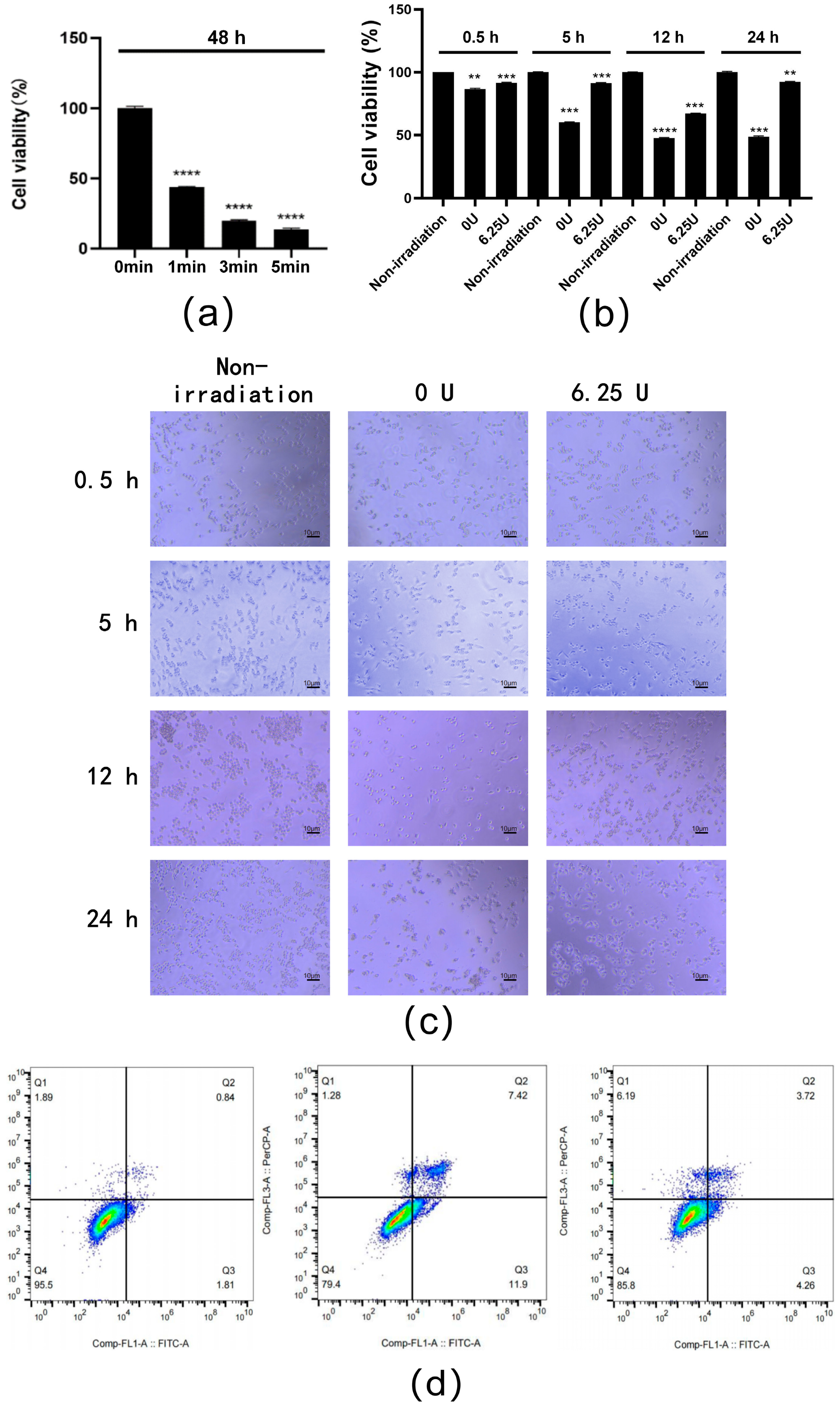
3.3. Stability of RsSOD Protein
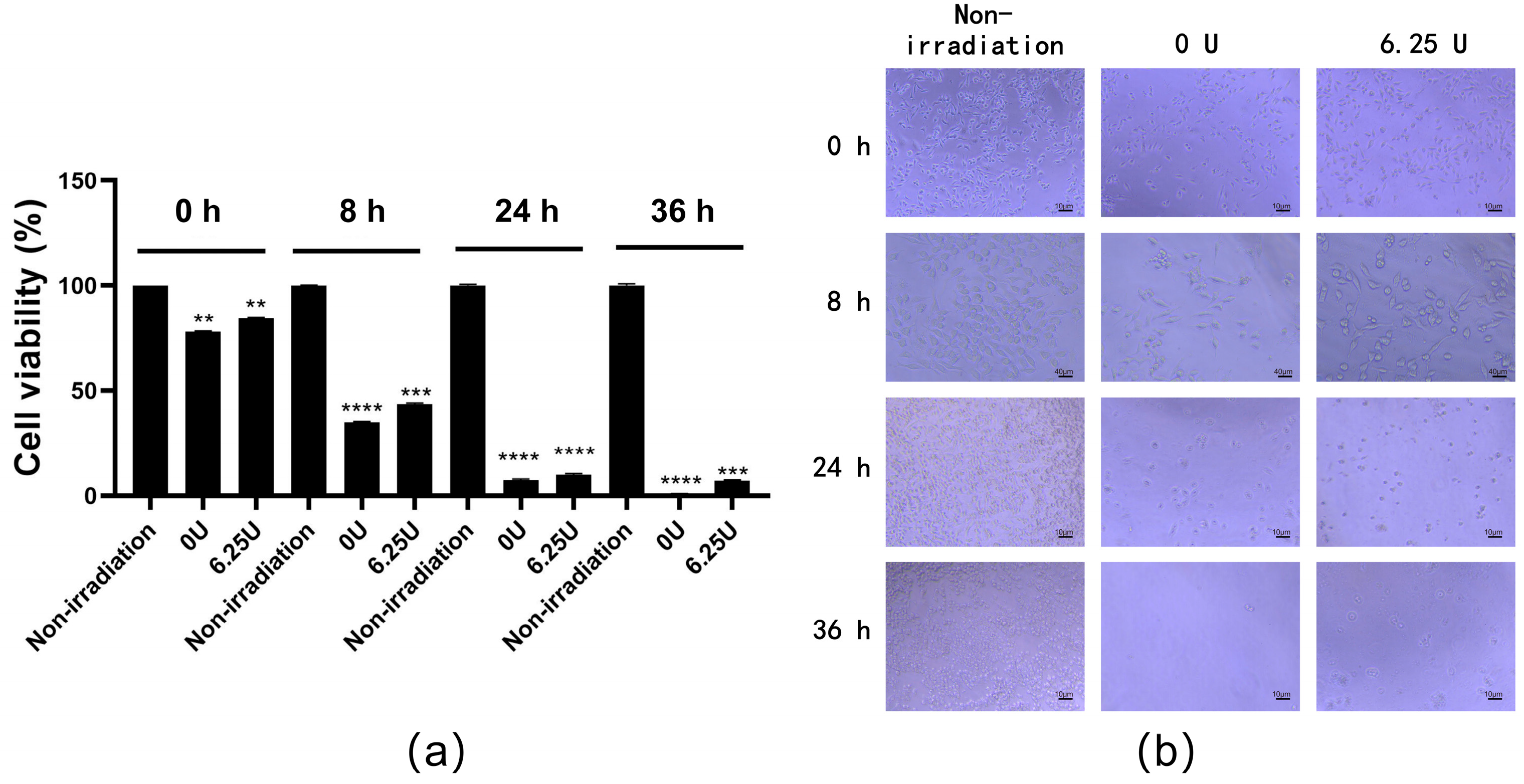
3.4. Determination of Optimal Dose of RsSOD against UV Irradiation on HCE-T Cells
3.5. Effect of RsSOD against Long-Term UV Irradiation in HCE-T Cells
3.6. Effect of RsSOD against Short-Term UV Irradiation in HCE-T Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SOD | Superoxide dismutase |
| MnSOD | Manganese superoxide dismutase |
| RsSOD | Radiobacillus sp. SOD |
| IPTG | Isopropyl β-D-1-thiogalactopyranoside |
| UV | Ultraviolet |
| MDA | Maleicdialdehyde |
| HCE-T | Human corneal epithelial cells-transformed |
| ROS | Reactive oxygen species |
| ORF | Open reading frame |
| mtDNA | Mitochondrial DNA |
| DEAE | Diethylaminoethyl |
| WST-8 | 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium sodium salt |
References
- Zheng, M.; Liu, Y.; Zhang, G.; Yang, Z.; Xu, W.; Chen, Q. The Applications and Mechanisms of Superoxide Dismutase in Medicine, Food, and Cosmetics. Antioxidants 2023, 12, 1675. [Google Scholar] [CrossRef]
- Eghtedari, M.; Jafari Porzani, S.; Javanmardi, M.; Ganjali, M.R.; Hosseinkhani, S. Etching of AuNPs Through Superoxide Radical Dismutation by Cu-Zn Superoxide Dismutase Resulted in Remarkable Changes of its Localized Surface Plasmon Resonance. Iran J. Biotechnol. 2021, 19, e2741. [Google Scholar] [CrossRef]
- Chen, D.; Ai, X.; Li, Y.; Li, Y.; Ao, Y.; Rong, J.; Li, G. Protective effects of Cu/Zn-SOD and Mn-SOD on UVC radiation-induced damage in NIH/3T3 cells and murine skin. Acta Histochem. 2023, 125, 152030. [Google Scholar] [CrossRef]
- Chen, Y.; Li, B.; Li, K.; Lin, Y. Superoxide dismutase nanozymes: Current status and future perspectives on brain disease treatment and diagnosis. Chem. Commun. 2024, 60, 4140–4147. [Google Scholar] [CrossRef] [PubMed]
- Vaneev, A.N.; Kost, O.A.; Eremeev, N.L.; Beznos, O.V.; Alova, A.V.; Gorelkin, P.V.; Erofeev, A.S.; Chesnokova, N.B.; Kabanov, A.V.; Klyachko, N.L. Superoxide Dismutase 1 Nanoparticles (Nano-SOD1) as a Potential Drug for the Treatment of Inflammatory Eye Diseases. Biomedicines 2021, 9, 396. [Google Scholar] [CrossRef] [PubMed]
- Kost, O.A.; Beznos, O.V.; Davydova, N.G.; Manickam, D.S.; Nikolskaya, I.I.; Guller, A.E.; Binevski, P.V.; Chesnokova, N.B.; Shekhter, A.B.; Klyachko, N.L.; et al. Superoxide Dismutase 1 Nanozyme for Treatment of Eye Inflammation. Oxid. Med. Cell. Longev. 2015, 2015, 5194239. [Google Scholar] [CrossRef] [PubMed]
- Kopincova, J.; Kolomaznik, M.; Mikolka, P.; Kosutova, P.; Topercerova, J.; Matasova, K., Jr.; Calkovska, A.; Mokra, D. Recombinant Human Superoxide Dismutase and N-Acetylcysteine Addition to Exogenous Surfactant in the Treatment of Meconium Aspiration Syndrome. Molecules 2019, 24, 905. [Google Scholar] [CrossRef]
- Yam, J.C.; Kwok, A.K. Ultraviolet light and ocular diseases. Int. Ophthalmol. 2014, 34, 383–400. [Google Scholar] [CrossRef]
- Bohm, E.W.; Buonfiglio, F.; Voigt, A.M.; Bachmann, P.; Safi, T.; Pfeiffer, N.; Gericke, A. Oxidative stress in the eye and its role in the pathophysiology of ocular diseases. Redox Biol. 2023, 68, 102967. [Google Scholar] [CrossRef] [PubMed]
- Zigman, S.; Datiles, M.; Torczynski, E. Sunlight and human cataracts. Invest. Ophthalmol. Vis. Sci. 1979, 18, 462–467. [Google Scholar] [PubMed]
- Rosenthal, F.S.; Bakalian, A.E.; Lou, C.Q.; Taylor, H.R. The effect of sunglasses on ocular exposure to ultraviolet radiation. Am. J. Public Health 1988, 78, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Podskochy, A.; Fagerholm, P. Cellular response and reactive hyaluronan production in UV-exposed rabbit corneas. Cornea 1998, 17, 640–645. [Google Scholar] [CrossRef]
- Matsui, H.; Lin, L.R.; Ho, Y.S.; Reddy, V.N. The effect of up- and downregulation of MnSOD enzyme on oxidative stress in human lens epithelial cells. Invest. Ophthalmol. Vis. Sci. 2003, 44, 3467–3475. [Google Scholar] [CrossRef]
- Epperly, M.W.; Wang, H.; Jones, J.A.; Dixon, T.; Montesinos, C.A.; Greenberger, J.S. Antioxidant-chemoprevention diet ameliorates late effects of total-body irradiation and supplements radioprotection by MnSOD-plasmid liposome administration. Radiat. Res. 2011, 175, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Epperly, M.W.; Bray, J.A.; Esocobar, P.; Bigbee, W.L.; Watkins, S.; Greenberger, J.S. Overexpression of the human manganese superoxide dismutase (MnSOD) transgene in subclones of murine hematopoietic progenitor cell line 32D cl 3 decreases irradiation-induced apoptosis but does not alter G2/M or G1/S phase cell cycle arrest. Radiat. Oncol. Investig. 1999, 7, 331–342. [Google Scholar] [CrossRef]
- Sasaki, H.; Akamatsu, H.; Horio, T. Effects of a single exposure to UVB radiation on the activities and protein levels of copper-zinc and manganese superoxide dismutase in cultured human keratinocytes. Photochem. Photobiol. 1997, 65, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Guan, J.; Pi, J. Protective effects of exogenous superoxide dismutase on rabbit retinal injury by acute intraocular hypertension. Zhonghua Yan Ke Za Zhi 1997, 33, 429–432. [Google Scholar]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Liu, X.; Yu, Y.; Garcia, L.A.; Au, M.L.; Tran, M.; Zhang, J.; Lou, A.; Liu, Y.; Wu, H. A grape-supplemented diet prevented ultraviolet (UV) radiation-induced cataract by regulating Nrf2 and XIAP pathways. J. Nutr. Biochem. 2024, 129, 109636. [Google Scholar] [CrossRef] [PubMed]
- Gadi, W.; Townsend, K.A.; Michael, W.E.; Joel, S.S.; Joel, S.G. Manganese Superoxide Dismutase (MnSOD) in vivo Effect on Post-Radiation Cataract. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1461. [Google Scholar]
- Maria Grazia, R.; Maria Laura, P.; Antonella, S.; Antonella, B.; Aldo, M.; Alessandra, P.; Patrizia, F. Effects of a New Human Recombinant MnSOD in the Treatment of Photoaging and Actinic Keratosis. J. Cancer Ther. 2013, 04, 56–59. [Google Scholar] [CrossRef]
- Fischer, T.W.; Scholz, G.; Knoll, B.; Hipler, U.C.; Elsner, P. Melatonin suppresses reactive oxygen species induced by UV irradiation in leukocytes. J. Pineal Res. 2004, 37, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Meinke, M.C.; Muller, R.; Bechtel, A.; Haag, S.F.; Darvin, M.E.; Lohan, S.B.; Ismaeel, F.; Lademann, J. Evaluation of carotenoids and reactive oxygen species in human skin after UV irradiation: A critical comparison between in vivo and ex vivo investigations. Exp. Dermatol. 2015, 24, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Takai, D.; Park, S.H.; Takada, Y.; Ichinose, S.; Kitagawa, M.; Akashi, M. UV-irradiation induces oxidative damage to mitochondrial DNA primarily through hydrogen peroxide: Analysis of 8-oxodGuo by HPLC. Free Radic. Res. 2006, 40, 1138–1148. [Google Scholar] [CrossRef]
- Kienhofer, J.; Haussler, D.J.; Ruckelshausen, F.; Muessig, E.; Weber, K.; Pimentel, D.; Ullrich, V.; Burkle, A.; Bachschmid, M.M. Association of mitochondrial antioxidant enzymes with mitochondrial DNA as integral nucleoid constituents. FASEB J. 2009, 23, 2034–2044. [Google Scholar] [CrossRef] [PubMed]
- Bakthavatchalu, V.; Dey, S.; Xu, Y.; Noel, T.; Jungsuwadee, P.; Holley, A.K.; Dhar, S.K.; Batinic-Haberle, I.; St Clair, D.K. Manganese superoxide dismutase is a mitochondrial fidelity protein that protects Polgamma against UV-induced inactivation. Oncogene 2012, 31, 2129–2139. [Google Scholar] [CrossRef]
- Sugiura, M.; Adachi, T.; Inoue, H.; Ito, Y.; Hirano, K. Purification and properties of two superoxide dismutases from human placenta. J. Pharmacobiodyn. 1981, 4, 235–244. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.; Jiang, Z.; Bi, W.; Yang, Z.; Lu, W.; Chen, J.; Lyu, Z.; Nie, Z. Expression, Purification, and Anti-UV Irradiation Effect of RsSOD on HCE-T Human Corneal Epithelial Cells. Genes 2024, 15, 1147. https://doi.org/10.3390/genes15091147
Fu X, Jiang Z, Bi W, Yang Z, Lu W, Chen J, Lyu Z, Nie Z. Expression, Purification, and Anti-UV Irradiation Effect of RsSOD on HCE-T Human Corneal Epithelial Cells. Genes. 2024; 15(9):1147. https://doi.org/10.3390/genes15091147
Chicago/Turabian StyleFu, Xucong, Zhuo Jiang, Wenhui Bi, Zhecheng Yang, Weina Lu, Jianqing Chen, Zhengbing Lyu, and Zuoming Nie. 2024. "Expression, Purification, and Anti-UV Irradiation Effect of RsSOD on HCE-T Human Corneal Epithelial Cells" Genes 15, no. 9: 1147. https://doi.org/10.3390/genes15091147
APA StyleFu, X., Jiang, Z., Bi, W., Yang, Z., Lu, W., Chen, J., Lyu, Z., & Nie, Z. (2024). Expression, Purification, and Anti-UV Irradiation Effect of RsSOD on HCE-T Human Corneal Epithelial Cells. Genes, 15(9), 1147. https://doi.org/10.3390/genes15091147





