NR0B2 Is a Key Factor for Gastric Diseases: A GEO Database Analysis Combined with Drug-Target Mendelian Randomization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Preprocessing
2.2. Bioinformatics Analysis
2.3. Mendelian Randomization Analysis
3. Results
3.1. NR0B2’s Down-Regulation in Gastric Cancer and Up-Regulation in Gastritis
3.2. NR0B2 Is Important for Lipid Metabolism and Oxidation-Related Processes
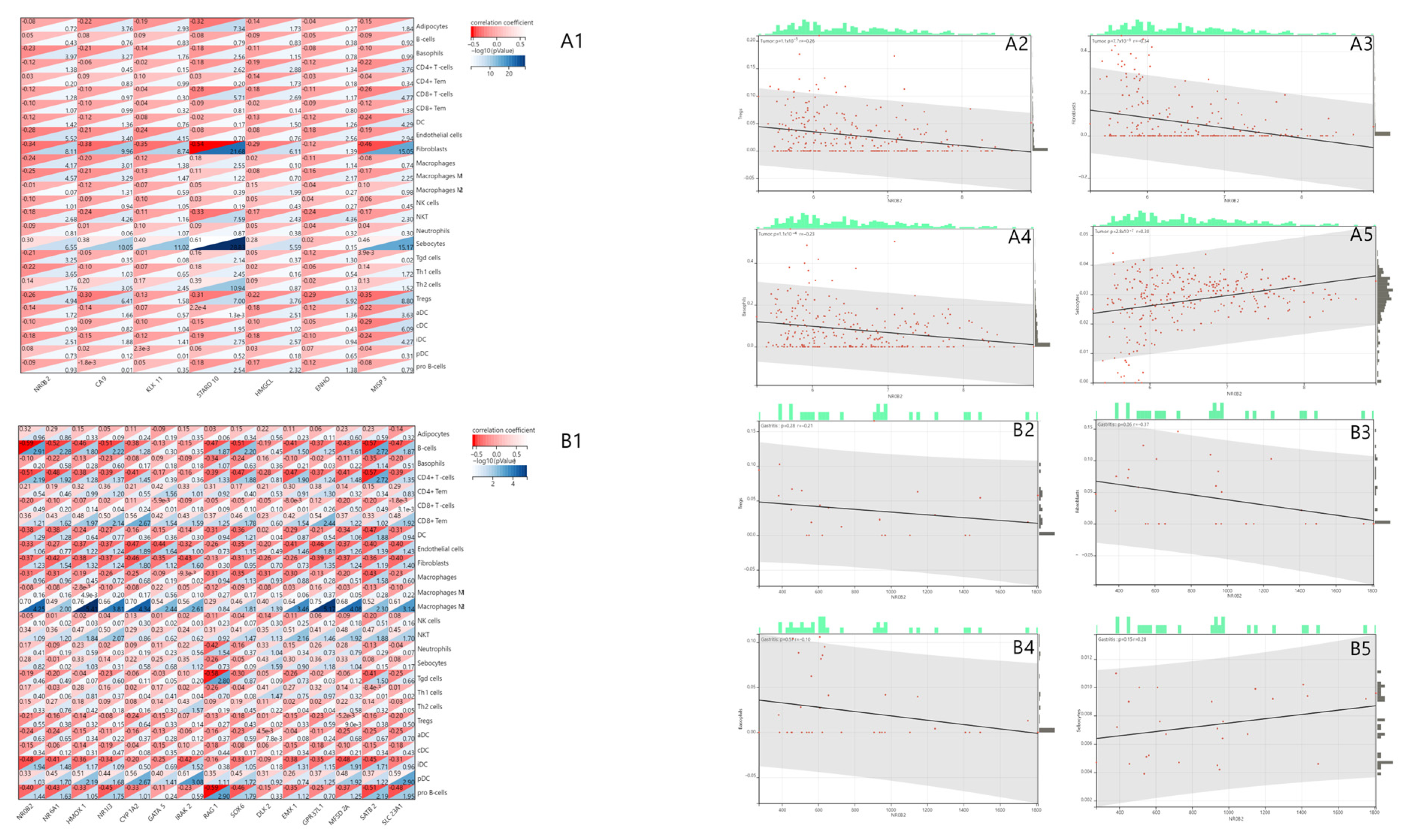
3.3. NR0B2 Is Associated with Several Immune Infiltrations
3.4. MR Analysis Results Show That NR0B2 Has a Causal Relationship with Gastric Diseases
3.5. Heterogeneity Analysis Results of MR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Hagedorn, C.H.; Wang, L. Role of nuclear receptor SHP in metabolism and cancer. Biochim. et Biophys. Acta Mol. Basis Dis. 2011, 1812, 893–908. [Google Scholar] [CrossRef] [PubMed]
- Garruti, G.; Wang, H.H.; Bonfrate, L.; de Bari, O.; Wang, D.Q.-H.; Portincasa, P. A pleiotropic role for the orphan nuclear receptor small heterodimer partner in lipid homeostasis and metabolic pathways. J. Lipids 2012, 2012, 304292. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Tu, Y.; Chang, J.; Xu, H.; Li, J.C.; Liu, W.; Do, A.-D.; Zhang, Y.; Wang, J.; Li, B. The orphan nuclear receptor gene NR0B2 is a favorite prognosis factor modulated by multiple cellular signal pathways in human liver cancers. Front. Oncol. 2021, 11, 691199. [Google Scholar] [CrossRef]
- Shahoei, S.H. The Immunomodulatory Roles of Small Heterodimer Partner and Their Implications in Breast Cancer Progression. Ph.D. Thesis, University of Illinois at Urbana-Champaign, Champaign, IL, USA, 2020. [Google Scholar]
- Shahoei, S.H.; Nelson, A.T.; Henn, M.A.; Mathews, A.E.; Chen, J.J.; Vembar, V.; Vardanyan, A.; Ma, L.; Wang, Y.; Apetoh, L. OR05-01 Small Heterodimer Partner Modulates Antigen Presenting Myeloid Cells to Impair Regulatory T Cell Expansion, Promoting Anti-Tumor Immunity in Models of Breast Cancer. J. Endocr. Soc. 2020, 4 (Suppl. S1), OR05-01. [Google Scholar] [CrossRef]
- Prestin, K.; Olbert, M.; Hussner, J.; Isenegger, T.L.; Gliesche, D.G.; Böttcher, K.; Zimmermann, U.; Meyer zu Schwabedissen, H.E. Modulation of expression of the nuclear receptor NR0B2 (small heterodimer partner 1) and its impact on proliferation of renal carcinoma cells. OncoTargets Ther. 2016, 9, 4867–4878. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtseva, A.; Nyushko, K.; Zaretsky, A.; Shagin, D.; Sadritdinova, A.; Fedorova, M.; Savvateeva, M.; Guvatova, Z.; Pudova, E.; Alekseev, B.Y. Suppression of NR0B2 gene in clear cell renal cell carcinoma is associated with hypermethylation of its promoter. Mol. Biol. 2018, 52, 414–418. [Google Scholar] [CrossRef]
- Hatch, J.; Liu, S.; Gayowski, T.; Sorensen, J.; Wang, L. Nuclear receptor SHP as a potential therapeutic target for liver cancer. Curr. Cancer Ther. Rev. 2010, 6, 317–322. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Q.; Guo, H.; Wang, N.; Fan, X.; Zhang, B.; Zhang, W.; Wang, W.; Fang, Z.; Wu, J. Dietary patterns and risk for gastric cancer: A case-control study in residents of the Huaihe River Basin, China. Front. Nutr. 2023, 10, 1118113. [Google Scholar] [CrossRef]
- He, F.; Wang, S.; Zheng, R.; Gu, J.; Zeng, H.; Sun, K.; Chen, R.; Li, L.; Han, B.; Li, X. Trends of gastric cancer burdens attributable to risk factors in China from 2000 to 2050. Lancet Reg. Health–West. Pac. 2024, 44, 101003. [Google Scholar] [CrossRef]
- Wei, R.; Zhang, W.; Yang, F.; Li, Q.; Wang, Q.; Liu, N.; Zhu, J.; Shan, Y. Dual targeting non-overlapping epitopes in HER2 domain IV substantially enhanced HER2/HER2 homodimers and HER2/EGFR heterodimers internalization leading to potent antitumor activity in HER2-positive human gastric cancer. J. Transl. Med. 2024, 22, 641. [Google Scholar] [CrossRef]
- Moore, J.T.; Collins, J.L.; Pearce, K.H. The nuclear receptor superfamily and drug discovery. ChemMedChem Chem. Enabling Drug Discov. 2006, 1, 504–523. [Google Scholar]
- Hegde, M.; Girisa, S.; Naliyadhara, N.; Kumar, A.; Alqahtani, M.S.; Abbas, M.; Mohan, C.D.; Warrier, S.; Hui, K.M.; Rangappa, K.S. Natural compounds targeting nuclear receptors for effective cancer therapy. Cancer Metastasis Rev. 2023, 42, 765–822. [Google Scholar] [CrossRef] [PubMed]
- Helleboid, S.; Haug, C.; Lamottke, K.; Zhou, Y.; Wei, J.; Daix, S.; Cambula, L.; Rigou, G.; Hum, D.W.; Walczak, R. The identification of naturally occurring neoruscogenin as a bioavailable, potent, and high-affinity agonist of the nuclear receptor RORα (NR1F1). J. Biomol. Screen. 2014, 19, 399–406. [Google Scholar] [CrossRef]
- Yang, Z.; Koehler, A.N.; Wang, L. A novel small molecule activator of nuclear receptor SHP inhibits HCC cell migration via suppressing Ccl2. Mol. Cancer Ther. 2016, 15, 2294–2301. [Google Scholar] [CrossRef]
- Clough, E.; Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M. NCBI GEO: Archive for gene expression and epigenomics data sets: 23-year update. Nucleic Acids Res. 2024, 52, D138–D144. [Google Scholar] [CrossRef]
- Sims, A.H.; Smethurst, G.J.; Hey, Y.; Okoniewski, M.J.; Pepper, S.D.; Howell, A.; Miller, C.J.; Clarke, R.B. The removal of multiplicative, systematic bias allows integration of breast cancer gene expression datasets–improving meta-analysis and prediction of prognosis. BMC Med. Genom. 2008, 1, 42. [Google Scholar] [CrossRef] [PubMed]
- Emdin, C.A.; Khera, A.V.; Kathiresan, S. Mendelian randomization. JAMA 2017, 318, 1925–1926. [Google Scholar] [CrossRef]
- Sanderson, E.; Glymour, M.M.; Holmes, M.V.; Kang, H.; Morrison, J.; Munafò, M.R.; Palmer, T.; Schooling, C.M.; Wallace, C.; Zhao, Q. Mendelian randomization. Nat. Rev. Methods Primers 2022, 2, 6. [Google Scholar] [CrossRef]
- Ban, H.-J.; Lee, S.; Jin, H.-J. Exploring Stroke Risk through Mendelian Randomization: A Comprehensive Study Integrating Genetics and Metabolic Traits in the Korean Population. Biomedicines 2024, 12, 1311. [Google Scholar] [CrossRef]
- Silva, S.; Fatumo, S.; Nitsch, D. Mendelian randomization studies on coronary artery disease: A systematic review and meta-analysis. Syst. Rev. 2024, 13, 29. [Google Scholar] [CrossRef]
- Mao, X.; Huang, C.; Wang, Y.; Mao, S.; Li, Z.; Zou, W.; Liao, Z. Association between dietary habits and pancreatitis among individuals of European ancestry: A two-sample Mendelian randomization study. Nutrients 2023, 15, 1153. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, Z.; Zhu, Y.; Yuan, G.; Yang, J.; Yu, W. Mendelian randomization and transcriptome-wide association analysis identified genes that were pleiotropically associated with intraocular pressure. Genes 2023, 14, 1027. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.F.; Finan, C.; Gordillo-Marañón, M.; Asselbergs, F.W.; Freitag, D.F.; Patel, R.S.; Tyl, B.; Chopade, S.; Faraway, R.; Zwierzyna, M. Genetic drug target validation using Mendelian randomisation. Nat. Commun. 2020, 11, 3255. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, W.; Zhang, D.; Mi, Y.; Zhang, J.; He, G. The causal relationship between PCSK9 inhibitors and malignant tumors: A mendelian randomization study based on drug targeting. Genes 2024, 15, 132. [Google Scholar] [CrossRef]
- Rosoff, D.B.; Bell, A.S.; Wagner, J.; Mavromatis, L.A.; Hamandi, A.; Park, L.; Jung, J.; Lohoff, F.W. Assessing the impact of PCSK9 and HMGCR inhibition on liver function: Drug-target Mendelian randomization analyses in four ancestries. Cell. Mol. Gastroenterol. Hepatol. 2024, 17, 29–40. [Google Scholar] [CrossRef]
- Aran, D.; Hu, Z.; Butte, A.J. xCell: Digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 2017, 18, 220. [Google Scholar] [CrossRef] [PubMed]
- Bartha, Á.; Győrffy, B. TNMplot. com: A web tool for the comparison of gene expression in normal, tumor and metastatic tissues. Int. J. Mol. Sci. 2021, 22, 2622. [Google Scholar] [CrossRef]
- Shahoei, S.H.; Kim, Y.-C.; Cler, S.J.; Ma, L.; Anakk, S.; Kemper, J.K.; Nelson, E.R. Small heterodimer partner regulates dichotomous T cell expansion by macrophages. Endocrinology 2019, 160, 1573–1589. [Google Scholar] [CrossRef]
- Carino, A.; Graziosi, L.; Marchianò, S.; Biagioli, M.; Marino, E.; Sepe, V.; Zampella, A.; Distrutti, E.; Donini, A.; Fiorucci, S. Analysis of gastric cancer transcriptome allows the identification of histotype specific molecular signatures with prognostic potential. Front. Oncol. 2021, 11, 663771. [Google Scholar] [CrossRef]
- Gamage, H.E.V.; Albright, S.T.; Smith, A.J.; Farmer, R.; Shahoei, S.H.; Wang, Y.; Fink, E.C.; Jacquin, E.; Weisser, E.; Bautista, R.O. Development of NR0B2 as a therapeutic target for the re-education of tumor associated myeloid cells. Cancer Lett. 2024, 597, 217086. [Google Scholar] [CrossRef]
- Xie, W.; Li, J.; Du, H.; Xia, J. Causal relationship between PCSK9 inhibitor and autoimmune diseases: A drug target Mendelian randomization study. Arthritis Res. Ther. 2023, 25, 148. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cao, D.; Zhang, Y.; Wu, Y.; Jia, Z.; Cui, Y.; Li, D.; Cao, X.; Jiang, J. Appraising associations between signature lipidomic biomarkers and digestive system cancer risk: Novel evidences from a prospective cohort study of UK Biobank and Mendelian randomization analyses. Lipids Health Dis. 2024, 23, 61. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Zhao, D. Association between serum total cholesterol and the development of gastric cancer: A two-way two-sample Mendelian randomization study. Medicine 2024, 103, e38900. [Google Scholar] [CrossRef]
- Batista-Gonzalez, A.; Vidal, R.; Criollo, A.; Carreño, L.J. New insights on the role of lipid metabolism in the metabolic reprogramming of macrophages. Front. Immunol. 2020, 10, 2993. [Google Scholar] [CrossRef]
- Remmerie, A.; Scott, C.L. Macrophages and lipid metabolism. Cell. Immunol. 2018, 330, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.K.-L.; Cheung, S.W.-M.; Cheng, K.K.-Y. NLRP3 inflammasome activation in adipose tissues and its implications on metabolic diseases. Int. J. Mol. Sci. 2020, 21, 4184. [Google Scholar] [CrossRef]
- Cobo, I.; Martinelli, P.; Flández, M.; Bakiri, L.; Zhang, M.; Carrillo-de-Santa-Pau, E.; Jia, J.; Sanchez-Arevalo Lobo, V.J.; Megías, D.; Felipe, I. Transcriptional regulation by NR5A2 links differentiation and inflammation in the pancreas. Nature 2018, 554, 533–537. [Google Scholar] [CrossRef]
- Lian, F.; Xing, X.; Yuan, G.; Schäfer, C.; Rauser, S.; Walch, A.; Röcken, C.; Ebeling, M.; Wright, M.B.; Schmid, R.M. Farnesoid X receptor protects human and murine gastric epithelial cells against inflammation-induced damage. Biochem. J. 2011, 438, 315–323. [Google Scholar] [CrossRef]
- Sun, R.; Xu, H.; Liu, F.; Zhou, B.; Li, M.; Sun, X. Unveiling the intricate causal nexus between pancreatic cancer and peripheral metabolites through a comprehensive bidirectional two-sample Mendelian randomization analysis. Front. Mol. Biosci. 2023, 10, 1279157. [Google Scholar] [CrossRef]


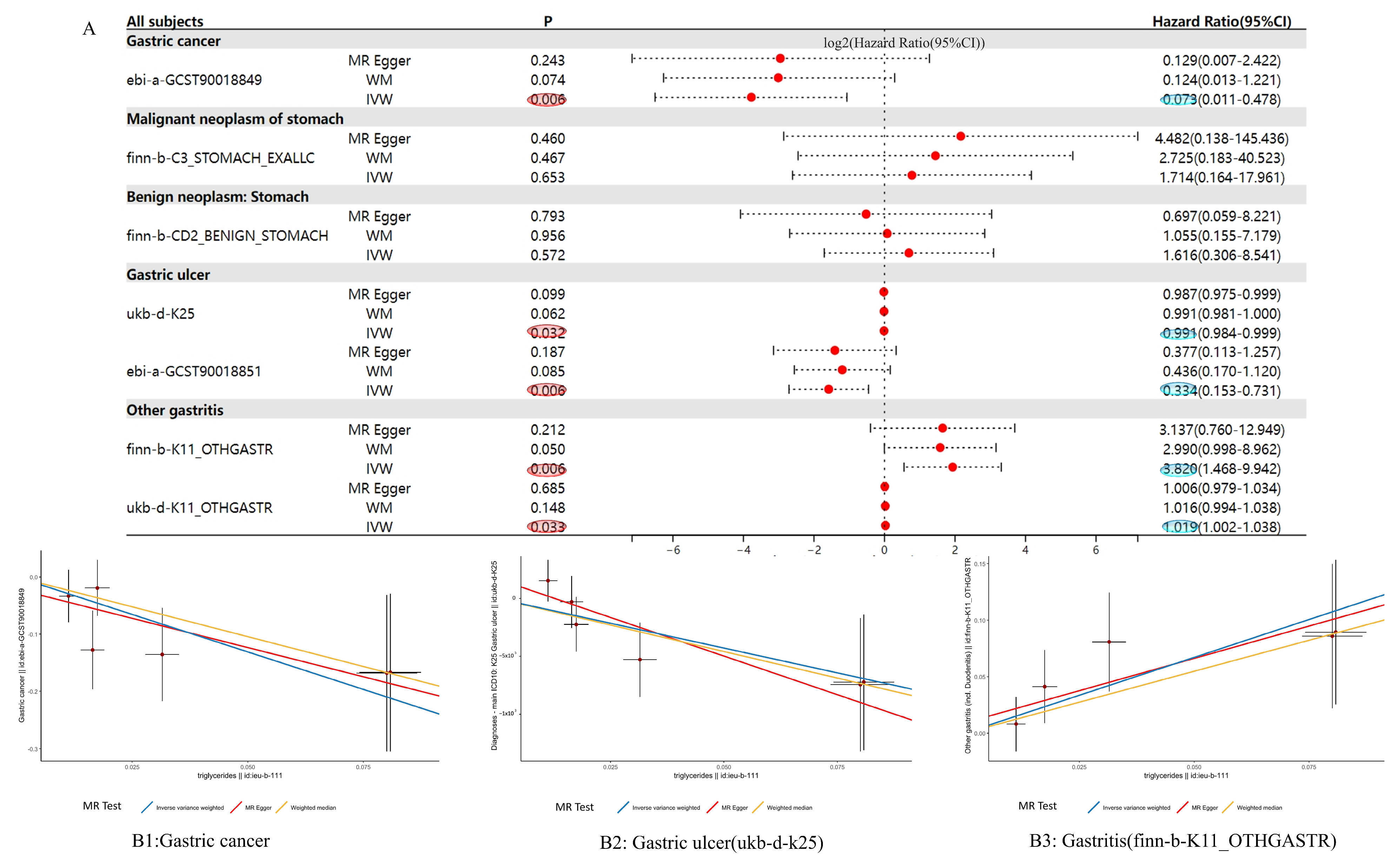

| Characteristics | GWASID | Type | Sample Size | Number of SNPs |
|---|---|---|---|---|
| Triglyceride | ieu-b-111 | Exposure | 441,060 | 12,321,875 |
| Gastric cancer | ebi-a-GCST90018849 | Outcome | 476,116 | 24,188,662 |
| Malignant neoplasm of stomach | finn-b-C3_STOMACH_EXALLC | Outcome | 174,639 | 16,380,305 |
| Benign neoplasm: stomach | finn-b-CD2_BENIGN_STOMACH | Outcome | 218,792 | 16,380,466 |
| Gastric ulcer | ukb-d-K25 | Outcome | 361,194 | 10,452,088 |
| Gastric ulcer | ebi-a-GCST90018851 | Outcome | 474,278 | 24,178,780 |
| Other gastritis | finn-b-K11_OTHGASTR | Outcome | 174,576 | 16,380,406 |
| Other gastritis | ukb-d-K11_OTHGASTR | Outcome | 361,194 | 13,356,120 |
| IVs | Chromosome | Effect Allele | Other Allele | β | SE | p |
|---|---|---|---|---|---|---|
| rs12727590 | 1 | G | A | 0.016437 | 0.0025535 | 1.2 × 10−10 |
| rs6659176 | 1 | G | C | 0.0314972 | 0.0036665 | 8.7 × 10−18 |
| rs11581460 | 1 | A | G | −0.011234 | 0.0020387 | 3.6 × 10−8 |
| rs79598313 | 1 | T | C | 0.0800707 | 0.0065634 | 3.1 × 10−34 |
| rs34928032 | 1 | G | A | 0.0174607 | 0.0026908 | 8.6 × 10−11 |
| rs75460349 | 1 | C | A | 0.0808376 | 0.0066624 | 7 × 10−34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Xu, L.; Huang, D.; Li, C.; Haenen, G.R.M.M.; Zhang, M. NR0B2 Is a Key Factor for Gastric Diseases: A GEO Database Analysis Combined with Drug-Target Mendelian Randomization. Genes 2024, 15, 1210. https://doi.org/10.3390/genes15091210
Li Z, Xu L, Huang D, Li C, Haenen GRMM, Zhang M. NR0B2 Is a Key Factor for Gastric Diseases: A GEO Database Analysis Combined with Drug-Target Mendelian Randomization. Genes. 2024; 15(9):1210. https://doi.org/10.3390/genes15091210
Chicago/Turabian StyleLi, Zhengwen, Lijia Xu, Dongliang Huang, Chujie Li, Guido R. M. M. Haenen, and Ming Zhang. 2024. "NR0B2 Is a Key Factor for Gastric Diseases: A GEO Database Analysis Combined with Drug-Target Mendelian Randomization" Genes 15, no. 9: 1210. https://doi.org/10.3390/genes15091210







