Genetic Modifiers of ALS: The Impact of Chromogranin B P413L in a Bulgarian ALS Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. SNP Selection and Genotyping
2.3. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; van den Berg, L.H. Amyotrophic Lateral Sclerosis. Nat. Rev. Dis. Primer 2017, 3, 17071. [Google Scholar] [CrossRef] [PubMed]
- Chia, R.; Chiò, A.; Traynor, B.J. Novel Genes Associated with Amyotrophic Lateral Sclerosis: Diagnostic and Clinical Implications. Lancet Neurol. 2018, 17, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Xu, R. Current Insights in the Molecular Genetic Pathogenesis of Amyotrophic Lateral Sclerosis. Front. Neurosci. 2023, 17, 1189470. [Google Scholar] [CrossRef]
- Al-Chalabi, A.; van den Berg, L.H.; Veldink, J. Gene Discovery in Amyotrophic Lateral Sclerosis: Implications for Clinical Management. Nat. Rev. Neurol. 2017, 13, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Webster, C.P.; Smith, E.F.; Shaw, P.J.; De Vos, K.J. Protein Homeostasis in Amyotrophic Lateral Sclerosis: Therapeutic Opportunities? Front. Mol. Neurosci. 2017, 10, 123. [Google Scholar] [CrossRef]
- Kim, J.; Hughes, E.G.; Shetty, A.S.; Arlotta, P.; Goff, L.A.; Bergles, D.E.; Brown, S.P. Changes in the Excitability of Neocortical Neurons in a Mouse Model of Amyotrophic Lateral Sclerosis Are Not Specific to Corticospinal Neurons and Are Modulated by Advancing Disease. J. Neurosci. Off. J. Soc. Neurosci. 2017, 37, 9037–9053. [Google Scholar] [CrossRef]
- Gao, J.; Wang, L.; Huntley, M.L.; Perry, G.; Wang, X. Pathomechanisms of TDP-43 in Neurodegeneration. J. Neurochem. 2018, 146, 7–20. [Google Scholar] [CrossRef]
- Stringer, R.N.; Weiss, N. Pathophysiology of Ion Channels in Amyotrophic Lateral Sclerosis. Mol. Brain 2023, 16, 82. [Google Scholar] [CrossRef]
- Takahashi, K.; Foster, J.B.; Lin, C.-L.G. Glutamate Transporter EAAT2: Regulation, Function, and Potential as a Therapeutic Target for Neurological and Psychiatric Disease. Cell. Mol. Life Sci. CMLS 2015, 72, 3489–3506. [Google Scholar] [CrossRef]
- Mejzini, R.; Flynn, L.L.; Pitout, I.L.; Fletcher, S.; Wilton, S.D.; Akkari, P.A. ALS Genetics, Mechanisms, and Therapeutics: Where Are We Now? Front. Neurosci. 2019, 13, 1310. [Google Scholar] [CrossRef]
- Chiò, A.; Calvo, A.; Mazzini, L.; Cantello, R.; Mora, G.; Moglia, C.; Corrado, L.; D’Alfonso, S.; Majounie, E.; Renton, A.; et al. Extensive Genetics of ALS. Neurology 2012, 79, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Logroscino, G.; Traynor, B.J.; Hardiman, O.; Chiò, A.; Mitchell, D.; Swingler, R.J.; Millul, A.; Benn, E.; Beghi, E. Incidence of Amyotrophic Lateral Sclerosis in Europe. J. Neurol. Neurosurg. Psychiatry 2010, 81, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Talbott, E.O.; Malek, A.M.; Lacomis, D. The Epidemiology of Amyotrophic Lateral Sclerosis. Handb. Clin. Neurol. 2016, 138, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Landers, J.E.; Melki, J.; Meininger, V.; Glass, J.D.; van den Berg, L.H.; van Es, M.A.; Sapp, P.C.; van Vught, P.W.J.; McKenna-Yasek, D.M.; Blauw, H.M.; et al. Reduced Expression of the Kinesin-Associated Protein 3 (KIFAP3) Gene Increases Survival in Sporadic Amyotrophic Lateral Sclerosis. Proc. Natl. Acad. Sci. USA 2009, 106, 9004–9009. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wei, Q.; Hou, Y.; Lin, J.; Ou, R.; Zhang, L.; Jiang, Q.; Xiao, Y.; Liu, K.; Chen, X.; et al. Genome-Wide Analyses Identify NEAT1 as Genetic Modifier of Age at Onset of Amyotrophic Lateral Sclerosis. Mol. Neurodegener. 2023, 18, 77. [Google Scholar] [CrossRef]
- Zhang, M.; Xi, Z.; Saez-Atienzar, S.; Chia, R.; Moreno, D.; Sato, C.; Montazer Haghighi, M.; Traynor, B.J.; Zinman, L.; Rogaeva, E. Combined Epigenetic/Genetic Study Identified an ALS Age of Onset Modifier. Acta Neuropathol. Commun. 2021, 9, 75. [Google Scholar] [CrossRef]
- Natori, S.; King, A.; Hellwig, A.; Weiss, U.; Iguchi, H.; Tsuchiya, B.; Kameya, T.; Takayanagi, R.; Nawata, H.; Huttner, W.B. Chromogranin B (Secretogranin I), a Neuroendocrine-Regulated Secretory Protein, Is Sorted to Exocrine Secretory Granules in Transgenic Mice. EMBO J. 1998, 17, 3277–3289. [Google Scholar] [CrossRef]
- Taupenot, L.; Harper, K.L.; O’Connor, D.T. The Chromogranin-Secretogranin Family. N. Engl. J. Med. 2003, 348, 1134–1149. [Google Scholar] [CrossRef]
- Gros-Louis, F.; Andersen, P.M.; Dupre, N.; Urushitani, M.; Dion, P.; Souchon, F.; D’Amour, M.; Camu, W.; Meininger, V.; Bouchard, J.-P.; et al. Chromogranin B P413L Variant as Risk Factor and Modifier of Disease Onset for Amyotrophic Lateral Sclerosis. Proc. Natl. Acad. Sci. USA 2009, 106, 21777–21782. [Google Scholar] [CrossRef]
- Helle, K.B. Chromogranins A and B and Secretogranin II as Prohormones for Regulatory Peptides from the Diffuse Neuroendocrine System. Results Probl. Cell Differ. 2010, 50, 21–44. [Google Scholar] [CrossRef]
- Bartolomucci, A.; Possenti, R.; Mahata, S.K.; Fischer-Colbrie, R.; Loh, Y.P.; Salton, S.R.J. The Extended Granin Family: Structure, Function, and Biomedical Implications. Endocr. Rev. 2011, 32, 755–797. [Google Scholar] [CrossRef] [PubMed]
- Bearrows, S.C.; Bauchle, C.J.; Becker, M.; Haldeman, J.M.; Swaminathan, S.; Stephens, S.B. Chromogranin B Regulates Early-Stage Insulin Granule Trafficking from the Golgi in Pancreatic Islet β-Cells. J. Cell Sci. 2019, 132, jcs231373. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-M.; Shui, H.-A.; Wu, Y.-T.; Chu, P.-W.; Lin, K.-G.; Kao, L.-S.; Chen, S.-T. Proteomic Analysis of Proteins in PC12 Cells before and after Treatment with Nerve Growth Factor: Increased Levels of a 43-kDa Chromogranin B-Derived Fragment during Neuronal Differentiation. Mol. Brain Res. 2001, 92, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Dahma, H.; Gourlet, P.; Vandermeers, A.; Vandermeers-Piret, M.-C.; Robberecht, P. Evidence That the Chromogranin B Fragment 368–417 Extracted from a Pheochromocytoma Is Phosphorylated. Peptides 2001, 22, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Benjannet, S.; Leduc, R.; Adrouche, N.; Falgueyret, J.P.; Marcinkiewicz, M.; Seidah, N.G.; Mbikay, M.; Lazure, C.; Chretien, M. Chromogranin B (Secretogranin I), a Putative Precursor of Two Novel Pituitary Peptides through Processing at Paired Basic Residues. FEBS Lett. 1987, 224, 142–148. [Google Scholar] [CrossRef]
- Benjannet, S.; Leduc, R.; Lazure, C.; Seidah, N.G.; Marcinkiewicz, M.; Chrétien, M. GAWK, a Novel Human Pituitary Polypeptide: Isolation, Immunocytochemical Localization and Complete Amino Acid Sequence. Biochem. Biophys. Res. Commun. 1985, 126, 602–609. [Google Scholar] [CrossRef]
- Kroesen, S.; Marksteiner, J.; Leitner, B.; Hogue-Angeletti, R.; Fischer-Colbrie, R.; Winkler, H. Rat Brain: Distribution of Immunoreactivity of PE-11, a Peptide Derived from Chromogranin B. Eur. J. Neurosci. 1996, 8, 2679–2689. [Google Scholar] [CrossRef]
- Scavello, F.; Kharouf, N.; Lavalle, P.; Haikel, Y.; Schneider, F.; Metz-Boutigue, M.-H. The Antimicrobial Peptides Secreted by the Chromaffin Cells of the Adrenal Medulla Link the Neuroendocrine and Immune Systems: From Basic to Clinical Studies. Front. Immunol. 2022, 13, 977175. [Google Scholar] [CrossRef]
- Strub, J.M.; Garcia-Sablone, P.; Lonning, K.; Taupenot, L.; Hubert, P.; Van Dorsselaer, A.; Aunis, D.; Metz-Boutigue, M.H. Processing of Chromogranin B in Bovine Adrenal Medulla. Identification of Secretolytin, the Endogenous C-terminal Fragment of Residues 614–626 with Antibacterial Activity. Eur. J. Biochem. 1995, 229, 356–368. [Google Scholar] [CrossRef]
- Yoo, S.H. Coupling of the IP3 Receptor/Ca2+ Channel with Ca2+ Storage Proteins Chromogranins A and B in Secretory Granules. Trends Neurosci. 2000, 23, 424–428. [Google Scholar] [CrossRef]
- Schmidt, S.; Mo, M.; Heidrich, F.M.; Ćelić, A.; Ehrlich, B.E. C-terminal Domain of Chromogranin B Regulates Intracellular Calcium Signaling. J. Biol. Chem. 2011, 286, 44888–44896. [Google Scholar] [CrossRef] [PubMed]
- Ricci, C.; Battistini, S.; Avemaria, F.; Benigni, M.; Tarlarini, C.; Giannini, F.; Corbo, M.; Lunetta, C.; Penco, S. Lack of Relationship between the P413L Chromogranin B Variant and a SALS Italian Cohort. Gene 2015, 568, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Blasco, H.; Corcia, P.; Veyrat-Durebex, C.; Coutadeur, C.; Fournier, C.; Camu, W.; Gordon, P.; Praline, J.; Andres, C.R.; Vourc’h, P.; et al. The P413L Chromogranin B Variation in French Patients with Sporadic Amyotrophic Lateral Sclerosis. Amyotroph. Lateral Scler. 2011, 12, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Soucy, G.; Phaneuf, D.; Audet, J.-N.; Gros-Louis, F.; Rouleau, G.A.; Blasco, H.; Corcia, P.; Andersen, P.M.; Nordin, F.; et al. Sex-Dependent Effects of Chromogranin B P413L Allelic Variant as Disease Modifier in Amyotrophic Lateral Sclerosis. Hum. Mol. Genet. 2016, 25, 4771–4786. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Xing, D.; Li, P.; Li, C.; Qi, L.; Xu, Y.; Ren, H. Lack of Association between the P413L Variant of Chromogranin B and ALS Risk or Age at Onset: A Meta-Analysis. Amyotroph. Lateral Scler. Front. Degener. 2018, 19, 80–86. [Google Scholar] [CrossRef]
- Nelis, M.; Esko, T.; Mägi, R.; Zimprich, F.; Zimprich, A.; Toncheva, D.; Karachanak, S.; Piskáčková, T.; Balaščák, I.; Peltonen, L.; et al. Genetic Structure of Europeans: A View from the North–East. PLoS ONE 2009, 4, e5472. [Google Scholar] [CrossRef]
- Kamisuki, S.; Himeno, N.; Tsurukawa, Y.; Kusayanagi, T.; Takeno, M.; Kamakura, T.; Kuramochi, K.; Sugawara, F. Identification of Proteins That Bind to the Neuroprotective Agent Neoechinulin A. Biosci. Biotechnol. Biochem. 2018, 82, 442–448. [Google Scholar] [CrossRef]
- Yadav, G.P.; Wang, H.; Ouwendijk, J.; Cross, S.; Wang, Q.; Qin, F.; Verkade, P.; Zhu, M.X.; Jiang, Q.-X. Chromogranin B (CHGB) Is Dimorphic and Responsible for Dominant Anion Channels Delivered to Cell Surface via Regulated Secretion. Front. Mol. Neurosci. 2023, 16, 1205516. [Google Scholar] [CrossRef]
- Lederer, C.W.; Torrisi, A.; Pantelidou, M.; Santama, N.; Cavallaro, S. Pathways and Genes Differentially Expressed in the Motor Cortex of Patients with Sporadic Amyotrophic Lateral Sclerosis. BMC Genom. 2007, 8, 26. [Google Scholar] [CrossRef]
- Peters, O.M.; Ghasemi, M.; Brown, R.H. Emerging Mechanisms of Molecular Pathology in ALS. J. Clin. Investig. 2015, 125, 1767–1779. [Google Scholar] [CrossRef]
- Tourtourikov, I.; Dabchev, K.; Todorov, T.; Angelov, T.; Chamova, T.; Tournev, I.; Kadiyska, T.; Mitev, V.; Todorova, A. Navigating the ALS Genetic Labyrinth: The Role of MAPT Haplotypes. Genes 2023, 14, 2023. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Francioli, L.C.; Goodrich, J.K.; Collins, R.L.; Kanai, M.; Wang, Q.; Alföldi, J.; Watts, N.A.; Vittal, C.; Gauthier, L.D.; et al. A Genomic Mutational Constraint Map Using Variation in 76,156 Human Genomes. Nature 2024, 625, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Kenna, K.P.; McLaughlin, R.L.; Byrne, S.; Elamin, M.; Heverin, M.; Kenny, E.M.; Cormican, P.; Morris, D.W.; Donaghy, C.G.; Bradley, D.G.; et al. Delineating the Genetic Heterogeneity of ALS Using Targeted High-Throughput Sequencing. J. Med. Genet. 2013, 50, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxid. Med. Cell. Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef] [PubMed]
- Rao, F.; Zhang, K.; Khandrika, S.; Mahata, M.; Fung, M.M.; Ziegler, M.G.; Rana, B.K.; O’Connor, D.T. Isoprostane, an “Intermediate Phenotype” for Oxidative Stress Heritability, Risk Trait Associations, and the Influence of Chromogranin B Polymorphism. J. Am. Coll. Cardiol. 2010, 56, 1338–1350. [Google Scholar] [CrossRef] [PubMed]
- Skillbäck, T.; Mattsson, N.; Hansson, K.; Mirgorodskaya, E.; Dahlén, R.; van der Flier, W.; Scheltens, P.; Duits, F.; Hansson, O.; Teunissen, C.; et al. A Novel Quantification-Driven Proteomic Strategy Identifies an Endogenous Peptide of Pleiotrophin as a New Biomarker of Alzheimer’s Disease. Sci. Rep. 2017, 7, 13333. [Google Scholar] [CrossRef]
- Turner, M.R.; Swash, M. The Expanding Syndrome of Amyotrophic Lateral Sclerosis: A Clinical and Molecular Odyssey. J. Neurol. Neurosurg. Psychiatry 2015, 86, 667–673. [Google Scholar] [CrossRef]
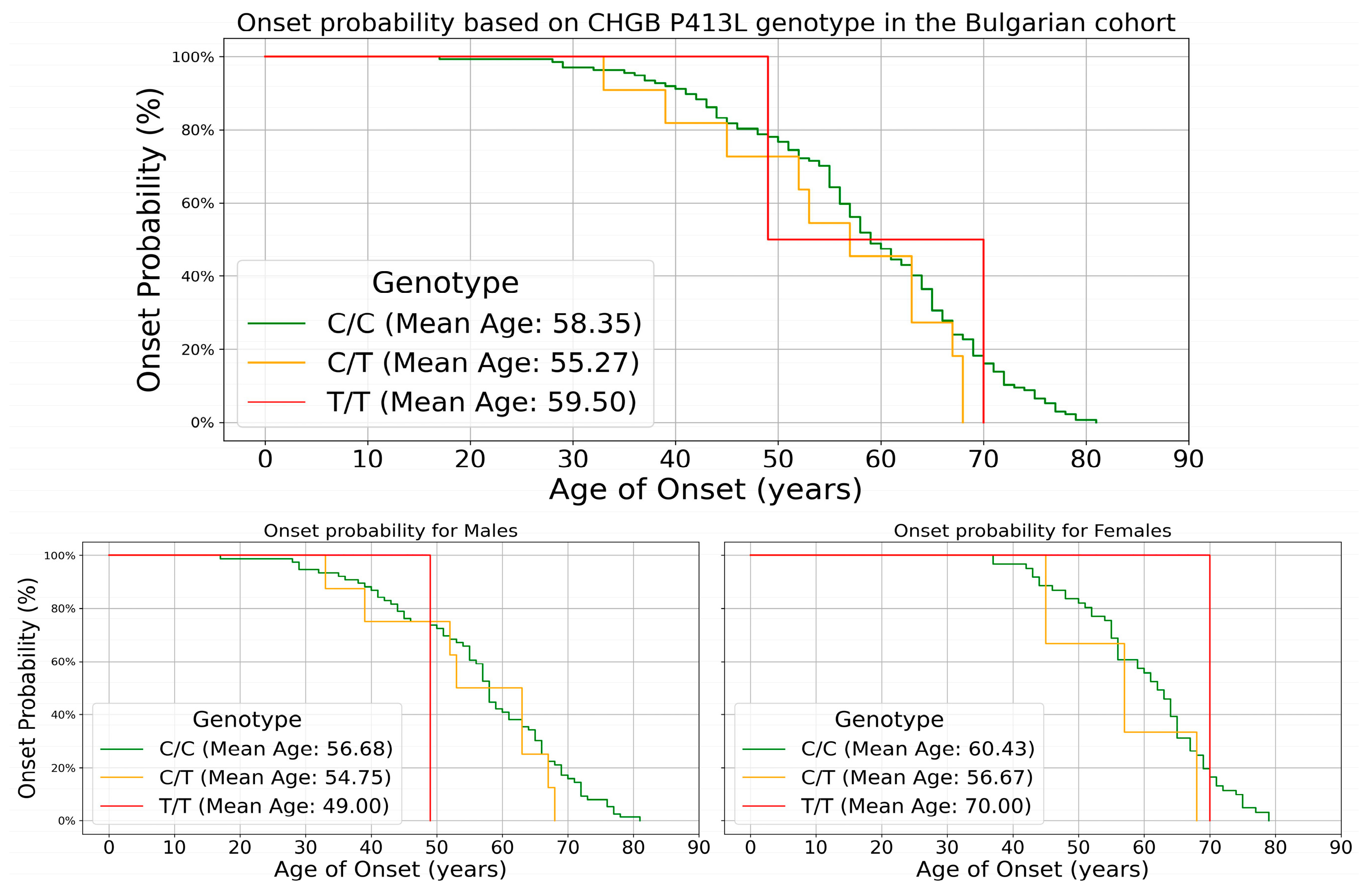

| Males | Females | |
|---|---|---|
| Count | 85 | 65 |
| Mean Age of Onset | 56.88 ± 12.83 | 60.4 ± 10.47 |
| Minimum Age of Onset | 28 | 37 |
| Maximum Age of Onset | 81 | 79 |
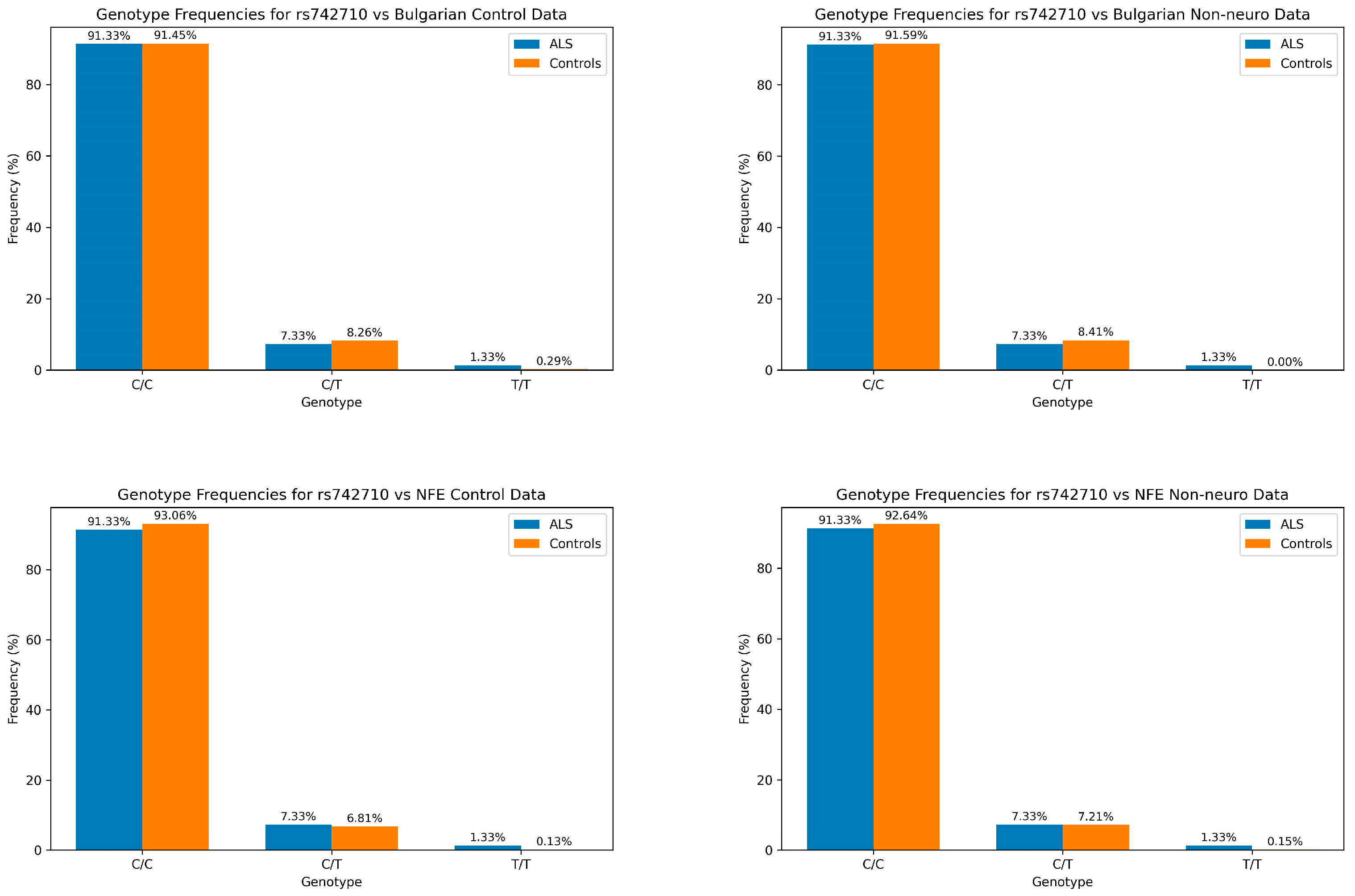
| Genotype Data | ||||||||
| Comparison Group | χ2 | p-Value | Control C/C | ALS C/C | Control C/T | ALS C/T | Control T/T | ALST/T |
| Bulgarian Control | 1.9395 | 3.79 × 10−1 | 91.45% | 91.33% | 8.26% | 7.33% | 0.29% | 1.33% |
| Bulgarian Non-Neuro | 3.1443 | 2.08 × 10−1 | 91.59% | 91.33% | 8.41% | 7.33% | 0.00% | 1.33% |
| NFE Control | 15.4572 | 4.40 × 10−4 | 93.06% | 91.33% | 6.81% | 7.33% | 0.13% | 1.33% |
| NFE Non-Neuro | 13.7498 | 1.03 × 10−3 | 92.64% | 91.33% | 7.21% | 7.33% | 0.15% | 1.33% |
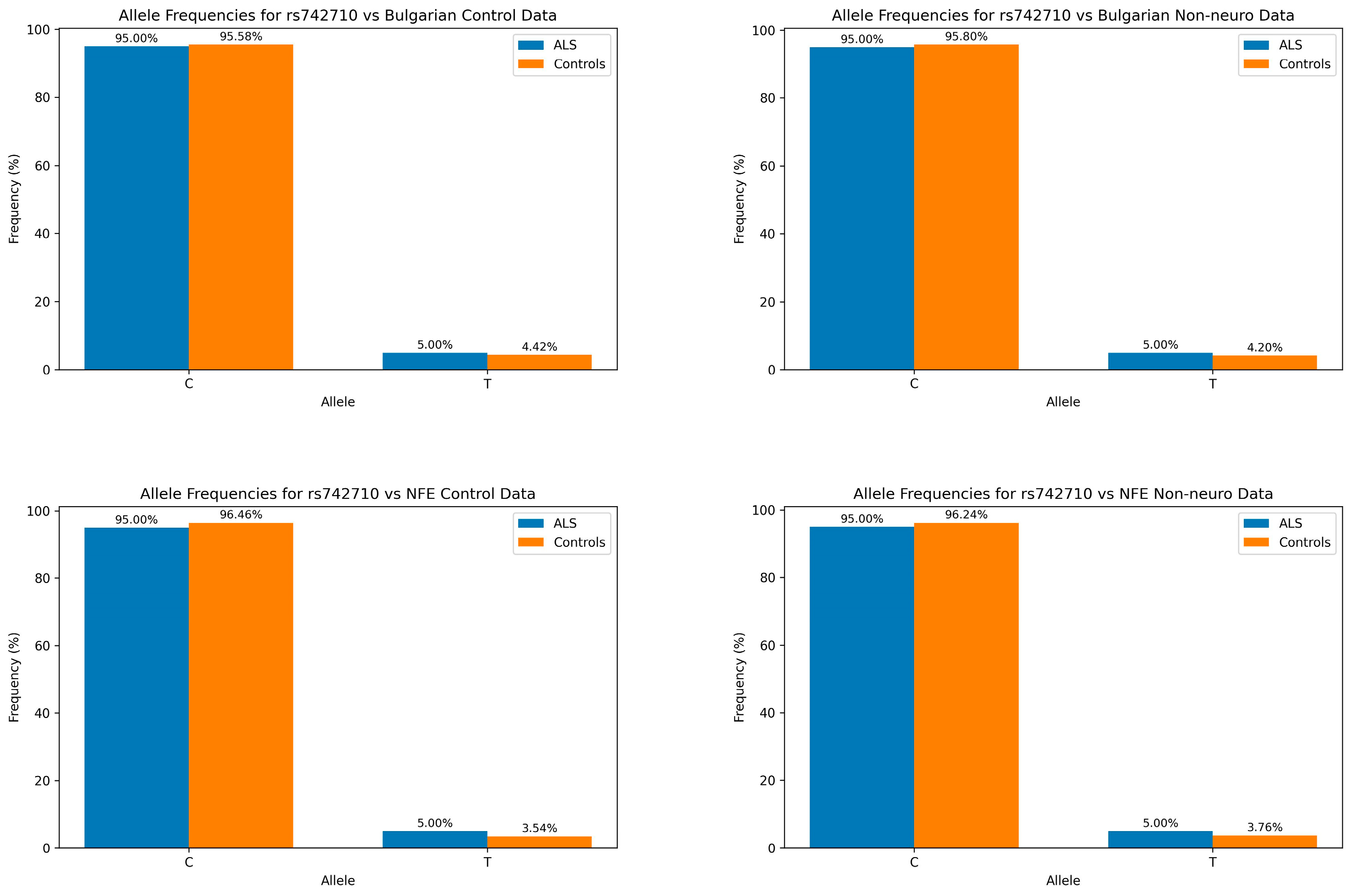
| Allele Data | ||||||||
| Comparison Group | χ2 | p-Value | Control C | ALS C | Control T | ALS T | ||
| Bulgarian Control | 0.0531 | 8.18 × 10−1 | 95.58% | 95.00% | 4.42% | 5.00% | ||
| Bulgarian Non-Neuro | 0.1126 | 7.37 × 10−1 | 95.80% | 95.00% | 4.20% | 5.00% | ||
| NFE Control | 1.458 | 2.27 × 10−1 | 96.46% | 95.00% | 3.54% | 5.00% | ||
| NFE Non-Neuro | 0.9571 | 3.28 × 10−1 | 96.24% | 95.00% | 3.76% | 5.00% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tourtourikov, I.; Todorov, T.; Angelov, T.; Chamova, T.; Tournev, I.; Mitev, V.; Todorova, A. Genetic Modifiers of ALS: The Impact of Chromogranin B P413L in a Bulgarian ALS Cohort. Genes 2024, 15, 1197. https://doi.org/10.3390/genes15091197
Tourtourikov I, Todorov T, Angelov T, Chamova T, Tournev I, Mitev V, Todorova A. Genetic Modifiers of ALS: The Impact of Chromogranin B P413L in a Bulgarian ALS Cohort. Genes. 2024; 15(9):1197. https://doi.org/10.3390/genes15091197
Chicago/Turabian StyleTourtourikov, Ivan, Tihomir Todorov, Teodor Angelov, Teodora Chamova, Ivailo Tournev, Vanyo Mitev, and Albena Todorova. 2024. "Genetic Modifiers of ALS: The Impact of Chromogranin B P413L in a Bulgarian ALS Cohort" Genes 15, no. 9: 1197. https://doi.org/10.3390/genes15091197
APA StyleTourtourikov, I., Todorov, T., Angelov, T., Chamova, T., Tournev, I., Mitev, V., & Todorova, A. (2024). Genetic Modifiers of ALS: The Impact of Chromogranin B P413L in a Bulgarian ALS Cohort. Genes, 15(9), 1197. https://doi.org/10.3390/genes15091197






