Impacts of Nucleosome Positioning Elements and Pre-Assembled Chromatin States on Expression and Retention of Transgenes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cloning of Reporter Plasmids
2.2. Site-Directed Mutagenesis of H3K9,14 and H4K16 to Mimic Acetylated States
2.3. Refolding of Histone Octamers and Assembly of Chromatin
2.4. Transfection by Calcium Phosphate
2.5. Flow Cytometry Analyses of Expression of Reporter eGFP
2.6. Statistical Analysis of Expression of Reporter eGFP
2.7. Rate of Loss of Expression of Reporter eGFP
3. Results
3.1. Cis-Sequences Impact the Efficiency of Expression of eGFP from Plasmids Transfected as Naked DNA
3.2. Pre-Assembled Chromatin Retain Expression of eGFP more Efficiently than Naked Plasmid DNA
3.3. Nucleosome Positioning Elements Affect the Efficiency of Expression of Reporter eGFP in Pre-Assembled Chromatin States
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glover, D.J.; Lipps, H.J.; Jans, D.A. Towards safe, non-viral therapeutic gene expression in humans. Nat. Rev. Genet. 2005, 6, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Naso, M.F.; Tomkowicz, B.; Perry, W.L., 3rd; Strohl, W.R. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs 2017, 31, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Berns, K.I.; Muzyczka, N. AAV: An Overview of Unanswered Questions. Hum. Gene. Ther. 2017, 28, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, G.A.R.; Paiva, R.M.A. Gene therapy: Advances, challenges and perspectives. Einstein 2017, 15, 369–375. [Google Scholar] [CrossRef]
- Shchaslyvyi, A.Y.; Antonenko, S.V.; Tesliuk, M.G.; Telegeev, G.D. Current State of Human Gene Therapy: Approved Products and Vectors. Pharmaceuticals 2023, 16, 1416. [Google Scholar] [CrossRef]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target Ther. 2021, 6, 53. [Google Scholar] [CrossRef]
- Wang, J.-H.; Gessler, D.J.; Zhan, W.; Gallagher, T.L.; Gao, G. Adeno-associated virus as a delivery vector for gene therapy of human diseases. Signal Transduct. Target Ther. 2024, 9, 78. [Google Scholar] [CrossRef]
- Komatsu, T.; Haruki, H.; Nagata, K. Cellular and viral chromatin proteins are positive factors in the regulation of adenovirus gene expression. Nucleic Acids Res. 2011, 39, 889–901. [Google Scholar] [CrossRef]
- Tate, V.E.; Philipson, L. Parental adenovirus DNA accumulates in nucleosome-like structures in infected cells. Nucleic Acids Res. 1979, 6, 2769–2785. [Google Scholar] [CrossRef]
- Wong, C.M.; McFall, E.R.; Burns, J.K.; Parks, R.J. The role of chromatin in adenoviral vector function. Viruses 2013, 5, 1500–1515. [Google Scholar] [CrossRef]
- Herweijer, H.; Wolff, J.A. Progress and prospects: Naked DNA gene transfer and therapy. Gene Ther. 2003, 10, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Togashi, R.; Harashima, H.; Kamiya, H. Correlation between transgen expression and plasmid DNA loss in mouse liver. J. Gene Med. 2013, 15, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Hobernik, D.; Bros, M. DNA Vaccines—How Far From Clinical Use? J. Mol. Med. 2018, 19, 3605. [Google Scholar] [CrossRef] [PubMed]
- Herweijer, H.; Zhang, G.; Subbotin, V.M.; Budker, V.; Williams, P.; Wolff, J.A. Time course of gene expression after plasmid DNA gene transfer to the liver. J. Gene Med. 2001, 3, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Ghazizadeh, S.; Kalish, R.S.; Taichman, L.B. Immune-mediated loss of transgene expression in skin: Implications for cutaneous gene therapy. Mol. Ther. 2003, 7, 296–303. [Google Scholar] [CrossRef]
- Carroll, A.C.; Wong, A. Plasmid persistence: Costs, benefits, and the plasmid paradox. Can. J. Microbiol. 2018, 64, 293–304. [Google Scholar] [CrossRef]
- Papapetrou, E.P.; Ziros, P.G.; Micheva, I.D.; Zoumbos, N.C.; Athanassiadou, A. Gene transfer into human hematopoietic progenitor cells with an episomal vector carrying an S/MAR element. Gene Ther. 2006, 13, 40–51. [Google Scholar] [CrossRef]
- Prösch, S.; Stein, J.; Staak, K.; Liebenthal, C.; Volk, H.D.; Krüger, D.H. Inactivation of the very strong HCMV immediate early promoter by DNA CpG methylation in vitro. Biol. Chem. Biol. Chem. Hoppe-Seyler 1996, 377, 195–201. [Google Scholar] [CrossRef]
- Hong, K.; Sherley, J.; Lauffenburger, D.A. Methylation of episomal plasmids as a barrier to transient gene expression via a synthetic delivery vector. Biomol. Eng. 2001, 18, 185–192. [Google Scholar] [CrossRef]
- Habib, O.; Mohd Sakri, R.; Ghazalli, N.; Chau, D.-M.; Ling, K.-H.; Abdullah, S. Limited expression of non-integrating CpG-free plasmid is associated with increased nucleosome enrichment. PLoS ONE 2020, 15, e0244386. [Google Scholar] [CrossRef]
- Riu, E.; Chen, Z.-Y.; Xu, H.; He, C.-Y.; Kay, M.A. Histone Modifications are Associated with the Persistence or Silencing of Vector-mediated Transgene Expression In Vivo. Mol. Ther. 2007, 15, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, H.; Fujimuro, M.; Yokosawa, H.; Harashima, H.; Kamiya, H. Transient activation of transgene expression by hydrodynamics-based injection may cause rapid decrease in plasmid DNA expression. Gene Ther. 2007, 14, 1152–1159. [Google Scholar] [CrossRef]
- Kornberg, R.D.; Lorch, Y. Twenty-Five Years of the Nucleosome, Fundamental Particle of the Eukaryote Chromosome. Cell 1999, 98, 285–294. [Google Scholar] [CrossRef]
- Liu, R.; Wu, J.; Guo, H.; Yao, W.; Li, S.; Lu, Y.; Jia, Y.; Liang, X.; Tang, J.; Zhang, H. Post-translational modifications of histones: Mechanisms, biological functions, and therapeutic targets. MedComm. 2023, 4, e292. [Google Scholar] [CrossRef]
- Zhang, P.; Torres, K.; Liu, X.; Liu, C.G.; Pollock, R.E. An Overview of Chromatin-Regulating Proteins in Cells. Curr. Protein Pept. Sci. 2016, 17, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Hayes, J.J.; Pruss, D.; Wolffe, A.P. A positive role for histone acetylation in transcription factor access to nucleosomal DNA. Cell 1993, 72, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Kundu, T.K.; Wang, Z.; Roeder, R.G. Human TFIIIC relieves chromatin-mediated repression of RNA polymerase III transcription and contains an intrinsic histone acetyltransferase activity. Mol. Cell Biol. 1999, 19, 1605–1615. [Google Scholar] [CrossRef]
- Dion, M.F.; Altschuler, S.J.; Wu, L.F.; Rando, O.J. Genomic characterization reveals a simple histone H4 acetylation code. PNAS 2005, 102, 5501–5506. [Google Scholar] [CrossRef]
- Shogren-Knaak, M.; Ishii, H.; Sun, J.M.; Pazin, M.J.; Davie, J.R.; Peterson, C.L. Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science 2006, 311, 844–847. [Google Scholar] [CrossRef]
- Braunstein, M.; Rose, A.B.; Holmes, S.G.; Allis, C.D.; Broach, J.R. Transcriptional silencing in yeast is associated with reduced nucleosome acetylation. Genes Dev. 1993, 7, 592–604. [Google Scholar] [CrossRef]
- Kouzarides, T. Chromatin Modifications and Their Function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Yu, L.R.; Wang, L.; Zhang, Z.; Kasper, L.H.; Lee, J.E.; Wang, C.; Brindle, P.K.; Dent, S.Y.R.; Ge, K. Distinct roles of GCN5/PCAF-mediated H3K9ac and CBP/p300-mediated H3K18/27ac in nuclear receptor transactivation. EMBO J. 2011, 30, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Karmodiya, K.; Krebs, A.R.; Oulad-Abdelghani, M.; Kimura, H.; Tora, L. H3K9 and H3K14 acetylation co-occur at many gene regulatory elements, while H3K14ac marks a subset of inactive inducible promoters in mouse embryonic stem cells. BMC Genom. 2012, 13, 424. [Google Scholar] [CrossRef]
- Cabrera, A.; Edelstein, H.I.; Glykofrydis, F.; Love, K.S.; Palacios, S.; Tycko, J.; Zhang, M.; Lensch, S.; Shields, C.E.; Livingston, M.; et al. The sound of silence: Transgene silencing in mammalian cell engineering. Cell Syst. 2022, 13, 950–973. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Ball, A.R., Jr.; Yokomori, K. HP1: Heterochromatin binding proteins working the genome. Epigenetics 2010, 5, 287–292. [Google Scholar] [CrossRef]
- Maeda, R.; Tachibana, M. HP1 maintains protein stability of H3K9 methyltransferases and demethylases. EMBO Rep. 2022, 23, e53581. [Google Scholar] [CrossRef]
- Barrett, C.M.; McCracken, R.; Elmer, J.; Haynes, K.A. Components from the Human c-myb Transcriptional Regulation System Reactivate Epigenetically Repressed Transgenes. Int. J. Mol. Sci. 2020, 21, 530. [Google Scholar] [CrossRef]
- George, O.L.; Ness, S.A. Situational awareness: Regulation of the myb transcription factor in differentiation, the cell cycle and oncogenesis. Cancers 2014, 6, 2049–2071. [Google Scholar] [CrossRef]
- Schmitz, M.L.; Baeuerle, P.A. The p65 subunit is responsible for the strong transcription activating potential of NF-kappa B. Embo J. 1991, 10, 3805–3817. [Google Scholar] [CrossRef]
- Neville, J.J.; Orlando, J.; Mann, K.; McCloskey, B.; Antoniou, M.N. Ubiquitous Chromatin-opening Elements (UCOEs): Applications in biomanufacturing and gene therapy. Biotechnol. Adv. 2017, 35, 557–564. [Google Scholar] [CrossRef]
- Saunders, F.; Sweeney, B.; Antoniou, M.N.; Stephens, P.; Cain, K. Chromatin function modifying elements in an industrial antibody production platform--comparison of UCOE, MAR, STAR and cHS4 elements. PLoS ONE 2015, 10, e0120096. [Google Scholar] [CrossRef] [PubMed]
- Sizer, R.E.; White, R.J. Use of ubiquitous chromatin opening elements (UCOE) as tools to maintain transgene expression in biotechnology. Comput. Struct. Biotechnol. J. 2023, 21, 275–283. [Google Scholar] [CrossRef]
- Betts, Z.; Dickson, A.J. Assessment of UCOE on Recombinant EPO Production and Expression Stability in Amplified Chinese Hamster Ovary Cells. Mol. Biotechnol. 2015, 57, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Betts, Z.; Croxford, A.S.; Dickson, A.J. Evaluating the interaction between UCOE and DHFR-linked amplification and stability of recombinant protein expression. Biotechnol. Prog. 2015, 31, 1014–1025. [Google Scholar] [CrossRef]
- Antonova, D.V.; Alekseenko, I.V.; Siniushina, A.K.; Kuzmich, A.I.; Pleshkan, V.V. Searching for Promoters to Drive Stable and Long-Term Transgene Expression in Fibroblasts for Syngeneic Mouse Tumor Models. Int. J. Mol. Sci 2020, 21, 6098. [Google Scholar] [CrossRef]
- Khabou, H.; Cordeau, C.; Pacot, L.; Fisson, S.; Dalkara, D. Dosage Thresholds and Influence of Transgene Cassette in Adeno-Associated Virus-Related Toxicity. Hum. Gene Ther. 2018, 29, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Kinjo, M. Monitoring intracellular degradation of exogenous DNA using diffusion properties. J. Control. Release 2010, 143, 104–111. [Google Scholar] [CrossRef]
- Kanazawa, T.; Yamazaki, M.; Fukuda, T.; Takashima, Y.; Okada, H. Versatile nuclear localization signal-based oligopeptide as a gene vector. Biol. Pharm. Bull. 2015, 38, 559–565. [Google Scholar] [CrossRef]
- Ross, N.L.; Sullivan, M.O. Importin-4 Regulates Gene Delivery by Enhancing Nuclear Retention and Chromatin Deposition by Polyplexes. Mol. Pharm. 2015, 12, 4488–4497. [Google Scholar] [CrossRef]
- Reilly, M.J.; Larsen, J.D.; Sullivan, M.O. Histone H3 Tail Peptides and Poly(ethylenimine) Have Synergistic Effects for Gene Delivery. Mol. Pharm. 2012, 9, 1031–1040. [Google Scholar] [CrossRef]
- Kamiya, H.; Goto, H.; Kanda, G.; Yamada, Y.; Harashima, H. Transgene expression efficiency from plasmid DNA delivered as a complex with histone H3. Int. J. Pharm. 2010, 392, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Kaouass, M.; Beaulieu, R.; Balicki, D. Histonefection: Novel and potent non-viral gene delivery. J. Control. Release 2006, 113, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Uetsuki, T.; Naito, A.; Nagata, S.; Kaziro, Y. Isolation and Characterization of the Human Chromosomal Gene for Polypeptide Chain Elongation Factor-1α. J. Biol. Chem. 1989, 264, 5791–5798. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi-Ito, N.; Nagata, S. Characterization of the regulatory elements in the promoter of the human elongation factor-1 alpha gene. J. Biol. Chem. 1994, 269, 29831–29837. [Google Scholar] [CrossRef] [PubMed]
- Haase, R.; Argyros, O.; Wong, S.-P.; Harbottle, R.P.; Lipps, H.J.; Ogris, M.; Magnusson, T.; Pinto, M.G.V.; Haas, J.; Baiker, A. pEPito: A significantly improved non-viral episomal expression vector for mammalian cells. BMC Biotechnol. 2010, 10, 20. [Google Scholar] [CrossRef]
- Kreppel, F.; Hagedorn, C. Episomes and Transposases—Utilities to Maintain Transgene Expression from Nonviral Vectors. Genes 2022, 13, 1872. [Google Scholar] [CrossRef]
- Mehta, A.K.; Majumdar, S.S.; Alam, P.; Gulati, N.; Brahmachari, V. Epigenetic regulation of cytomegalovirus major immediate-early promoter activity in transgenic mice. Gene 2009, 428, 20–24. [Google Scholar] [CrossRef]
- Lowary, P.T.; Widom, J. New DNA sequence rules for high affinity binding to histone octamer and sequence-directed nucleosome positioning. J. Mol. Biol. 1998, 276, 19–42. [Google Scholar] [CrossRef]
- Jimenez-Useche, I.; Yuan, C. The Effect of DNA CpG Methylation on the Dynamic Conformation of a Nucleosome. Biophys. J. 2012, 103, 2502–2512. [Google Scholar] [CrossRef]
- Nurse, N.P.; Jimenez-Useche, I.; Smith, I.T.; Yuan, C. Clipping of Flexible Tails of Histones H3 and H4 Affects the Structure and Dynamics of the Nucleosome. Biophys. J. 2013, 104, 1081–1088. [Google Scholar] [CrossRef]
- Nurse, N.P.; Yuan, C. Cis and trans internucleosomal interactions of H3 and H4 tails in tetranucleosomes. Biopolymers 2015, 103, 33–40. [Google Scholar] [CrossRef]
- Bednar, J.; Horowitz, R.A.; Grigoryev, S.A.; Carruthers, L.M.; Hansen, J.C.; Koster, A.J.; Woodcock, C.L. Nucleosomes, linker DNA, and linker histone form a unique structural motif that directs the higher-order folding and compaction of chromatin. Proc. Nat. Acad. Sci. USA 1998, 95, 14173–14178. [Google Scholar] [CrossRef]
- Cleri, F.; Giordano, S.; Blossey, R. Nucleosome Array Deformation in Chromatin is Sustained by Bending, Twisting and Kinking of Linker DNA. J. Mol. Biol. 2023, 435, 168263. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Useche, I.; Nurse, N.P.; Tian, Y.; Kansara, B.S.; Shim, D.; Yuan, C. DNA methylation effects on tetra-nucleosome compaction and aggregation. Biophys. J. 2014, 107, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Carroll, D. Continuous-flow salt gradient dialysis for the preparation of polynucleotide-polypeptide complexes. Anal. Biochem. 1971, 44, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.; Schallhorn, A.; Wurm, F.M. Transfecting mammalian cells: Optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucleic Acids Res. 1996, 24, 596–601. [Google Scholar] [CrossRef]
- Bewick, V.; Cheek, L.; Ball, J. Statistics review 9: One-way analysis of variance. Crit. Care 2004, 8, 130. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Tukey’s honestly significant difference (HSD) test. In Encyclopedia of Research Design; Sage: Thousand Oaks, CA, USA, 2010; Volume 3, pp. 1–5. [Google Scholar]
- Valouev, A.; Johnson, S.M.; Boyd, S.D.; Smith, C.L.; Fire, A.Z.; Sidow, A. Determinants of nucleosome organization in primary human cells. Nature 2011, 474, 516–520. [Google Scholar] [CrossRef]
- Makde, R.D.; England, J.R.; Yennawar, H.P.; Tan, S. Structure of RCC1 chromatin factor bound to the nucleosome core particle. Nature 2010, 467, 562–566. [Google Scholar] [CrossRef]
- Zhao, H.; Guo, M.; Zhang, F.; Shao, X.; Liu, G.; Xing, Y.; Zhao, X.; Luo, L.; Cai, L. Nucleosome Assembly and Disassembly in vitro Are Governed by Chemical Kinetic Principles. Front. Cell. Dev. Biol. 2021, 9, 762571. [Google Scholar] [CrossRef]
- Flaus, A. Principles and practice of nucleosome positioning in vitro. Front. Life Sci. 2011, 5, 5–27. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Z.; Tian, Z.; Zhang, X.; Xu, D.; Li, Q.; Zhang, J.; Wang, T. The EF-1α promoter maintains high-level transgene expression from episomal vectors in transfected CHO-K1 cells. J. Cell. Mol. Med. 2017, 21, 3044–3054. [Google Scholar] [CrossRef]
- Rogge, R.A.; Kalashnikova, A.A.; Muthurajan, U.M.; Porter-Goff, M.E.; Luger, K.; Hansen, J.C. Assembly of nucleosomal arrays from recombinant core histones and nucleosome positioning DNA. J. Vis. Exp. 2013, e50354. [Google Scholar] [CrossRef]
- Kirchmaier, A.L.; Sugden, B. Dominant-negative inhibitors of EBNA-1 of Epstein-Barr virus. J. Virol. 1997, 71, 1766–1775. [Google Scholar] [CrossRef]
- Kirchmaier, A.L.; Sugden, B. Rep*: A viral element that can partially replace the origin of plasmid DNA synthesis of Epstein-Barr virus. J. Virol. 1998, 72, 4657–4666. [Google Scholar] [CrossRef] [PubMed]
- Corish, P.; Tyler-Smith, C. Attenuation of green fluorescent protein half-life in mammalian cells. PEDS 1999, 12, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Leight, E.R.; Sugden, B. The cis-acting family of repeats can inhibit as well as stimulate establishment of an oriP replicon. J. Virol. 2001, 75, 10709–10720. [Google Scholar] [CrossRef]
- Leyes Porello, E.A.; Trudeau, R.T.; Lim, B. Transcriptional bursting: Stochasticity in deterministic development. Development 2023, 150, dev201546. [Google Scholar] [CrossRef]
- Vaquero, A.; Scher, M.B.; Lee, D.H.; Sutton, A.; Cheng, H.L.; Alt, F.W.; Serrano, L.; Sternglanz, R.; Reinberg, D. SirT2 is a histone deacetylase with preference for histone H4 Lys 16 during mitosis. Genes Dev. 2006, 20, 1256–1261. [Google Scholar] [CrossRef]
- Chen, H.P.; Zhao, Y.T.; Zhao, T.C. Histone deacetylases and mechanisms of regulation of gene expression. Crit. Rev. Oncog. 2015, 20, 35–47. [Google Scholar] [CrossRef]
- Shabane, P.S.; Onufriev, A.V. Significant compaction of H4 histone tail upon charge neutralization by acetylation and its mimics, possible effects on chromatin structure. J. Mol. Biol. 2021, 433, 166683. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hayes, J.J. Acetylation mimics within individual core histone tail domains indicate distinct roles in regulating the stability of higher-order chromatin structure. Mol. Cell. Biol. 2008, 28, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Mishima, Y.; Miyagi, S.; Saraya, A.; Negishi, M.; Endoh, M.; Endo, T.A.; Toyoda, T.; Shinga, J.; Katsumoto, T.; Chiba, T.; et al. The Hbo1-Brd1/Brpf2 complex is responsible for global acetylation of H3K14 and required for fetal liver erythropoiesis. Blood 2011, 118, 2443–2453. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zang, C.; Rosenfeld, J.A.; Schones, D.E.; Barski, A.; Cuddapah, S.; Cui, K.; Roh, T.Y.; Peng, W.; Zhang, M.Q.; et al. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat. Genet. 2008, 40, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.D.; Nitiyanandan, R.; Meraji, S.; Daer, R.; Godeshala, S.; Goklany, S.; Haynes, K.; Rege, K. An inhibitor screen identifies histone-modifying enzymes as mediators of polymer-mediated transgene expression from plasmid DNA. J. Control Release 2018, 286, 210–223. [Google Scholar] [CrossRef]
- Elmer, J.J.; Christensen, M.D.; Barua, S.; Lehrman, J.; Haynes, K.A.; Rege, K. The histone deacetylase inhibitor Entinostat enhances polymer-mediated transgene expression in cancer cell lines. Biotechnol. Bioeng. 2016, 113, 1345–1356. [Google Scholar] [CrossRef]
- Barua, S.; Rege, K. The influence of mediators of intracellular trafficking on transgene expression efficacy of polymer-plasmid DNA complexes. Biomaterials 2010, 31, 5894–5902. [Google Scholar] [CrossRef]
- Struhl, K.; Segal, E. Determinants of nucleosome positioning. Nat. Struct. Mol. Biol. 2013, 20, 267–273. [Google Scholar] [CrossRef]
- Gu, S.G.; Fire, A. Partitioning the C. elegans genome by nucleosome modification, occupancy, and positioning. Chromosoma 2010, 119, 73–87. [Google Scholar] [CrossRef]
- Saxton, D.S.; Rine, J. Nucleosome Positioning Regulates the Establishment, Stability, and Inheritance of Heterochromatin in Saccharomyces cerevisiae. Proc. Nat. Acad. Sci. USA 2020, 117, 27493–27501. [Google Scholar] [CrossRef]
- Taylor, G.C.; Eskeland, R.; Hekimoglu-Balkan, B.; Pradeepa, M.M.; Bickmore, W.A. H4K16 acetylation marks active genes and enhancers of embryonic stem cells, but does not alter chromatin compaction. Genome Res. 2013, 23, 2053–2065. [Google Scholar] [CrossRef] [PubMed]
- Reeves, R.; Gorman, C.M.; Howard, B. Minichromosome assembly of non-integrated plasmid DNA transfected into mammalian cells. Nucleic Acids Res. 1985, 13, 3599–3615. [Google Scholar] [CrossRef]
- O’Connor, L.; Gilmour, J.; Bonifer, C. The Role of the Ubiquitously Expressed Transcription Factor Sp1 in Tissue-specific Transcriptional Regulation and in Disease. Yale J. Biol. Med. 2016, 89, 513–525. [Google Scholar] [PubMed]
- Wu, Z.; Nicoll, M.; Ingham, R.J. AP-1 family transcription factors: A diverse family of proteins that regulate varied cellular activities in classical hodgkin lymphoma and ALK+ ALCL. Exp. Hematol. Oncol. 2021, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Elgin, S.C.; Reuter, G. Position-effect variegation, heterochromatin formation, and gene silencing in Drosophila. Cold Spring Harb. Perspect. Biol. 2013, 5, a017780. [Google Scholar] [CrossRef]
- Gates, L.A.; Shi, J.; Rohira, A.D.; Feng, Q.; Zhu, B.; Bedford, M.T.; Sagum, C.A.; Jung, S.Y.; Qin, J.; Tsai, M.J.; et al. Acetylation on histone H3 lysine 9 mediates a switch from transcription initiation to elongation. J. Biol. Chem. 2017, 292, 14456–14472. [Google Scholar] [CrossRef]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.b.1–A3.b.3. [Google Scholar] [CrossRef]
- Li, C.; Goryaynov, A.; Yang, W. The selective permeability barrier in the nuclear pore complex. Nucleus 2016, 7, 430–446. [Google Scholar] [CrossRef]
- van der Aa, M.A.; Mastrobattista, E.; Oosting, R.S.; Hennink, W.E.; Koning, G.A.; Crommelin, D.J. The nuclear pore complex: The gateway to successful nonviral gene delivery. Pharm Res. 2006, 23, 447–459. [Google Scholar] [CrossRef]
- Shimozono, S.; Tsutsui, H.; Miyawaki, A. Diffusion of large molecules into assembling nuclei revealed using an optical highlighting technique. Biophys J. 2009, 97, 1288–1294. [Google Scholar] [CrossRef]
- Bernardes, N.E.; Fung, H.Y.J.; Li, Y.; Chen, Z.; Chook, Y.M. Structure of IMPORTIN-4 bound to the H3–H4–ASF1 histone–histone chaperone complex. Proc. Nat. Acad. Sci. USA 2022, 119, e2207177119. [Google Scholar] [CrossRef] [PubMed]
- Sumida, N.; Nishikawa, J.; Kishi, H.; Amano, M.; Furuya, T.; Sonobe, H.; Ohyama, T. A designed curved DNA segment that is a remarkable activator of eukaryotic transcription. FEBS J. 2006, 273, 5691–5702. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, H.; Fukunaga, S.; Ohyama, T.; Harashima, H. The location of the left-handedly curved DNA sequence affects exogenous DNA expression in vivo. Arch. Biochem. Biophys. 2007, 461, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, H.; Goto, H.; Harashima, H. Effects of non-B DNA sequences on transgene expression. J. Biosci. Bioeng. 2009, 108, 20–23. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, S.; Yang, W.; Li, S. Enhanced transgene expression using two β-globin MARs flanking expression cassettes in stably transfected CHO-K1 cells. 3 Biotech. 2019, 9, 435. [Google Scholar] [CrossRef]

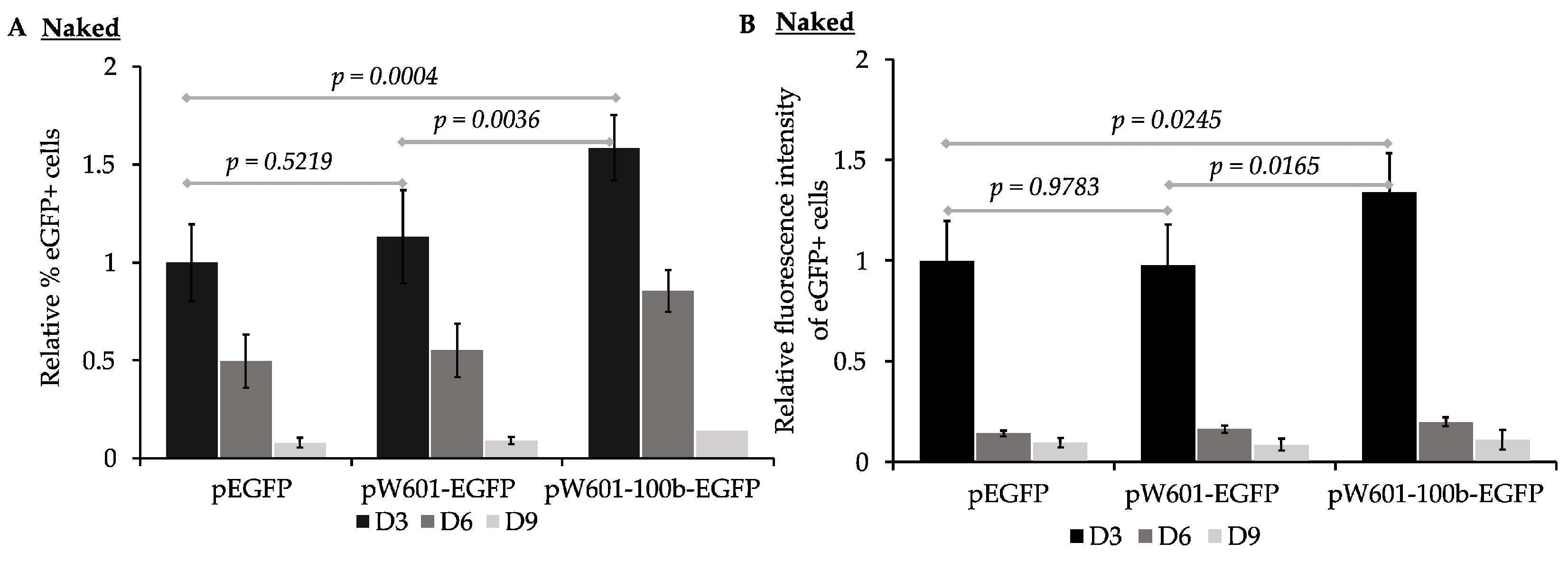
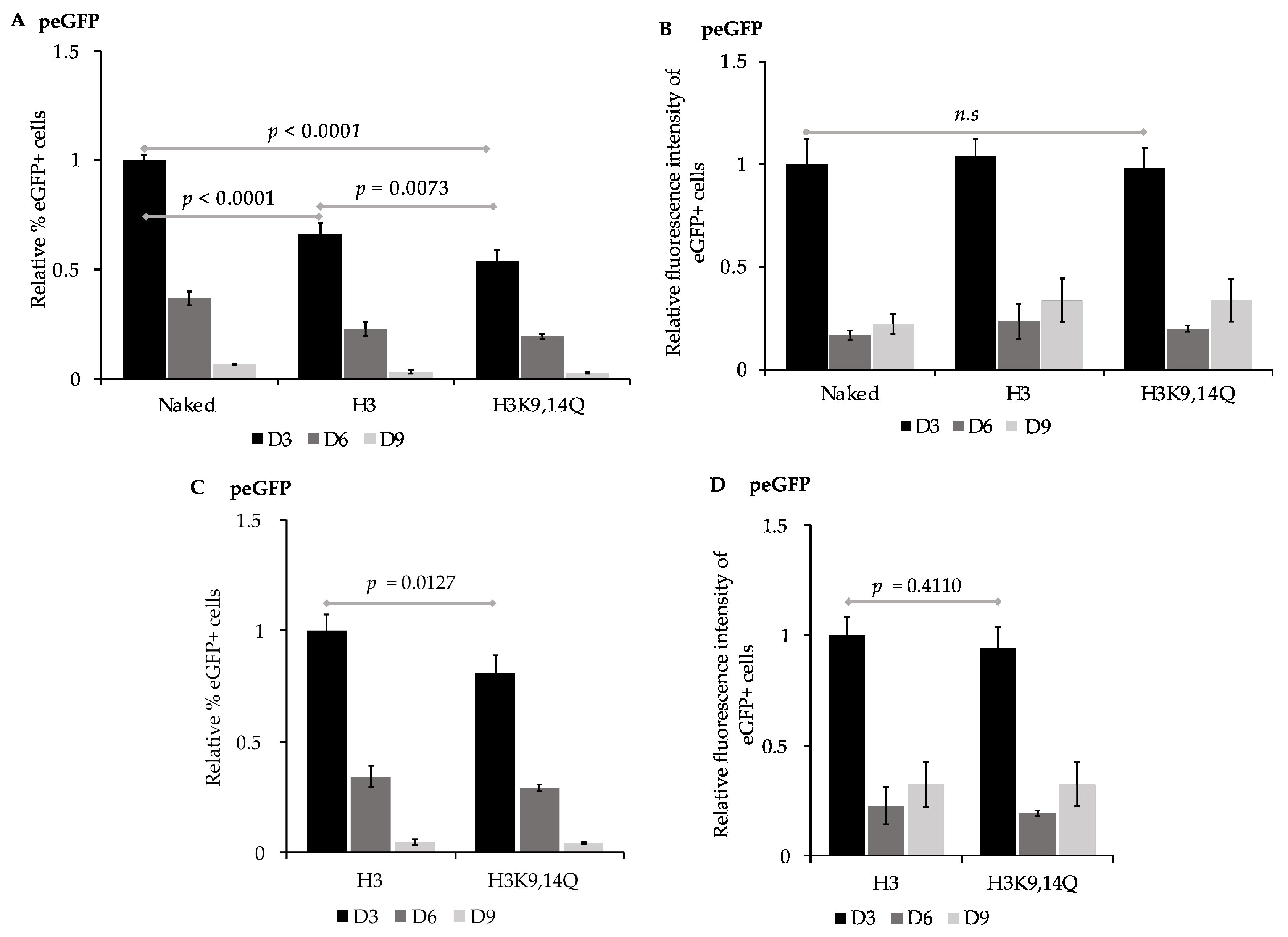
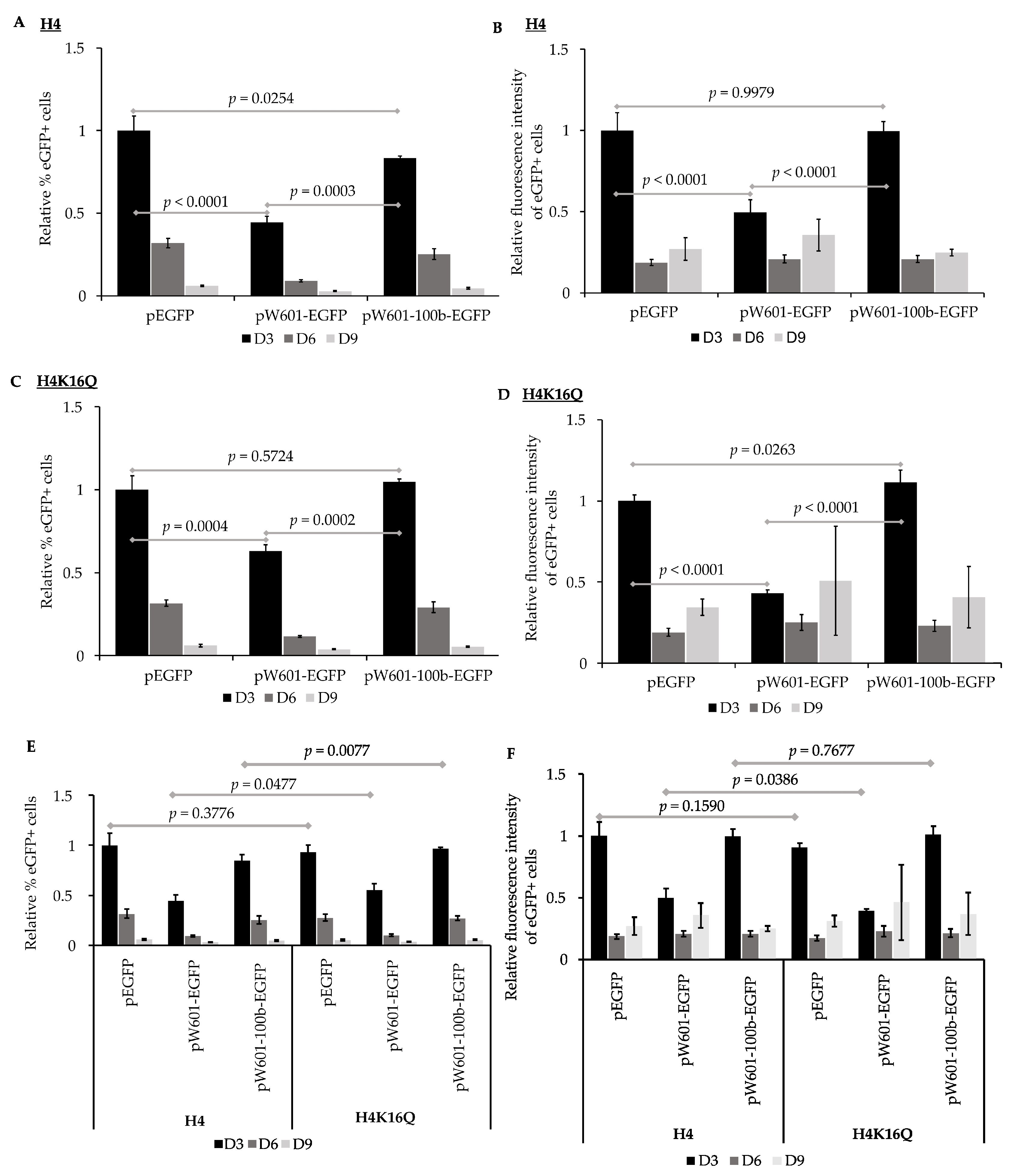
| Unassembled Plasmids | % Loss of eGFP+ Cells/Generation a | % Loss of Fluorescence Intensity/ Generation a |
|---|---|---|
| peGFP | 18.4 ± 4 b | 17 ± 4 e |
| pW601-eGFP | 20.3 ± 5 c | 15.4 ± 4 f |
| pW601-100b-eGFP | 25.7 ± 3 d | 22.2 ± 7.6 g |
| Chromatin State of peGFP | % Loss of eGFP+ Cells/ Generation a | % Loss of Fluorescence Intensity/ Generation a |
|---|---|---|
| Unassembled | 10.6 ± 0 b | 9.4 ± 1.3 e |
| H3 | 7.7 ± 1 c | 9.1 ± 1.8 f |
| H3K9,14Q | 6.0 ± 0 d | 8.3 ± 1.8 g |
| Pre-Assembled with Unmodified Histones | % Loss of eGFP+ Cells/Generation a | % Loss of eGFP Fluorescence/Generation a |
|---|---|---|
| peGFP | 11.3 ± 1 b | 9.1 ± 2 e |
| pW601-eGFP | 5.3 ± 0 c | 2.0 ± 1 f |
| pW601-100b-eGFP | 9.8 ± 0 d | 9.5 ± 0 g |
| Pre-Assembled with H4K16Q | % Loss of eGFP+ Cells/Generation a | % Loss of eGFP Fluorescence/Generation a |
|---|---|---|
| peGFP | 11.2 ±1 b | 8.4 ± 0 e |
| pW601-eGFP | 6.7 ± 0 c | 1 ± 3.8 f |
| pW601-100b-eGFP | 12.1 ± 0 d | 9.1 ± 1.8 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwizera, R.; Xie, J.; Nurse, N.; Yuan, C.; Kirchmaier, A.L. Impacts of Nucleosome Positioning Elements and Pre-Assembled Chromatin States on Expression and Retention of Transgenes. Genes 2024, 15, 1232. https://doi.org/10.3390/genes15091232
Kwizera R, Xie J, Nurse N, Yuan C, Kirchmaier AL. Impacts of Nucleosome Positioning Elements and Pre-Assembled Chromatin States on Expression and Retention of Transgenes. Genes. 2024; 15(9):1232. https://doi.org/10.3390/genes15091232
Chicago/Turabian StyleKwizera, Ronard, Junkai Xie, Nathan Nurse, Chongli Yuan, and Ann L. Kirchmaier. 2024. "Impacts of Nucleosome Positioning Elements and Pre-Assembled Chromatin States on Expression and Retention of Transgenes" Genes 15, no. 9: 1232. https://doi.org/10.3390/genes15091232







