Causal Effect of Immunocytes, Plasma Metabolites, and Hepatocellular Carcinoma: A Bidirectional Two-Sample Mendelian Randomization Study and Mediation Analysis in East Asian Populations
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Data Sources
2.3. Instrument Variables
2.4. Statistical Analysis
2.4.1. Two-Sample MR
2.4.2. Reverse MR Analysis
2.4.3. Analysis of Metabolic Pathways
2.4.4. Mediation Analysis
2.4.5. Sensitivity Analysis
3. Results
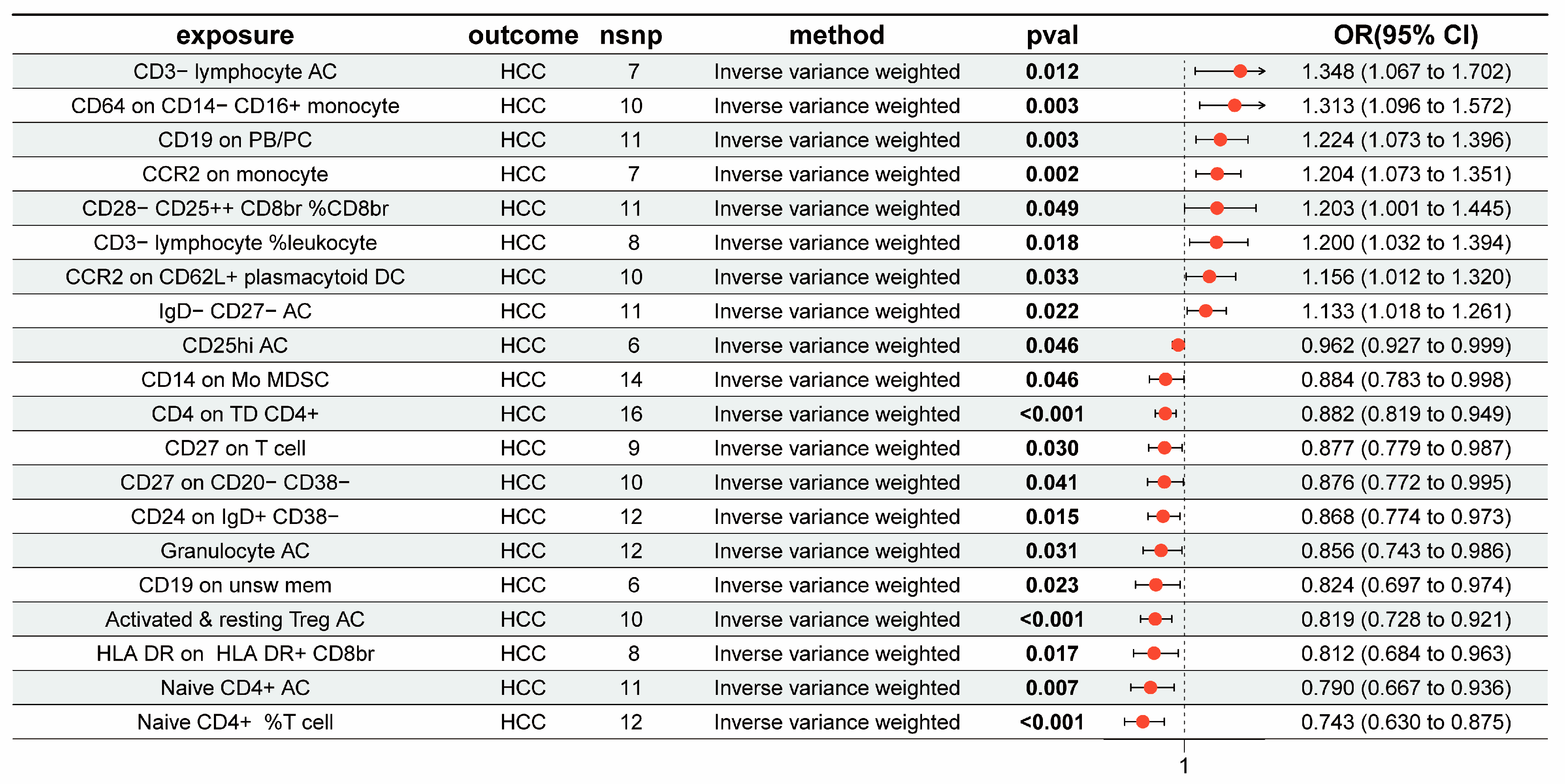
3.1. The Overall Causal Impact of Immunocytes on HCC
3.2. The Overall Causal Impact of Plasma Metabolites on HCC
3.3. The Results of the Mediation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maluccio, M.; Covey, A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J. Clin. 2012, 62, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xie, H.; Hu, M.; Huang, T.; Hu, Y.; Sang, N.; Zhao, Y. Recent progress in the treatment of hepatocellular carcinoma. Am. J. Cancer Res. 2020, 10, 2993–3036. [Google Scholar]
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491.e1. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Hollebecque, A.; Malka, D.; Ferté, C.; Ducreux, M.; Boige, V. Systemic treatment of advanced hepatocellular carcinoma: From disillusions to new horizons. Eur. J. Cancer 2015, 51, 327–339. [Google Scholar] [CrossRef]
- Krishnan, M.S.; Kd, A.R.; Park, J.; Arjunan, V.; Marques, F.J.G.; Bermudez, A.; Girvan, O.A.; Hoang, N.S.; Yin, J.; Nguyen, M.H.; et al. Genomic Analysis of Vascular Invasion in HCC Reveals Molecular Drivers and Predictive Biomarkers. Hepatology 2021, 73, 2342–2360. [Google Scholar] [CrossRef]
- Li, D.-F.; Yang, M.-F.; Xu, J.; Xu, H.-M.; Zhu, M.-Z.; Liang, Y.-J.; Zhang, Y.; Tian, C.-M.; Nie, Y.-Q.; Shi, R.-Y.; et al. Extracellular Vesicles: The Next Generation Theranostic Nanomedicine for Inflammatory Bowel Disease. Int. J. Nanomed. 2022, 17, 3893–3911. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Yan, K.-K.; Ding, L.; Qian, C.; Chi, H.; Yu, J. Network Approaches for Dissecting the Immune System. iScience 2020, 23, 101354. [Google Scholar] [CrossRef]
- Xia, L.; Oyang, L.; Lin, J.; Tan, S.; Han, Y.; Wu, N.; Yi, P.; Tang, L.; Pan, Q.; Rao, S.; et al. The cancer metabolic reprogramming and immune response. Mol. Cancer 2021, 20, 28. [Google Scholar] [CrossRef]
- Gabrielson, A.; Wu, Y.; Wang, H.; Jiang, J.; Kallakury, B.; Gatalica, Z.; Reddy, S.; Kleiner, D.; Fishbein, T.; Johnson, L.; et al. Intratumoral CD3 and CD8 T-cell Densities Associated with Relapse-Free Survival in, H.C.C. Cancer Immunol. Res. 2016, 4, 419–430. [Google Scholar] [CrossRef]
- Chew, V.; Tow, C.; Teo, M.; Wong, H.L.; Chan, J.; Gehring, A.; Loh, M.; Bolze, A.; Quek, R.; Lee, V.K.; et al. Inflammatory tumour microenvironment is associated with superior survival in hepatocellular carcinoma patients. J. Hepatol. 2010, 52, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Hokuto, D.; Sho, M.; Yamato, I.; Yasuda, S.; Obara, S.; Nomi, T.; Nakajima, Y. Clinical impact of herpesvirus entry mediator expression in human hepatocellular carcinoma. Eur. J. Cancer 2015, 51, 157–165. [Google Scholar] [CrossRef]
- Llovet, J.M.; Castet, F.; Heikenwalder, M.; Maini, M.K.; Mazzaferro, V.; Pinato, D.J.; Pikarsky, E.; Zhu, A.X.; Finn, R.S. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2022, 19, 151–172. [Google Scholar] [CrossRef]
- Zhu, X.; Qiu, Z.; Yang, M.; Yang, J.; Kong, L.; Li, L.; Huang, Y.; Xie, L. Causal associations of BAFF-R on IgD+ CD24− B cell immune cell trait with hepatocellular carcinoma and the mediating role of phenylacetylglutamate levels: A Mendelian randomization study. J. Cancer 2024, 15, 4591–4603. [Google Scholar] [CrossRef]
- Tao, X.; Mao, S.; Wang, J.; Li, G.; Sun, B. Causal Effects and Immune Cell Mediators of Prescription Analgesic Use and Risk of Liver Cancer and Precancerosis in European Population: A Mendelian Randomization Study. Biomedicines 2024, 12, 1537. [Google Scholar] [CrossRef]
- Chen, H.; Yang, R.; Zhang, J.; Tang, J.; Yu, X.; Zhou, W.; Li, K.; Peng, W.; Zeng, P. Causal relationships between immune cells, inflammatory factors, serum metabolites, and hepatic cancer: A two-sample Mendelian randomization study. Heliyon 2024, 10, e35003. [Google Scholar] [CrossRef] [PubMed]
- Emdin, C.A.; Khera, A.V.; Kathiresan, S. Mendelian Randomization. JAMA 2017, 318, 1925–1926. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.R.; Sanderson, E.; Hammerton, G.; Richmond, R.C.; Smith, G.D.; Heron, J.; Taylor, A.E.; Davies, N.M.; Howe, L.D. Mendelian randomisation for mediation analysis: Current methods and challenges for implementation. Eur. J. Epidemiol. 2021, 36, 465–478. [Google Scholar] [CrossRef]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Yarmolinsky, J.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Higgins, J.P.T.; Timpson, N.J.; Dimou, N.; et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA 2021, 326, 1614–1621. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, T.; Pettersson-Kymmer, U.; Stewart, I.D.; Butler-Laporte, G.; Nakanishi, T.; Cerani, A.; Liang, K.Y.H.; Yoshiji, S.; Willett, J.D.S.; et al. Genomic atlas of the plasma metabolome prioritizes metabolites implicated in human diseases. Nat. Genet. 2023, 55, 44–53. [Google Scholar] [CrossRef]
- Orrù, V.; Steri, M.; Sidore, C.; Marongiu, M.; Serra, V.; Olla, S.; Sole, G.; Lai, S.; Dei, M.; Mulas, A.; et al. Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat. Genet. 2020, 52, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Ishigaki, K.; Akiyama, M.; Kanai, M.; Takahashi, A.; Kawakami, E.; Sugishita, H.; Sakaue, S.; Matoba, N.; Low, S.-K.; Okada, Y.; et al. Large-scale genome-wide association study in a Japanese population identifies novel susceptibility loci across different diseases. Nat. Genet. 2020, 52, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Benn, M.; Nordestgaard, B.G. From genome-wide association studies to Mendelian randomization: Novel opportunities for understanding cardiovascular disease causality, pathogenesis, prevention, and treatment. Cardiovasc. Res. 2018, 114, 1192–1208. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.-Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Thompson, S.G.; CRP CHD Genetics Collaboration. Avoiding bias from weak instruments in Mendelian randomization studies. Int. J. Epidemiol. 2011, 40, 755–764. [Google Scholar] [CrossRef]
- Pierce, B.L.; Ahsan, H.; Vanderweele, T.J. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int. J. Epidemiol. 2011, 40, 740–752. [Google Scholar] [CrossRef]
- Roerecke, M.; Vafaei, A.; Hasan, O.S.; Chrystoja, B.R.; Cruz, M.; Lee, R.; Neuman, M.G.; Rehm, J. Alcohol Consumption and Risk of Liver Cirrhosis: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2019, 114, 1574–1586. [Google Scholar] [CrossRef]
- Marti-Aguado, D.; Clemente-Sanchez, A.; Bataller, R. Cigarette smoking and liver diseases. J. Hepatol. 2022, 77, 191–205. [Google Scholar] [CrossRef]
- Yi, S.-W.; Choi, J.-S.; Yi, J.-J.; Lee, Y.-H.; Han, K.J. Risk factors for hepatocellular carcinoma by age, sex, and liver disorder status: A prospective cohort study in Korea. Cancer 2018, 124, 2748–2757. [Google Scholar] [CrossRef]
- Benjamini, Y.; Drai, D.; Elmer, G.; Kafkafi, N.; Golani, I. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 2001, 125, 279–284. [Google Scholar] [CrossRef]
- Yarmolinsky, J.; Robinson, J.W.; Mariosa, D.; Karhunen, V.; Huang, J.; Dimou, N.; Murphy, N.; Burrows, K.; Bouras, E.; Smith-Byrne, K.; et al. Association between circulating inflammatory markers and adult cancer risk: A Mendelian randomization analysis. EBioMedicine 2024, 100, 104991. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; E MacDonald, P.; Wishart, D.S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 2024, 52, gkae253. [Google Scholar] [CrossRef]
- Frolkis, A.; Knox, C.; Lim, E.; Jewison, T.; Law, V.; Hau, D.D.; Liu, P.; Gautam, B.; Ly, S.; Guo, A.C.; et al. SMPDB: The Small Molecule Pathway Database. Nucleic Acids Res. 2010, 38, D480–D487. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Sato, Y.; Furumichi, M.; Tanabe, M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012, 40, D109–D114. [Google Scholar] [CrossRef]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 2018, 7, e34408. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Ramapriyan, R.; Caetano, M.S.; Barsoumian, H.B.; Mafra, A.C.P.; Zambalde, E.P.; Menon, H.; Tsouko, E.; Welsh, J.W.; Cortez, M.A. Altered cancer metabolism in mechanisms of immunotherapy resistance. Pharmacol. Ther. 2019, 195, 162–171. [Google Scholar] [CrossRef]
- Cronin, S.J.F.; Seehus, C.; Weidinger, A.; Talbot, S.; Reissig, S.; Seifert, M.; Pierson, Y.; McNeill, E.; Longhi, M.S.; Turnes, B.L.; et al. The metabolite BH4 controls T cell proliferation in autoimmunity and cancer. Nature 2018, 563, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.d.M.; Teixeira, F.M.E.; Sato, M.N. Impact of Retinoic Acid on Immune Cells and Inflammatory Diseases. Mediators Inflamm. 2018, 2018, 3067126. [Google Scholar] [CrossRef]
- Calder, P.C. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 197–202. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, H.; Wang, S.; Tu, Z.; Zhang, L.; Wang, X.; Hou, Y.; Wang, C.; Chen, J.; Liu, Y. Flaxseed Oil Attenuates Intestinal Damage and Inflammation by Regulating Necroptosis and TLR4/NOD Signaling Pathways Following Lipopolysaccharide Challenge in a Piglet Model. Mol. Nutr. Food Res. 2018, 62, e1700814. [Google Scholar] [CrossRef]
- Feng, S.; Xie, X.; Chen, C.; Zuo, S.; Zhao, X.; Li, H. Alpha-linolenic acid inhibits hepatocellular carcinoma cell growth through Farnesoid X receptor/β-catenin signaling pathway. Nutr. Metab. 2022, 19, 57. [Google Scholar] [CrossRef]
- Cui, H.; Han, F.; Zhang, L.; Wang, L.; Kumar, M. Gamma linolenic acid regulates PHD2 mediated hypoxia and mitochondrial apoptosis in DEN induced hepatocellular carcinoma. Drug Des. Devel Ther. 2018, 12, 4241–4252. [Google Scholar] [CrossRef]
- Campbell, C.; McKenney, P.T.; Konstantinovsky, D.; Isaeva, O.I.; Schizas, M.; Verter, J.; Mai, C.; Jin, W.-B.; Guo, C.-J.; Violante, S.; et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature 2020, 581, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Sadelain, M.; Rivière, I.; Riddell, S. Therapeutic T cell engineering. Nature 2017, 545, 423–431. [Google Scholar] [CrossRef]
- Tu, X.; Chen, L.; Zheng, Y.; Mu, C.; Zhang, Z.; Wang, F.; Ren, Y.; Duan, Y.; Zhang, H.; Tong, Z.; et al. S100A9+CD14+ monocytes contribute to anti-PD-1 immunotherapy resistance in advanced hepatocellular carcinoma by attenuating T cell-mediated antitumor function. J. Exp. Clin. Cancer Res. 2024, 43, 72. [Google Scholar] [CrossRef] [PubMed]
- Hoeks, C.; Duran, G.; Hellings, N.; Broux, B. When Helpers Go Above and Beyond: Development and Characterization of Cytotoxic CD4+ T Cells. Front. Immunol. 2022, 13, 951900. [Google Scholar] [CrossRef] [PubMed]
- Alspach, E.; Lussier, D.M.; Miceli, A.P.; Kizhvatov, I.; DuPage, M.; Luoma, A.M.; Meng, W.; Lichti, C.F.; Esaulova, E.; Vomund, A.N.; et al. MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature 2019, 574, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Marzo, A.L.; Kinnear, B.F.; Lake, R.A.; Frelinger, J.J.; Collins, E.J.; Robinson, B.W.S.; Scott, B. Tumor-specific CD4+ T cells have a major “post-licensing” role in CTL mediated anti-tumor immunity. J. Immunol. 2000, 165, 6047–6055. [Google Scholar] [CrossRef]
- Xue, H.; Lin, F.; Tan, H.; Zhu, Z.-Q.; Zhang, Z.-Y.; Zhao, L. Overrepresentation of IL-10-Expressing B Cells Suppresses Cytotoxic CD4+ T Cell Activity in HBV-Induced Hepatocellular Carcinoma. PLoS ONE 2016, 11, e0154815. [Google Scholar] [CrossRef]
- Levan, S.R.; Stamnes, K.A.; Lin, D.L.; Panzer, A.R.; Fukui, E.; McCauley, K.; Fujimura, K.E.; McKean, M.; Ownby, D.R.; Zoratti, E.M.; et al. Elevated faecal 12,13-diHOME concentration in neonates at high risk for asthma is produced by gut bacteria and impedes immune tolerance. Nat. Microbiol. 2019, 4, 1851–1861. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.; Dobretsov, M.; Kurten, R.C.; Grant, D.F.; Stimers, J.R. Effect of linoleic acid metabolites on Na+/K+ pump current in N20.1 oligodendrocytes: Role of membrane fluidity. Toxicol. Appl. Pharmacol. 2002, 182, 76–83. [Google Scholar] [CrossRef]
- Shiratori, H.; Oguchi, H.; Isobe, Y.; Han, K.-H.; Sen, A.; Yakebe, K.; Takahashi, D.; Fukushima, M.; Arita, M.; Hase, K. Gut microbiota-derived lipid metabolites facilitate regulatory T cell differentiation. Sci. Rep. 2023, 13, 8903. [Google Scholar] [CrossRef]
- Li, X.; Yao, W.; Yuan, Y.; Chen, P.; Li, B.; Li, J.; Chu, R.; Song, H.; Xie, D.; Jiang, X.; et al. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut 2017, 66, 157–167. [Google Scholar] [CrossRef]
- Xue, C.; Gu, X.; Zheng, Q.; Shi, Q.; Yuan, X.; Chu, Q.; Jia, J.; Su, Y.; Bao, Z.; Lu, J.; et al. Effects of 3-HAA on HCC by Regulating the Heterogeneous Macrophages-A scRNA-Seq Analysis. Adv. Sci. 2023, 10, e2207074. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.-Y.; Gao, Q.; Wang, Z.-C.; Zhou, J.; Wang, X.-Y.; Min, Z.-H.; Shi, Y.-H.; Shi, G.-M.; Ding, Z.-B.; Ke, A.-W.; et al. Margin-infiltrating CD20+ B cells display an atypical memory phenotype and correlate with favorable prognosis in hepatocellular carcinoma. Clin. Cancer Res. 2013, 19, 5994–6005. [Google Scholar] [CrossRef]
- Jin, D.; Hui, Y.; Liu, D.; Li, N.; Leng, J.; Wang, G.; Wang, Q.; Lu, Z. LINC00942 inhibits ferroptosis and induces the immunosuppression of regulatory T cells by recruiting IGF2BP3/SLC7A11 in hepatocellular carcinoma. Funct. Integr. Genom. 2024, 24, 29. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-J.; Dai, Z.-Z.; Hu, M.-Z.; Liu, J.-N.; Liang, H.; Shen, M.-M.; Zhu, S.-J.; Sheng, H.-J.; Gao, J.; Huang, A.-L.; et al. Upregulation of TUBG1 expression promotes hepatocellular carcinoma development. Med. Oncol. 2023, 40, 96. [Google Scholar] [CrossRef]
- Lewinska, M.; Santos-Laso, A.; Arretxe, E.; Alonso, C.; Zhuravleva, E.; Jimenez-Agüero, R.; Eizaguirre, E.; Pareja, M.J.; Romero-Gómez, M.; Arrese, M.; et al. The altered serum lipidome and its diagnostic potential for Non-Alcoholic Fatty Liver (NAFL)-associated hepatocellular carcinoma. EBioMedicine 2021, 73, 103661. [Google Scholar] [CrossRef]
- Stepien, M.; Keski-Rahkonen, P.; Kiss, A.; Robinot, N.; Duarte-Salles, T.; Murphy, N.; Perlemuter, G.; Viallon, V.; Tjønneland, A.; Rostgaard-Hansen, A.L.; et al. Metabolic perturbations prior to hepatocellular carcinoma diagnosis: Findings from a prospective observational cohort study. Int. J. Cancer 2021, 148, 609–625. [Google Scholar] [CrossRef]
- Wishart, D.S. Emerging applications of metabolomics in drug discovery and precision medicine. Nat. Rev. Drug Discov. 2016, 15, 473–484. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, H.; Chen, Z.; Deng, C.; Zhou, L.; Chen, S.; Kang, J.; Chen, Y.; He, S.; Zhou, Z. Identification of a novel plasma metabolite panel as diagnostic biomarker for hepatocellular carcinoma. Clin. Chim. Acta 2023, 543, 117302. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Zhang, Z.; Chen, J.; Fan, Y.; Guo, J.; Su, Y.; Wang, H.; Zhang, X.; Wu, X.; Jiang, Q.; et al. High-fat diet promotes liver tumorigenesis via palmitoylation and activation of AKT. Gut 2024, 73, 1156–1168. [Google Scholar] [CrossRef]
- Sun, J.; Ding, J.; Shen, Q.; Wang, X.; Wang, M.; Huang, Y.; Zhang, X.; Zhu, H.; Zhang, F.; Wu, D.; et al. Decreased propionyl-CoA metabolism facilitates metabolic reprogramming and promotes hepatocellular carcinoma. J. Hepatol. 2023, 78, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jin, M.; Meng, Y.; Dai, Y.; Chen, S.; Zhou, Y.; Li, Y.; Tang, L. Involvement and targeted intervention of benzo(a)pyrene-regulated apoptosis related proteome modification and muti-drug resistance in hepatocellular carcinoma. Cell Death Dis. 2023, 14, 265. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Feng, Y.; Liu, Y.; Ye, X.; Ji, X.; Sun, L.; Gao, F.; Zhang, Q.; Li, Y.; Zhu, B.; et al. Fuzheng Jiedu Xiaoji formulation inhibits hepatocellular carcinoma progression in patients by targeting the AKT/CyclinD1/p21/p27 pathway. Phytomedicine 2021, 87, 153575. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Wang, X.; Huang, F.; Zhao, A.; Chen, W.; Yan, J.; Zhang, Y.; Lei, S.; Ge, K.; Zheng, X.; et al. Dysregulated hepatic bile acids collaboratively promote liver carcinogenesis. Int. J. Cancer 2016, 139, 1764–1775. [Google Scholar] [CrossRef]
- Hong, J.; Wang, X.; Jin, H.; Chen, Y.; Jiang, Y.; Du, K.; Chen, D.; Zheng, S.; Cao, L. Environment relevant exposure of perfluorooctanoic acid accelerates the growth of hepatocellular carcinoma cells through mammalian target of rapamycin (mTOR) signal pathway. Environ. Pollut. 2024, 341, 122910. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Zhang, Z.; Shi, L.; Cheng, L.; Zhang, X. Effect of the gut microbiome, plasma metabolome, peripheral cells, and inflammatory cytokines on obesity: A bidirectional two-sample Mendelian randomization study and mediation analysis. Front. Immunol. 2024, 15, 1348347. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, Y.; Cao, L.; Ji, B.; Ge, Z.; Zheng, Q.; Qi, Z.; Ding, S. PC 18:1/18:1 mediates the anti-inflammatory effects of exercise and remodels tumor microenvironment of hepatocellular carcinoma. Life Sci. 2024, 336, 122335. [Google Scholar] [CrossRef]
- Li, K.; Shi, W.; Song, Y.; Qin, L.; Zang, C.; Mei, T.; Li, A.; Song, Q.; Zhang, Y. Reprogramming of lipid metabolism in hepatocellular carcinoma resulting in downregulation of phosphatidylcholines used as potential markers for diagnosis and prediction. Expert. Rev. Mol. Diagn. 2023, 23, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liao, X.; Hu, Y.; Li, M.; Tang, M.; Zhang, S.; Mo, S.; Li, X.; Chen, S.; Qian, W.; et al. SLC27A4-mediated selective uptake of mono-unsaturated fatty acids promotes ferroptosis defense in hepatocellular carcinoma. Free Radic. Biol. Med. 2023, 201, 41–54. [Google Scholar] [CrossRef] [PubMed]


| Exposure | Mediator | Outcome | Mediated Effect (95% Cl) | Exposure | p-Value |
|---|---|---|---|---|---|
| CCR2 on CD62L+ plasmacytoid DC | 3-hydroxyhexanoate levels | HCC | 0.0131 (0.0024, 0.0238) | 9.03% (1.64%, 16.4%) | 0.0166 |
| CCR2 on CD62L+ plasmacytoid DC | Dodecenedioate (C12:1-DC) levels | HCC | 0.0132 (0.0033, 0.0232) | 9.14% (2.25%, 16%) | 0.0093 |
| CD3− lymphocyte %leukocyte | X-24811 levels | HCC | 0.0128 (0.0018, 0.0239) | 7.04% (0.96%, 13.1%) | 0.0232 |
| CD4 on TD CD4+ | 9,10-DiHOME levels | HCC | −0.00596 (−0.0108, −0.0011) | 4.73% (8.56%, 0.90%) | 0.0154 |
| CD4 on TD CD4+ | X-24306 levels | HCC | −0.00844 (−0.0169, −2.2× 10−5) | 6.71% (13.4%, 0.02%) | 0.0494 |
| CD4 on TD CD4+ | X-24307 levels | HCC | −0.0188 (−0.0335, −0.0040) | 14.9% (26.6%, 3.19%) | 0.0126 |
| CD14 on Mo MDSC | Linolenate [α or γ; (18:3n3 or 6)] levels | HCC | −0.0128 (−0.0247, −0.0009) | 10.4% (19.9%, 0.78%) | 0.0339 |
| CD19 on PB/PC | Linolenate [α or γ; (18:3n3 or 6)] levels | HCC | 0.0389 (0.012, 0.0658) | 19.3% (5.94%, 32.6%) | 0.0046 |
| CD19 on unsw mem | N-acetyl-isoputreanine levels | HCC | −0.00829 (−0.0163, −0.0002) | 4.27% (8.42%, 0.13%) | 0.0434 |
| CD24 on IgD+ CD38- | 3-methyl-2-oxovalerate to 4-methyl-2-oxopentanoate ratio | HCC | −0.00862 (−0.0162, −0.0010) | 6.08% (11.4%, 0.72%) | 0.0262 |
| CD28− CD25++ CD8br %CD8br | 5alpha-pregnan-3beta,20beta-diol monosulfate (1) levels | HCC | 0.00593 (−0.0002, 0.0121) | 3.21% (−0.10%, 6.52%) | 0.0579 |
| CD28− CD25++ CD8br %CD8br | N-acetyl-isoputreanine levels | HCC | 0.00745 (−0.0001, 0.015) | 4.03% (−0.07%, 8.13%) | 0.0538 |
| CD64 on CD14− CD16+ monocyte | Citrate to 4-hydroxyphenylpyruvate ratio | HCC | 0.0327 (0.0045, 0.0607) | 12% (1.69%, 22.3%) | 0.0226 |
| Granulocyte AC | Serotonin levels | HCC | −0.0155 (−0.0288, −0.0022) | 9.96% (18.5%, 1.43%) | 0.0221 |
| HLA DR on HLA DR+ CD8br | Phenyllactate levels in elite athletes | HCC | −0.014 (−0.0281, 0.0001) | 6.7% (13.5%, −0.07%) | 0.0523 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Xue, J.; Zhang, J.; Zhou, J. Causal Effect of Immunocytes, Plasma Metabolites, and Hepatocellular Carcinoma: A Bidirectional Two-Sample Mendelian Randomization Study and Mediation Analysis in East Asian Populations. Genes 2024, 15, 1183. https://doi.org/10.3390/genes15091183
Tang X, Xue J, Zhang J, Zhou J. Causal Effect of Immunocytes, Plasma Metabolites, and Hepatocellular Carcinoma: A Bidirectional Two-Sample Mendelian Randomization Study and Mediation Analysis in East Asian Populations. Genes. 2024; 15(9):1183. https://doi.org/10.3390/genes15091183
Chicago/Turabian StyleTang, Xilong, Jianjin Xue, Jie Zhang, and Jiajia Zhou. 2024. "Causal Effect of Immunocytes, Plasma Metabolites, and Hepatocellular Carcinoma: A Bidirectional Two-Sample Mendelian Randomization Study and Mediation Analysis in East Asian Populations" Genes 15, no. 9: 1183. https://doi.org/10.3390/genes15091183
APA StyleTang, X., Xue, J., Zhang, J., & Zhou, J. (2024). Causal Effect of Immunocytes, Plasma Metabolites, and Hepatocellular Carcinoma: A Bidirectional Two-Sample Mendelian Randomization Study and Mediation Analysis in East Asian Populations. Genes, 15(9), 1183. https://doi.org/10.3390/genes15091183






