Mitochondrial Dysfunctions: Genetic and Cellular Implications Revealed by Various Model Organisms
Abstract
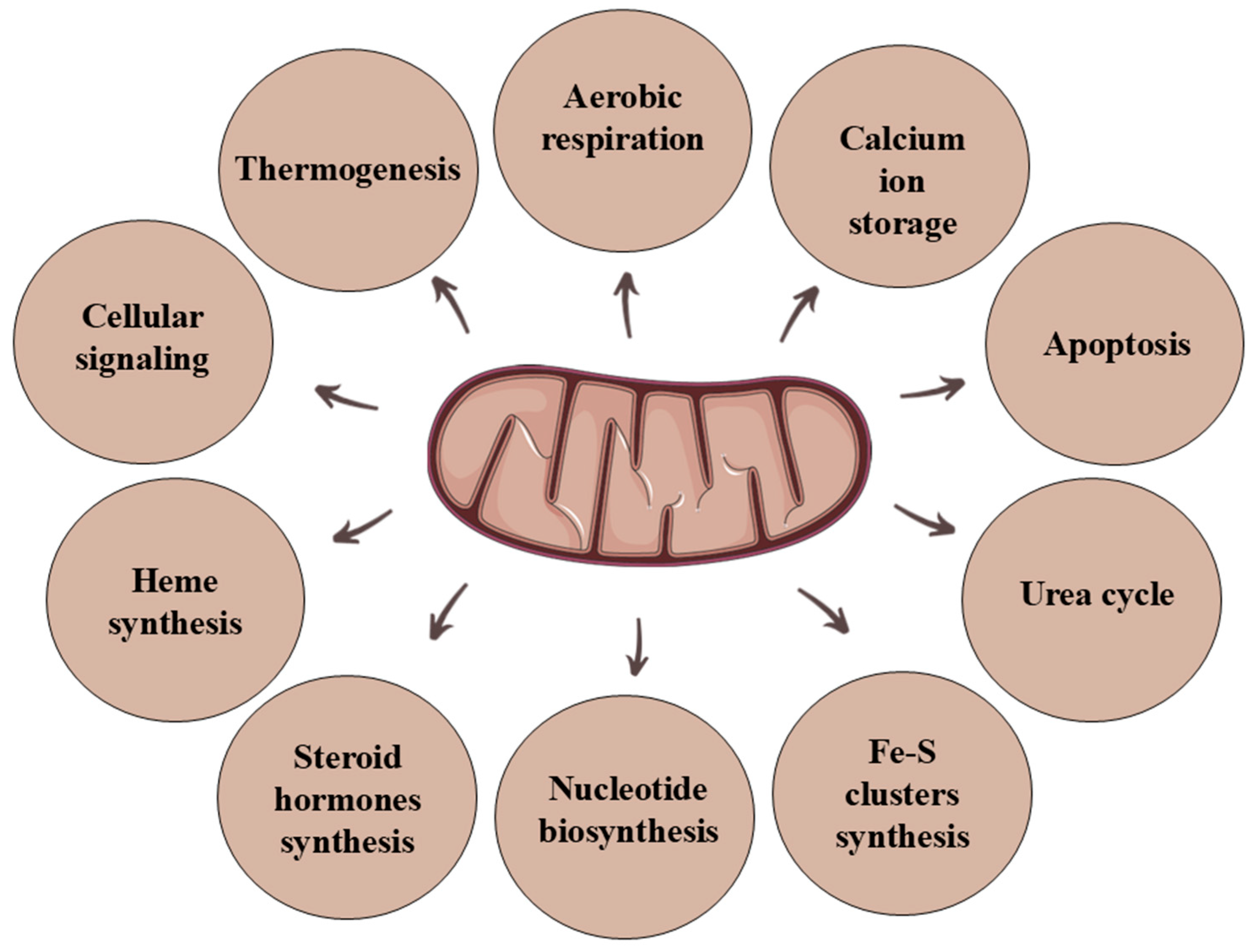
1. Introduction
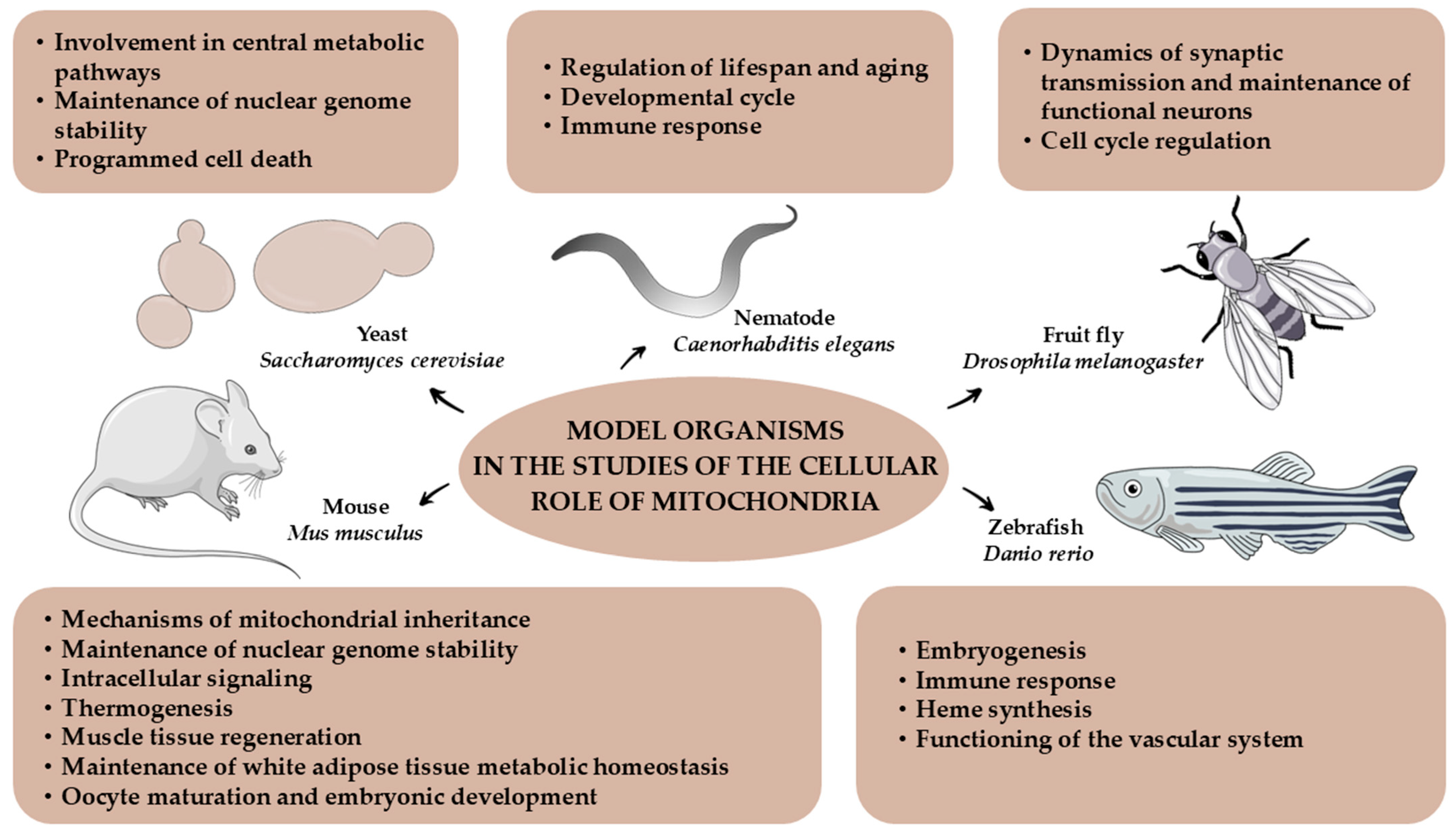
2. Model Organisms Used in Studies of the Cellular Role of Mitochondria
3. Mitochondrial Dysfunctions
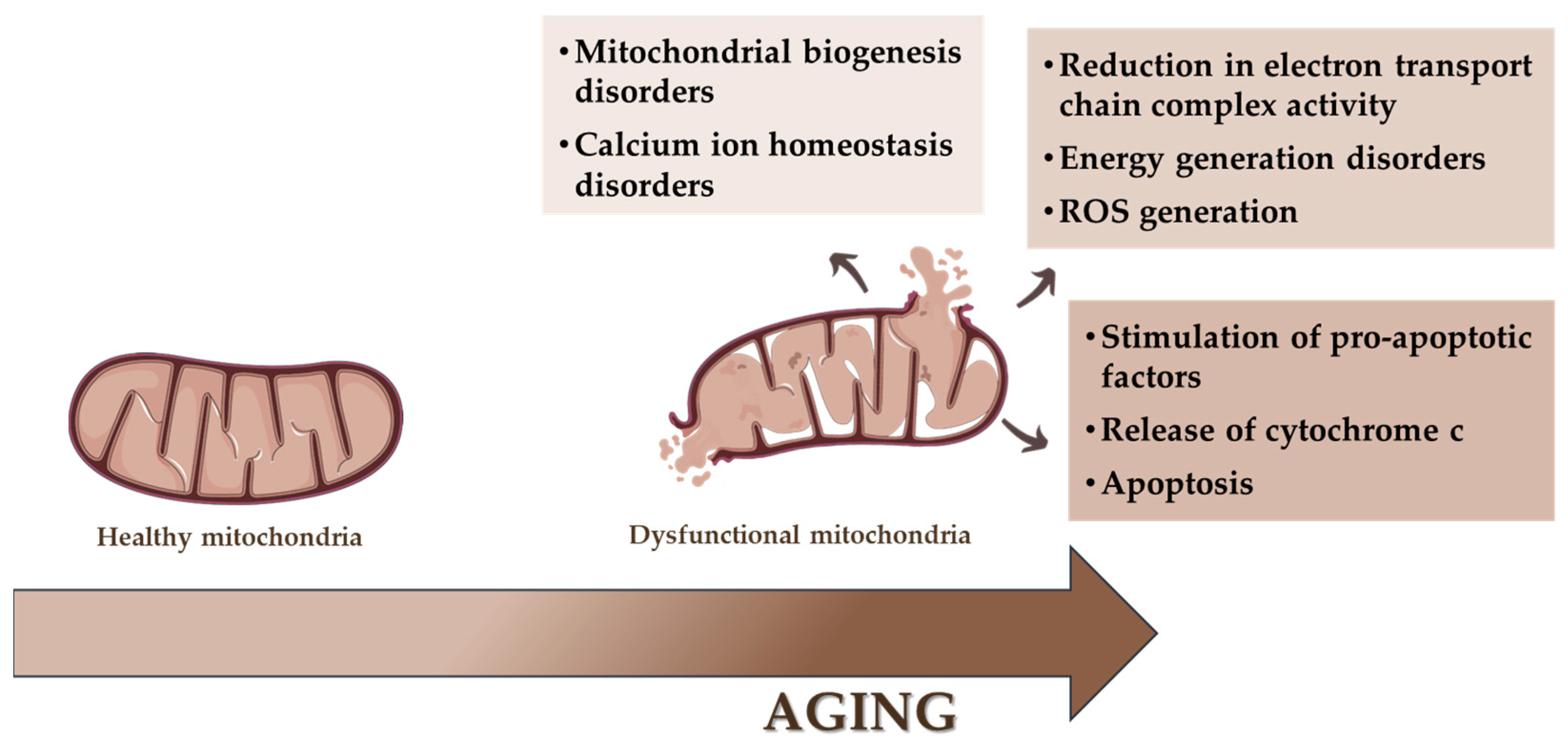
4. Consequence of Mitochondrial Dysfunctions
4.1. Cellular Senescence and Aging Process
4.2. Mitochondrial Diseases
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Malina, C.; Larsson, C.; Nielsen, J. Yeast mitochondria: An overview of mitochondrial biology and the potential of mitochondrial systems biology. FEMS Yeast Res. 2018, 18, foy040. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, T.W. Properties of human mitochondrial ribosomes. IUBMB Life 2003, 55, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Kühlbrandt, W. Structure and function of mitochondrial membrane protein complexes. BMC Biol. 2015, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Rizzuto, R.; Hajnoczky, G.; Su, T.P. MAM: More than just a housekeeper. Trends Cell Biol. 2009, 19, 81–88. [Google Scholar] [CrossRef]
- Daum, G.; Vance, J.E. Import of lipids into mitochondria. Prog. Lipid Res. 1997, 36, 103–130. [Google Scholar] [CrossRef]
- Ruhle, T.; Leister, D. Assembly of F1F0-ATP synthases. Biochim. Biophys. Acta (BBA) Bioenerg. 2015, 1847, 849–860. [Google Scholar] [CrossRef]
- Wolters, J.F.; Chiu, K.; Fiumera, H.L. Population structure of mitochondrial genomes in Saccharomyces cerevisiae. BMC Genom. 2015, 16, 451. [Google Scholar] [CrossRef]
- Osellame, L.D.; Blacker, T.S.; Duchen, M.R. Cellular and molecular mechanisms of mitochondrial function. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 711–723. [Google Scholar] [CrossRef]
- Chen, X.J.; Clark-Walker, G.D. The petite mutation in yeasts: 50 Years on. Int. Rev. Cytol. 2000, 194, 197–238. [Google Scholar] [CrossRef]
- Goffeau, A.; Barrell, B.G.; Bussey, H.; Davis, R.W.; Dujon, B.; Feldmann, H.; Galibert, F.; Hoheisel, J.D.; Jacq, C.; Johnston, M.; et al. Life with 6000 genes. Science 1996, 274, 546, 563-547. [Google Scholar] [CrossRef]
- Shibata, T.; Ling, F. DNA recombination protein-dependent mechanism of homoplasmy and its proposed functions. Mitochondrion 2007, 7, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Tenreiro, S.; Outeiro, T.F. Simple is good: Yeast models of neurodegeneration. FEMS Yeast Res. 2010, 10, 970–979. [Google Scholar] [CrossRef]
- Corsi, A.K.; Wightman, B.; Chalfie, M. A Transparent Window into Biology: A Primer on Caenorhabditis elegans. Genetics 2015, 200, 387–407. [Google Scholar] [CrossRef]
- Rea, S.L.; Graham, B.H.; Nakamaru-Ogiso, E.; Kar, A.; Falk, M.J. Bacteria, yeast, worms, and flies: Exploiting simple model organisms to investigate human mitochondrial diseases. Dev. Disabil. Res. Rev. 2010, 16, 200–218. [Google Scholar] [CrossRef]
- Roussos, A.; Kitopoulou, K.; Borbolis, F.; Palikaras, K. Caenorhabditis elegans as a Model System to Study Human Neurodegenerative Disorders. Biomolecules 2023, 13, 478. [Google Scholar] [CrossRef]
- Schmitt, F.; Eckert, G.P. Caenorhabditis elegans as a Model for the Effects of Phytochemicals on Mitochondria and Aging. Biomolecules 2022, 12, 1550. [Google Scholar] [CrossRef] [PubMed]
- Brischigliaro, M.; Fernandez-Vizarra, E.; Viscomi, C. Mitochondrial Neurodegeneration: Lessons from Drosophila melanogaster Models. Biomolecules 2023, 13, 378. [Google Scholar] [CrossRef]
- Fernández-Moreno, M.A.; Farr, C.L.; Kaguni, L.S.; Garesse, R. Drosophila melanogaster as a model system to study mitochondrial biology. Methods Mol. Biol. 2007, 372, 33–49. [Google Scholar] [CrossRef]
- Khan, F.R.; Alhewairini, S.S. Zebrafish (Danio rerio) as a Model Organism. Curr. Trends Cancer Manag. 2018, 27, 318. [Google Scholar]
- Phifer-Rixey, M.; Nachman, M.W. Insights into mammalian biology from the wild house mouse Mus musculus. eLife 2015, 4, e05959. [Google Scholar] [CrossRef] [PubMed]
- Rydell-Törmänen, K.; Johnson, J.R. The Applicability of Mouse Models to the Study of Human Disease. Methods Mol. Biol. 2019, 1940, 3–22. [Google Scholar] [CrossRef]
- Hiltunen, J.K.; Chen, Z.; Haapalainen, A.M.; Wierenga, R.K.; Kastaniotis, A.J. Mitochondrial fatty acid synthesis--an adopted set of enzymes making a pathway of major importance for the cellular metabolism. Prog. Lipid Res. 2010, 49, 27–45. [Google Scholar] [CrossRef]
- Aerts, A.M.; Zabrocki, P.; François, I.E.; Carmona-Gutierrez, D.; Govaert, G.; Mao, C.; Smets, B.; Madeo, F.; Winderickx, J.; Cammue, B.P.; et al. Ydc1p ceramidase triggers organelle fragmentation, apoptosis and accelerated ageing in yeast. Cell. Mol. Life Sci. 2008, 65, 1933–1942. [Google Scholar] [CrossRef]
- Horvath, S.E.; Daum, G. Lipids of mitochondria. Prog. Lipid Res. 2013, 52, 590–614. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.K. Mitochondria damage checkpoint in apoptosis and genome stability. FEMS Yeast Res. 2004, 5, 127–132. [Google Scholar] [CrossRef]
- Cardoso, A.R.; Queliconi, B.B.; Kowaltowski, A.J. Mitochondrial ion transport pathways: Role in metabolic diseases. Biochim. Biophys. Acta (BBA) Bioenerg. 2010, 1797, 832–838. [Google Scholar] [CrossRef]
- Tran, U.C.; Clarke, C.F. Endogenous synthesis of coenzyme Q in eukaryotes. Mitochondrion 2007, 7, S62–S71. [Google Scholar] [CrossRef]
- Sheftel, A.; Stehling, O.; Lill, R. Iron-sulfur proteins in health and disease. Trends Endocrinol. Metab. 2010, 21, 302–314. [Google Scholar] [CrossRef]
- Koppen, M.; Langer, T. Protein degradation within mitochondria: Versatile activities of AAA proteases and other peptidases. Crit. Rev. Biochem. Mol. Biol. 2007, 42, 221–242. [Google Scholar] [CrossRef]
- Ludovico, P.; Sousa, M.J.; Silva, M.T.; Leão, C.L.; Côrte-Real, M. Saccharomyces cerevisiae commits to a programmed cell death process in response to acetic acid. Microbiology (Reading) 2001, 147, 2409–2415. [Google Scholar] [CrossRef]
- Tsang, W.Y.; Lemire, B.D. The role of mitochondria in the life of the nematode, Caenorhabditis elegans. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2003, 1638, 91–105. [Google Scholar] [CrossRef]
- Kwon, S.; Kim, E.J.E.; Lee, S.V. Mitochondria-mediated defense mechanisms against pathogens in Caenorhabditis elegans. BMB Rep. 2018, 51, 274–279. [Google Scholar] [CrossRef]
- Hales, K.G.; Fuller, M.T. Developmentally regulated mitochondrial fusion mediated by a conserved, novel, predicted GTPase. Cell 1997, 90, 121–129. [Google Scholar] [CrossRef]
- Verstreken, P.; Ly, C.V.; Venken, K.J.; Koh, T.W.; Zhou, Y.; Bellen, H.J. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron 2005, 47, 365–378. [Google Scholar] [CrossRef]
- Mandal, S.; Guptan, P.; Owusu-Ansah, E.; Banerjee, U. Mitochondrial regulation of cell cycle progression during development as revealed by the tenured mutation in Drosophila. Dev. Cell 2005, 9, 843–854. [Google Scholar] [CrossRef]
- Pinho, B.R.; Santos, M.M.; Fonseca-Silva, A.; Valentão, P.; Andrade, P.B.; Oliveira, J.M. How mitochondrial dysfunction affects zebrafish development and cardiovascular function: An in vivo model for testing mitochondria-targeted drugs. Br. J. Pharmacol. 2013, 169, 1072–1090. [Google Scholar] [CrossRef]
- do Amaral, M.A.; Paredes, L.C.; Padovani, B.N.; Mendonça-Gomes, J.M.; Montes, L.F.; Câmara, N.O.S.; Morales Fénero, C. Mitochondrial connections with immune system in Zebrafish. Fish Shellfish Immunol. Rep. 2021, 2, 100019. [Google Scholar] [CrossRef]
- Shaw, G.C.; Cope, J.J.; Li, L.; Corson, K.; Hersey, C.; Ackermann, G.E.; Gwynn, B.; Lambert, A.J.; Wingert, R.A.; Traver, D.; et al. Mitoferrin is essential for erythroid iron assimilation. Nature 2006, 440, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Willaert, A.; Khatri, S.; Callewaert, B.L.; Coucke, P.J.; Crosby, S.D.; Lee, J.G.; Davis, E.C.; Shiva, S.; Tsang, M.; De Paepe, A.; et al. GLUT10 is required for the development of the cardiovascular system and the notochord and connects mitochondrial function to TGFβ signaling. Hum. Mol. Genet. 2012, 21, 1248–1259. [Google Scholar] [CrossRef] [PubMed]
- Boudina, S.; Graham, T.E. Mitochondrial function/dysfunction in white adipose tissue. Exp. Physiol. 2014, 99, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, A.; Oh, K.J.; Lee, S.C.; Kim, W.K.; Bae, K.H. The Role of Adipose Tissue Mitochondria: Regulation of Mitochondrial Function for the Treatment of Metabolic Diseases. Int. J. Mol. Sci. 2019, 20, 4924. [Google Scholar] [CrossRef] [PubMed]
- Babayev, E.; Seli, E. Oocyte mitochondrial function and reproduction. Curr. Opin. Obstet. Gynecol. 2015, 27, 175–181. [Google Scholar] [CrossRef]
- Chen, H.; Detmer, S.A.; Ewald, A.J.; Griffin, E.E.; Fraser, S.E.; Chan, D.C. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 2003, 160, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Samper, E.; Nicholls, D.G.; Melov, S. Mitochondrial oxidative stress causes chromosomal instability of mouse embryonic fibroblasts. Aging Cell 2003, 2, 277–285. [Google Scholar] [CrossRef]
- Alway, S.E.; Paez, H.G.; Pitzer, C.R. The Role of Mitochondria in Mediation of Skeletal Muscle Repair. Muscles 2023, 2, 119–163. [Google Scholar] [CrossRef]
- Cummins, J.M.; Wakayama, T.; Yanagimachi, R. Fate of microinjected spermatid mitochondria in the mouse oocyte and embryo. Zygote 1998, 6, 213–222. [Google Scholar] [CrossRef]
- Chandel, N.S. Mitochondria as signaling organelles. BMC Biol. 2014, 12, 34. [Google Scholar] [CrossRef]
- Pieczenik, S.R.; Neustadt, J. Mitochondrial dysfunction and molecular pathways of disease. Exp. Mol. Pathol. 2007, 83, 84–92. [Google Scholar] [CrossRef]
- Busch, K.B.; Kowald, A.; Spelbrink, J.N. Quality matters: How does mitochondrial network dynamics and quality control impact on mtDNA integrity? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130442. [Google Scholar] [CrossRef]
- Povea-Cabello, S.; Villanueva-Paz, M.; Suárez-Rivero, J.M.; Álvarez-Córdoba, M.; Villalón-García, I.; Talaverón-Rey, M.; Suárez-Carrillo, A.; Munuera-Cabeza, M.; Sánchez-Alcázar, J.A. Advances in mt-tRNA Mutation-Caused Mitochondrial Disease Modeling: Patients’ Brain in a Dish. Front. Genet. 2020, 11, 610764. [Google Scholar] [CrossRef]
- Park, C.B.; Larsson, N.G. Mitochondrial DNA mutations in disease and aging. J. Cell Biol. 2011, 193, 809–818. [Google Scholar] [CrossRef]
- Palmer, C.S.; Osellame, L.D.; Stojanovski, D.; Ryan, M.T. The regulation of mitochondrial morphology: Intricate mechanisms and dynamic machinery. Cell. Signal. 2011, 23, 1534–1545. [Google Scholar] [CrossRef]
- Chen, H.; Chomyn, A.; Chan, D.C. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J. Biol. Chem. 2005, 280, 26185–26192. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.V.; Margreiter, R. Heterogeneity of mitochondria and mitochondrial function within cells as another level of mitochondrial complexity. Int. J. Mol. Sci. 2009, 10, 1911–1929. [Google Scholar] [CrossRef] [PubMed]
- Parone, P.A.; Da Cruz, S.; Tondera, D.; Mattenberger, Y.; James, D.I.; Maechler, P.; Barja, F.; Martinou, J.C. Preventing mitochondrial fission impairs mitochondrial function and leads to loss of mitochondrial DNA. PLoS ONE 2008, 3, e3257. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Harman, D. The biologic clock: The mitochondria? J. Am. Geriatr. Soc. 1972, 20, 145–147. [Google Scholar] [CrossRef]
- Martini, H.; Passos, J.F. Cellular senescence: All roads lead to mitochondria. FEBS J. 2023, 290, 1186–1202. [Google Scholar] [CrossRef]
- Velarde, M.C.; Flynn, J.M.; Day, N.U.; Melov, S.; Campisi, J. Mitochondrial oxidative stress caused by Sod2 deficiency promotes cellular senescence and aging phenotypes in the skin. Aging 2012, 4, 3–12. [Google Scholar] [CrossRef] [PubMed]
- von Zglinicki, T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002, 27, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Saretzki, G.; Murphy, M.P.; von Zglinicki, T. MitoQ counteracts telomere shortening and elongates lifespan of fibroblasts under mild oxidative stress. Aging Cell 2003, 2, 141–143. [Google Scholar] [CrossRef]
- Treiber, N.; Maity, P.; Singh, K.; Kohn, M.; Keist, A.F.; Ferchiu, F.; Sante, L.; Frese, S.; Bloch, W.; Kreppel, F.; et al. Accelerated aging phenotype in mice with conditional deficiency for mitochondrial superoxide dismutase in the connective tissue. Aging Cell 2011, 10, 239–254. [Google Scholar] [CrossRef]
- Schaar, C.E.; Dues, D.J.; Spielbauer, K.K.; Machiela, E.; Cooper, J.F.; Senchuk, M.; Hekimi, S.; Van Raamsdonk, J.M. Mitochondrial and cytoplasmic ROS have opposing effects on lifespan. PLoS Genet. 2015, 11, e1004972. [Google Scholar] [CrossRef]
- Chocron, E.S.; Munkácsy, E.; Pickering, A.M. Cause or casualty: The role of mitochondrial DNA in aging and age-associated disease. Biochim. Et Biophys. Acta (BBA) Mol. Basis Dis. 2019, 1865, 285–297. [Google Scholar] [CrossRef]
- Sanchez-Contreras, M.; Kennedy, S.R. The Complicated Nature of Somatic mtDNA Mutations in Aging. Front. Aging 2022, 2, 805126. [Google Scholar] [CrossRef] [PubMed]
- Edgar, D.; Shabalina, I.; Camara, Y.; Wredenberg, A.; Calvaruso, M.A.; Nijtmans, L.; Nedergaard, J.; Cannon, B.; Larsson, N.G.; Trifunovic, A. Random point mutations with major effects on protein-coding genes are the driving force behind premature aging in mtDNA mutator mice. Cell Metab. 2009, 10, 131–138. [Google Scholar] [CrossRef]
- Trifunovic, A.; Wredenberg, A.; Falkenberg, M.; Spelbrink, J.N.; Rovio, A.T.; Bruder, C.E.; Bohlooly-Y, M.; Gidlöf, S.; Oldfors, A.; Wibom, R.; et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 2004, 429, 417–423. [Google Scholar] [CrossRef]
- Ross, J.M.; Stewart, J.B.; Hagström, E.; Brené, S.; Mourier, A.; Coppotelli, G.; Freyer, C.; Lagouge, M.; Hoffer, B.J.; Olson, L.; et al. Germline mitochondrial DNA mutations aggravate ageing and can impair brain development. Nature 2013, 501, 412–415. [Google Scholar] [CrossRef]
- Chapman, J.; Fielder, E.; Passos, J.F. Mitochondrial dysfunction and cell senescence: Deciphering a complex relationship. FEBS Lett. 2019, 593, 1566–1579. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Yun, J.; Liu, J.; Malide, D.; Liu, C.; Rovira, I.I.; Holmström, K.M.; Fergusson, M.M.; Yoo, Y.H.; Combs, C.A.; et al. Measuring In Vivo Mitophagy. Mol. Cell 2015, 60, 685–696. [Google Scholar] [CrossRef]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans. Nature 2015, 521, 525–528. [Google Scholar] [CrossRef]
- Sebastián, D.; Palacín, M.; Zorzano, A. Mitochondrial Dynamics: Coupling Mitochondrial Fitness with Healthy Aging. Trends Mol. Med. 2017, 23, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Westermann, B.; Neupert, W. Mitochondria-targeted green fluorescent proteins: Convenient tools for the study of organelle biogenesis in Saccharomyces cerevisiae. Yeast 2000, 16, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.G.; Hong, S.; Huh, W.K. Mitochondrial dysfunction reduces yeast replicative lifespan by elevating RAS-dependent ROS production by the ER-localized NADPH oxidase Yno1. PLoS ONE 2018, 13, e0198619. [Google Scholar] [CrossRef]
- Braeckman, B.P.; Houthoofd, K.; De Vreese, A.; Vanfleteren, J.R. Assaying metabolic activity in ageing Caenorhabditis elegans. Mech. Ageing Dev. 2002, 123, 105–119. [Google Scholar] [CrossRef]
- Rana, A.; Oliveira, M.P.; Khamoui, A.V.; Aparicio, R.; Rera, M.; Rossiter, H.B.; Walker, D.W. Promoting Drp1-mediated mitochondrial fission in midlife prolongs healthy lifespan of Drosophila melanogaster. Nat. Commun. 2017, 8, 448. [Google Scholar] [CrossRef]
- Song, Y.; Yang, J.; Law, A.D.; Hendrix, D.A.; Kretzschmar, D.; Robinson, M.; Giebultowicz, J.M. Age-dependent effects of blue light exposure on lifespan, neurodegeneration, and mitochondria physiology in Drosophila melanogaster. npj Aging 2022, 8, 11. [Google Scholar] [CrossRef]
- Sun, J.; Folk, D.; Bradley, T.J.; Tower, J. Induced overexpression of mitochondrial Mn-superoxide dismutase extends the life span of adult Drosophila melanogaster. Genetics 2002, 161, 661–672. [Google Scholar] [CrossRef]
- Walker, D.W.; Benzer, S. Mitochondrial “swirls” induced by oxygen stress and in the Drosophila mutant hyperswirl. Proc. Natl. Acad. Sci. USA 2004, 101, 10290–10295. [Google Scholar] [CrossRef] [PubMed]
- Almaida-Pagán, P.F.; Lucas-Sánchez, A.; Tocher, D.R. Changes in mitochondrial membrane composition and oxidative status during rapid growth, maturation and aging in zebrafish, Danio Rerio. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2014, 1841, 1003–1011. [Google Scholar] [CrossRef]
- Wang, N.; Luo, Z.; Jin, M.; Sheng, W.; Wang, H.T.; Long, X.; Wu, Y.; Hu, P.; Xu, H.; Zhang, X. Exploration of age-related mitochondrial dysfunction and the anti-aging effects of resveratrol in zebrafish retina. Aging 2019, 11, 3117–3137. [Google Scholar] [CrossRef] [PubMed]
- Gorman, G.S.; Chinnery, P.F.; DiMauro, S.; Hirano, M.; Koga, Y.; McFarland, R.; Suomalainen, A.; Thorburn, D.R.; Zeviani, M.; Turnbull, D.M. Mitochondrial diseases. Nat. Rev. Dis. Primers 2016, 2, 16080. [Google Scholar] [CrossRef] [PubMed]
- Nunnari, J.; Suomalainen, A. Mitochondria: In sickness and in health. Cell 2012, 148, 1145–1159. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S.; Baek, G.; Kim, A.; Kang, B.-C.; Seo, H.; Kim, J.-S. Mitochondrial DNA editing in mice with DddA-TALE fusion deaminases. Nat. Commun. 2021, 12, 1190. [Google Scholar] [CrossRef]
- Sabharwal, A.; Kar, B.; Restrepo-Castillo, S.; Holmberg, S.R.; Mathew, N.D.; Kendall, B.L.; Cotter, R.P.; WareJoncas, Z.; Seiler, C.; Nakamaru-Ogiso, E.; et al. The FusX TALE Base Editor (FusXTBE) for Rapid Mitochondrial DNA Programming of Human Cells In Vitro and Zebrafish Disease Models In Vivo. CRISPR J. 2021, 4, 799–821. [Google Scholar] [CrossRef]
- Tetsuka, S.; Ogawa, T.; Hashimoto, R.; Kato, H. Clinical features, pathogenesis, and management of stroke-like episodes due to MELAS. Metab. Brain Dis. 2021, 36, 2181–2193. [Google Scholar] [CrossRef]
- Finsterer, J.; Zarrouk-Mahjoub, S.; Shoffner, J.M. MERRF Classification: Implications for Diagnosis and Clinical Trials. Pediatr. Neurol. 2018, 80, 8–23. [Google Scholar] [CrossRef]
- Shimizu, A.; Mito, T.; Hashizume, O.; Yonekawa, H.; Ishikawa, K.; Nakada, K.; Hayashi, J. G7731A mutation in mouse mitochondrial tRNALys regulates late-onset disorders in transmitochondrial mice. Biochem. Biophys. Res. Commun. 2015, 459, 66–70. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, X.; Chen, X.; Sun, H.; Dai, Y.; Wang, J.; Qian, X.; Tan, L.; Lou, X.; Shen, B. Precision modeling of mitochondrial diseases in zebrafish via DdCBE-mediated mtDNA base editing. Cell Discov. 2021, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Hage, R.; Vignal-Clermont, C. Leber Hereditary Optic Neuropathy: Review of Treatment and Management. Front. Neurol. 2021, 12, 651639. [Google Scholar] [CrossRef] [PubMed]
- Claeys, K.G.; Abicht, A.; Häusler, M.; Kleinle, S.; Wiesmann, M.; Schulz, J.B.; Horvath, R.; Weis, J. Novel genetic and neuropathological insights in neurogenic muscle weakness, ataxia, and retinitis pigmentosa (NARP). Muscle Nerve 2016, 54, 328–333. [Google Scholar] [CrossRef]
- Rak, M.; Tetaud, E.; Duvezin-Caubet, S.; Ezkurdia, N.; Bietenhader, M.; Rytka, J.; di Rago, J.P. A yeast model of the neurogenic ataxia retinitis pigmentosa (NARP) T8993G mutation in the mitochondrial ATP synthase-6 gene. J. Biol. Chem. 2007, 282, 34039–34047. [Google Scholar] [CrossRef] [PubMed]
- Heighton, J.N.; Brady, L.I.; Newman, M.C.; Tarnopolsky, M.A. Clinical and demographic features of chronic progressive external ophthalmoplegia in a large adult-onset cohort. Mitochondrion 2019, 44, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Morena, J.; Gupta, A.; Hoyle, J.C. Charcot-Marie-Tooth: From Molecules to Therapy. Int. J. Mol. Sci. 2019, 20, 3419. [Google Scholar] [CrossRef]
- Vettori, A.; Bergamin, G.; Moro, E.; Vazza, G.; Polo, G.; Tiso, N.; Argenton, F.; Mostacciuolo, M.L. Developmental defects and neuromuscular alterations due to mitofusin 2 gene (MFN2) silencing in zebrafish: A new model for Charcot-Marie-Tooth type 2A neuropathy. Neuromuscul. Disord. 2011, 21, 58–67. [Google Scholar] [CrossRef]
- Xu, W.Y.; Zhu, H.; Shen, Y.; Wan, Y.H.; Tu, X.D.; Wu, W.T.; Tang, L.; Zhang, H.X.; Lu, S.Y.; Jin, X.L.; et al. DHTKD1 Deficiency Causes Charcot-Marie-Tooth Disease in Mice. Mol. Cell. Biol. 2018, 38, e00085-18. [Google Scholar] [CrossRef] [PubMed]
- Facchinello, N.; Laquatra, C.; Locatello, L.; Beffagna, G.; Brañas Casas, R.; Fornetto, C.; Dinarello, A.; Martorano, L.; Vettori, A.; Risato, G.; et al. Efficient clofilium tosylate-mediated rescue of POLG-related disease phenotypes in zebrafish. Cell Death Dis. 2021, 12, 100. [Google Scholar] [CrossRef]
- Saneto, R.P. Alpers-Huttenlocher syndrome: The role of a multidisciplinary health care team. J. Multidiscip. Healthc. 2016, 9, 323–333. [Google Scholar] [CrossRef]
- Cook, A.; Giunti, P. Friedreich’s ataxia: Clinical features, pathogenesis and management. Br. Med. Bull. 2017, 124, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Dominska, M.; Gawel, M.; Greenwell, P.W.; Petes, T.D. Genomic deletions and point mutations induced in Saccharomyces cerevisiae by the trinucleotide repeats (GAA·TTC) associated with Friedreich’s ataxia. DNA Repair 2013, 12, 10–17. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, Z.; Qi, Y.; French, S.; Zhang, G.; Covian Garcia, R.; Balaban, R.; Xu, H. Genetic mosaic analysis of a deleterious mitochondrial DNA mutation in Drosophila reveals novel aspects of mitochondrial regulation and function. Mol. Biol. Cell 2015, 26, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Kishita, Y.; Ishikawa, K.; Nakada, K.; Hayashi, J.I.; Fushimi, T.; Shimura, M.; Kohda, M.; Ohtake, A.; Murayama, K.; Okazaki, Y. A high mutation load of m.14597A>G in MT-ND6 causes Leigh syndrome. Sci. Rep. 2021, 11, 11123. [Google Scholar] [CrossRef]
- Clarke, S.L.; Bowron, A.; Gonzalez, I.L.; Groves, S.J.; Newbury-Ecob, R.; Clayton, N.; Martin, R.P.; Tsai-Goodman, B.; Garratt, V.; Ashworth, M.; et al. Barth syndrome. Orphanet J. Rare Dis. 2013, 8, 23. [Google Scholar] [CrossRef]
- Xu, Y.; Condell, M.; Plesken, H.; Edelman-Novemsky, I.; Ma, J.; Ren, M.; Schlame, M. A Drosophila model of Barth syndrome. Proc. Natl. Acad. Sci. USA 2006, 103, 11584–11588. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Nie, J.; Shi, Y. Restoration of mitophagy ameliorates cardiomyopathy in Barth syndrome. Autophagy 2022, 18, 2134–2149. [Google Scholar] [CrossRef]

| Organism | Description | Advantages of the Model Organism | Limitations of the Model Organism | References |
|---|---|---|---|---|
| Yeast Saccharomyces cerevisiae |
|
|
| [9,10,11,12] |
| Nematode Caenorhabditis elegans |
|
|
| [13,14,15,16] |
| Fruit fly Drosophila melanogaster |
|
|
| [17,18] |
| Zebrafish Danio rerio |
|
|
| [19] |
| Mouse Mus musculus |
|
|
| [20,21] |
| Model Organism | Mitochondrial Aspect Related to the Aging Process | References |
|---|---|---|
| Yeast Saccharomyces cerevisiae |
| [75,76] |
| Nematode Caenorhabditis elegans |
| [65,73,77] |
| Fruit fly Drosophila melanogaster |
| [78,79,80,81] |
| Zebrafish Danio rerio |
| [82,83] |
| Mouse Mus musculus |
| [61,68,69,70] |
| Disease | Genetic Change | Mitochondrial Dysfunctions | Sign and Symptoms of Disease | Model Organism for Studying Disease | References |
|---|---|---|---|---|---|
| Diseases caused by mutations in mtDNA | |||||
| MELAS syndrome | MT-TL1 gene mutation |
| encephalopathy, lactic acidosis (due to increased glycolysis) | Saccharomyces cerevisiae, Danio rerio, Mus musculus | [86,87,88] |
| MERRF syndrome | MT-TK gene mutation |
| progressive myoclonic epilepsy, muscle weakness, and muscle cells appear as frayed fibers | Danio rerio Mus musculus | [89,90] |
| Leber’s hereditary optic neuropathy (LHON) | MT-ND1, MT-ND4, MT-ND4L, MT-ND6 genes mutation |
| loss of optic nerves due to degeneration of retinal ganglion cells and their axons | Danio rerio Mus musculus | [86,91,92] |
| NARP syndrome | MT-ATP6 gene mutation |
| neuropathy, ataxia, retinitis pigmentosa | Saccharomyces cerevisiae | [93,94] |
| Chronic progressive external ophthalmoplegia syndrome (CPEO) | deletions in mtDNA |
| myopathy, ptosis, retinitis pigmentosa, central nervous system dysfunction | Danio rerio | [91,95] |
| Diseases caused by mutations in nuclear DNA | |||||
| Charcot-Marie-Tooth disease type 2A (CMT2A) | MFN2 gene mutation |
| degeneration and loss of axons of myelin fibers in peripheral nerves | Saccharomyces cerevisiae, Danio rerio, Mus musculus | [96,97,98] |
| Alpers-Huttenloch disease | POLG gene mutation |
| progressive loss of cognitive and motor skills | Caenorhabditis elegans, Danio rerio | [99,100] |
| Friedreich’s ataxia | FXN gene mutation (encoding frataxin) |
| progressive ataxia of gait and limbs | Saccharomyces cerevisiae | [101,102] |
| Leigh syndrome | mutations of genes encoding proteins of the respiratory chain complexes |
| necrotic changes in the basal ganglia, brainstem and midbrain, hypotonia, epilepsy, ataxia, lactic acidosis | Saccharomyces cerevisiae, Drosophila melanogaster, Danio rerio, Mus musculus | [86,91,103,104] |
| Barth syndrome | TAZ gene mutation (encoding tafazzin) |
| decreased muscle tone, dilated cardiomyopathy, neutropenia | Saccharomyces cerevisiae, Drosophila melanogaster, Mus musculus | [105,106,107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stańczyk, M.; Szubart, N.; Maslanka, R.; Zadrag-Tecza, R. Mitochondrial Dysfunctions: Genetic and Cellular Implications Revealed by Various Model Organisms. Genes 2024, 15, 1153. https://doi.org/10.3390/genes15091153
Stańczyk M, Szubart N, Maslanka R, Zadrag-Tecza R. Mitochondrial Dysfunctions: Genetic and Cellular Implications Revealed by Various Model Organisms. Genes. 2024; 15(9):1153. https://doi.org/10.3390/genes15091153
Chicago/Turabian StyleStańczyk, Monika, Natalia Szubart, Roman Maslanka, and Renata Zadrag-Tecza. 2024. "Mitochondrial Dysfunctions: Genetic and Cellular Implications Revealed by Various Model Organisms" Genes 15, no. 9: 1153. https://doi.org/10.3390/genes15091153
APA StyleStańczyk, M., Szubart, N., Maslanka, R., & Zadrag-Tecza, R. (2024). Mitochondrial Dysfunctions: Genetic and Cellular Implications Revealed by Various Model Organisms. Genes, 15(9), 1153. https://doi.org/10.3390/genes15091153






