Clinical Actionability of Genes in Gastrointestinal Tumors
Abstract
1. Introduction
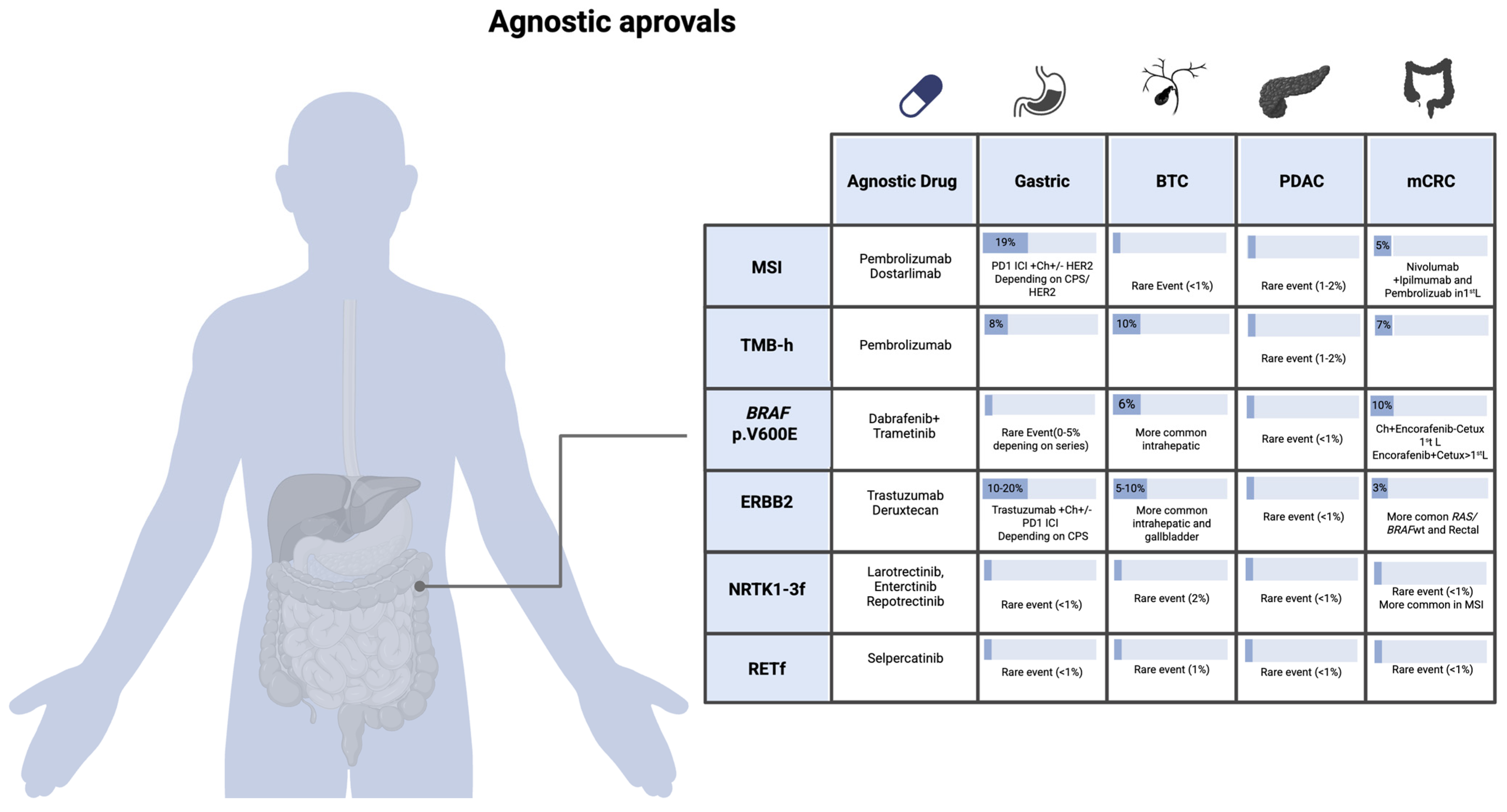
2. Tumor-Agnostic Biomarkers and Therapeutic Implications in GI Oncology
2.1. MSI-High/dMMR (Microsatellite Instability–High/Deficient Mismatch Repair)
2.1.1. Therapeutic Implications of MSI in CRC
2.1.2. Therapeutic Implications of MSI in Other GI Tumors
2.2. TMB-High (Tumor Mutational Burden ≥ 10 Mut/Mb)
2.3. BRAF p.V600E Mutation
2.4. HER2 Overexpression/ERBB2 Amplification
2.5. Fusions in NTRK1, NTRK2, and NTRK3 Genes
2.6. RET Fusions
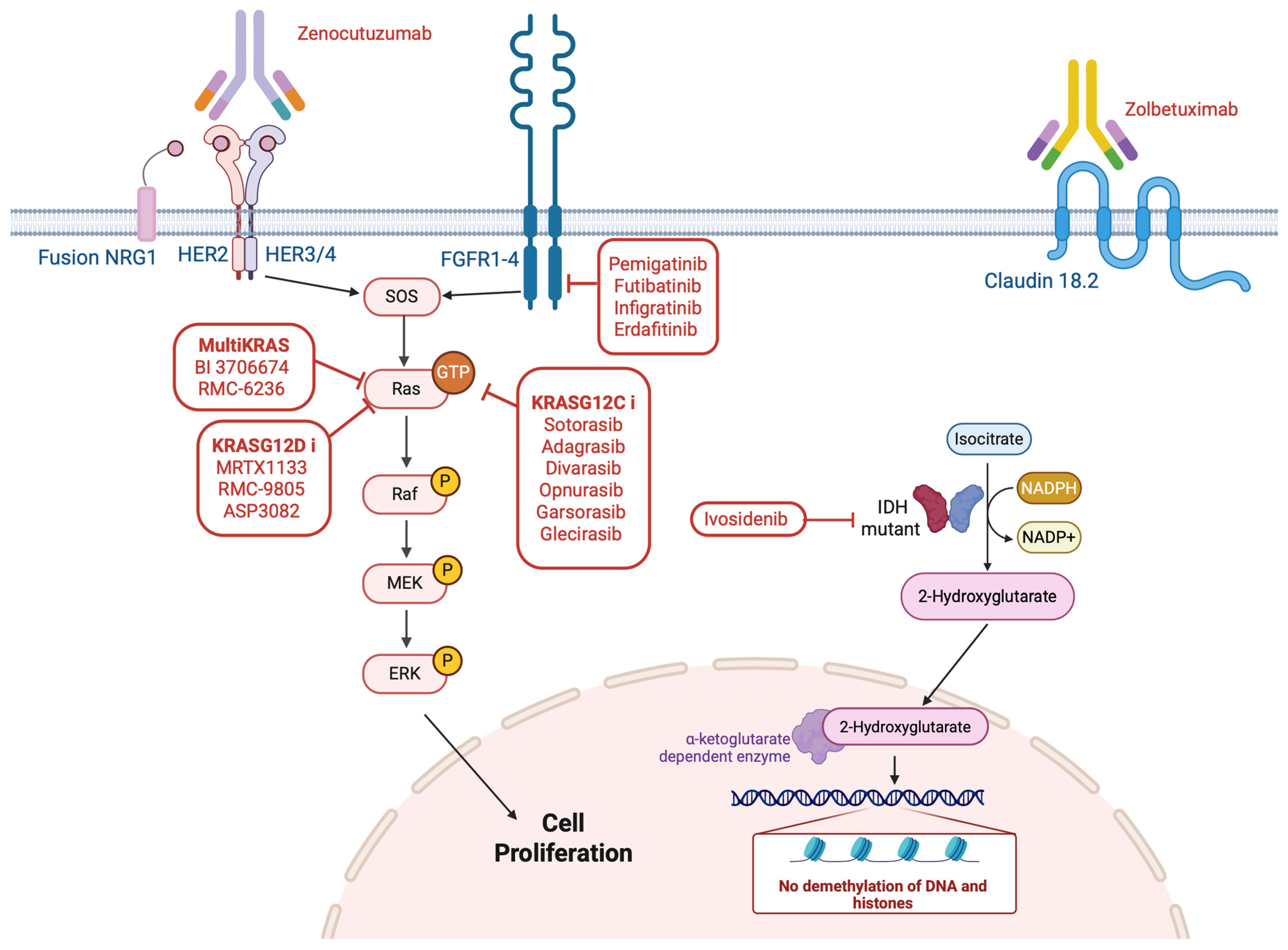
3. Non-Agnostic Biomarkers and Therapeutic Implications in GI Oncology
3.1. NRG1
3.2. Claudin 18.2
3.3. Advances in Targeting KRAS for GI Tumors
3.4. Genetic Alteration of BRCA1/2 Genes in Pancreas
3.5. Fibroblast Growth Factor Receptor (FGFR) Alterations
3.6. Other Clinically Actionable Genes
4. Clinical Tumor Molecular Boards in the Interpretation of Genomic Alterations
5. Discussion and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CIMP | CpG Island Methylator Phenotype |
| CRC | Colorectal Cancer |
| CR | Complete Response |
| CTLs | Cytotoxic T Lymphocytes |
| dMMR | Deficient Mismatch Repair |
| EMA | European Medicines Agency |
| FDA | US Food and Drug Administration |
| FISH | Fluorescence In Situ Hybridization |
| GEJ | Gastroesophageal Junction |
| GI | Gastrointestinal |
| ICI | Immune Checkpoint Inhibitor |
| IHC | Immunohistochemistry |
| MMR | DNA Mismatch Repair |
| MSI-H | Microsatellite Instability—High |
| NGS | Next-Generation Sequencing |
| NSCLC | Non-Small-Cell Lung Cancer |
| ORR | Objective Response Rate |
| OS | Overall Survival |
| PD-1 | Programmed Death-1 |
| PD-L1 | Programmed Death-Ligand 1 |
| PDAC | Pancreatic Ductal Adenocarcinoma |
| PFS | Progression-Free Survival |
| PR | Partial Response |
| TMB | Tumor Mutational Burden |
| TK | Tyrosine Kinase |
References
- Chin-Yee, B.; Plutynski, A. Concepts of Actionability in Precision Oncology. Philos. Sci. 2024, 91, 1349–1360. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zheng, R.; Li, J.; Zeng, H.; Li, L.; Chen, R.; Sun, K.; Han, B.; Bray, F.; Wei, W.; et al. Global, regional, and national lifetime risks of developing and dying from gastrointestinal cancers in 185 countries: A population-based systematic analysis of GLOBOCAN. Lancet Gastroenterol. Hepatol. 2024, 9, 229–237. [Google Scholar] [CrossRef]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef]
- Jayakrishnan, T.; Ng, K. Early-Onset Gastrointestinal Cancers: A Review. JAMA, 2025; ahead of print. [Google Scholar] [CrossRef]
- Ben-Aharon, I.; van Laarhoven, H.W.M.; Fontana, E.; Obermannova, R.; Nilsson, M.; Lordick, F. Early-Onset Cancer in the Gastrointestinal Tract Is on the Rise—Evidence and Implications. Cancer Discov. 2023, 13, 538–551. [Google Scholar] [CrossRef]
- Mauri, G.; Patelli, G.; Sartore-Bianchi, A.; Abrignani, S.; Bodega, B.; Marsoni, S.; Costanzo, V.; Bachi, A.; Siena, S.; Bardelli, A. Early-onset cancers: Biological bases and clinical implications. Cell Rep. Med. 2024, 5, 101737. [Google Scholar] [CrossRef]
- Bentley, D.R.; Balasubramanian, S.; Swerdlow, H.P.; Smith, G.P.; Milton, J.; Brown, C.G.; Hall, K.P.; Evers, D.J.; Barnes, C.L.; Bignell, H.R.; et al. Accurate whole human genome sequencing using reversible terminator chemistry. Nature 2008, 456, 53–59. [Google Scholar] [CrossRef]
- Margulies, M.; Egholm, M.; Altman, W.E.; Attiya, S.; Bader, J.S.; Bemben, L.A.; Berka, J.; Braverman, M.S.; Chen, Y.J.; Chen, Z.; et al. Genome sequencing in microfabricated high-density picolitre reactors. Nature 2005, 437, 376–380. [Google Scholar] [CrossRef]
- Venter, J.C.; Adams, M.D.; Myers, E.W.; Li, P.W.; Mural, R.J.; Sutton, G.G.; Smith, H.O.; Yandell, M.; Evans, C.A.; Holt, R.A.; et al. The sequence of the human genome. Science 2001, 291, 1304–1351. [Google Scholar] [CrossRef]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef]
- Subbiah, V.; Gouda, M.A.; Ryll, B.; Burris, H.A.; Kurzrock, R. The evolving landscape of tissue-agnostic therapies in precision oncology. CA Cancer J. Clin. 2024, 74, 433–452. [Google Scholar] [CrossRef]
- Sartore-Bianchi, A.; Agostara, A.G.; Patelli, G.; Mauri, G.; Pizzutilo, E.G.; Siena, S. Application of histology-agnostic treatments in metastatic colorectal cancer. Dig. Liver Dis. 2022, 54, 1291–1303. [Google Scholar] [CrossRef] [PubMed]
- Mosele, M.F.; Westphalen, C.B.; Stenzinger, A.; Barlesi, F.; Bayle, A.; Bièche, I.; Bonastre, J.; Castro, E.; Dienstmann, R.; Krämer, A.; et al. Recommendations for the use of next-generation sequencing (NGS) for patients with advanced cancer in 2024: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2024, 35, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.; Chakravarty, D.; Dienstmann, R.; Jezdic, S.; Gonzalez-Perez, A.; Lopez-Bigas, N.; Ng, C.K.Y.; Bedard, P.L.; Tortora, G.; Douillard, J.Y.; et al. A framework to rank genomic alterations as targets for cancer precision medicine: The ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann. Oncol. 2018, 29, 1895–1902. [Google Scholar] [CrossRef]
- Woodcock, J.; LaVange, L.M. Master Protocols to Study Multiple Therapies, Multiple Diseases, or Both. N. Engl. J. Med. 2017, 377, 62–70. [Google Scholar] [CrossRef]
- First Tissue-Agnostic Drug Approval Issued. Cancer Discov. 2017, 7, 656. [CrossRef]
- Lyer, R.R.; Pluciennik, A.; Burdett, V.; Modrich, P.L. DNA mismatch repair: Functions and mechanisms. Chem. Rev. 2006, 106, 302–323. [Google Scholar]
- Kane, M.F.; Loda, M.; Gaida, G.M.; Lipman, J.; Mishra, R.; Goldman, H.; Jessup, J.M.; Kolodner, R. Methylation of the hMLH1 Promoter Correlates with Lack of Expression of hMLH1 in Sporadic Colon Tumors and Mismatch Repair-defective Human Tumor Cell Lines. Cancer Res. 1997, 57, 808–811. [Google Scholar]
- Papadopoulos, N.; Nicolaides, N.C.; Wei, Y.F.; Ruben, S.M.; Carter, K.C.; Rosen, C.A.; Haseltine, W.A.; Fleischmann, R.D.; Fraser, C.M.; Adams, M.D.; et al. Mutation of a mutL homolog in hereditary colon cancer. Science 1994, 263, 1625–1629. [Google Scholar] [CrossRef]
- Bonneville, R.; Krook, M.A.; Chen, H.Z.; Smith, A.; Samorodnitsky, E.; Wing, M.R.; Reeser, J.W.; Roychowdhury, S. Detection of Microsatellite Instability Biomarkers via Next-Generation Sequencing. Methods Mol. Biol. 2020, 2055, 119–132. [Google Scholar]
- Garrido-Ramos, M.A. Satellite DNA: An evolving topic. Genes 2017, 8, 230. [Google Scholar] [CrossRef] [PubMed]
- Bacher, J.W.; Flanagan, L.A.; Smalley, R.L.; Nassif, N.A.; Burgart, L.J.; Halberg, R.B.; Megid, W.M.; Thibodeau, S.N. Development of a fluorescent multiplex assay for detection of MSI-high tumors. Dis. Markers 2004, 20, 237–250. [Google Scholar]
- Poulogiannis, G.; Frayling, I.M.; Arends, M.J. DNA mismatch repair deficiency in sporadic colorectal cancer and Lynch syndrome. Histopathology 2010, 56, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Bonneville, R.; Krook, M.A.; Kautto, E.A.; Miya, J.; Wing, M.R.; Chen, H.Z.; Reeser, J.W.; Yu, L.; Roychowdhury, S. Landscape of Microsatellite Instability Across 39 Cancer Types. JCO Precis. Oncol. 2017, 2017, 1–15. [Google Scholar] [CrossRef]
- Bhamidipati, D.; Subbiah, V. Impact of tissue-agnostic approvals for patients with gastrointestinal malignancies. Trends Cancer 2023, 9, 237–249. [Google Scholar] [CrossRef]
- Abu-Ghazaleh, N.; Kaushik, V.; Gorelik, A.; Jenkins, M.; Macrae, F. Worldwide prevalence of Lynch syndrome in patients with colorectal cancer: Systematic review and meta-analysis. Genet. Med. 2022, 24, 971–985. [Google Scholar] [CrossRef]
- Fang, M.; Ou, J.; Hutchinson, L.; Green, M.R. The BRAF Oncoprotein Functions through the Transcriptional Repressor MAFG to Mediate the CpG Island Methylator Phenotype. Mol. Cell 2014, 55, 904–915. [Google Scholar] [CrossRef]
- Findeisen, P.; Kloor, M.; Merx, S.; Sutter, C.; Woerner, S.M.; Dostmann, N.; Benner, A.; Dondog, B.; Pawlita, M.; Dippold, W.; et al. T25 repeat in the 3′ untranslated region of the CASP2 gene: A sensitive and specific marker for microsatellite instability in colorectal cancer. Cancer Res. 2005, 65, 8072–8078. [Google Scholar] [CrossRef]
- Llosa, N.J.; Cruise, M.; Tam, A.; Wicks, E.C.; Hechenbleikner, E.M.; Taube, J.M.; Blosser, R.L.; Fan, H.; Wang, H.; Luber, B.S.; et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015, 5, 43–51. [Google Scholar] [CrossRef]
- Hechtman, J.F.; Rana, S.; Middha, S.; Stadler, Z.K.; Latham, A.; Benayed, R.; Soslow, R.; Ladanyi, M.; Yaeger, R.; Zehir, A.; et al. Retained mismatch repair protein expression occurs in approximately 6% of microsatellite instability-high cancers and is associated with missense mutations in mismatch repair genes. Mod. Pathol. 2020, 33, 871–879. [Google Scholar] [PubMed]
- Tosi, F.; Salvatore, L.; Tamburini, E.; Artale, S.; Lonardi, S.; Marchetti, S.; Pastorino, A.; Pietrantonio, F.; Puccini, A.; Rojas-Llimpe, F.L.; et al. Curative immune checkpoint inhibitors therapy in patients with mismatch repair-deficient locally advanced rectal cancer: A real-world observational study. ESMO Open 2024, 9, 103929. [Google Scholar] [CrossRef]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 Blockade in Mismatch Repair–Deficient, Locally Advanced Rectal Cancer. N. Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef]
- Cercek, A.; Foote, M.B.; Rousseau, B.; Smith, J.J.; Shia, J.; Sinopoli, J.; Weiss, J.; Lumish, M.; Temple, L.; Patel, M.; et al. Nonoperative Management of Mismatch Repair–Deficient Tumors. N. Engl. J. Med. 2025, 392, 2297–2308. [Google Scholar] [CrossRef]
- Marcus, L.; Lemery, S.J.; Keegan, P.; Pazdur, R. FDA approval summary: Pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin. Cancer Res. 2019, 25, 3753–3758. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Chow, L.Q.M.; Haddad, R.; Gupta, S.; Mahipal, A.; Mehra, R.; Tahara, M.; Berger, R.; Eder, J.P.; Burtness, B.; Lee, S.H.; et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: Results from the phase Ib KEYNOTE-012 expansion cohort. J. Clin. Oncol. 2016, 34, 3838–3845. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results from the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2019, 38, 1. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Kim, T.W.; Van Cutsem, E.; Geva, R.; Jäger, D.; Hara, H.; Burge, M.; O’Neil, B.; Kavan, P.; Yoshino, T.; et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability–high/mismatch repair–deficient metastatic colorectal cancer: KEYNOTE-164. J. Clin. Oncol. 2020, 38, 11–19. [Google Scholar] [CrossRef]
- Frenel, J.S.; Le Tourneau, C.; O’Neil, B.; Ott, P.A.; Piha-Paul, S.A.; Gomez-Roca, C.; van Brummelen, E.M.J.; Rugo, H.S.; Thomas, S.; Saraf, S.; et al. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1-positive cervical cancer: Results from the phase IB KEYNOTE-028 trial. J. Clin. Oncol. 2017, 35, 4035–4041. [Google Scholar] [CrossRef]
- André, T.; Berton, D.; Curigliano, G.; Sabatier, R.; Tinker, A.V.; Oaknin, A.; Ellard, S.; de Braud, F.; Arkenau, H.T.; Trigo, J.; et al. Antitumor Activity and Safety of Dostarlimab Monotherapy in Patients with Mismatch Repair Deficient Solid Tumors: A Nonrandomized Controlled Trial. JAMA Netw. Open 2023, 6, e2341165. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Bertagnolli, M.M.; Redston, M.; Compton, C.C.; Niedzwiecki, D.; Mayer, R.J.; Goldberg, R.M.; Colacchio, T.A.; Saltz, L.B.; Warren, R.S. Microsatellite Instability and Loss of Heterozygosity at Chromosomal Location 18q: Prospective Evaluation of Biomarkers for Stages II and III Colon Cancer—A Study of CALGB 9581 and 89803. J. Clin. Oncol. 2011, 29, 3153. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, G.; Southward, K.; Handley, K.; Magill, L.; Beaumont, C.; Stahlschmidt, J.; Richman, S.; Chambers, P.; Seymour, M.; Kerr, D.; et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J. Clin. Oncol. 2011, 29, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Taieb, J.; Fiskum, J.; Yothers, G.; Goldberg, R.; Yoshino, T.; Alberts, S.; Allegra, C.; de Gramont, A.; Seitz, J.F.; et al. Microsatellite Instability in Patients with Stage III Colon Cancer Receiving Fluoropyrimidine with or Without Oxaliplatin: An ACCENT Pooled Analysis of 12 Adjuvant Trials. J. Clin. Oncol. 2021, 39, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Sargent, D.J.; Marsoni, S.; Monges, G.; Thibodeau, S.N.; Labianca, R.; Hamilton, S.R.; French, A.J.; Kabat, B.; Foster, N.R.; Torri, V.; et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J. Clin. Oncol. 2010, 28, 3219–3226. [Google Scholar] [CrossRef] [PubMed]
- Vilar, E.; Gruber, S.B. Microsatellite instability in colorectal cancer—The stable evidence. Nat. Rev. Clin. Oncol. 2010, 7, 153. [Google Scholar] [CrossRef]
- André, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Andre, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.J.; Smith, D.M.; Garcia-Carbonero, R.; Alcaide, J.; Gibbs, P.; et al. Final overall survival for the phase III KN177 study: Pembrolizumab versus chemotherapy in microsatellite instability-high/mismatch repair deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC). J. Clin. Oncol. 2021, 39 (Suppl. S15), 3500. [Google Scholar] [CrossRef]
- Diaz, L.A., Jr.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): Final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022, 23, 659–670. [Google Scholar] [CrossRef]
- André, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.J.A.; Smith, D.; Garcia-Carbonero, R.; Alcaide-Garcia, J.; Gibbs, P.; et al. Pembrolizumab versus chemotherapy in microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer: 5-year follow-up from the randomized phase III KEYNOTE-177 study. Ann. Oncol. 2025, 36, 277–284. [Google Scholar] [CrossRef]
- Andre, T.; Elez, E.; Van Cutsem, E.; Jensen, L.H.; Bennouna, J.; Mendez, G.; Schenker, M.; de la Fouchardiere, C.; Limon, M.L.; Yoshino, T.; et al. Nivolumab plus Ipilimumab in Microsatellite-Instability–High Metastatic Colorectal Cancer. N. Engl. J. Med. 2024, 391, 2014–2026. [Google Scholar]
- André, T.; Elez, E.; Lenz, H.J.; Jensen, L.H.; Touchefeu, Y.; Van Cutsem, E.; Garcia-Carbonero, R.; Tougeron, D.; Mendez, G.A.; Schenker, M.; et al. Nivolumab plus ipilimumab versus nivolumab in microsatellite instability-high metastatic colorectal cancer (CheckMate 8HW): A randomised, open-label, phase 3 trial. Lancet 2025, 405, 383–395. [Google Scholar] [CrossRef]
- André, T.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; et al. Nivolumab plus low-dose ipilimumab in previously treated patients with microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: 4-year follow-up from CheckMate 142. Ann. Oncol. 2022, 33, 1052–1060. [Google Scholar] [CrossRef]
- Chalabi, M.; Verschoor, Y.L.; Tan, P.B.; Balduzzi, S.; Van Lent, A.U.; Grootscholten, C.; Dokter, S.; Büller, N.V.; Grotenhuis, B.A.; Kuhlmann, K.; et al. Neoadjuvant Immunotherapy in Locally Advanced Mismatch Repair–Deficient Colon Cancer. N. Engl. J. Med. 2024, 390, 1949–1958. [Google Scholar] [CrossRef]
- de Gooyer, P.G.M.; Verschoor, Y.L.; van den Dungen, L.D.W.; Balduzzi, S.; Marsman, H.A.; Geukes Foppen, M.H.; Grootscholten, C.; Dokter, S.; den Hartog, A.G.; Verbeek, W.H.M.; et al. Neoadjuvant nivolumab and relatlimab in locally advanced MMR-deficient colon cancer: A phase 2 trial. Nat. Med. 2024, 30, 3284–3290. [Google Scholar] [CrossRef] [PubMed]
- Cercek, A. Neoadjuvant Treatment of Mismatch Repair–Deficient Colon Cancer—Clinically Meaningful? N. Engl. J. Med. 2024, 390, 2024–2025. [Google Scholar] [CrossRef] [PubMed]
- Sinicrope, F.A.; Ou, F.S.; Zemla, T.; Nixon, A.B.; Mody, K.; Levasseur, A.; Dueck, A.C.; Dhanarajan, A.R.; Lieu, C.H.; Cohen, D.J.; et al. Randomized trial of standard chemotherapy alone or combined with atezolizumab as adjuvant therapy for patients with stage III deficient DNA mismatch repair (dMMR) colon cancer (Alliance A021502; ATOMIC). J. Clin. Oncol. 2025, 43 (Suppl. S17), LBA1. [Google Scholar] [CrossRef]
- Ikoma, N.; Cloyd, J.; Badgwell, B.D.; Agnes, A.; Rodriguez-Bigas, M.; Ajani, J.A.; You, Y.N. Clinical features and survival of gastric cancer patients with DNA mismatch repair deficiency. J. Surg. Oncol. 2018, 117, 707–709. [Google Scholar]
- Bass, A.J.; Thorsson, V.; Shmulevich, I.; Reynolds, S.M.; Miller, M.; Bernard, B.; Hinoue, T.; Laird, P.W.; Curtis, C.; Shen, H.; et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef]
- Russi, S.; Marano, L.; Laurino, S.; Calice, G.; Scala, D.; Marino, G.; Sgambato, A.; Mazzone, P.; Carbone, L.; Napolitano, G.; et al. Gene Regulatory Network Characterization of Gastric Cancer’s Histological Subtypes: Distinctive Biological and Clinically Relevant Master Regulators. Cancers 2022, 14, 4961. [Google Scholar] [CrossRef]
- Marano, L.; Sorrenti, S.; Malerba, S.; Skokowski, J.; Polom, K.; Girnyi, S.; Cwalinski, T.; Prete, F.P.; González-Ojeda, A.; Fuentes-Orozco, C.; et al. Different Master Regulators Define Proximal and Distal Gastric Cancer: Insights into Prognosis and Opportunities for Targeted Therapy. Curr. Oncol. 2025, 32, 424. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Özgüroğlu, M.; Bang, Y.J.; Di Bartolomeo, M.; Mandalà, M.; Ryu, M.H.; Fornaro, L.; Olesiński, T.; Caglevic, C.; Chung, H.C.; et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): A randomised, open-label, controlled, phase 3 trial. Lancet 2018, 392, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Janjigian, Y.Y.; Ajani, J.; Moehler, M.; Yao, J.; Wang, X.; Chhibber, A.; Pandya, D.; Shen, L.; Garrido, M.; et al. Nivolumab plus chemotherapy or ipilimumab in gastroesophageal cancer: Exploratory biomarker analyses of a randomized phase 3 trial. Nat. Med. 2025, 31, 1519–1530. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.; Fuchs, C.S.; Shitara, K.; Tabernero, J.; Muro, K.; Van Cutsem, E.; Bang, Y.J.; De Vita, F.; Landers, G.; Yen, C.J.; et al. Assessment of Pembrolizumab Therapy for the Treatment of Microsatellite Instability-High Gastric or Gastroesophageal Junction Cancer among Patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 Clinical Trials. JAMA Oncol. 2021, 7, 895–902. [Google Scholar] [CrossRef]
- Smyth, E.C.; Wotherspoon, A.; Peckitt, C.; Gonzalez, D.; Hulkki-Wilson, S.; Eltahir, Z.; Fassan, M.; Rugge, M.; Valeri, N.; Okines, A.; et al. Mismatch repair deficiency, microsatellite instability, and survival: An exploratory analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial. JAMA Oncol. 2017, 3, 1197–1203. [Google Scholar] [CrossRef]
- André, T.; Tougeron, D.; Piessen, G.; de la Fouchardière, C.; Louvet, C.; Adenis, A.; Jary, M.; Tournigand, C.; Aparicio, T.; Desrame, J.; et al. Neoadjuvant Nivolumab Plus Ipilimumab and Adjuvant Nivolumab in Localized Deficient Mismatch Repair/Microsatellite Instability-High Gastric or Esophagogastric Junction Adenocarcinoma: The GERCOR NEONIPIGA Phase II Study. J. Clin. Oncol. 2023, 41, 255–265. [Google Scholar] [CrossRef]
- Raimondi, A.; Lonardi, S.; Murgioni, S.; Cardellino, G.G.; Tamberi, S.; Strippoli, A.; Palermo, F.; De Manzoni, G.; Bencivenga, M.; Bittoni, A.; et al. Tremelimumab and durvalumab as neoadjuvant or non-operative management strategy of patients with microsatellite instability-high resectable gastric or gastroesophageal junction adenocarcinoma: The INFINITY study by GONO. Ann. Oncol. 2025, 36, 285–296. [Google Scholar] [CrossRef]
- Philip, P.A.; Azar, I.; Xiu, J.; Hall, M.J.; Hendifar, A.E.; Lou, E.; Hwang, J.J.; Gong, J.; Feldman, R.; Ellis, M.; et al. Molecular Characterization of KRAS Wild-type Tumors in Patients with Pancreatic Adenocarcinoma. Clin. Cancer Res. 2022, 28, 2704–2714. [Google Scholar] [CrossRef]
- Wang, Y.; Cuggia, A.; Pacis, A.; Boileau, J.C.; Marcus, V.A.; Gao, Z.H.; Chong, G.; Foulkes, W.D.; Zogopoulos, G. Pancreatic Cancer Progression in a Patient with Lynch Syndrome Receiving Immunotherapy: A Cautionary Tale. J. Natl. Compr. Cancer Netw. 2021, 19, 883–887. [Google Scholar] [CrossRef]
- Taïeb, J.; Sayah, L.; Heinrich, K.; Kunzmann, V.; Boileve, A.; Cirkel, G.; Lonardi, S.; Chibaudel, B.; Turpin, A.; Beller, T.; et al. Efficacy of immune checkpoint inhibitors in microsatellite unstable/mismatch repair-deficient advanced pancreatic adenocarcinoma: An AGEO European Cohort. Eur. J. Cancer 2023, 188, 90–97. [Google Scholar] [CrossRef]
- Vogel, A.; Chan, S.L.; Dawson, L.A.; Kelley, R.K.; Llovet, J.M.; Meyer, T.; Ricke, J.; Rimassa, L.; Sapisochin, G.; Vilgrain, V.; et al. Hepatocellular carcinoma: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2025, 36, 491–506. [Google Scholar] [CrossRef]
- Klempner, S.J.; Fabrizio, D.; Bane, S.; Reinhart, M.; Peoples, T.; Ali, S.M.; Sokol, E.S.; Frampton, G.; Schrock, A.B.; Anhorn, R.; et al. Tumor Mutational Burden as a Predictive Biomarker for Response to Immune Checkpoint Inhibitors: A Review of Current Evidence. Oncologist 2020, 25, e147–e159. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.M.; Kato, S.; Bazhenova, L.; Patel, S.P.; Frampton, G.M.; Miller, V.; Stephens, P.J.; Daniels, G.A.; Kurzrock, R. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol. Cancer Ther. 2017, 16, 2598–2608. [Google Scholar] [CrossRef] [PubMed]
- Vega, D.M.; Yee, L.M.; McShane, L.M.; Williams, P.M.; Chen, L.; Vilimas, T.; Fabrizio, D.; Funari, V.; Newberg, J.; Bruce, L.K.; et al. Aligning tumor mutational burden (TMB) quantification across diagnostic platforms: Phase II of the Friends of Cancer Research TMB Harmonization Project. Ann. Oncol. 2021, 32, 1626–1636. [Google Scholar] [CrossRef]
- Merino, D.M.; McShane, L.M.; Fabrizio, D.; Funari, V.; Chen, S.J.; White, J.R.; Wenz, P.; Baden, J.; Barrett, J.C.; Chaudhary, R.; et al. Establishing guidelines to harmonize tumor mutational burden (TMB): In silico assessment of variation in TMB quantification across diagnostic platforms: Phase I of the Friends of Cancer Research TMB Harmonization Project. J. Immunother. Cancer 2020, 8, e000147. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, G. Mutational Signatures in Colorectal Cancer: Translational Insights, Clinical Applications, and Limitations. Cancers 2024, 16, 2956. [Google Scholar] [CrossRef]
- Salem, M.E.; Puccini, A.; Grothey, A.; Raghavan, D.; Goldberg, R.M.; Xiu, J.; Korn, W.M.; Weinberg, B.A.; Hwang, J.J.; Shields, A.F.; et al. Landscape of tumor mutation load, mismatch repair deficiency, and PD-L1 expression in a large patient cohort of gastrointestinal cancers. Mol. Cancer Res. 2018, 16, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, Z.R.; Connelly, C.F.; Fabrizio, D.; Gay, L.; Ali, S.M.; Ennis, R.; Schrock, A.; Campbell, B.; Shlien, A.; Chmielecki, J.; et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017, 9, 34. [Google Scholar] [CrossRef]
- Sha, D.; Jin, Z.; Budczies, J.; Kluck, K.; Stenzinger, A.; Sinicrope, F.A. Tumor mutational burden as a predictive biomarker in solid tumors. Cancer Discov. 2020, 10, 1808–1825. [Google Scholar] [CrossRef]
- Keshinro, A.; Vanderbilt, C.; Kim, J.K.; Firat, C.; Chen, C.T.; Yaeger, R.; Ganesh, K.; Segal, N.H.; Gonen, M.; Shia, J.; et al. Tumor-Infiltrating Lymphocytes, Tumor Mutational Burden, and Genetic Alterations in Microsatellite Unstable, Microsatellite Stable, or Mutant POLE/POLD1 Colon Cancer. JCO Precis. Oncol. 2021, 5, 817–826. [Google Scholar] [CrossRef]
- Battuello, P.; Corti, G.; Bartolini, A.; Lorenzato, A.; Sogari, A.; Russo, M.; Di Nicolantonio, F.; Bardelli, A.; Crisafulli, G. Mutational signatures of colorectal cancers according to distinct computational workflows. Brief. Bioinform. 2024, 25, 249. [Google Scholar] [CrossRef]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H., Jr.; et al. Real-world application of tumor mutational burden-high (TMB-high) and microsatellite instability (MSI) confirms their utility as immunotherapy biomarkers. ESMO Open 2022, 7, 100336. [Google Scholar]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H., Jr.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- Rousseau, B.; Foote, M.B.; Maron, S.B.; Diplas, B.H.; Lu, S.; Argilés, G.; Cercek, A.; Diaz, L.A., Jr. The Spectrum of Benefit from Checkpoint Blockade in Hypermutated Tumors. N. Engl. J. Med. 2021, 384, 1168–1170. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Jeon, Y.; Jeong, S.Y.; Lim, S.H.; Kang, W.K.; Lee, J.; Kim, S.T. The Optimal Tumor Mutational Burden Cutoff Value as a Novel Marker for Predicting the Efficacy of Programmed Cell Death-1 Checkpoint Inhibitors in Advanced Gastric Cancer. J. Gastric Cancer 2023, 23, 476. [Google Scholar] [CrossRef] [PubMed]
- Friedman, C.F.; Hainsworth, J.D.; Kurzrock, R.; Spigel, D.R.; Burris, H.A.; Sweeney, C.J.; Meric-Bernstam, F.; Wang, Y.; Levy, J.; Grindheim, J.; et al. Atezolizumab Treatment of Tumors with High Tumor Mutational Burden from MyPathway, a Multicenter, Open-Label, Phase IIa Multiple Basket Study. Cancer Discov. 2022, 12, 654–669. [Google Scholar] [CrossRef]
- Crisafulli, G.; Sartore-Bianchi, A.; Lazzari, L.; Pietrantonio, F.; Amatu, A.; Macagno, M.; Racca, F.; Ghezzi, S.; Belli, V.; Truini, M.; et al. Temozolomide Treatment Alters Mismatch Repair and Boosts Mutational Burden in Tumor and Blood of Colorectal Cancer Patients. Cancer Discov. 2022, 12, 1656–1675. [Google Scholar] [CrossRef]
- Crisafulli, G. Liquid Biopsy and Challenge of Assay Heterogeneity for Minimal Residual Disease Assessment in Colon Cancer Treatment. Genes 2025, 16, 71. [Google Scholar] [CrossRef]
- Bando, H.; Nakamura, Y.; Taniguchi, H.; Shiozawa, M.; Yasui, H.; Esaki, T.; Kagawa, Y.; Denda, T.; Satoh, T.; Yamazaki, K.; et al. Effects of Metastatic Sites on Circulating Tumor DNA in Patients with Metastatic Colorectal Cancer. JCO Precis. Oncol. 2022, 6, e2100535. [Google Scholar] [CrossRef]
- Hanrahan, A.J.; Chen, Z.; Rosen, N.; Solit, D.B. BRAF—A tumour-agnostic drug target with lineage-specific dependencies. Nat. Rev. Clin. Oncol. 2024, 21, 224–247. [Google Scholar] [CrossRef]
- Hyman, D.M.; Puzanov, I.; Subbiah, V.; Faris, J.E.; Chau, I.; Blay, J.Y.; Wolf, J.; Raje, N.S.; Diamond, E.L.; Hollebecque, A.; et al. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N. Engl. J. Med. 2015, 373, 726–736. [Google Scholar] [CrossRef]
- Prahallad, A.; Sun, C.; Huang, S.; Di Nicolantonio, F.; Salazar, R.; Zecchin, D.; Beijersbergen, R.L.; Bardelli, A.; Bernards, R. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature 2012, 483, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, R.B.; Ebi, H.; Turke, A.B.; Coffee, E.M.; Nishino, M.; Cogdill, A.P.; Brown, R.D.; Della Pelle, P.; Dias-Santagata, D.; Hung, K.E.; et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2012, 2, 227–235. [Google Scholar] [CrossRef]
- Gouda, M.A.; Subbiah, V. Expanding the Benefit: Dabrafenib/Trametinib as Tissue-Agnostic Therapy for BRAF V600E–Positive Adult and Pediatric Solid Tumors. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e404770. [Google Scholar] [CrossRef]
- Salama, A.K.S.; Li, S.; Macrae, E.R.; Park, J.-I.; Mitchell, E.P.; Zwiebel, J.A.; Chen, H.X.; Gray, R.J.; McShane, L.M.; Rubinstein, L.V.; et al. Dabrafenib and trametinib in patients with tumors with BRAFV600E mutations: Results of the NCI-MATCH trial subprotocol, H. J. Clin. Oncol. 2020, 38, 3895–3904. [Google Scholar] [CrossRef]
- Tabernero, J.; Ros, J.; Élez, E. The Evolving Treatment Landscape in BRAF-V600E-Mutated Metastatic Colorectal Cancer. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 254–263. [Google Scholar] [CrossRef]
- Tabernero, J.; Grothey, A.; Van Cutsem, E.; Yaeger, R.; Wasan, H.; Yoshino, T.; Desai, J.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib Plus Cetuximab as a New Standard of Care for Previously Treated BRAF V600E-Mutant Metastatic Colorectal Cancer: Updated Survival Results and Subgroup Analyses from the BEACON Study. J. Clin. Oncol. 2021, 39, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Kopetz, S.; Grothey, A.; Yaeger, R.; Van Cutsem, E.; Desai, J.; Yoshino, T.; Wasan, H.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E–Mutated Colorectal Cancer. N. Engl. J. Med. 2019, 381, 1632–1643. [Google Scholar] [CrossRef] [PubMed]
- Elez, E.; Yoshino, T.; Shen, L.; Lonardi, S.; Van Cutsem, E.; Eng, C.; Kim, T.W.; Wasan, H.S.; Desai, J.; Ciardiello, F.; et al. Encorafenib, Cetuximab, and mFOLFOX6 in BRAF -Mutated Colorectal Cancer. N. Engl. J. Med. 2025, 392, 2425–2437. [Google Scholar] [CrossRef]
- Subbiah, V.; Kreitman, R.J.; Wainberg, Z.A.; Gazzah, A.; Lassen, U.; Stein, A.; Wen, P.Y.; Dietrich, S.; de Jonge, M.J.A.; Blay, J.Y.; et al. Dabrafenib plus trametinib in BRAFV600E-mutated rare cancers: The phase 2 ROAR trial. Nat. Med. 2023, 29, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- FDA Grants Accelerated Approval to Fam-Trastuzumab Deruxtecan-Nxki for Unresectable or Metastatic HER2-Positive Solid Tumors|FDA. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-fam-trastuzumab-deruxtecan-nxki-unresectable-or-metastatic-her2 (accessed on 22 July 2025).
- Raghav, K.P.S.; Siena, S.; Takashima, A.; Kato, T.; Van den Eynde, M.; Pietrantonio, F.; Komatsu, Y.; Kawakami, H.; Peeters, M.; Andre, T.; et al. Trastuzumab deruxtecan in patients with HER2-positive advanced colorectal cancer (DESTINY-CRC02): Primary results from a multicentre, randomised, phase 2 trial. Lancet Oncol. 2024, 25, 1147–1162. [Google Scholar] [CrossRef]
- Smit, E.F.; Felip, E.; Uprety, D.; Nagasaka, M.; Nakagawa, K.; Paz-Ares Rodríguez, L.; Pacheco, J.M.; Li, B.T.; Planchard, D.; Baik, C.; et al. Trastuzumab deruxtecan in patients with metastatic non-small-cell lung cancer (DESTINY-Lung01): Primary results of the HER2-overexpressing cohorts from a single-arm, phase 2 trial. Lancet Oncol. 2024, 25, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Makker, V.; Oaknin, A.; Oh, D.-Y.; Banerjee, S.; González-Martín, A.; Jung, K.H.; Ługowska, I.; Manso, L.; Manzano, A.; et al. Efficacy and Safety of Trastuzumab Deruxtecan in Patients With HER2-Expressing Solid Tumors: Primary Results From the DESTINY-PanTumor02 Phase II Trial. J. Clin. Oncol. 2024, 42, 47–58. [Google Scholar] [CrossRef]
- Lucas, F.A.M.; Cristovam, S.N. HER2 testing in gastric cancer: An update. World J. Gastroenterol. 2016, 22, 4619. [Google Scholar] [CrossRef]
- Gravalos, C.; Jimeno, A. HER2 in gastric cancer: A new prognostic factor and a novel therapeutic target. Ann. Oncol. 2008, 19, 1523–1529. [Google Scholar] [CrossRef]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Van Cutsem, E.; di Bartolomeo, M.; Smyth, E.; Chau, I.; Park, H.; Siena, S.; Lonardi, S.; Wainberg, Z.A.; Ajani, J.; Chao, J.; et al. Trastuzumab deruxtecan in patients in the USA and Europe with HER2-positive advanced gastric or gastroesophageal junction cancer with disease progression on or after a trastuzumab-containing regimen (DESTINY-Gastric02): Primary and updated analyses from a single-arm, phase 2 study. Lancet Oncol. 2023, 24, 744–756. [Google Scholar] [PubMed]
- Shitara, K.; Bang, Y.-J.; Iwasa, S.; Sugimoto, N.; Ryu, M.-H.; Sakai, D.; Nishina, T.; Yamaguchi, K.; Chao, Y.; Muro, K.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N. Engl. J. Med. 2020, 382, 2419–2430. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Rha, S.Y.; Hamilton, E.; Kang, Y.K.; Hanna, D.L.; Iqbal, S.; Lee, K.W.; Lee, J.; Beeram, M.; Oh, D.Y.; et al. Zanidatamab monotherapy or combined with chemotherapy in HER2-expressing gastroesophageal adenocarcinoma: A phase 1 trial. Nat. Commun. 2025, 16, 4293. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Kawazoe, A.; Bai, Y.; Xu, J.; Lonardi, S.; Metges, J.-P.; Ilson, D.H.; Kato, K.; Cleary, J.M.; Boku, N.; et al. Pembrolizumab in HER2-Positive Gastric Cancer. N. Engl. J. Med. 2024, 391, 1360–1362. [Google Scholar] [CrossRef]
- Ciardiello, F.; Normanno, N. HER2 Signaling and Resistance to the Anti-EGFR Monoclonal Antibody Cetuximab: A Further Step toward Personalized Medicine for Patients with Colorectal Cancer. Cancer Discov. 2011, 1, 472–474. [Google Scholar] [CrossRef] [PubMed]
- Siena, S.; Sartore-Bianchi, A.; Marsoni, S.; Hurwitz, H.I.; McCall, S.J.; Penault-Llorca, F.; Srock, S.; Bardelli, A.; Trusolino, L. Targeting the human epidermal growth factor receptor 2 (HER2) oncogene in colorectal cancer. Ann. Oncol. 2018, 29, 1108. [Google Scholar] [CrossRef]
- Sartore-Bianchi, A.; Trusolino, L.; Martino, C.; Bencardino, K.; Lonardi, S.; Bergamo, F.; Zagonel, V.; Leone, F.; Depetris, I.; Martinelli, E.; et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): A proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 738–746. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Hurwitz, H.; Raghav, K.P.S.; McWilliams, R.R.; Fakih, M.; VanderWalde, A.; Swanton, C.; Kurzrock, R.; Burris, H.; Sweeney, C.J.; et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): An updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019, 20, 518–530. [Google Scholar] [CrossRef]
- Nakamura, Y.; Okamoto, W.; Kato, T.; Esaki, T.; Kato, K.; Komatsu, Y.; Yuki, S.; Masuishi, T.; Nishina, T.; Ebi, H.; et al. Circulating tumor DNA-guided treatment with pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer: A phase 2 trial. Nat. Med. 2021, 27, 1899–1903. [Google Scholar] [CrossRef]
- Sartore-Bianchi, A.; Lonardi, S.; Martino, C.; Fenocchio, E.; Tosi, F.; Ghezzi, S.; Leone, F.; Bergamo, F.; Zagonel, V.; Ciardiello, F.; et al. Pertuzumab and trastuzumab emtansine in patients with HER2-amplified metastatic colorectal cancer: The phase II HERACLES-B trial. ESMO Open 2020, 5, e000911. [Google Scholar] [CrossRef] [PubMed]
- Bekaii-Saab, T.S.; Van Cutsem, E.; Tabernero, J.; Siena, S.; Yoshino, T.; Nakamura, Y.; Raghav, K.P.S.; Cercek, A.; Heinemann, V.; Adelberg, D.E.; et al. MOUNTAINEER-03: Phase 3 study of tucatinib, trastuzumab, and mFOLFOX6 as first-line treatment in HER2+ metastatic colorectal cancer—Trial in progress. J. Clin. Oncol. 2023, 41 (Suppl. S4), TPS261. [Google Scholar] [CrossRef]
- Demetri, G.D.; De Braud, F.; Drilon, A.; Siena, S.; Patel, M.R.; Cho, B.C.; Liu, S.V.; Ahn, M.J.; Chiu, C.H.; Lin, J.J.; et al. Updated Integrated Analysis of the Efficacy and Safety of Entrectinib in Patients with NTRK Fusion-Positive Solid Tumors. Clin. Cancer Res. 2022, 28, 1302–1312. [Google Scholar] [CrossRef]
- Ross, J.S.; Wang, K.; Gay, L.; Al-Rohil, R.; Rand, J.V.; Jones, D.M.; Lee, H.J.; Sheehan, C.E.; Otto, G.A.; Palmer, G.; et al. New Routes to Targeted Therapy of Intrahepatic Cholangiocarcinomas Revealed by Next-Generation Sequencing. Oncologist 2014, 19, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.J.; Zhang, A.; Bavi, P.; Kim, J.C.; Jang, G.H.; Kelly, D.; Perera, S.; Denroche, R.E.; Notta, F.; Wilson, J.M.; et al. Molecular characterisation of pancreatic ductal adenocarcinoma with NTRK fusions and review of the literature. J. Clin. Pathol. 2023, 76, 158–165. [Google Scholar] [CrossRef]
- Hong, D.S.; Shen, L.; van Tilburg, C.M.; Tan, D.S.W.; Kummar, S.; Lin, J.J.; Doz, F.P.; McDermott, R.S.; Albert, C.M.; Berlin, J.; et al. Long-term efficacy and safety of larotrectinib in an integrated dataset of patients with TRK fusion cancer. J. Clin. Oncol. 2021, 39 (Suppl. S15), 3108. [Google Scholar] [CrossRef]
- Varghese, A.M.; Singh, I.; Singh, R.; Kunte, S.; Chou, J.F.; Capanu, M.; Wong, W.; Lowery, M.A.; Stadler, Z.K.; Salo-Mullen, E.; et al. Early-Onset Pancreas Cancer: Clinical Descriptors, Genomics, and Outcomes. J. Natl. Cancer Inst. 2021, 113, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Hechtman, J.F.; Benayed, R.; Hyman, D.M.; Drilon, A.; Zehir, A.; Frosina, D.; Arcila, M.E.; Dogan, S.; Klimstra, D.S.; Ladanyi, M.; et al. Pan-Trk Immunohistochemistry Is an Efficient and Reliable Screen for the Detection of NTRK Fusions. Am. J. Surg. Pathol. 2017, 41, 1547–1551. [Google Scholar] [CrossRef] [PubMed]
- Corti, G.; Bartolini, A.; Crisafulli, G.; Novara, L.; Rospo, G.; Montone, M.; Negrino, C.; Mussolin, B.; Buscarino, M.; Isella, C.; et al. A Genomic Analysis Workflow for Colorectal Cancer Precision Oncology. Clin Color. Cancer 2019, 18, 91–101.e3. [Google Scholar] [CrossRef]
- Russo, M.; Siravegna, G.; Blaszkowsky, L.S.; Corti, G.; Crisafulli, G.; Ahronian, L.G.; Mussolin, B.; Kwak, E.L.; Buscarino, M.; Lazzari, L.; et al. Tumor heterogeneity and Lesion-Specific response to targeted therapy in colorectal cancer. Cancer Discov. 2016, 6, 147–153. [Google Scholar] [CrossRef]
- Li, A.Y.; McCusker, M.G.; Russo, A.; Scilla, K.A.; Gittens, A.; Arensmeyer, K.; Mehra, R.; Adamo, V.; Rolfo, C. RET fusions in solid tumors. Cancer Treat. Rev. 2019, 81, 101911. [Google Scholar] [CrossRef]
- Subbiah, V.; Wolf, J.; Konda, B.; Kang, H.; Spira, A.; Weiss, J.; Takeda, M.; Ohe, Y.; Khan, S.; Ohashi, K.; et al. Tumour-agnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001): A phase 1/2, open-label, basket trial. Lancet Oncol. 2022, 23, 1261–1273. [Google Scholar] [CrossRef]
- Subbiah, V.; Cassier, P.A.; Siena, S.; Garralda, E.; Paz-Ares, L.; Garrido, P.; Nadal, E.; Vuky, J.; Lopes, G.; Kalemkerian, G.P.; et al. Pan-cancer efficacy of pralsetinib in patients with RET fusion–positive solid tumors from the phase 1/2 ARROW trial. Nat. Med. 2022, 28, 1640–1645. [Google Scholar] [CrossRef]
- FDA Approves Selpercatinib for Locally Advanced or Metastatic RET Fusion-Positive Solid Tumors|FDA. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-selpercatinib-locally-advanced-or-metastatic-ret-fusion-positive-solid-tumors (accessed on 22 July 2025).
- Nagasaka, M.; Ou, S.H.I. NRG1 and NRG2 fusion positive solid tumor malignancies: A paradigm of ligand-fusion oncogenesis. Trends Cancer 2022, 8, 242–258. [Google Scholar] [CrossRef]
- Jonna, S.; Feldman, R.A.; Swensen, J.; Gatalica, Z.; Korn, W.M.; Borghaei, H.; Ma, P.C.; Nieva, J.J.; Spira, A.I.; Vanderwalde, A.M.; et al. Detection of NRG1 gene fusions in solid tumors. Clin. Cancer Res. 2019, 25, 4966–4972. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.R.; Williamson, L.M.; Topham, J.T.; Lee, M.K.C.; Goytain, A.; Ho, J.; Denroche, R.E.; Jang, G.; Pleasance, E.; Shen, Y.; et al. NRG1 gene fusions are recurrent, clinically actionable gene rearrangements in KRAS wild-type pancreatic ductal adenocarcinoma. Clin. Cancer Res. 2019, 25, 4674–4681. [Google Scholar] [CrossRef]
- Heining, C.; Horak, P.; Uhrig, S.; Codo, P.L.; Klink, B.; Hutter, B.; Fröhlich, M.; Bonekamp, D.; Richter, D.; Steiger, K.; et al. NRG1 fusions in KRAS wild-type pancreatic cancer. Cancer Discov. 2018, 8, 1087–1095. [Google Scholar] [CrossRef]
- Laskin, J.; Liu, S.V.; Tolba, K.; Heining, C.; Schlenk, R.F.; Cheema, P.; Cadranel, J.; Jones, M.R.; Drilon, A.; Cseh, A.; et al. NRG1 fusion-driven tumors: Biology, detection, and the therapeutic role of afatinib and other ErbB-targeting agents. Ann. Oncol. 2020, 31, 1693–1703. [Google Scholar] [CrossRef]
- Schram, A.M.; Goto, K.; Kim, D.W.; Macarulla, T.; Hollebecque, A.; O’Reilly, E.M.; Ou, S.I.; Rodon, J.; Rha, S.Y.; Nishino, K.; et al. Efficacy of Zenocutuzumab in NRG1 Fusion–Positive Cancer. N. Engl. J. Med. 2025, 392, 566–576. [Google Scholar] [CrossRef]
- Coati, I.; Lotz, G.; Fanelli, G.N.; Brignola, S.; Lanza, C.; Cappellesso, R.; Pellino, A.; Pucciarelli, S.; Spolverato, G.; Guzzardo, V.; et al. Claudin-18 expression in oesophagogastric adenocarcinomas: A tissue microarray study of 523 molecularly profiled cases. Br. J. Cancer 2019, 121, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Kawazoe, A.; Mishima, S.; Nakamura, Y.; Kotani, D.; Kuboki, Y.; Bando, H.; Kojima, T.; Doi, T.; Yoshino, T.; et al. Comprehensive clinical and molecular characterization of claudin 18.2 expression in advanced gastric or gastroesophageal junction cancer. ESMO Open 2023, 8, 100762. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Lordick, F.; Bang, Y.J.; Enzinger, P.; Ilson, D.; Shah, M.A.; Van Cutsem, E.; Xu, R.H.; Aprile, G.; Xu, J.; et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): A multicentre, randomised, double-blind, phase 3 trial. Lancet 2023, 401, 1655–1668. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Shitara, K.; Ajani, J.A.; Bang, Y.J.; Enzinger, P.; Ilson, D.; Lordick, F.; Van Cutsem, E.; Gallego Plazas, J.; Huang, J.; et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: The randomized, phase 3 GLOW trial. Nat. Med. 2023, 29, 2133–2141. [Google Scholar] [CrossRef]
- Lu, S.; Jang, H.; Nussinov, R.; Zhang, J. The Structural Basis of Oncogenic Mutations G12, G13 and Q61 in Small GTPase K-Ras4B. Sci. Rep. 2016, 6, 21949. [Google Scholar] [CrossRef]
- Rubinson, D.A.; Tanaka, N.; Fece de la Cruz, F.; Kapner, K.S.; Rosenthal, M.H.; Norden, B.L.; Barnes, H.; Ehnstrom, S.; Morales-Giron, A.A.; Brais, L.K.; et al. Sotorasib Is a Pan-RASG12C Inhibitor Capable of Driving Clinical Response in NRASG12C Cancers. Cancer Discov. 2024, 14, 727–736. [Google Scholar] [CrossRef]
- Hofmann, M.H.; Gerlach, D.; Misale, S.; Petronczki, M.; Kraut, N. Expanding the Reach of Precision Oncology by Drugging All KRAS Mutants. Cancer Discov. 2022, 12, 924–937. [Google Scholar] [CrossRef]
- Patelli, G.; Tosi, F.; Amatu, A.; Mauri, G.; Curaba, A.; Patanè, D.A.; Pani, A.; Scaglione, F.; Siena, S.; Sartore-Bianchi, A. Strategies to tackle RAS-mutated metastatic colorectal cancer. ESMO Open 2021, 6, 100156. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.S.; Yaeger, R.; Spira, A.I.; Pelster, M.S.; Sabari, J.K.; Hafez, N.; Barve, M.; Velastegui, K.; Yan, X.; Shetty, A.; et al. Adagrasib in Advanced Solid Tumors Harboring a KRAS G12CMutation. J. Clin. Oncol. 2023, 41, 4097–4106. [Google Scholar] [CrossRef]
- Jänne, P.A.; Riely, G.J.; Gadgeel, S.M.; Heist, R.S.; Ou, S.I.; Pacheco, J.M.; Johnson, M.L.; Sabari, J.K.; Leventakos, K.; Yau, E.; et al. Adagrasib in Non–Small-Cell Lung Cancer Harboring a KRAS G12C Mutation. N. Engl. J. Med. 2022, 387, 120–131. [Google Scholar]
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F.; et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef]
- Yaeger, R.; Weiss, J.; Pelster, M.S.; Spira, A.I.; Barve, M.; Ou, S.I.; Leal, T.A.; Bekaii-Saab, T.S.; Paweletz, C.P.; Heavey, G.A. Adagrasib with or without Cetuximab in Colorectal Cancer with Mutated KRAS G12C. N. Engl. J. Med. 2023, 388, 44–54. [Google Scholar] [CrossRef]
- Hong, D.S.; Fakih, M.G.; Strickler, J.H.; Desai, J.; Durm, G.A.; Shapiro, G.I.; Falchook, G.S.; Price, T.J.; Sacher, A.; Denlinger, C.S.; et al. KRAS G12C Inhibition with Sotorasib in Advanced Solid Tumors. N. Engl. J. Med. 2020, 383, 1207–1217. [Google Scholar] [CrossRef]
- Ryan, M.B.; Fece de la Cruz, F.; Phat, S.; Myers, D.T.; Wong, E.; Shahzade, H.A.; Hong, C.B.; Corcoran, R.B. Vertical pathway inhibition overcomes adaptive feedback resistance to KrasG12C inhibition. Clin. Cancer Res. 2020, 26, 1617–1643. [Google Scholar] [CrossRef] [PubMed]
- Kuboki, Y.; Yaeger, R.; Fakih, M.; Strickler, J.H.; Masuishi, T.; Kim, E.H.; Bestvina, C.M.; Langer, C.J.; Krauss, J.C.; Puri, S.; et al. 45MO Sotorasib in combination with panitumumab in refractory KRAS G12C-mutated colorectal cancer: Safety and efficacy for phase Ib full expansion cohort. Ann. Oncol. 2022, 33, S1445–S1446. [Google Scholar] [CrossRef]
- Klempner, S.J.; Weiss, J.; Pelster, M.; Spira, A.; Barve, M.; Ou, S.I.; Leal, T.A.; Bekaii-Saab, T.; Christensen, J.G.; Kheoh, T.; et al. LBA24 KRYSTAL-1: Updated efficacy and safety of adagrasib (MRTX849) with or without cetuximab in patients with advanced colorectal cancer (CRC) harboring a KRASG12C mutation. Ann. Oncol. 2022, 33, S1391. [Google Scholar] [CrossRef]
- Desai, J.; Alonso, G.; Kim, S.H.; Cervantes, A.; Karasic, T.; Medina, L.; Shacham-Shmueli, E.; Cosman, R.; Falcon, A.; Gort, E. Divarasib plus cetuximab in KRAS G12C-positive colorectal cancer: A phase 1b trial. Nat. Med. 2024, 30, 271–278. [Google Scholar] [CrossRef]
- Amodio, V.; Yaeger, R.; Arcella, P.; Cancelliere, C.; Lamba, S.; Lorenzato, A.; Arena, S.; Montone, M.; Mussolin, B.; Bian, Y.; et al. EGFR Blockade Reverts Resistance to KRASG12C Inhibition in Colorectal Cancer. Cancer Discov. 2020, 10, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Fakih, M.G.; Salvatore, L.; Esaki, T.; Modest, D.P.; Lopez-Bravo, D.P.; Taieb, J.; Karamouzis, M.V.; Ruiz-Garcia, E.; Kim, T.W.; Kuboki, Y.; et al. Sotorasib plus Panitumumab in Refractory Colorectal Cancer with Mutated KRAS G12C. N. Engl. J. Med. 2023, 389, 2125–2139. [Google Scholar] [CrossRef]
- Corcoran, R.B. A single inhibitor for all KRAS mutations. Nat. Cancer 2023, 4, 1060–1062. [Google Scholar] [CrossRef]
- Buscail, L.; Bournet, B.; Cordelier, P. Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.R.; Rosenberg, S.C.; McCormick, F.; Malek, S. RAS-targeted therapies: Is the undruggable drugged? Nat. Rev. Drug Discov. 2020, 19, 533–552. [Google Scholar] [CrossRef]
- Neumann, J.; Zeindl-Eberhart, E.; Kirchner, T.; Jung, A. Frequency and type of KRAS mutations in routine diagnostic analysis of metastatic colorectal cancer. Pathol. Res. Pract. 2009, 205, 858–862. [Google Scholar] [CrossRef]
- Spira, A.I.; Papadopoulos, K.P.; Kim, D.W.; Parikh, A.R.; Barve, M.A.; Powderly, J.D.; Starodub, A.; Strickler, J.H.; Li, B.T.; Oberstein, P.E.; et al. Preliminary safety, antitumor activity, and circulating tumor DNA (ctDNA) changes with RMC-9805, an oral, RAS(ON) G12D-selective tri-complex inhibitor in patients with KRAS G12D pancreatic ductal adenocarcinoma (PDAC) from a phase 1 study in advanced solid tumors. J. Clin. Oncol. 2025, 43 (Suppl. S4), 724. [Google Scholar]
- Wei, D.; Wang, L.; Zuo, X.; Maitra, A.; Bresalier, R.S. A Small Molecule with Big Impact: MRTX1133 Targets the KRASG12D Mutation in Pancreatic Cancer. Clin. Cancer Res. 2024, 30, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Hallin, J.; Bowcut, V.; Calinisan, A.; Briere, D.M.; Hargis, L.; Engstrom, L.D.; Laguer, J.; Medwid, J.; Vanderpool, D.; Lifset, E.; et al. Anti-tumor efficacy of a potent and selective non-covalent KRASG12D inhibitor. Nat. Med. 2022, 28, 2171–2182. [Google Scholar] [CrossRef]
- Koltun, E.; Lin, W. RMC-6236, a RAS(ON) Multi-Selective Tri-Complex Inhibitor. In Proceedings of the 2024 AACR Annual Meeting, San Diego, CA, USA, 5–10 April 2024. [Google Scholar]
- Holderfield, M.; Lee, B.J.; Jiang, J.; Tomlinson, A.; Seamon, K.J.; Mira, A.; Patrucco, E.; Goodhart, G.; Dilly, J.; Gindin, Y.; et al. Concurrent inhibition of oncogenic and wild-type RAS-GTP for cancer therapy. Nature 2024, 629, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Arbour, K.C.; Punekar, S.; Garrido-Laguna, I.; Hong, D.S.; Wolpin, B.; Pelster, M.S.; Barve, M.; Starodub, A.; Sommerhalder, D.; Chang, S.; et al. 652O Preliminary clinical activity of RMC-6236, a first-in-class, RAS-selective, tri-complex RAS-MULTI (ON) inhibitor in patients with KRAS mutant pancreatic ductal adenocarcinoma (PDAC) and non-small cell lung cancer (NSCLC). Ann. Oncol. 2023, 34, S458. [Google Scholar] [CrossRef]
- Kim, D.; Herdeis, L.; Rudolph, D.; Zhao, Y.; Böttcher, J.; Vides, A.; Ayala-Santos, C.I.; Pourfarjam, Y.; Cuevas-Navarro, A.; Xue, J.Y.; et al. Pan-KRAS inhibitor disables oncogenic signalling and tumour growth. Nature 2023, 619, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Lowery, M.A.; Jordan, E.J.; Basturk, O.; Ptashkin, R.N.; Zehir, A.; Berger, M.F.; Leach, T.; Herbst, B.; Askan, G.; Maynard, H.; et al. Real-time genomic profiling of pancreatic ductal adenocarcinoma: Potential actionability and correlation with clinical phenotype. Clin. Cancer Res. 2017, 23, 6094–6100. [Google Scholar] [CrossRef]
- Mandelker, D.; Marra, A.; Zheng-Lin, B.; Selenica, P.; Blanco-Heredia, J.; Zhu, Y.; Gazzo, A.; Wong, D.; Yelskaya, Z.; Rai, V.; et al. Genomic Profiling Reveals Germline Predisposition and Homologous Recombination Deficiency in Pancreatic Acinar Cell Carcinoma. J. Clin. Oncol. 2023, 41, 5151–5162. [Google Scholar] [CrossRef]
- Kindler, H.L.; Hammel, P.; Reni, M.; Van Cutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.O.; Hochhauser, D.; Arnold, D.; Oh, D.Y.; et al. Overall Survival Results from the POLO Trial: A Phase III Study of Active Maintenance Olaparib Versus Placebo for Germline BRCA-Mutated Metastatic Pancreatic Cancer. J. Clin. Oncol. 2022, 40, 3929–3939. [Google Scholar] [CrossRef]
- Golan, T.; Hammel, P.; Reni, M.; Van Cutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.O.; Hochhauser, D.; Arnold, D.; Oh, D.Y.; et al. Maintenance Olaparib for Germline BRCA -Mutated Metastatic Pancreatic Cancer. N. Engl. J. Med. 2019, 381, 317–327. [Google Scholar] [CrossRef]
- O’Reilly, E.M.; Lee, J.W.; Zalupski, M.; Capanu, M.; Park, J.; Golan, T.; Tahover, E.; Lowery, M.A.; Chou, J.F.; Sahai, V.; et al. Randomized, multicenter, phase II trial of gemcitabine and cisplatin with or without veliparib in patients with pancreas adenocarcinoma and a germline BRCA/ PALB2 mutation. J. Clin. Oncol. 2020, 38, 1378–1388. [Google Scholar] [CrossRef]
- Park, W.; O’Connor, C.; Chou, J.F.; Schwartz, C.; Varghese, A.M.; Larsen, M.; Balogun, F.; Brenner, R.; Yu, K.H.; Diguglielmo, E.; et al. Phase 2 trial of pembrolizumab and olaparib (POLAR) maintenance for patients (pts) with metastatic pancreatic cancer (mPDAC): Two cohorts B non-core homologous recombination deficiency (HRD) and C exceptional response to platinum-therapy. J. Clin. Oncol. 2023, 41 (Suppl. S16), 4140. [Google Scholar] [CrossRef]
- Helsten, T.; Elkin, S.; Arthur, E.; Tomson, B.N.; Carter, J.; Kurzrock, R. The FGFR landscape in cancer: Analysis of 4,853 tumors by next-generation sequencing. Clin. Cancer Res. 2016, 22, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Grose, R. Fibroblast growth factor signalling: From development to cancer. Nat. Rev. Cancer 2010, 10, 116–129. [Google Scholar] [CrossRef]
- Goyal, L.; Meric-Bernstam, F.; Hollebecque, A.; Valle, J.W.; Morizane, C.; Karasic, T.B.; Abrams, T.A.; Furuse, J.; Kelley, R.K.; Cassier, P.A.; et al. Futibatinib for FGFR2 -Rearranged Intrahepatic Cholangiocarcinoma. N. Engl. J. Med. 2023, 388, 228–239. [Google Scholar] [CrossRef]
- Ahn, S.; Lee, J.; Hong, M.; Kim, S.T.; Park, S.H.; Choi, M.G.; Lee, J.H.; Sohn, T.S.; Bae, J.M.; Kim, S.; et al. FGFR2 in gastric cancer: Protein overexpression predicts gene amplification and high H-index predicts poor survival. Mod. Pathol. 2016, 29, 1095–1103. [Google Scholar] [CrossRef]
- Dienstmann, R.; Salazar, R.; Tabernero, J. Molecular Subtypes and the Evolution of Treatment Decisions in Metastatic Colorectal Cancer. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 231–238. [Google Scholar] [CrossRef]
- Lyu, X.; Cai, R.; Han, B.; Deng, Y. Comprehensive landscape of FGFR variations and prognosis revelance in colorectal cancer from circulating tumor DNA and tissue gene analyses in 2083 patients. J. Clin. Oncol. 2025, 43 (Suppl. S4), 278. [Google Scholar] [CrossRef]
- Dienstmann, R.; Rodon, J.; Prat, A.; Perez-Garcia, J.; Adamo, B.; Felip, E.; Cortes, J.; Iafrate, A.J.; Nuciforo, P.; Tabernero, J. Genomic aberrations in the FGFR pathway: Opportunities for targeted therapies in solid tumors. Ann. Oncol. 2013, 25, 552. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef]
- WITHDRAWN: FDA Grants Accelerated Approval to Infigratinib for Metastatic Cholangiocarcinoma|FDA. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/withdrawn-fda-grants-accelerated-approval-infigratinib-metastatic-cholangiocarcinoma (accessed on 23 July 2025).
- Loriot, Y.; Matsubara, N.; Park, S.H.; Huddart, R.A.; Burgess, E.F.; Houede, N.; Banek, S.; Guadalupi, V.; Ku, J.H.; Valderrama, B.P.; et al. Erdafitinib or Chemotherapy in Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2023, 389, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Pant, S.; Schuler, M.; Iyer, G.; Witt, O.; Doi, T.; Qin, S.; Tabernero, J.; Reardon, D.A.; Massard, C.; Minchom, A.; et al. Erdafitinib in patients with advanced solid tumours with FGFR alterations (RAGNAR): An international, single-arm, phase 2 study. Lancet Oncol. 2023, 24, 925–935. [Google Scholar] [CrossRef]
- Gong, J.; Mita, A.C.; Wei, Z.; Cheng, H.H.; Mitchell, E.P.; Wright, J.J.; Ivy, S.P.; Wang, V.; Gray, R.C.; McShane, L.M.; et al. Phase II Study of Erdafitinib in Patients with Tumors with FGFR Amplifications: Results from the NCI-MATCH ECOG-ACRIN Trial (EAY131) Subprotocol K1. JCO Precis. Oncol. 2024, 8, e2300406. [Google Scholar] [CrossRef] [PubMed]
- Ellis, H.; Goyal, L. Are FGFR Fusions and Mutations the Next Tumor-Agnostic Targets in Oncology? JCO Precis. Oncol. 2024, 8, e2400113. [Google Scholar] [CrossRef]
- Gujarathi, R.; Peshin, S.; Zhang, X.; Bachini, M.; Meeks, M.N.; Shroff, R.T.; Pillai, A. Intrahepatic cholangiocarcinoma: Insights on molecular testing, targeted therapies, and future directions from a multidisciplinary panel. Hepatol. Commun. 2025, 9, e0743. [Google Scholar] [CrossRef]
- Vogel, A.; Bridgewater, J.; Edeline, J.; Kelley, R.K.; Klümpen, H.J.; Malka, D.; Primrose, J.N.; Rimassa, L.; Stenzinger, A.; Valle, J.W.; et al. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up 5 behalf of the ESMO Guidelines Committee. Ann. Oncol. 2022, 34, 127–140. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef]
- Nakamura, H.; Arai, Y.; Totoki, Y.; Shirota, T.; Elzawahry, A.; Kato, M.; Hama, N.; Hosoda, F.; Urushidate, T.; Ohashi, S.; et al. Genomic spectra of biliary tract cancer. Nat. Genet. 2015, 47, 1003–1010. [Google Scholar] [CrossRef]
- Jusakul, A.; Cutcutache, I.; Yong, C.H.; Lim, J.Q.; Huang, M.N.; Padmanabhan, N.; Nellore, V.; Kongpetch, S.; Ng, A.W.T.; Ng, L.M.; et al. Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discov. 2017, 7, 1116–1135. [Google Scholar] [CrossRef]
- Zhu, A.X.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.T.; Borad, M.J.; Bridgewater, J.A.; et al. Final Overall Survival Efficacy Results of Ivosidenib for Patients with Advanced Cholangiocarcinoma with IDH1 Mutation: The Phase 3 Randomized Clinical ClarIDHy Trial. JAMA Oncol. 2021, 7, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Luo, S.; Liu, D.; Lu, X.; Wang, M.; Liu, X.; Jia, F.; Pang, Y.; Shen, Y.; Zeng, C.; et al. Genomic and transcriptomic landscape of human gastrointestinal stromal tumors. Nat. Commun. 2024, 15, 9495. [Google Scholar] [CrossRef]
- Heinrich, M.C.; Jones, R.L.; von Mehren, M.; Schöffski, P.; Serrano, C.; Kang, Y.K.; Cassier, P.A.; Mir, O.; Eskens, F.; Tap, W.D.; et al. Avapritinib in advanced PDGFRA D842V-mutant gastrointestinal stromal tumour (NAVIGATOR): A multicentre, open-label, phase 1 trial. Lancet Oncol. 2020, 21, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Tap, W.D.; Wagner, A.J.; Bauer, S.; Heinrich, M.C.; Jones, R.L.; Serrano, C.; von Mehren, M.; Somaiah, N.; Andor, T.; Zou, L.; et al. Safety, pharmacokinetics (PK), and clinical activity of bezuclastinib + sunitinib in previously-treated gastrointestinal stromal tumor (GIST): Results from part 1 of the phase 3 Peak study. J. Clin. Oncol. 2023, 41 (Suppl. S16), 11537. [Google Scholar] [CrossRef]
- Casali, P.G.; Blay, J.Y.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.V.M.G.; et al. Gastrointestinal stromal tumours: ESMO–EURACAN–GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 20–33. [Google Scholar] [CrossRef]
- Serrano, C.; Bauer, S.; Gómez-Peregrina, D.; Kang, Y.K.; Jones, R.L.; Rutkowski, P.; Mir, O.; Heinrich, M.C.; Tap, W.D.; Newberry, K.; et al. Circulating tumor DNA analysis of the phase III VOYAGER trial: KIT mutational landscape and outcomes in patients with advanced gastrointestinal stromal tumor treated with avapritinib or regorafenib. Ann. Oncol. 2023, 34, 615–625. [Google Scholar] [CrossRef]
- Alhalabi, O.; Zhu, Y.; Hamza, A.; Qiao, W.; Lin, Y.; Wang, R.M.; Shah, A.Y.; Campbell, M.T.; Holla, V.; Kamat, A.; et al. Integrative Clinical and Genomic Characterization of MTAP-deficient Metastatic Urothelial Cancer. Eur. Urol. Oncol. 2023, 6, 228–232. [Google Scholar] [CrossRef]
- Marjon, K.; Cameron, M.J.; Quang, P.; Clasquin, M.F.; Mandley, E.; Kunii, K.; McVay, M.; Choe, S.; Kernytsky, A.; Gross, S.; et al. MTAP Deletions in Cancer Create Vulnerability to Targeting of the MAT2A/PRMT5/RIOK1 Axis. Cell Rep. 2016, 15, 574–587. [Google Scholar]
- Mavrakis, K.J.; McDonald, E.R., III; Schlabach, M.R.; Billy, E.; Hoffman, G.R.; deWeck, A.; Ruddy, D.A.; Venkatesan, K.; Yu, J.; McAllister, G.; et al. Disordered methionine metabolism in MTAP/CDKN2A-deleted cancers leads to dependence on PRMT5. Science 2016, 351, 1208–1213. [Google Scholar]
- Kryukov, G.V.; Wilson, F.H.; Ruth, J.R.; Paulk, J.; Tsherniak, A.; Marlow, S.E.; Vazquez, F.; Weir, B.A.; Fitzgerald, M.E.; Tanaka, M.; et al. MTAP deletion confers enhanced dependency on the PRMT5 arginine methyltransferase in cancer cells. Science 2016, 351, 1214–1218. [Google Scholar] [CrossRef] [PubMed]
- Arbour, K.C.; George, B.; Henry, J.T.; Leventakos, K.; Rodon Ahnert, J.; Johnson, M.L.; Papadopoulos, K.P.; Spira, A.I.; Perez, C.A.; Zuniga, R.; et al. BMS-986504 in patients (pts) with advanced solid tumors with homozygous MTAP deletion (MTAP-del): Clinical update and first report of pharmacokinetics (PK) and pharmacodynamic (PD) analyses from CA240-0007. J. Clin. Oncol. 2025, 43 (Suppl. S16), 3011. [Google Scholar] [CrossRef]
- van de Haar, J.; Roepman, P.; Andre, F.; Balmaña, J.; Castro, E.; Chakravarty, D.; Curigliano, G.; Czarnecka, A.M.; Dienstmann, R.; Horak, P.; et al. ESMO Recommendations on clinical reporting of genomic test results for solid cancers. Ann. Oncol. 2024, 35, 954–967. [Google Scholar] [CrossRef]
- Larson, K.L.; Huang, B.; Weiss, H.L.; Hull, P.; Westgate, P.M.; Miller, R.W.; Arnold, S.M.; Kolesar, J.M. Clinical Outcomes of Molecular Tumor Boards: A Systematic Review. JCO Precis. Oncol. 2021, 5, 1122–1132. [Google Scholar] [CrossRef]
- Cicala, C.M.; Olivares-Rivas, I.; Aguirre-Carrillo, J.A.; Serrano, C. KIT/PDGFRA inhibitors for the treatment of gastrointestinal stromal tumors: Getting to the gist of the problem. Expert Opin. Investig. Drugs 2024, 33, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Kuboki, Y.; Fakih, M.; Strickler, J.; Yaeger, R.; Masuishi, T.; Kim, E.J.; Bestvina, C.M.; Kopetz, S.; Falchook, G.S.; Langer, C.; et al. Sotorasib with panitumumab in chemotherapy-refractory KRAS G12C-mutated colorectal cancer: A phase 1b trial. Nat. Med. 2024, 30, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Lebofsky, R.; Decraene, C.; Bernard, V.; Kamal, M.; Blin, A.; Leroy, Q.; Rio Frio, T.; Pierron, G.; Callens, C.; Bieche, I.; et al. Circulating tumor DNA as a non-invasive substitute to metastasis biopsy for tumor genotyping and personalized medicine in a prospective trial across all tumor types. Mol. Oncol. 2014, 9, 783. [Google Scholar] [CrossRef] [PubMed]
- Sartore-Bianchi, A.; Pietrantonio, F.; Lonardi, S.; Mussolin, B.; Rua, F.; Crisafulli, G.; Bartolini, A.; Fenocchio, E.; Amatu, A.; Manca, P.; et al. Circulating tumor DNA to guide rechallenge with panitumumab in metastatic colorectal cancer: The phase 2 CHRONOS trial. Nat. Med. 2022, 28, 1612–1618. [Google Scholar] [CrossRef]
- Kopetz, S.; Murphy, D.A.; Pu, J.; Ciardiello, F.; Desai, J.; Van Cutsem, E.; Wasan, H.S.; Yoshino, T.; Saffari, H.; Zhang, X.; et al. Molecular profiling of BRAF-V600E-mutant metastatic colorectal cancer in the phase 3 BEACON CRC trial. Nat. Med. 2024, 30, 3261–3271. [Google Scholar] [CrossRef]
- Dickson, D.; Johnson, J.; Bergan, R.; Owens, R.; Subbiah, V.; Kurzrock, R. The Master Observational Trial: A New Class of Master Protocol to Advance Precision Medicine. Cell 2020, 180, 9–14. [Google Scholar] [CrossRef]
- Subbiah, V. The next generation of evidence-based medicine. Nat. Med. 2023, 29, 49–58. [Google Scholar] [CrossRef] [PubMed]
| Study | Study Design | Nº of Patients | MSI-H/dMMR Testing Method | ICI Regimen | Clinical Outcomes |
|---|---|---|---|---|---|
| KN-016 | Phase II, mCRC and non-mCRC MSI/dMMR patients | 86 | Local PCR or IHC | Pembrolizumab 10 mg/kg every 2 weeks | General: ORR: 50%/CR: 21%/PR: 28/86 (33%) Colon n = 40; ORR 53% (5 CR, 16 PR) Ampullary n = 4; ORR 25% (1 CR, 1 SD, 1 PD) Cholangiocarcinoma n = 4; ORR 25% (1 CR, 3 SD) Gastroesophageal n = 5; ORR 60% (3 CR, 2 PD) Pancreas n = 8; ORR 63% (2 CR, 3 PR, 1 SD) Small intestine: 5; ORR 80% (2 CR, 2 PR, 1 PD) |
| KN-164 | Phase II, refractory MSI/dMMR CRC | 124 | Local PCR or IHC | Pembrolizumab 200 mg every 3 weeks | ORR: 41/124 (33%) All CRC |
| KN-158 | Phase II, non mCRC dMMR | 233 | Local PCR/IHC or central PCR | Pembrolizumab 200 mg every 3 weeks | General: ORR: 34% CR: 10 /PR: 24%/ SD: 18% Gastric n = 24; ORR 46% (4 CR, 7 PR) Cholangiocarcinoma n = 22; ORR 41% (2 CR, 7 PR) Pancreatic n = 22; ORR 18% (1 CR, 3 PR) Small intestine n = 19; ORR 26% (3 CR, 2 PR) Anal n = 1 |
| GARNET | Phase I | 327 | General: ORR: 39% Colon n = 115; ORR = 43,5% Small intestine n = 23; ORR = 39% Pancreatic n = 12; ORR:41% Gastric n = 22 ORR 45% |
| Drug Name | Target | Development Stage |
|---|---|---|
| Sotorasib (AMG510 Amgen) | KRAS p.G12C (OFF) | Approved |
| Adagrasib (MRTX849 Mirati) | KRAS p.G12C (OFF) | Approved |
| Divarasib (GDC-6036 Genentech/Roche) | KRAS p.G12C (OFF) | Phase III |
| Opnurasib (JDQ443 Novartis) | KRAS p.G12C (OFF) | Phase III |
| Garsorasib (D-1553 InventisBio) | KRAS p.G12C (OFF) | Phase II |
| Glecirasib (JAB-21822 Jacobio) | KRAS p.G12C (OFF) | Phase II |
| GFH925 (GenFleet) | KRAS p.G12C (OFF) | Phase II |
| YL-15293 (Shanghai Yingli) | KRAS p.G12C (OFF) | Phase II |
| HS-10370 (Jiangsu Hansoh) | KRAS p.G12C (OFF) | Phase II |
| LY3537982 (Lilly) | KRAS p.G12C (OFF) | Phase II |
| BI 1823911 (Boehringer Ingelheim) | KRAS p.G12C (OFF) | Phase II |
| BPI0421286 (Belta) | KRAS p.G12C (OFF) | Phase II |
| GH35 (Suzhou Genhouse Bio) | KRAS p.G12C (OFF) | Phase II |
| GEC255 (GenEros Biopharma) | KRAS p.G12C (OFF) | Phase II |
| MK-1084 (Merck) | KRAS p.G12C (OFF) | Phase II |
| D3S-001 (D3 Bio) | KRAS p.G12C (OFF) | Phase II |
| HBI-2438 (HuyaBio) | KRAS p.G12C (OFF) | Phase II |
| SY-5933 (Shouyao Holdings) | KRAS p.G12C (OFF) | Phase II |
| JNJ-74699157 (Janssen) | KRAS p.G12C (OFF) | Discontinued |
| RMC-6291 (Revolution Medicines) | KRAS p.G12C (ON) | Phase I |
| MRTX1133 (Mirati) | KRAS p.G12D | Phase I |
| RMC-9805 (Revolution Medicines) | KRAS p.G12D | Phase I |
| ASP3082 (Astellas) | KRAS p.G12D | Phase I |
| HRS-4642 (Jiangsu Hengrui) | KRAS p.G12D | Phase I |
| INCB161731 (Incyte) | KRAS p.G12D | Phase I |
| BI 3706674 (Boehringer Ingelheim) | Multi-KRAS | Phase I |
| RMC-6236 (Revolution Medicines) | Multi-KRAS | Phase I |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saoudi Gonzalez, N.; Patelli, G.; Crisafulli, G. Clinical Actionability of Genes in Gastrointestinal Tumors. Genes 2025, 16, 1130. https://doi.org/10.3390/genes16101130
Saoudi Gonzalez N, Patelli G, Crisafulli G. Clinical Actionability of Genes in Gastrointestinal Tumors. Genes. 2025; 16(10):1130. https://doi.org/10.3390/genes16101130
Chicago/Turabian StyleSaoudi Gonzalez, Nadia, Giorgio Patelli, and Giovanni Crisafulli. 2025. "Clinical Actionability of Genes in Gastrointestinal Tumors" Genes 16, no. 10: 1130. https://doi.org/10.3390/genes16101130
APA StyleSaoudi Gonzalez, N., Patelli, G., & Crisafulli, G. (2025). Clinical Actionability of Genes in Gastrointestinal Tumors. Genes, 16(10), 1130. https://doi.org/10.3390/genes16101130







