The Association of Genetic Variants Within the Type XII Collagen and Tenascin C Genes with Knee Joint Laxity Measurements
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. General Characteristics
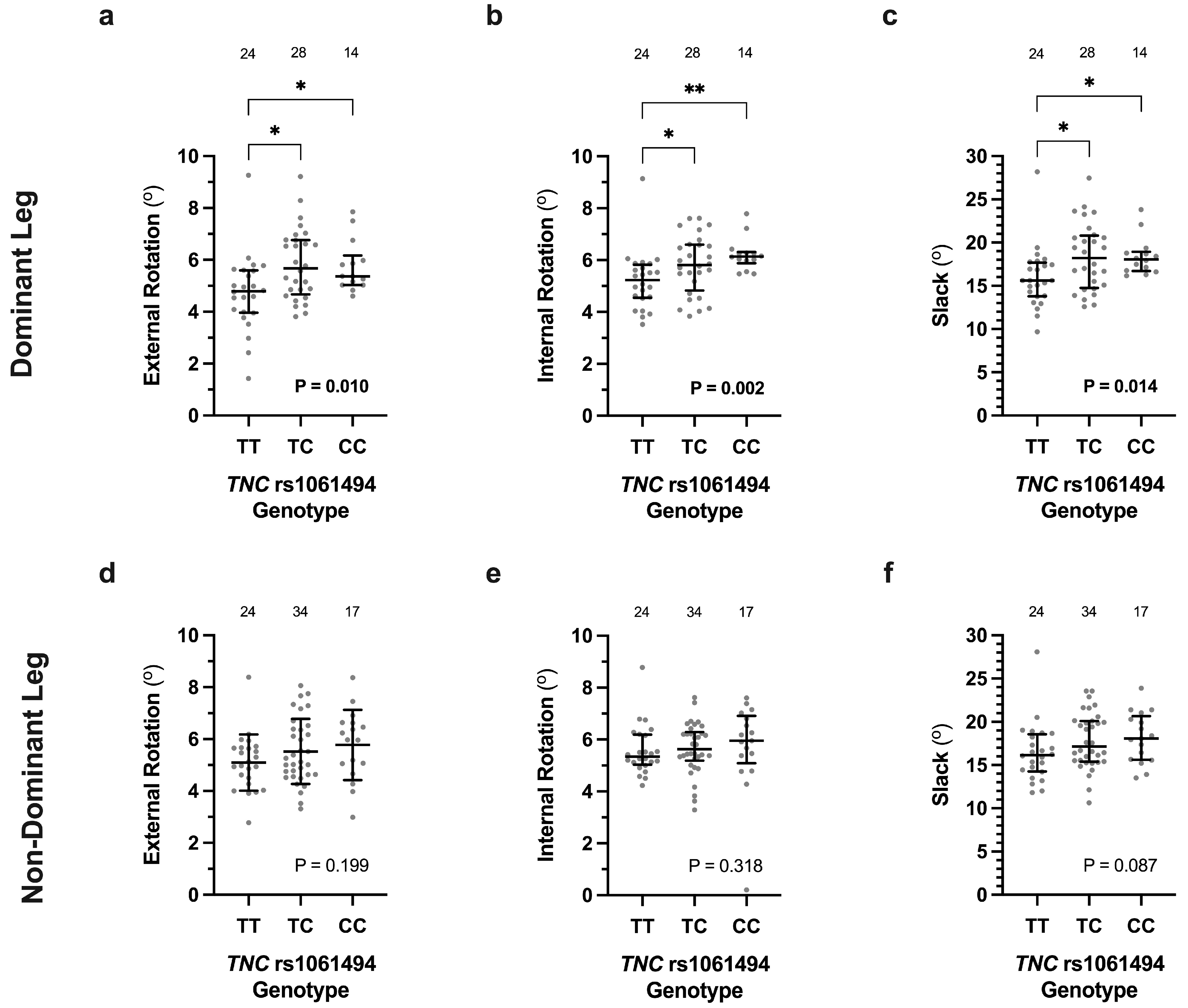
3.2. Association of COL12A1 and TNC Genotypes with Knee Laxity Measurements
3.3. Contribution of COL12A1, TNC and COL1A1 Genotypes to Multiple Linear Regression Models
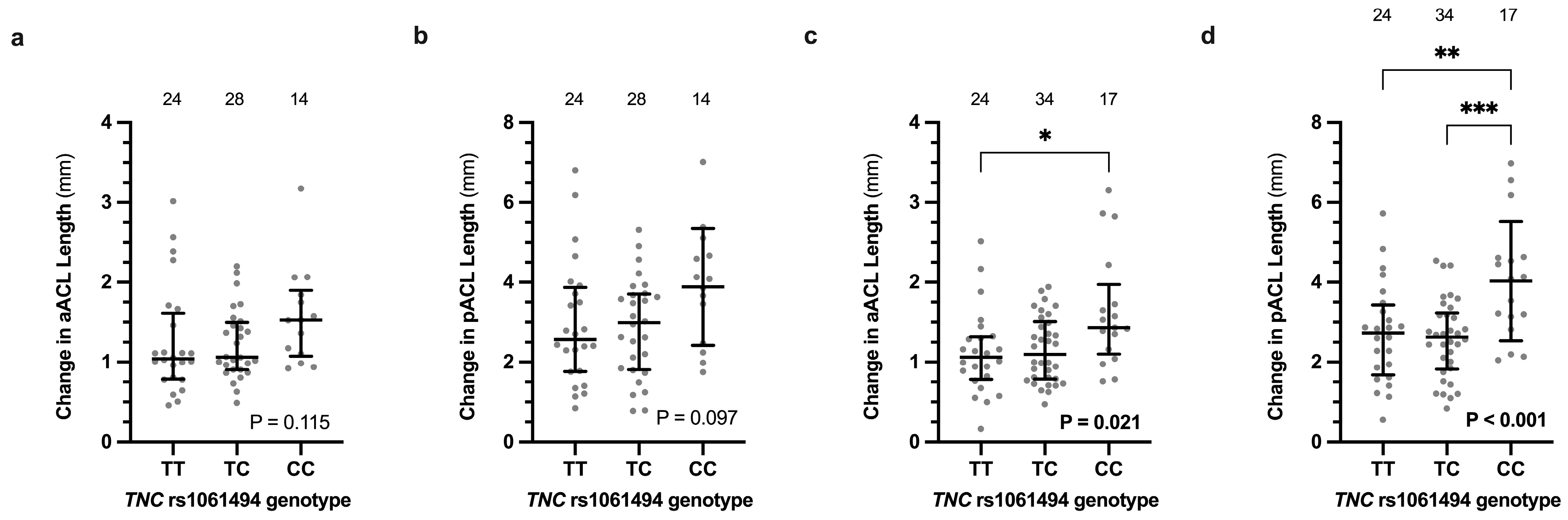
3.4. Ligament Length Changes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cross, M. Clinical terminology for describing knee instability. Sports Med. Arthrosc. Rev. 1996, 4, 313–318. [Google Scholar]
- Beighton, P.; Solomon, L.; Soskolne, C.L. Articular mobility in an African population. Ann. Rheum. Dis. 1973, 32, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Jobling, R.; D’Souza, R.; Baker, N.; Lara-Corrales, I.; Mendoza-Londono, R.; Dupuis, L.; Savarirayan, R.; Ala-Kokko, L.; Kannu, P. The collagenopathies: Review of clinical phenotypes and molecular correlations. Curr. Rheumatol. Rep. 2014, 16, 394. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.D.; Shultz, S.J.; Wideman, L.; Henrich, V.C. Collagen gene variants previously associated with anterior cruciate ligament injury risk are also associated with joint laxity. Sports Health 2012, 4, 312–318. [Google Scholar] [CrossRef]
- Beckley, S.; Dey, R.; Stinton, S.; van der Merwe, W.; Branch, T.; September, A.V.; Posthumus, M.; Collins, M. Investigating the association between COL1A1 and COL3A1 gene variants and knee joint laxity and ligament measurements. Clin. Biomech. 2022, 100, 105822. [Google Scholar] [CrossRef]
- Beckley, S.; Dey, R.; Stinton, S.; van der Merwe, W.; Branch, T.; September, A.V.; Posthumus, M.; Collins, M. The Association of Variants within Types V and XI Collagen Genes with Knee Joint Laxity Measurements. Genes 2022, 13, 2359. [Google Scholar] [CrossRef]
- Nishiyama, T.; Mcdonough, A.M.; Bruns, R.R.; Burgeson, R.E. Type-XII and Type-XIV Collagens Mediate Interactions between Banded Collagen-Fibers in-Vitro and May Modulate Extracellular-Matrix Deformability. J. Biol. Chem. 1994, 269, 28193–28199. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef]
- Delbaere, S.; Dhooge, T.; Syx, D.; Petit, F.; Goemans, N.; Destree, A.; Vanakker, O.; De Rycke, R.; Symoens, S.; Malfait, F. Novel defects in collagen XII and VI expand the mixed myopathy/Ehlers-Danlos syndrome spectrum and lead to variant-specific alterations in the extracellular matrix. Genet. Med. 2020, 22, 112–123. [Google Scholar] [CrossRef]
- Malfait, F.; Francomano, C.; Byers, P.; Belmont, J.; Berglund, B.; Black, J.; Bloom, L.; Bowen, J.M.; Brady, A.F.; Burrows, N.P.; et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am. J. Med. Genet. C Semin. Med. Genet. 2017, 175, 8–26. [Google Scholar] [CrossRef]
- Zou, Y.; Zwolanek, D.; Izu, Y.; Gandhy, S.; Schreiber, G.; Brockmann, K.; Devoto, M.; Tian, Z.; Hu, Y.; Veit, G.; et al. Recessive and dominant mutations in COL12A1 cause a novel EDS/myopathy overlap syndrome in humans and mice. Hum. Mol. Genet. 2014, 23, 2339–2352. [Google Scholar] [CrossRef] [PubMed]
- Midwood, K.S.; Chiquet, M.; Tucker, R.P.; Orend, G. Tenascin-C at a glance. J. Cell Sci. 2016, 129, 4321–4327. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, C.S.; Butler, M.G. Mutation in TNXB gene causes moderate to severe Ehlers-Danlos syndrome. World J. Med. Genet. 2016, 6, 17–21. [Google Scholar] [CrossRef]
- Rymen, D.; Ritelli, M.; Zoppi, N.; Cinquina, V.; Giunta, C.; Rohrbach, M.; Colombi, M. Clinical and Molecular Characterization of Classical-Like Ehlers-Danlos Syndrome Due to a Novel TNXB Variant. Genes 2019, 10, 843. [Google Scholar] [CrossRef]
- Chiquet, M. Regulation of extracellular matrix gene expression by mechanical stress. Matrix Biol. 1999, 18, 417–426. [Google Scholar] [CrossRef]
- Warden, S.J.; Carballido-Gamio, J.; Weatherholt, A.M.; Keyak, J.H.; Yan, C.; Kersh, M.E.; Lang, T.F.; Fuchs, R.K. Heterogeneous Spatial and Strength Adaptation of the Proximal Femur to Physical Activity: A Within-Subject Controlled Cross-Sectional Study. J. Bone Miner. Res. 2020, 35, 681–690. [Google Scholar] [CrossRef]
- Brown, J.C.; Miller, C.J.; Schwellnus, M.P.; Collins, M. Range of motion measurements diverge with increasing age for COL5A1 genotypes. Scand. J. Med. Sci. Sports 2011, 21, e266–e272. [Google Scholar] [CrossRef]
- Shultz, S.J.; Nguyen, A.D.; Levine, B.J. The Relationship Between Lower Extremity Alignment Characteristics and Anterior Knee Joint Laxity. Sports Health 2009, 1, 54–60. [Google Scholar] [CrossRef]
- Beckley, S.; Stinton, S.; Lesosky, M.; September, A.; Collins, M.; Branch, T.; Posthumus, M. Reliability of a Robotic Knee Testing Tool to Assess Rotational Stability of the Knee Joint in Healthy Female and Male Volunteers. Sports Med. Open 2020, 6, 33. [Google Scholar] [CrossRef]
- Xu, H.; Bloswick, D.; Merryweather, A. An improved OpenSim gait model with multiple degrees of freedom knee joint and knee ligaments. Comput. Methods Biomech. Biomed. Eng. 2015, 18, 1217–1224. [Google Scholar] [CrossRef]
- Mokone, G.G.; Gajjar, M.; September, A.V.; Schwellnus, M.P.; Greenberg, J.; Noakes, T.D.; Collins, M. The guanine-thymine dinucleotide repeat polymorphism within the tenascin-C gene is associated with achilles tendon injuries. Am. J. Sports Med. 2005, 33, 1016–1021. [Google Scholar] [CrossRef]
- Shultz, S.J.; Nguyen, A.D.; Schmitz, R.J. Differences in lower extremity anatomical and postural characteristics in males and females between maturation groups. J. Orthop. Sports Phys. Ther. 2008, 38, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.J.; Jazrawi, L.M. Secondary Stabilizers of Tibial Rotation in the Intact and Anterior Cruciate Ligament Deficient Knee. Clin. Sports Med. 2018, 37, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.W.; Shefelbine, S.J.; Buehler, M.J. Structural and mechanical differences between collagen homo- and heterotrimers: Relevance for the molecular origin of brittle bone disease. Biophys. J. 2012, 102, 640–648. [Google Scholar] [CrossRef]
- Gibbon, A.; Raleigh, S.M.; Ribbans, W.J.; Posthumus, M.; Collins, M.; September, A.V. Functional COL1A1 variants are associated with the risk of acute musculoskeletal soft tissue injuries. J. Orthop. Res. 2020, 38, 2290–2298. [Google Scholar] [CrossRef] [PubMed]
- Izu, Y.; Adams, S.M.; Connizzo, B.K.; Beason, D.P.; Soslowsky, L.J.; Koch, M.; Birk, D.E. Collagen XII mediated cellular and extracellular mechanisms regulate establishment of tendon structure and function. Matrix Biol. 2021, 95, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Izu, Y.; Birk, D.E. Collagen XII mediated cellular and extracellular mechanisms in development, regeneration, and disease. Front. Cell Dev. Biol. 2023, 11, 1129000. [Google Scholar] [CrossRef]
- Fukusato, S.; Nagao, M.; Fujihara, K.; Yoneda, T.; Arai, K.; Koch, M.; Kaneko, K.; Ishijima, M.; Izu, Y. Collagen XII Deficiency Increases the Risk of Anterior Cruciate Ligament Injury in Mice. J. Clin. Med. 2021, 10, 4051. [Google Scholar] [CrossRef]
- Kania, A.M.; Reichenberger, E.; Baur, S.T.; Karimbux, N.Y.; Taylor, R.W.; Olsen, B.R.; Nishimura, I. Structural variation of type XII collagen at its carboxyl-terminal NC1 domain generated by tissue-specific alternative splicing. J. Biol. Chem. 1999, 274, 22053–22059. [Google Scholar] [CrossRef]
- Tsuzuki, T.; Kajiya, H.; T-Goto, K.; Tsutsumi, T.; Nemoto, T.; Okabe, K.; Takahashi, Y. Hyperocclusion stimulates the expression of collagen type XII in periodontal ligament. Arch. Oral Biol. 2016, 66, 86–91. [Google Scholar] [CrossRef]
- Posthumus, M.; September, A.V.; O’Cuinneagain, D.; van der Merwe, W.; Schwellnus, M.P.; Collins, M. The association between the COL12A1 gene and anterior cruciate ligament ruptures. Br. J. Sports Med. 2010, 44, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, D.; Bope, C.D.; Patricios, J.; Chimusa, E.R.; Collins, M.; September, A.V. A whole genome sequencing approach to anterior cruciate ligament rupture-a twin study in two unrelated families. PLoS ONE 2022, 17, e0274354. [Google Scholar] [CrossRef] [PubMed]
- Webb, C.M.; Zaman, G.; Mosley, J.R.; Tucker, R.P.; Lanyon, L.E.; Mackie, E.J. Expression of tenascin-C in bones responding to mechanical load. J. Bone Miner. Res. 1997, 12, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Jarvinen, T.A.; Jozsa, L.; Kannus, P.; Jarvinen, T.L.; Hurme, T.; Kvist, M.; Pelto-Huikko, M.; Kalimo, H.; Jarvinen, M. Mechanical loading regulates the expression of tenascin-C in the myotendinous junction and tendon but does not induce de novo synthesis in the skeletal muscle. J. Cell Sci. 2003, 116 Pt 5, 857–866. [Google Scholar] [CrossRef]
- Matsuda, A.; Hirota, T.; Akahoshi, M.; Shimizu, M.; Tamari, M.; Miyatake, A.; Takahashi, A.; Nakashima, K.; Takahashi, N.; Obara, K.; et al. Coding SNP in tenascin-C Fn-III-D domain associates with adult asthma. Hum. Mol. Genet. 2005, 14, 2779–2786. [Google Scholar] [CrossRef]
- Chockalingam, P.S.; Glasson, S.S.; Lohmander, L.S. Tenascin-C levels in synovial fluid are elevated after injury to the human and canine joint and correlate with markers of inflammation and matrix degradation. Osteoarthr. Cartil. 2013, 21, 339–345. [Google Scholar] [CrossRef]
- Gibbon, A.; Saunders, C.J.; Collins, M.; Gamieldien, J.; September, A.V. Defining the molecular signatures of Achilles tendinopathy and anterior cruciate ligament ruptures: A whole-exome sequencing approach. PLoS ONE 2018, 13, e0205860. [Google Scholar] [CrossRef]
- Davis, H.G. Conservative Surgery, as Exhibited in Remedying Some of the Mechanical Causes That Operate Injuriously Both in Health and Disease; with Illustrations; D. Appleton & Company: New York, NY, USA, 1867; pp. 1–314. [Google Scholar]
- Lin, H.C.; Lai, W.H.; Shih, Y.F.; Chang, C.M.; Lo, C.Y.; Hsu, H.C. Physiological anterior laxity in healthy young females: The effect of knee hyperextension and dominance. Knee Surg. Sports Traumatol. Arthrosc. 2009, 17, 1083–1088. [Google Scholar] [CrossRef]
- Bravo-Sanchez, A.; Abian, P.; Jimenez, F.; Abian-Vicen, J. Myotendinous asymmetries derived from the prolonged practice of badminton in professional players. PLoS ONE 2019, 14, e0222190. [Google Scholar] [CrossRef]
- Pfeifer, C.E.; Beattie, P.F.; Sacko, R.S.; Hand, A. Risk Factors Associated with Non-Contact Anterior Cruciate Ligament Injury: A Systematic Review. Int. J. Sports Phys. Ther. 2018, 13, 575–587. [Google Scholar] [CrossRef]
- Somerson, J.S.; Isby, I.J.; Hagen, M.S.; Kweon, C.Y.; Gee, A.O. The Menstrual Cycle May Affect Anterior Knee Laxity and the Rate of Anterior Cruciate Ligament Rupture A Systematic Review and Meta-Analysis. JBJS Rev. 2019, 7, e2. [Google Scholar] [CrossRef]



| Estimate | Standard Error | 95% CI | p-Value | |||
|---|---|---|---|---|---|---|
| External Tibial Rotation (°) | Constant | 6.66 | 0.30 | 6.07 to 7.26 | <0.001 | R2 = 0.29 Adjusted R2 = 0.26 p < 0.001 |
| Sex (Male) | −0.74 | 0.31 | −1.36 to −0.12 | 0.021 | ||
| COL12A1 rs970547 (AA) | −0.80 | 0.31 | −1.41 to −0.19 | 0.011 | ||
| TNC rs1061494 (TT) | −1.04 | 0.32 | −1.67 to −0.41 | 0.002 | ||
| Internal Tibial Rotation (°) | Constant | 8.78 | 0.96 | 6.87 to 10.70 | <0.001 | R2 = 0.36 Adjusted R2 = 0.32 p < 0.001 |
| BMI (kg·m−2) | −0.12 | 0.04 | −0.20 to −0.04 | 0.004 | ||
| COL12A1 rs970547 (AA) | −0.47 | 0.23 | −0.94 to 0.00 | 0.049 | ||
| TNC rs1061494 (TT) | −0.69 | 0.23 | −1.16 to −0.22 | 0.005 | ||
| COL1A1 (GG and GG) | 0.63 | 0.23 | 0.18 to 1.09 | 0.007 | ||
| Slack (°) | Constant | 28.7 | 3.3 | 22.1 to 35.3 | <0.001 | R2 = 0.27 Adjusted R2 = 0.23 p < 0.001 |
| Sex (male) | −1.71 | 0.80 | −3.31 to −0.10 | 0.037 | ||
| BMI (kg·m−2) | −0.38 | 0.14 | −0.65 to −0.11 | 0.006 | ||
| TNC rs1061494 (TT) | −2.39 | 0.82 | −4.02 to −0.75 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beckley, S.; Dey, R.; Stinton, S.; van der Merwe, W.; Branch, T.; September, A.V.; Posthumus, M.; Collins, M. The Association of Genetic Variants Within the Type XII Collagen and Tenascin C Genes with Knee Joint Laxity Measurements. Genes 2025, 16, 164. https://doi.org/10.3390/genes16020164
Beckley S, Dey R, Stinton S, van der Merwe W, Branch T, September AV, Posthumus M, Collins M. The Association of Genetic Variants Within the Type XII Collagen and Tenascin C Genes with Knee Joint Laxity Measurements. Genes. 2025; 16(2):164. https://doi.org/10.3390/genes16020164
Chicago/Turabian StyleBeckley, Samantha, Roopam Dey, Shaun Stinton, Willem van der Merwe, Thomas Branch, Alison V. September, Michael Posthumus, and Malcolm Collins. 2025. "The Association of Genetic Variants Within the Type XII Collagen and Tenascin C Genes with Knee Joint Laxity Measurements" Genes 16, no. 2: 164. https://doi.org/10.3390/genes16020164
APA StyleBeckley, S., Dey, R., Stinton, S., van der Merwe, W., Branch, T., September, A. V., Posthumus, M., & Collins, M. (2025). The Association of Genetic Variants Within the Type XII Collagen and Tenascin C Genes with Knee Joint Laxity Measurements. Genes, 16(2), 164. https://doi.org/10.3390/genes16020164






