Genetic Overlap of Thoracic Aortic Aneurysms and Intracranial Aneurysms
Abstract
1. Introduction
2. Methods
3. Results
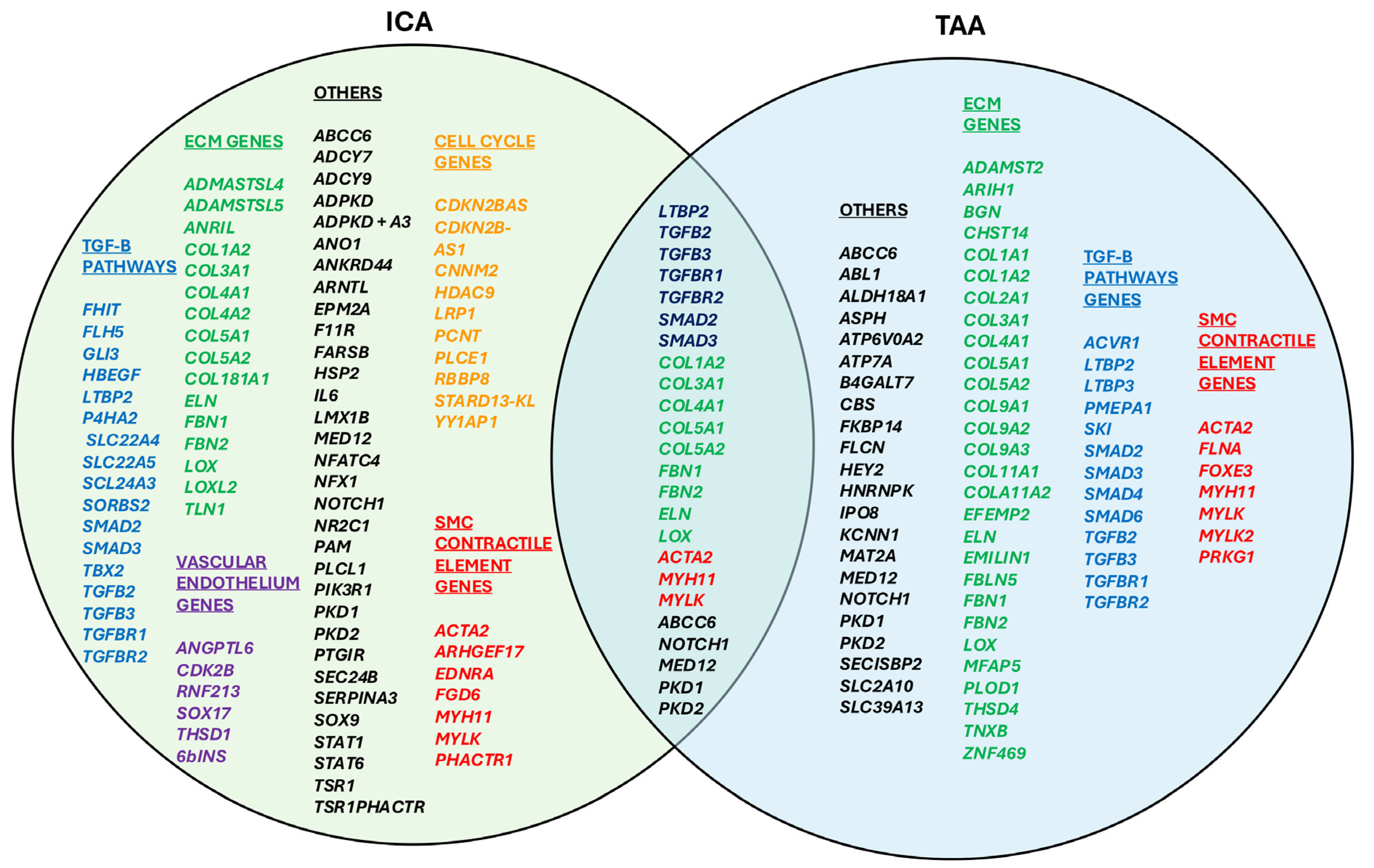
3.1. TAA Genes
3.2. ICA Genes
3.3. Overlapping Genes
- TGF-β Signaling Pathway: TBP2, TGFB2, TGFB3, TGFBR1, TGFBR2, SMAD2, SMAD3;
- Extracellular Matrix (ECM) Organization: COL1A2, COL3A1, COL4A1, COL5A1, COL5A2, FBN1, FBN2, ELN, LOX;
- Smooth Muscle Contraction and Cytoskeletal Organization: ACTA2, MYH11, MYLK.
- Other Pathways:
- ○
- ABCC6: Encodes for a member of the ATP-binding cassette (ABC) transporter family, implicated in the transport of various molecules across membranes. Mutations in ABCC6 are associated with pseudoxanthoma elasticum, affecting elastic fibers in tissues.
- ○
- NOTCH1: Part of the Notch signaling pathway, which influences cell fate decisions, proliferation, and apoptosis.
- ○
- MED12: Encodes for a subunit of the Mediator complex, involved in transcriptional regulation by serving as a bridge between gene-specific transcription factors and the RNA polymerase II machinery.
- ○
- PKD1, PKD2: Encode for polycystin-1 and polycystin-2, respectively, which are involved in calcium signaling pathways and are associated with autosomal dominant polycystic kidney disease.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TAA | Thoracic Aortic Aneurysm |
| ICA | Intracranial Aneurysm |
| ATAA | Ascending Thoracic Aortic Aneurysm |
| ATAD | Ascending Thoracic Aortic Dissection |
| FIA | Familial Intracranial Aneurysm |
| GWAS | Genome-Wide Association Studies |
References
- Johnston, K.W.; Rutherford, R.B.; Tilson, M.D.; Shah, D.M.; Hollier, L.; Stanley, J.C. Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery. J. Vasc. Surg. 1991, 13, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Elefteriades, J.A.; Farkas, E.A. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J. Am. Coll. Cardiol. 2010, 55, 841–857. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.H.; LeMaire, S.A.; Webb, N.R.; Cassis, L.A.; Daugherty, A.; Lu, H.S. Aortic Aneurysms and Dissections Series: Part II: Dynamic Signaling Responses in Aortic Aneurysms and Dissections. Arterioscler. Thromb. Vasc. Biol. 2020, 40, e78–e86. [Google Scholar] [CrossRef] [PubMed]
- Ostberg, N.; Zafar, M. Thoracic Aortic Aneurysms and Dissection. Encyclopedia. Available online: https://encyclopedia.pub/entry/11331 (accessed on 25 May 2024).
- Melo, R.G.E.; Duarte, G.S.; Lopes, A.; Alves, M.; Caldeira, D.; e Fernandes, R.F.; Pedro, L.M. Incidence and Prevalence of Thoracic Aortic Aneurysms: A Systematic Review and Meta-analysis of Population-Based Studies. Semin. Thorac. Cardiovasc. Surg. 2022, 34, 1–16. [Google Scholar] [CrossRef]
- Bickerstaff, L.; Pairolero, P.; Hollier, L.; Melton, L.; Vanpeenen, H.; Cherry, K.; Joyce, J.; Lie, J. Thoracic aortic aneurysms: A population-based study. Surgery 1982, 92, 1103–1108. [Google Scholar] [PubMed]
- Rivera, P.A.; Dattilo, J.B. Pseudoaneurysm. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Etminan, N.; Chang, H.-S.; Hackenberg, K.; de Rooij, N.K.; Vergouwen, M.D.I.; Rinkel, G.J.E.; Algra, A. Worldwide Incidence of Aneurysmal Subarachnoid Hemorrhage According to Region, Time Period, Blood Pressure, and Smoking Prevalence in the Population: A Systematic Review and Meta-analysis. JAMA Neurol. 2019, 76, 588–597. [Google Scholar] [CrossRef]
- Asikainen, A.; Korja, M.; Kaprio, J.; Rautalin, I. Case Fatality in Patients With Aneurysmal Subarachnoid Hemorrhage in Finland: A Nationwide Register-Based Study. Neurology 2023, 100, e348–e356. [Google Scholar] [CrossRef]
- Home—OMIM. Omim.org. Available online: https://omim.org/ (accessed on 25 May 2024).
- ClinVar. (n.d.). Nih.gov. Available online: https://www.ncbi.nlm.nih.gov/clinvar/ (accessed on 4 June 2024).
- Milewicz, D.M.; Guo, D.; Hostetler, E.; Marin, I.; Pinard, A.C.; Cecchi, A.C. Update on the genetic risk for thoracic aortic aneurysms and acute aortic dissections: Implications for clinical care. J. Cardiovasc. Surg. 2021, 62, 203–210. [Google Scholar] [CrossRef]
- Brownstein, A.J.; Ziganshin, B.A.; Kuivaniemi, H.; Body, S.C.; Bale, A.E.; Elefteriades, J.A. Genes Associated with Thoracic Aortic Aneurysm and Dissection: An Update and Clinical Implications. Aorta 2017, 5, 11–20. [Google Scholar] [CrossRef]
- Milewicz, D.M.; Regalado, E.S.; Shendure, J.; Nickerson, D.A.; Guo, D.C. Successes and challenges of using whole exome sequencing to identify novel genes underlying an inherited predisposition for thoracic aortic aneurysms and acute aortic dissections. Trends Cardiovasc. Med. 2014, 24, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Jeoffrey, S.M.H.; Kalyanasundaram, A.; Zafar, M.A.; Ziganshin, B.A.; Elefteriades, J.A. Genetic Overlap of Spontaneous Dissection of Either the Thoracic Aorta or the Coronary Arteries. Am. J. Cardiol. 2023, 205, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Czerny, M.; Grabenwöger, M.; Berger, T.; Aboyans, V.; Della Corte, A.; Chen, E.P.; Desai, N.D.; Dumfarth, J.; Elefteriades, J.A.; Etz, C.D.; et al. EACTS/STS Guidelines for Diagnosing and Treating Acute and Chronic Syndromes of the Aortic Organ. Ann. Thorac. Surg. 2024, 118, 5–115. [Google Scholar] [CrossRef]
- Dietz, H.C.; Cutting, C.R.; Pyeritz, R.E.; Maslen, C.L.; Sakai, L.Y.; Corson, G.M.; Puffenberger, E.G.; Hamosh, A.; Nanthakumar, E.J.; Curristin, S.M.; et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature 1991, 352, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Gordon, K.J.; Blobe, G.C. Role of transforming growth factor-β superfamily signaling pathways in human disease. Biochim. Biophys. Acta 2008, 1782, 197–228. [Google Scholar] [CrossRef] [PubMed]
- Malfait, F.; Francomano, C.; Byers, P.; Belmont, J.; Berglund, B.; Black, J.; Bloom, L.; Bowen, J.M.; Brady, A.F.; Burrows, N.P.; et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am. J. Med. Genet. C Semin. Med. Genet. 2017, 175, 8–26. [Google Scholar] [CrossRef]
- Elefteriades, J.A.; Zafar, M.A.; Ziganshin, B.A. Genetics of aortic aneurysm disease: 10 key points for the practitioner. JTCVS Open 2024, 21, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Gene Ontology Resource. Available online: https://geneontology.org/ (accessed on 25 May 2024).
- Genecards.org. Available online: https://www.genecards.org/ (accessed on 25 May 2024).
- Bakker, M.K.; Ruigrok, Y.M. Genetics of Intracranial Aneurysms. Stroke 2021, 52, 3004–3012. [Google Scholar] [CrossRef]
- Wang, P.S.; Longstreth, W.T., Jr.; Koepsell, T.D. Subarachnoid hemorrhage and family history. A population-based case-control study. Arch. Neurol. 1995, 52, 202–204. [Google Scholar] [CrossRef]
- Schievink, W.I.; Schaid, D.J.; Michels, V.V.; Piepgras, D.G. Familial aneurysmal subarachnoid hemorrhage: A community-based study. J. Neurosurg. 1995, 83, 426–429. [Google Scholar] [CrossRef]
- Ronkainen, A.; Hernesniemi, J.; Puranen, M.; Niemitukia, L.; Vanninen, R.; Ryynänen, M.; Kuivaniemi, H.; Tromp, G. Familial intracranial aneurysms. Lancet 1997, 349, 380–384. [Google Scholar] [CrossRef]
- Slot, E.M.H.; Rinkel, G.J.E.; Algra, A.; Ruigrok, Y.M. Patient and aneurysm characteristics in familial intracranial aneurysms. A systematic review and meta-analysis. PLoS ONE 2019, 14, e0213372. [Google Scholar] [CrossRef] [PubMed]
- Collins, F.S.; Fink, L. The Human Genome Project. Alcohol. Health Res. World 1995, 19, 190–195. [Google Scholar] [CrossRef] [PubMed]
- LeMaire, S.A.; McDonald, M.-L.N.; Guo, D.-C.; Russell, L.; Miller, C.C.; Johnson, R.J.; Bekheirnia, M.R.; Franco, L.M.; Nguyen, M.; Pyeritz, R.E.; et al. Genome-wide association study identifies a susceptibility locus for thoracic aortic aneurysms and aortic dissections spanning FBN1 at 15q21.1. Nat. Genet. 2011, 43, 996–1000. [Google Scholar] [CrossRef]
- Guo, D.-C.; Grove, M.L.; Prakash, S.K.; Eriksson, P.; Hostetler, E.M.; LeMaire, S.A.; Body, S.C.; Shalhub, S.; Estrera, A.L.; Safi, H.J.; et al. Genetic Variants in LRP1 and ULK4 Are Associated with Acute Aortic Dissections. Am. J. Hum. Genet. 2016, 99, 762–769. [Google Scholar] [CrossRef]
- Xu, H.; Chen, S.; Zhang, H.; Zou, Y.; Zhao, J.; Yu, J.; Le, S.; Cui, J.; Jiang, L.; Wu, J.; et al. Network-based analysis reveals novel gene signatures in the peripheral blood of patients with sporadic nonsyndromic thoracic aortic aneurysm. J. Cell. Physiol. 2020, 235, 2478–2491. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.-C.; Hostetler, E.M.; Fan, Y.; Kulmacz, R.J.; Zhang, D.; Nickerson, D.A.; Leal, S.M.; LeMaire, S.A.; Regalado, E.S.; Milewicz, D.M. Heritable Thoracic Aortic Disease Genes in Sporadic Aortic Dissection. J. Am. Coll. Cardiol. 2017, 70, 2728–2730. [Google Scholar] [CrossRef] [PubMed]
- Klarin, D.; Devineni, P.; Sendamarai, A.K.; Angueira, A.R.; Graham, S.E.; Shen, Y.H.; Levin, M.G.; Pirruccello, J.P.; Surakka, I.; Karnam, P.R.; et al. Genome-wide association study of thoracic aortic aneurysm and dissection in the Million Veteran Program. Nat. Genet. 2023, 55, 1106–1115. [Google Scholar] [CrossRef]
- Mahlmann, A.; Elzanaty, N.; Saleh, M.; Irqsusi, M.; Rastan, A.; Leip, J.L.; Behrendt, C.-A.; Ghazy, T. Prevalence of Genetic Variants and Deep Phenotyping in Patients with Thoracic Aortic Aneurysm and Dissection: A Cross-Sectional Single-Centre Cohort Study. J. Clin. Med. 2024, 13, 461. [Google Scholar] [CrossRef]
- Asatryan, B.; Yee, L.; Ben-Haim, Y.; Dobner, S.; Servatius, H.; Roten, L.; Tanner, H.; Crotti, L.; Skinner, J.R.; Remme, C.A.; et al. Sex-Related Differences in Cardiac Channelopathies: Implications for Clinical Practice. Circulation 2021, 143, 739–752, Erratum in Circulation 2021, 143, e1028. [Google Scholar] [CrossRef]
- Smedberg, C.; Steuer, J.; Leander, K.; Hultgren, R. Sex differences and temporal trends in aortic dissection: A population-based study of incidence, treatment strategies, and outcome in Swedish patients during 15 years. Eur. Heart J. 2020, 41, 2430–2438. [Google Scholar] [CrossRef] [PubMed]
- Rieß, H.C.; Debus, E.S.; Schwaneberg, T.; Sedrakyan, A.; Kölbel, T.; Tsilimparis, N.; Larena-Avellaneda, A.; Behrendt, C.-A. Gender disparities in fenestrated and branched endovascular aortic repair. Eur. J. Cardiothorac. Surg. 2019, 55, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, L.; Xu, X.; Li, K.; Sun, Y.; Wang, Y.; Wang, D.W. Genetic architecture of thoracic aortic dissection in the female population. Gene 2023, 887, 147727. [Google Scholar] [CrossRef] [PubMed]
- Bilguvar, K.; Yasuno, K.; Niemelä, M.; Ruigrok, Y.M.; Fraunberg, M.U.; van Duijn, C.M.; Berg, L.H.D.; Mane, S.; Mason, C.E.; Choi, M.; et al. Susceptibility loci for intracranial aneurysm in European and Japanese populations. Nat. Genet. 2008, 40, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- Yasuno, K.; Bilguvar, K.; Bijlenga, P.; Low, S.-K.; Krischek, B.; Auburger, G.; Simon, M.; Krex, D.; Arlier, Z.; Nayak, N.; et al. Genome-wide association study of intracranial aneurysm identifies three new risk loci. Nat. Genet. 2010, 42, 420–425. [Google Scholar] [CrossRef]
- Yasuno, K.; Bakırcıoğlu, M.; Low, S.-K.; Bilgüvar, K.; Gaál, E.; Ruigrok, Y.M.; Niemelä, M.; Hata, A.; Bijlenga, P.; Kasuya, H.; et al. Common variant near the endothelin receptor type A (EDNRA) gene is associated with intracranial aneurysm risk. Proc. Natl. Acad. Sci. USA 2011, 108, 19707–19712. [Google Scholar] [CrossRef] [PubMed]
- Stroke, H.A.-I.; Bakker, M.K.; China Kadoorie Biobank Collaborative Group; BioBank Japan Project Consortium; The ICAN Study Group; CADISP Group; Genetics and Observational Subarachnoid Haemorrhage (GOSH) Study investigators; International Stroke Genetics Consortium (ISGC); van der Spek, R.A.A.; van Rheenen, W.; et al. Genome-wide association study of intracranial aneurysms identifies 17 risk loci and genetic overlap with clinical risk factors. Nat. Genet. 2020, 52, 1303–1313, Erratum in Nat. Genet. 2020, 53, 254. [Google Scholar] [CrossRef]
- Korja, M.; Silventoinen, K.; McCarron, P.; Zdravkovic, S.; Skytthe, A.; Haapanen, A.; de Faire, U.; Pedersen, N.L.; Christensen, K.; Koskenvuo, M.; et al. Genetic epidemiology of spontaneous subarachnoid hemorrhage: Nordic Twin Study. Stroke 2010, 41, 2458–2462. [Google Scholar] [CrossRef]
- Song, Y.; Lee, J.K.; Lee, J.O.; Kwon, B.; Seo, E.J.; Suh, D.C. Whole Exome Sequencing in Patients with Phenotypically Associated Familial Intracranial Aneurysm. Korean J. Radiol. 2022, 23, 101–111. [Google Scholar] [CrossRef]
- Bakker, M.K.; Cobyte, S.; Hennekam, F.A.M.; Rinkel, G.J.E.; Veldink, J.H.; Ruigrok, Y.M. Genome-wide linkage analysis combined with genome sequencing in large families with intracranial aneurysms. Eur. J. Hum. Genet. 2022, 30, 833–840. [Google Scholar] [CrossRef]
- Shima, Y.; Sasagawa, S.; Ota, N.; Oyama, R.; Tanaka, M.; Kubota-Sakashita, M.; Kawakami, H.; Kobayashi, M.; Takubo, N.; Ozeki, A.N.; et al. Increased PDGFRB and NF-κB signaling caused by highly prevalent somatic mutations in intracranial aneurysms. Sci. Transl. Med. 2023, 15, eabq7721. [Google Scholar] [CrossRef]
- Maimaiti, A.; Turhon, M.; Abulaiti, A.; Dilixiati, Y.; Zhang, F.; Axieer, A.; Kadeer, K.; Zhang, Y.; Maimaitili, A.; Yang, X. DNA methylation regulator-mediated modification patterns and risk of intracranial aneurysm: A multi-omics and epigenome-wide association study integrating machine learning, Mendelian randomization, eQTL and mQTL data. J. Transl. Med. 2023, 21, 660. [Google Scholar] [CrossRef]
- Kim, B.J.; Hong, E.P.; Youn, D.H.; Jeon, J.P.; First Korean Stroke Genetics Association Research. Genome-Wide Association Study of the Relationship Between Matrix Metalloproteinases and Intracranial Aneurysms. J. Clin. Neurol. 2022, 18, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.P.; Cho, S.M.; Rhim, J.K.; Park, J.J.; Ahn, J.H.; Youn, D.H.; Kim, J.-T.; Park, C.H.; Lee, Y.; Jeon, J.P.; et al. Updated Trans-Ethnic Meta-Analysis of Associations between Inflammation-Related Genes and Intracranial Aneurysm. J. Korean Neurosurg. Soc. 2023, 66, 525–535. [Google Scholar] [CrossRef]
- Gyftopoulos, A.; Ziganshin, B.A.; Elefteriades, J.A.; Ochoa Chaar, C.I. Comparison of Genes Associated with Thoracic and Abdominal Aortic Aneurysms. Aorta 2023, 11, 125–134. [Google Scholar] [CrossRef]
- Brownstein, A.J.; Bin Mahmood, S.U.; Saeyeldin, A.; Velasquez Mejia, C.; Zafar, M.A.; Li, Y.; Rizzo, J.A.; Dahl, N.K.; Erben, Y.; Ziganshin, B.A.; et al. Simple renal cysts and bovine aortic arch: Markers for aortic disease. Open Heart 2019, 6, e000862. [Google Scholar] [CrossRef]
- Malhotra, A.; Seifert, K.; Wu, X.; Matouk, C.; Elefteriades, J.A. Screening for Intracranial Aneurysms in Patients with Thoracic Aortic Aneurysms. Cerebrovasc. Dis. 2019, 47, 253–259. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Changez, M.I.K.; Nasir, A.; Sonsino, A.; Jeoffrey, S.M.; Kalyanasundaram, A.; Zafar, M.A.; Ziganshin, B.A.; Elefteriades, J.A. Genetic Overlap of Thoracic Aortic Aneurysms and Intracranial Aneurysms. Genes 2025, 16, 154. https://doi.org/10.3390/genes16020154
Changez MIK, Nasir A, Sonsino A, Jeoffrey SM, Kalyanasundaram A, Zafar MA, Ziganshin BA, Elefteriades JA. Genetic Overlap of Thoracic Aortic Aneurysms and Intracranial Aneurysms. Genes. 2025; 16(2):154. https://doi.org/10.3390/genes16020154
Chicago/Turabian StyleChangez, Mah I Kan, Afsheen Nasir, Alexandra Sonsino, Syeda Manahil Jeoffrey, Asanish Kalyanasundaram, Mohammad A. Zafar, Bulat A. Ziganshin, and John A. Elefteriades. 2025. "Genetic Overlap of Thoracic Aortic Aneurysms and Intracranial Aneurysms" Genes 16, no. 2: 154. https://doi.org/10.3390/genes16020154
APA StyleChangez, M. I. K., Nasir, A., Sonsino, A., Jeoffrey, S. M., Kalyanasundaram, A., Zafar, M. A., Ziganshin, B. A., & Elefteriades, J. A. (2025). Genetic Overlap of Thoracic Aortic Aneurysms and Intracranial Aneurysms. Genes, 16(2), 154. https://doi.org/10.3390/genes16020154




