17α-Ethynylestradiol and Levonorgestrel Exposure of Rainbow Trout RTL-W1 Cells at 18 °C and 21 °C Mainly Reveals Thermal Tolerance, Absence of Estrogenic Effects, and Progestin-Induced Upregulation of Detoxification Genes
Abstract
:1. Introduction
2. Materials and Methods
2.1. RTL-W1 Cell Culture
2.2. Exposure to EDCs
2.3. RNA Extraction and cDNA Synthesis
2.4. Gene Expression Analysis
2.5. Statistical Analysis
3. Results
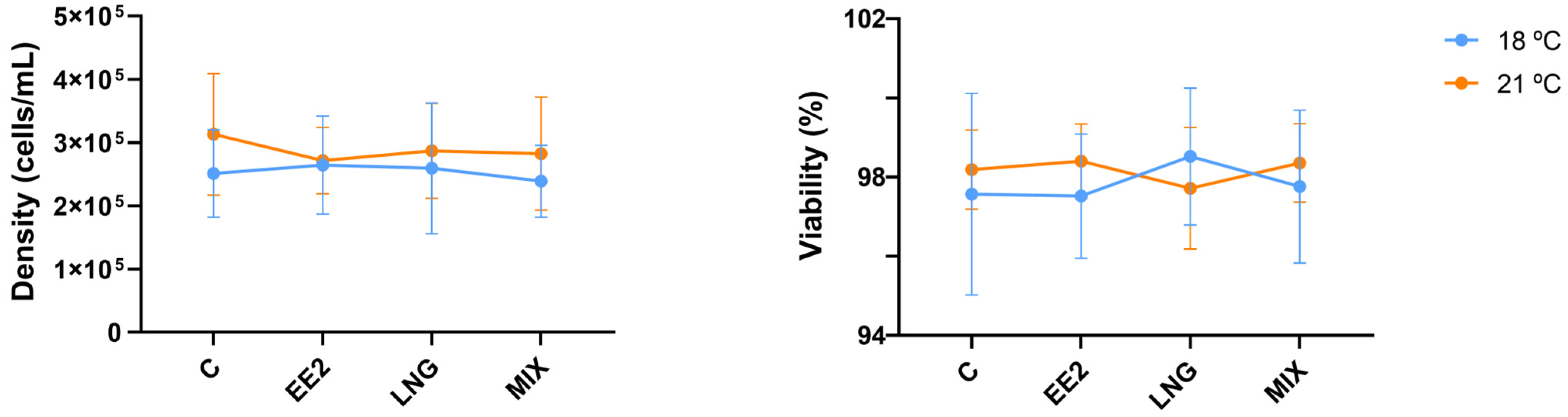
3.1. Cell Density and Viability
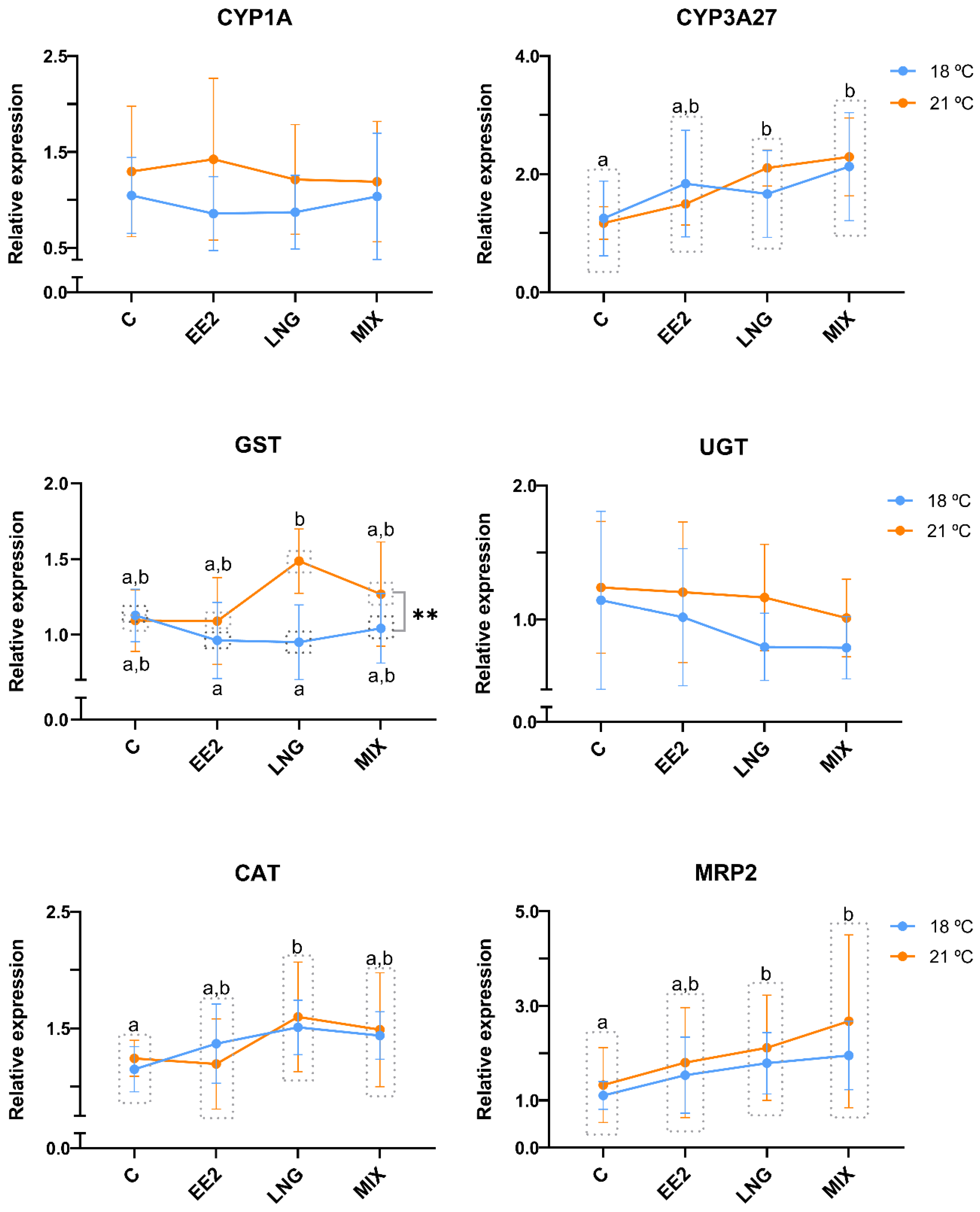
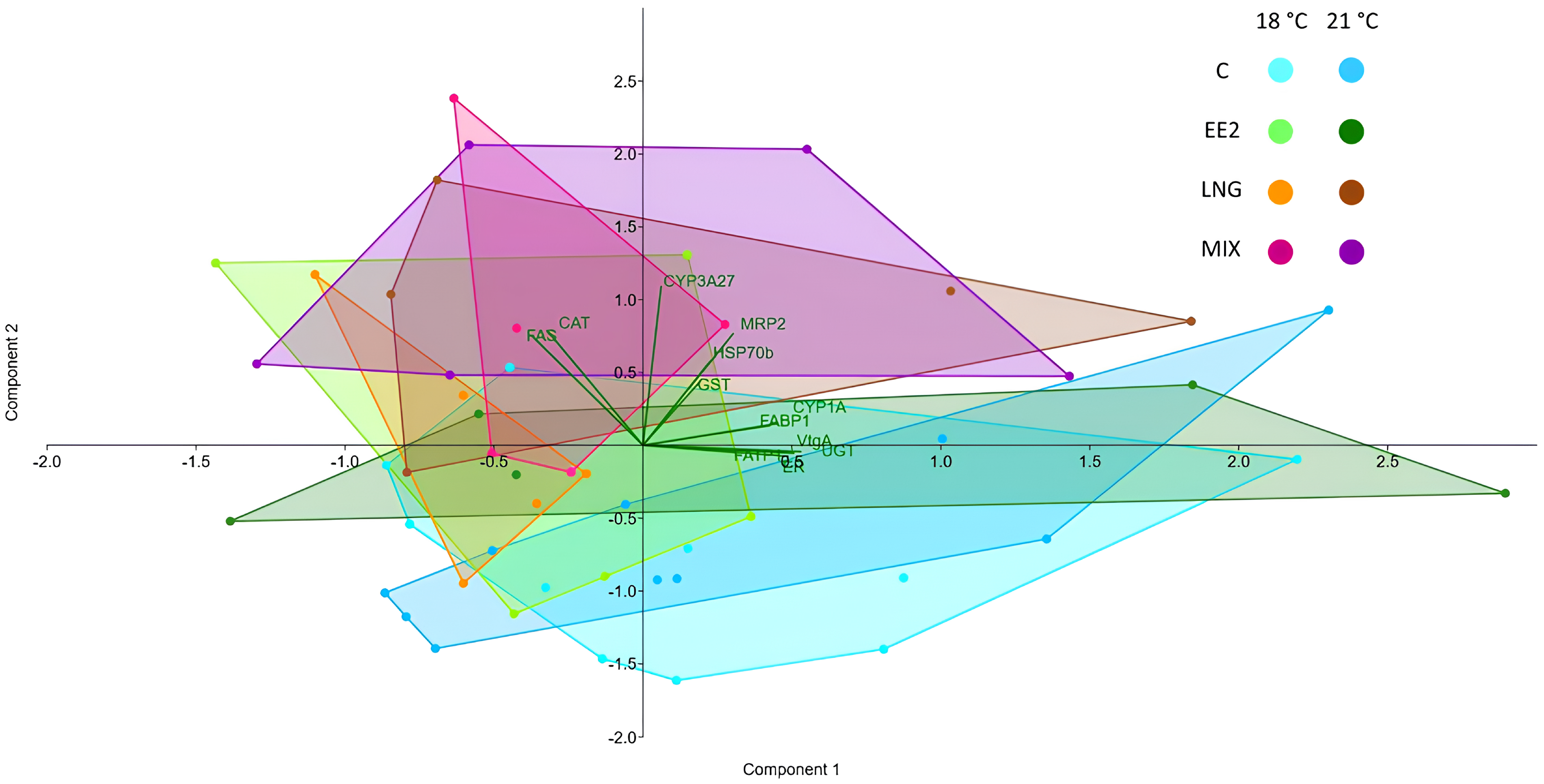
3.2. Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flaws, J.; Damdimopoulou, P.; Patisaul, H.B.; Gore, A.; Raetzman, L.; Vandenberg, L.N. Plastics, EDCs & Health: A Guide for Public Interest Organizations and Policy-Makers on Endocrine Disrupting Chemicals & Plastics; Endocrine Society: Washington, DC, USA, 2020. [Google Scholar]
- United Nations Environment Programme, World Health Organization. State of the Science of Endocrine Disrupting Chemicals 2012. 2012. Available online: https://www.who.int/publications/i/item/9789241505031 (accessed on 2 January 2024).
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Almazrouei, B.; Islayem, D.; Alskafi, F.; Catacutan, M.K.; Amna, R.; Nasrat, S.; Sizirici, B.; Yildiz, I. Steroid Hormones in Wastewater: Sources, Treatments, Environmental Risks, and Regulations. Emerg. Contam. 2023, 9, 100210. [Google Scholar] [CrossRef]
- Jenila, J.S.; Issac, P.K.; Lam, S.S.; Oviya, J.C.; Jones, S.; Munusamy-Ramanujam, G.; Chang, S.W.; Ravindran, B.; Mannacharaju, M.; Ghotekar, S.; et al. Deleterious Effect of Gestagens from Wastewater Effluent on Fish Reproduction in Aquatic Environment: A Review. Environ. Res. 2023, 236, 116810. [Google Scholar] [CrossRef] [PubMed]
- Martyniuk, C.J.; Feswick, A.; Munkittrick, K.R.; Dreier, D.A.; Denslow, N.D. Twenty Years of Transcriptomics, 17Alpha-Ethinylestradiol, and Fish. Gen. Comp. Endocrinol. 2020, 286, 113325. [Google Scholar] [CrossRef]
- Rocha, M.J.; Rocha, E. Synthetic Progestins in Waste and Surface Waters: Concentrations, Impacts and Ecological Risk. Toxics 2022, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Shon, J.; Kim, M.; Yu, C.; Zhang, L.; Huang, S.; Lee, L.; Tran, D.; Li, L. Role of CYP3A in Oral Contraceptives Clearance. Clin. Transl. Sci. 2018, 11, 251–260. [Google Scholar] [CrossRef]
- European Commission, Joint Research Centre; Gomez Cortes, L.; Marinov, D.; Sanseverino, I. Selection of Substances for the 4th Watch List under the Water Framework Directive; Publications Office of the European Union: Luxembourg, 2022. [Google Scholar] [CrossRef]
- Zucchi, S.; Castiglioni, S.; Fent, K. Progestins and Antiprogestins Affect Gene Expression in Early Development in Zebrafish (Danio rerio) at Environmental Concentrations. Environ. Sci. Technol. 2012, 46, 5183–5192. [Google Scholar] [CrossRef]
- Celino-Brady, F.T.; Lerner, D.T.; Seale, A.P. Experimental Approaches for Characterizing the Endocrine-Disrupting Effects of Environmental Chemicals in Fish. Front. Endocrinol. 2021, 11, 619361. [Google Scholar] [CrossRef]
- Zeilinger, J.; Steger-Hartmann, T.; Maser, E.; Goller, S.; Vonk, R.; Länge, R. Effects of Synthetic Gestagens on Fish Reproduction. Environ. Toxicol. Chem. 2009, 28, 2663–2670. [Google Scholar] [CrossRef]
- Moncaut, N.; Nostro, F.L.; Maggese, M.C. Vitellogenin Detection in Surface Mucus of the South American Cichlid Fish Cichlasoma dimerus (Heckel, 1840) Induced by Estradiol-17beta. Effects on Liver and Gonads. Aquat. Toxicol. 2003, 63, 127–137. [Google Scholar] [CrossRef]
- Van der Ven, L.T.; Holbech, H.; Fenske, M.; Van den Brandhof, E.J.; Gielis-Proper, F.K.; Wester, P.W. Vitellogenin Expression in Zebrafish Danio rerio: Evaluation by Histochemistry, Immunohistochemistry, and In Situ mRNA Hybridisation. Aquat. Toxicol. 2003, 65, 1–11. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Arterburn, J.B.; Smith, H.O.; Oprea, T.I.; Sklar, L.A.; Hathaway, H.J. Estrogen Signaling Through the Transmembrane G Protein-Coupled Receptor GPR30. Annu. Rev. Physiol. 2008, 70, 165–190. [Google Scholar] [CrossRef]
- Stefkovich, M.L.; Arao, Y.; Hamilton, K.J.; Korach, K.S. Experimental Models for Evaluating Non-Genomic Estrogen Signaling. Steroids 2018, 133, 34–37. [Google Scholar] [CrossRef]
- Hou, L.; Xu, H.; Ying, G.; Yang, Y.; Shu, H.; Zhao, J.; Cheng, X. Physiological Responses and Gene Expression Changes in the Western Mosquitofish (Gambusia affinis) Exposed to Progesterone at Environmentally Relevant Concentrations. Aquat. Toxicol. 2017, 192, 69–77. [Google Scholar] [CrossRef]
- Hou, L.; Chen, S.; Chen, H.; Ying, G.; Chen, D.; Liu, J.; Liang, Y.; Wu, R.; Fang, X.; Zhang, C.; et al. Rapid Masculinization and Effects on the Liver of Female Western Mosquitofish (Gambusia affinis) by Norethindrone. Chemosphere 2019, 216, 94–102. [Google Scholar] [CrossRef]
- Cardoso, P.G.; Resende-de-Oliveira, R.; Rocha, E. Combined Effects of Increased Temperature and Levonorgestrel Exposure on Zebrafish Female Liver, Using Stereology and Immunohistochemistry Against Catalase, CYP1A, HSP90 and Vitellogenin. Environ. Pollut. 2019, 252, 1059–1067. [Google Scholar] [CrossRef]
- Feswick, A.; Munkittrick, K.R.; Martyniuk, C.J. Estrogen-Responsive Gene Networks in the Teleost Liver: What Are the Key Molecular Indicators? Environ. Toxicol. Pharmacol. 2017, 56, 366–374. [Google Scholar] [CrossRef]
- Yadetie, F.; Brun, N.R.; Vieweg, I.; Nahrgang, J.; Karlsen, O.A.; Goksøyr, A. Transcriptome Responses in Polar Cod (Boreogadus saida) Liver Slice Culture Exposed to Benzo[a]pyrene and Ethynylestradiol: Insights into Anti-Estrogenic Effects. Toxicol. Vitr. 2021, 75, 105193. [Google Scholar] [CrossRef]
- Mortensen, A.S.; Arukwe, A. Effects of 17Alpha-Ethynylestradiol on Hormonal Responses and Xenobiotic Biotransformation System of Atlantic Salmon (Salmo salar). Aquat. Toxicol. 2007, 85, 113–123. [Google Scholar] [CrossRef]
- Thilagam, H.; Gopalakrishnan, S.; Qu, H.D.; Bo, J.; Wang, K.J. 17β Estradiol Induced ROS Generation, DNA Damage and Enzymatic Responses in the Hepatic Tissue of Japanese Sea Bass. Ecotoxicology 2010, 19, 1258–1267. [Google Scholar] [CrossRef]
- Weiserova, Z.; Blahova, J.; Dobukova, V.; Marsalek, P.; Hodkovicova, N.; Lenz, J.; Tichy, F.; Franek, R.; Psenicka, M.; Franc, A.; et al. Does Dietary Exposure to 17α-Ethinylestradiol Alter Biomarkers Related with Endocrine Disruption and Oxidative Stress in the Adult Triploid of Danio rerio? Sci. Total Environ. 2023, 870, 161911. [Google Scholar] [CrossRef] [PubMed]
- Brockmeier, E.K.; Scott, P.D.; Denslow, N.D.; Leusch, F.D. Transcriptomic and Physiological Changes in Eastern Mosquitofish (Gambusia holbrooki) After Exposure to Progestins and Anti-Progestagens. Aquat. Toxicol. 2016, 179, 8–17. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2022: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.-O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022; p. 3056. [Google Scholar] [CrossRef]
- Bănăduc, D.; Curtean-Bănăduc, A.; Barinova, S.; Lozano, V.L.; Afanasyev, S.; Leite, T.; Branco, P.; Gomez Isaza, D.F.; Geist, J.; Tegos, A.; et al. Multi-Interacting Natural and Anthropogenic Stressors on Freshwater Ecosystems: Their Current Status and Future Prospects for 21st Century. Water 2024, 16, 1483. [Google Scholar] [CrossRef]
- Neuheimer, A.B.; Thresher, R.E.; Lyle, J.M.; Semmens, J.M. Tolerance Limit for Fish Growth Exceeded by Warming Waters. Nat. Clim. Chang. 2011, 1, 110–113. [Google Scholar] [CrossRef]
- Kang, Y.; Liu, Z.; Shi, H.; Wang, J.; Huang, J.; Li, Y.; Li, J.; Wang, Y. Label-Free Quantification of Protein Expression in the Rainbow Trout (Oncorhynchus mykiss) in Response to Short-Term Exposure to Heat Stress. Comp. Biochem. Physiology. Part D Genom. Proteom. 2019, 30, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Nuez-Ortín, W.G.; Carter, C.G.; Nichols, P.D.; Cooke, I.R.; Wilson, R. Liver Proteome Response of Pre-Harvest Atlantic Salmon Following Exposure to Elevated Temperature. BMC Genom. 2018, 19, 133. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, É.R.; França, L.R. Effects of Different Temperatures on Testis Structure and Function, with Emphasis on Somatic Cells, in Sexually Mature Nile Tilapias (Oreochromis niloticus). Biol. Reprod. 2009, 80, 537–544. [Google Scholar] [CrossRef]
- Dzikowski, R.; Gideon, H.; Karplus, I.; Harpaz, S. Effect of Temperature and Dietary L-Carnitine Supplementation on Reproductive Performance of Female Guppy (Poecilia reticulata). Aquaculture 2001, 199, 323–332. [Google Scholar] [CrossRef]
- Liu, B.; Xu, P.; Brown, P.B.; Xie, J.; Ge, X.; Miao, L.; Pan, L. The Effect of Hyperthermia on Liver Histology, Oxidative Stress and Disease Resistance of the Wuchang Bream, Megalobrama amblycephala. Fish Shellfish Immunol. 2016, 52, 317–324. [Google Scholar] [CrossRef]
- Zhao, T.; Ma, A.; Huang, Z.; Liu, Z.; Sun, Z.; Zhu, C.; Yang, J.; Li, Y.; Wang, Q.; Qiao, X.; et al. Transcriptome Analysis Reveals that High Temperatures Alter Modes of Lipid Metabolism in Juvenile Turbot (Scophthalmus maximus) Liver. Comp. Biochem. Physiology. Part D Genom. Proteom. 2021, 40, 100887. [Google Scholar] [CrossRef]
- Sárria, M.P.; Santos, M.M.; Reis-Henriques, M.A.; Vieira, N.M.; Monteiro, N.M. The Unpredictable Effects of Mixtures of Androgenic and Estrogenic Chemicals on Fish Early Life. Environ. Int. 2011, 37, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.; Rajapakse, N.; Kortenkamp, A. Something From “Nothing”-Eight Weak Estrogenic Chemicals Combined at Concentrations Below NOECs Produce Significant Mixture Effects. Environ. Sci. Technol. 2002, 36, 1751–1756. [Google Scholar] [CrossRef]
- Ács, A.; Liang, X.; Bock, I.; Griffitts, J.; Ivánovics, B.; Vásárhelyi, E.; Ferincz, Á.; Pirger, Z.; Urbányi, B.; Csenki, Z. Chronic Effects of Carbamazepine, Progesterone and Their Mixtures at Environmentally Relevant Concentrations on Biochemical Markers of Zebrafish (Danio rerio). Antioxidants 2022, 11, 1776. [Google Scholar] [CrossRef]
- Hinfray, N.; Tebby, C.; Garoche, C.; Piccini, B.; Bourgine, G.; Aït-Aïssa, S.; Kah, O.; Pakdel, F.; Brion, F. Additive Effects of Levonorgestrel and Ethinylestradiol on Brain Aromatase (cyp19a1b) in Zebrafish Specific In Vitro and In Vivo Bioassays. Toxicol. Appl. Pharmacol. 2016, 307, 108–114. [Google Scholar] [CrossRef]
- Hua, J.; Han, J.; Wang, X.; Guo, Y.; Zhou, B. The Binary Mixtures of Megestrol Acetate and 17α-Ethynylestradiol Adversely Affect Zebrafish Reproduction. Environ. Pollut. 2016, 213, 776–784. [Google Scholar] [CrossRef]
- DeCourten, B.; Romney, A.; Brander, S. Chapter 2—The Heat Is On: Complexities of Aquatic Endocrine Disruption in a Changing Global Climate. Sep. Sci. Technol. 2019, 11, 13–49. [Google Scholar] [CrossRef]
- Noyes, P.D.; McElwee, M.K.; Miller, H.D.; Clark, B.W.; Van Tiem, L.A.; Walcott, K.C.; Erwin, K.N.; Levin, E.D. The Toxicology of Climate Change: Environmental Contaminants in a Warming World. Environ. Int. 2009, 35, 971–986. [Google Scholar] [CrossRef]
- Gandar, A.; Laffaille, P.; Canlet, C.; Tremblay-Franco, M.; Gautier, R.; Perrault, A.; Gress, L.; Mormède, P.; Tapie, N.; Budzinski, H.; et al. Adaptive Response under Multiple Stress Exposure in Fish: From the Molecular to Individual Level. Chemosphere 2017, 188, 60–72. [Google Scholar] [CrossRef]
- Gandar, A.; Laffaille, P.; Marty-Gasset, N.; Viala, D.; Molette, C.; Jean, S. Proteome Response of Fish under Multiple Stress Exposure: Effects of Pesticide Mixtures and Temperature Increase. Aquat. Toxicol. 2017, 184, 61–77. [Google Scholar] [CrossRef]
- DeCourten, B.M.; Brander, S.M. Combined Effects of Increased Temperature and Endocrine Disrupting Pollutants on Sex Determination, Survival, and Development Across Generations. Sci. Rep. 2017, 7, 9310. [Google Scholar] [CrossRef]
- Castaño, A.; Bols, N.; Braunbeck, T.; Dierickx, P.; Halder, M.; Isomaa, B.; Kawahara, K.; Lee, L.E.; Mothersill, C.; Pärt, P.; et al. The Use of Fish Cells in Ecotoxicology. The Report and Recommendations of ECVAM Workshop 47. Altern. Lab. Anim. ATLA 2003, 31, 317–351. [Google Scholar] [CrossRef]
- Lee, L.E.; Clemons, J.H.; Bechtel, D.G.; Caldwell, S.J.; Han, K.B.; Pasitschniak-Arts, M.; Mosser, D.D.; Bols, N.C. Development and Characterization of a Rainbow Trout Liver Cell Line Expressing Cytochrome P450-Dependent Monooxygenase Activity. Cell Biol. Toxicol. 1993, 9, 279–294. [Google Scholar] [CrossRef]
- Bancel, S.; Cachot, J.; Bon, C.; Rochard, É.; Geffard, O. A Critical Review of Pollution Active Biomonitoring using Sentinel Fish: Challenges and Opportunities. Environ. Pollut. 2024, 360, 124661. [Google Scholar] [CrossRef]
- Malhão, F.; Urbatzka, R.; Navas, J.M.; Cruzeiro, C.; Monteiro, R.A.; Rocha, E. Cytological, Immunocytochemical, Ultrastructural and Growth Characterization of the Rainbow Trout Liver Cell Line RTL-W1. Tissue Cell 2013, 45, 159–174. [Google Scholar] [CrossRef]
- Thibaut, R.; Schnell, S.; Porte, C. Assessment of Metabolic Capabilities of PLHC-1 and RTL-W1 Fish Liver Cell Lines. Cell Biol. Toxicol. 2009, 25, 611–622. [Google Scholar] [CrossRef]
- Pannetier, P.; Fuster, L.; Clérandeau, C.; Lacroix, C.; Gourves, P.Y.; Cachot, J.; Morin, B. Usefulness of RTL-W1 and OLCAB-e3 Fish Cell Lines and Multiple Endpoint Measurements for Toxicity Evaluation of Unknown or Complex Mixture of Chemicals. Ecotoxicol. Environ. Saf. 2018, 150, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Fent, K. Fish Cell Lines as Versatile Tools in Ecotoxicology: Assessment of Cytotoxicity, Cytochrome P4501A Induction Potential and Estrogenic Activity of Chemicals and Environmental Samples. Toxicol. Vitr. 2001, 15, 477–488. [Google Scholar] [CrossRef]
- Pereira, F.C.R.M. Characterization of the Trout Liver Cell Line RTL-W1 and Its Potential for Carcinogenesis-Related Studies. Master’s Thesis, University of Porto, Porto, Portugal, 2010. [Google Scholar]
- Dimastrogiovanni, G.; Córdoba, M.; Navarro, I.; Jáuregui, O.; Porte, C. Alteration of Cellular Lipids and Lipid Metabolism Markers in RTL-W1 Cells Exposed to Model Endocrine Disrupters. Aquat. Toxicol. 2015, 165, 277–285. [Google Scholar] [CrossRef]
- Sun, J.; Liu, Z.; Quan, J.; Li, L.; Zhao, G.; Lu, J. Protective Effects of Different Concentrations of Selenium Nanoparticles on Rainbow Trout (Oncorhynchus mykiss) Primary Hepatocytes under Heat Stress. Ecotoxicol. Environ. Saf. 2022, 230, 113121. [Google Scholar] [CrossRef]
- Estrela-Segrelles, C.; Gómez-Martínez, G.; Pérez-Martín, M.Á. Climate Change Risks on Mediterranean River Ecosystems and Adaptation Measures (Spain). Water Resour. Manag. 2023, 37, 2757–2770. [Google Scholar] [CrossRef]
- Reddin, I.G.; Fenton, T.R.; Wass, M.N.; Michaelis, M. Large Inherent Variability in Data Derived from Highly Standardised Cell Culture Experiments. Pharmacol. Res. 2023, 188, 106671. [Google Scholar] [CrossRef]
- Navas, J.M.; Segner, H. Modulation of Trout 7-Ethoxyresorufin-O-Deethylase (EROD) Activity by Estradiol and Octylphenol. Mar. Environ. Res. 2000, 50, 157–162. [Google Scholar] [CrossRef]
- Madureira, T.V.; Malhão, F.; Pinheiro, I.; Lopes, C.; Ferreira, N.; Urbatzka, R.; Castro, L.F.; Rocha, E. Estrogenic and Anti-Estrogenic Influences in Cultured Brown Trout Hepatocytes: Focus on the Expression of Some Estrogen and Peroxisomal Related Genes and Linked Phenotypic Anchors. Aquat. Toxicol. 2015, 169, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef]
- Uchea, C.; Owen, S.F.; Chipman, J.K. Functional Xenobiotic Metabolism and Efflux Transporters in Trout Hepatocyte Spheroid Cultures. Toxicol. Res. 2015, 4, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Ojima, N.; Yamashita, M.; Watabe, S. Quantitative mRNA Expression Profiling of Heat-Shock Protein Families in Rainbow Trout Cells. Biochem. Biophys. Res. Commun. 2005, 329, 51–57. [Google Scholar] [CrossRef] [PubMed]
- De Anna, J.S.; Darraz, L.A.; Painefilú, J.C.; Cárcamo, J.G.; Moura-Alves, P.; Venturino, A.; Luquet, C.M. The Insecticide Chlorpyrifos Modifies the Expression of Genes Involved in the PXR and AhR Pathways in the Rainbow Trout, Oncorhynchus mykiss. Pestic. Biochem. Physiol. 2021, 178, 104920. [Google Scholar] [CrossRef] [PubMed]
- Batista-Pinto, C. Peroxisomes in Brown Trout (Salmo trutta f. fario): Regulation by Estrogens; Institute of Biomedical Sciences Abel Salazar: Porto, Portugal, 2007; pp. 121–135. [Google Scholar]
- Körner, O.; Kohno, S.; Schönenberger, R.; Suter, M.J.-F.; Knauer, K.; Guillette Jr, L.J.; Burkhardt-Holm, P. Water Temperature and Concomitant Waterborne Ethinylestradiol Exposure Affects the Vitellogenin Expression in Juvenile Brown Trout (Salmo trutta). Aquat. Toxicol. 2008, 90, 188–196. [Google Scholar] [CrossRef]
- Minghetti, M.; Leaver, M.J.; Tocher, D.R. Transcriptional Control Mechanisms of Genes of Lipid and Fatty Acid Metabolism in the Atlantic salmon (Salmo salar L.) Established Cell Line, SHK-1. Biochim. Biophys. Acta 2011, 1811, 194–202. [Google Scholar] [CrossRef]
- Madureira, T.V.; Pinheiro, I.; Malhão, F.; Lopes, C.; Urbatzka, R.; Castro, L.F.C.; Rocha, E. Cross-Interference of Two Model Peroxisome Proliferators in Peroxisomal and Estrogenic Pathways in Brown Trout Hepatocytes. Aquat. Toxicol. 2017, 187, 153–162. [Google Scholar] [CrossRef]
- Sánchez-Gurmaches, J.; Cruz-Garcia, L.; Gutiérrez, J.; Navarro, I. mRNA Expression of Fatty Acid Transporters in Rainbow Trout: In Vivo and In Vitro Regulation by Insulin, Fasting and Inflammation and Infection Mediators. Comp. Biochem. Physiology. Part A Mol. Integr. Physiol. 2012, 163, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Madureira, T.V.; Pinheiro, I.; Freire, R.P.; Rocha, E.; Castro, L.F.; Urbatzka, R. Genome Specific PPARαB Duplicates in Salmonids and Insights into Estrogenic Regulation in Brown Trout. Comp. Biochem. Physiol. Part B 2017, 208–209, 94–101. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT-PCR. Nucleic Acids Res. 2001, 29, 2002–2007. [Google Scholar] [CrossRef]
- The Jamovi Project. Jamovi (Version 2.3) [Computer Software]; 2023. Available online: https://www.jamovi.org (accessed on 2 January 2024).
- GraphPad Prism, Version 9.0.0 for Windows; GraphPad Software: San Diego, CA, USA, 2023. Available online: https://www.graphpad.com (accessed on 2 January 2024).
- Green, J.; Wheeler, J.R. The Use of Carrier Solvents in Regulatory Aquatic Toxicology Testing: Practical, Statistical and Regulatory Considerations. Aquat. Toxicol. 2013, 144–145, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological Statistics Software Package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Klaunig, J.E.; Ruch, R.J.; Goldblatt, P.J. Trout Hepatocyte Culture: Isolation and Primary Culture. Vitr. Cell. Dev. Biol. 1985, 21, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.I.; Hayes, S.F.; Coe, J.E. Estrogen Induces Cytokeratin Aggregation in Primary Cultures of Armenian Hamster Hepatocytes. Cell Motil. Cytoskelet. 1999, 43, 35–42. [Google Scholar] [CrossRef]
- Christianson-Heiska, I.; Isomaa, B. The Use of Primary Hepatocytes from Brown Trout (Salmo trutta lacustris) and the Fish Cell Lines RTH-149 and ZF-L for In Vitro Screening of (Anti)Estrogenic Activity of Wood Extractives. Toxicol. Vitr. 2008, 22, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, S.; Islinger, M.; Völkl, A.; Braunbeck, T. Temperature-Dependent Vitellogenin-mRNA Expression in Primary Cultures of Rainbow Trout (Oncorhynchus mykiss) Hepatocytes at 14 and 18 °C. Toxicol. Vitr. 2000, 14, 531–540. [Google Scholar] [CrossRef]
- Olin, T.; Von Der Decken, A. Vitellogenin Synthesis in Atlantic Salmon (Salmo Salar) at Different Acclimation Temperatures. Aquaculture 1989, 79, 397–402. [Google Scholar] [CrossRef]
- Sato, N.; Kawazoe, I.; Suzuki, Y.; Aida, K. Effects of Temperature on Vitellogenesis in Japanese Eel Anguilla japonica. Fish. Sci. 2006, 72, 961–966. [Google Scholar] [CrossRef]
- DeQuattro, Z.A.; Peissig, E.J.; Antkiewicz, D.S.; Lundgren, E.J.; Hedman, C.J.; Hemming, J.D.; Barry, T.P. Effects of Progesterone on Reproduction and Embryonic Development in the Fathead Minnow (Pimephales promelas). Environ. Toxicol. Chem. 2012, 31, 851–856. [Google Scholar] [CrossRef]
- Overturf, M.D.; Overturf, C.L.; Carty, D.R.; Hala, D.; Huggett, D.B. Levonorgestrel Exposure to Fathead Minnows (Pimephales promelas) Alters Survival, Growth, Steroidogenic Gene Expression and Hormone Production. Aquat. Toxicol. 2014, 148, 152–161. [Google Scholar] [CrossRef]
- Kroupova, H.; Trubiroha, A.; Lorenz, C.; Contardo-Jara, V.; Lutz, I.; Grabic, R.; Kocour, M.; Kloas, W. The Progestin Levonorgestrel Disrupts Gonadotropin Expression and Sex Steroid Levels in Pubertal Roach (Rutilus rutilus). Aquat. Toxicol. 2014, 154, 154–162. [Google Scholar] [CrossRef]
- Sun, H.; Sivasubramanian, R.; Vaidya, S.; Barve, A.; Jarugula, V. Drug-Drug Interaction Studies with Oral Contraceptives: Pharmacokinetic/Pharmacodynamic and Study Design Considerations. J. Clin. Pharmacol. 2020, 60, 49–62. [Google Scholar] [CrossRef]
- Bols, N.; Schirmer, K.; Joyce, E.; Dixon, D.; Greenberg, B.; Whyte, J. Ability of Polycyclic Aromatic Hydrocarbons to Induce 7-Ethoxyresorufin-o-deethylase Activity in a Trout Liver Cell Line. Ecotoxicol. Environ. Saf. 1999, 44, 118–128. [Google Scholar] [CrossRef]
- Schirmer, K.; Chan, A.G.J.; Bols, N.C. Transitory Metabolic Disruption and Cytotoxicity Elicited by Benzo[a]pyrene In Two Cell Lines from Rainbow Trout Liver. J. Biochem. Mol. Toxicol. 2000, 14, 262–276. [Google Scholar] [CrossRef]
- Valdehita, A.; Luisa, M.; Navas, J.M. The Potentiating Effect of Graphene Oxide on the Arylhydrocarbon Receptor (AhR)–Cytochrome P4501A (Cyp1A) System Activated by Benzo(k)fluoranthene (BkF) in Rainbow Trout Cell Line. Nanomaterials 2023, 13, 2501. [Google Scholar] [CrossRef]
- Svensson, J.; Fick, J.; Brandt, I.; Brunström, B. The Synthetic Progestin Levonorgestrel Is a Potent Androgen in the Three-Spined Stickleback (Gasterosteus aculeatus). Environ. Sci. Technol. 2013, 47, 2043–2051. [Google Scholar] [CrossRef]
- Svensson, J.; Fick, J.; Brandt, I.; Brunström, B. Environmental Concentrations of an Androgenic Progestin Disrupts the Seasonal Breeding Cycle in Male Three-Spined Stickleback (Gasterosteus aculeatus). Aquat. Toxicol. 2014, 147, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Contardo-Jara, V.; Lorenz, C.; Pflugmacher, S.; Nützmann, G.; Kloas, W.; Wiegand, C. Molecular Effects and Bioaccumulation of Levonorgestrel in the Non-Target Organism Dreissena polymorpha. Environ. Pollut. 2011, 159, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Christen, V.; Fent, K. Tissue-, Sex- and Development-Specific Transcription Profiles of Eight UDP-Glucuronosyltransferase Genes in Zebrafish (Danio rerio) and Their Regulation by Activator of Aryl Hydrocarbon Receptor. Aquat. Toxicol. 2014, 150, 93–102. [Google Scholar] [CrossRef] [PubMed]
- George, S.G.; Taylor, B. Molecular Evidence for Multiple UDP-Glucuronosyltransferase Gene Families in Fish. Mar. Environ. Res. 2002, 54, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Choi, S.; Song, J.W.; Chen, H.; Fischer, J.H. Regulation of UDP-Glucuronosyltransferase (UGT) 1A1 by Progesterone and Its Impact on Labetalol Elimination. Xenobiotica 2008, 38, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Ronisz, D.; Larsson, D.; Förlin, L. Seasonal Variations in the Activities of Selected Hepatic Biotransformation and Antioxidant Enzymes in Eelpout (Zoarces viviparus). Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1999, 124, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Loncar, J.; Zaja, R.; Schnell, S.; Schirmer, K.; Smital, T.; Luckenbach, T. Constitutive mRNA Expression and Protein Activity Levels of Nine ABC Efflux Transporters in Seven Permanent Cell Lines Derived from Different Tissues of Rainbow Trout (Oncorhynchus mykiss). Aquat. Toxicol. 2011, 101, 438–446. [Google Scholar] [CrossRef]
- Wojnarowski, K.; Cholewińska, P.; Palić, D.; Bednarska, M.; Jarosz, M.; Wiśniewska, I. Estrogen Receptors Mediated Negative Effects of Estrogens and Xenoestrogens in Teleost Fishes-Review. Int. J. Mol. Sci. 2022, 23, 2605. [Google Scholar] [CrossRef]
- Alves, R.F.; Lopes, C.; Rocha, E.; Madureira, T.V. Estrogenic Responsiveness of Brown Trout Primary Hepatocyte Spheroids to Environmental Levels of 17α-Ethinylestradiol. J. Xenobiotics 2024, 14, 1064–1078. [Google Scholar] [CrossRef]
- Madureira, T.V.; Malhão, F.; Simões, T.; Pinheiro, I.; Lopes, C.; Gonçalves, J.F.; Urbatzka, R.; Castro, L.F.C.; Lemos, M.F.L.; Rocha, E. Sex-Steroids and Hypolipidemic Chemicals Impacts on Brown Trout Lipid and Peroxisome Signaling—Molecular, Biochemical and Morphological Insights. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2018, 212, 1–17. [Google Scholar] [CrossRef]
- Lammel, T.; Tsoukatou, G.; Jellinek, J.; Sturve, J. Development of Three-Dimensional (3D) Spheroid Cultures of the Continuous Rainbow Trout Liver Cell Line RTL-W1. Ecotoxicol. Environ. Saf. 2019, 167, 250–258. [Google Scholar] [CrossRef]
- Gupta, S.C.; Sharma, A.; Mishra, M.; Mishra, R.K.; Chowdhuri, D.K. Heat Shock Proteins in Toxicology: How Close and How Far? Life Sci. 2010, 86, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, R.I.; Sarge, K.D.; Abravaya, K. Transcriptional Regulation of Heat Shock Genes. A Paradigm for Inducible Genomic Responses. J. Biol. Chem. 1992, 267, 21987–21990. [Google Scholar] [CrossRef] [PubMed]
- Ojima, N.; Yamashita, M.; Watabe, S. Comparative Expression Analysis of Two Paralogous Hsp70s in Rainbow Trout Cells Exposed to Heat Stress. Biochim. Biophys. Acta 2005, 1681, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Lutze, P.; Brenmoehl, J.; Tesenvitz, S.; Ohde, D.; Wanka, H.; Meyer, Z.; Grunow, B. Effects of Temperature Adaptation on the Metabolism and Physiological Properties of Sturgeon Fish Larvae Cell Line. Cells 2024, 13, 269. [Google Scholar] [CrossRef]

| Gene * | Primer (5′-3′) | Protocol | E (%) | Reference |
|---|---|---|---|---|
| CYP1A | F: GATGTCAGTGGCAGCTTTGA R: TCCTGGTCATCATGGCTGTA | 95 °C—3 min (95 °C—20 s; 60.0 °C—20 s; 72 °C—20 s) 40× 95 °C—1 min | 97.7 | [60] |
| CYP3A27 | F: GACGGTGGAGATCAACG R: GAGGATCTCGACCATGG | 95 °C—3 min (95 °C—20 s; 60.0 °C—20 s; 72 °C—20 s) 40× 95 °C—1 min | 99.0 | [60] |
| HSP70b | F: AGGCCCAACCATTGAAGAGA R: GCAATGTCCAGCAATGCAATA | 95 °C—3 min (95 °C—10 s; 60.0 °C—30 s; 72 °C—30 s) 40× 95 °C—1 min | 92.2 | [61] |
| UGT | F: ATAAGGACCGTCCCATCGAG R: ATCCAGTTGAGGTCGTGAGC | 94 °C—3 min (94 °C—20 s; 60.0 °C—20 s; 72 °C—20 s) 40× 94 °C—1 min | 94.9 | [60] |
| GST | F: AGCTGCTCCCAGCTGATCC R: CAAACCACGGCCACATCATGTAATC | 94 °C—3 min (94 °C—20 s; 60.0 °C—20 s; 72 °C—20 s) 40× 94 °C—1 min | 93.9 | [62] |
| CAT | F: CACTGATGAGGGCAACTGGG R: CTTGAAGTGGAACTTGCAG | 95 °C—3 min (95 °C—10 s; 58.0 °C—30 s; 72 °C—30 s) 40× 95 °C—30 s | 104.5 | [63] |
| MRP2 | F: CCATTCTGTTCGCTGTCTCA R: CTCGTAGCAGGGTCTGGAAG | 94 °C—3 min (94 °C—20 s; 60.0 °C—20 s; 72 °C—20 s) 40× 94 °C—1 min | 100.2 | [60] |
| ERα | F: GACATGCTCCTGGCCACTGT R: TGGCTTTGAGGCACACAAAC | 95 °C—4 min (95 °C—1 min; 61.6 °C—1 min) 40× 95 °C—1 min | 95.6 | [64] |
| VtgA | F: AACGGTGCTGAATGTCCATAG R: ATTGAGATCCTTGCTCTTGGTC | 95 °C—3 min (95 °C—10 s; 62.9 °C—1 min) 40× 95 °C—30 s | 102.5 | [64] |
| FAS | F: ACCGCCAAGCTCAGTGTGC R: CAGGCCCCAAAGGAGTAGC | 95 °C—3 min (95 °C—20 s; 60.0 °C—20 s; 72 °C—20 s) 40× 95 °C—1 min | 95.0 | [65] |
| FABP1 | F: GTCCGTCACCAACTCCTTC R: GCGTCTCAACCATCTCTCC | 94 °C—3 min (94 °C—20 s; 57.0 °C—20 s; 72 °C—20 s) 40× 95 °C—1 min | 102.5 | [66] |
| FATP1 | F: AGGAGAGAACGTCTCCACCA R: CGCATCACAGTCAAATGTCC | 95 °C—3 min (95 °C—20 s; 60.0 °C—20 s; 72 °C—20 s) 40× 95 °C—1 min | 92.9 | [67] |
| β-act | F: TCTGGCATCACACCTTCTAC R: TTCTCCCTGTTGGCTTTGG | 94 °C—3 min (94 °C—20 s; 55.0 °C—20 s; 72 °C—20 s) 40× 94 °C—1 min | 99.7 | [66] |
| ef1α | F: TGCCACACTGCTCACATC R: TCTCCAGACTTCAGGAACTTG | 94 °C—3 min (94 °C—20 s; 55.0 °C—20 s; 72 °C—20 s) 40× 94 °C—1 min | 97.4 | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilaça, M.; Lopes, C.; Seabra, R.; Rocha, E. 17α-Ethynylestradiol and Levonorgestrel Exposure of Rainbow Trout RTL-W1 Cells at 18 °C and 21 °C Mainly Reveals Thermal Tolerance, Absence of Estrogenic Effects, and Progestin-Induced Upregulation of Detoxification Genes. Genes 2024, 15, 1189. https://doi.org/10.3390/genes15091189
Vilaça M, Lopes C, Seabra R, Rocha E. 17α-Ethynylestradiol and Levonorgestrel Exposure of Rainbow Trout RTL-W1 Cells at 18 °C and 21 °C Mainly Reveals Thermal Tolerance, Absence of Estrogenic Effects, and Progestin-Induced Upregulation of Detoxification Genes. Genes. 2024; 15(9):1189. https://doi.org/10.3390/genes15091189
Chicago/Turabian StyleVilaça, Margarida, Célia Lopes, Rosária Seabra, and Eduardo Rocha. 2024. "17α-Ethynylestradiol and Levonorgestrel Exposure of Rainbow Trout RTL-W1 Cells at 18 °C and 21 °C Mainly Reveals Thermal Tolerance, Absence of Estrogenic Effects, and Progestin-Induced Upregulation of Detoxification Genes" Genes 15, no. 9: 1189. https://doi.org/10.3390/genes15091189






