The p12 Subunit Choreographs the Regulation and Functions of Two Forms of DNA Polymerase δ in Mammalian Cells
Abstract
:1. Introduction
2. Historical Perspective—The Subunit Structure of Human DNA Pol δ
3. Emergence of the Concept That Two Forms of Pol δ Are Involved in Human DNA Replication and Repair
3.1. Two Forms of Pol δ
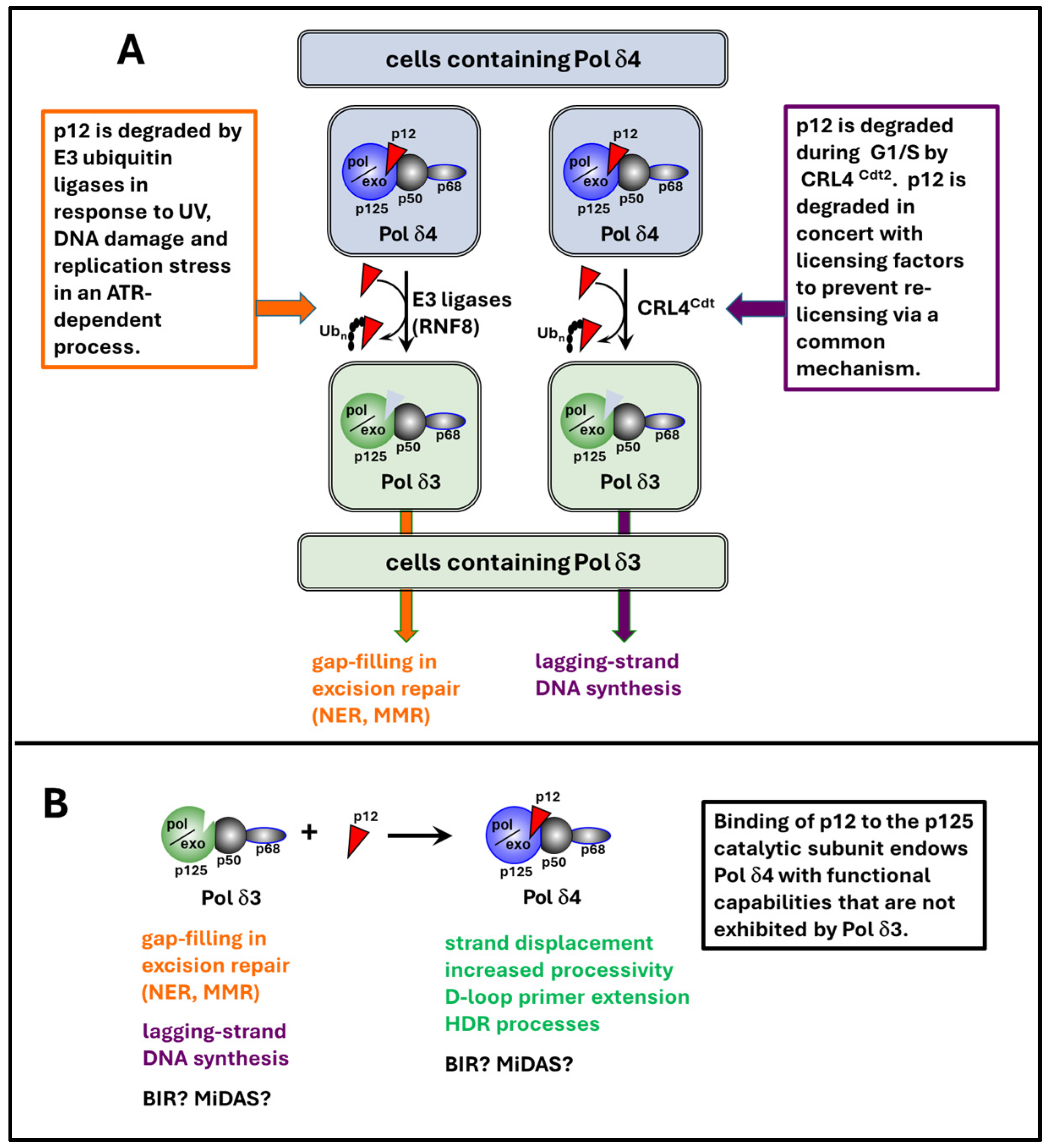
3.2. Interconversion of the Two Forms of Pol δ by Regulating p12 Availability
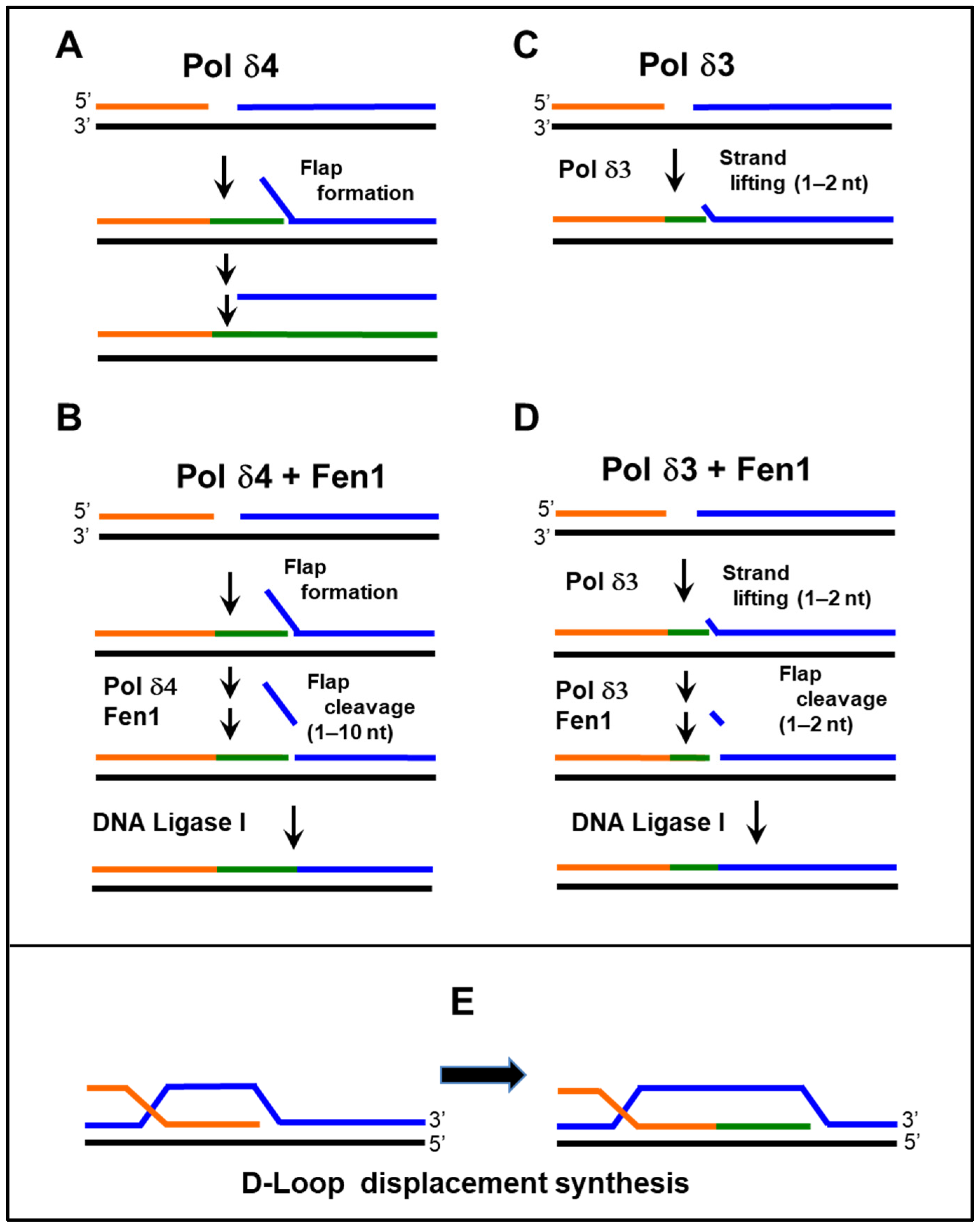
3.3. Functional Differences Between Pol δ3 and Pol δ4 That Are Conferred by p12
3.4. Potential Division of Functions of the Two Forms of Pol δ—Expanding the Range of Pol δ-Specific Functions
3.5. Mechanisms for Orchestrating the Division of Functions of Pol δ3 and Pol δ4
4. Implications for Pol δ4 in Cancer
5. Future Prospectives
Author Contributions
Funding
Conflicts of Interest
References
- Lee, M.; Wang, X.; Zhang, S.; Zhang, Z.; Lee, E.Y.C. Regulation and Modulation of Human DNA Polymerase δ Activity and Function. Genes 2017, 8, 190. [Google Scholar] [CrossRef]
- Lee, M.; Zhang, S.; Wang, X.; Chao, H.H.; Zhao, H.; Darzynkiewicz, Z.; Zhang, Z.; Lee, E.Y.C. Two forms of human DNA polymerase δ: Who does what and why? DNA Repair 2019, 81, 102656. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Zhang, S.; Lin, S.H.; Chea, J.; Wang, X.; LeRoy, C.; Wong, A.; Zhang, Z.; Lee, E.Y. Regulation of human DNA polymerase δ in the cellular responses to DNA damage. Environ. Mol. Mutagen. 2012, 53, 683–698. [Google Scholar] [CrossRef]
- Lee, M.Y.; Zhang, S.; Lin, S.H.; Wang, X.; Darzynkiewicz, Z.; Zhang, Z.; Lee, E.Y. The tail that wags the dog: p12, the smallest subunit of DNA polymerase δ, is degraded by ubiquitin ligases in response to DNA damage and during cell cycle progression. Cell Cycle 2014, 13, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhou, Y.; Sarkeshik, A.; Yates, J.R., 3rd; Thomson, T.M.; Zhang, Z.; Lee, E.Y.; Lee, M.Y. Identification of RNF8 as a ubiquitin ligase involved in targeting the p12 subunit of DNA polymerase δ for degradation in response to DNA damage. J. Biol. Chem. 2013, 288, 2941–2950. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, H.; Darzynkiewicz, Z.; Zhou, P.; Zhang, Z.; Lee, E.Y.; Lee, M.Y. A novel function of CRL4Cdt2: Regulation of the subunit structure of DNA polymerase δ in response to DNA damage and during the S phase. J. Biol. Chem. 2013, 288, 29550–29561. [Google Scholar] [CrossRef]
- Terai, K.; Shibata, E.; Abbas, T.; Dutta, A. Degradation of p12 subunit by CRL4Cdt2 E3 ligase inhibits fork progression after DNA damage. J. Biol. Chem. 2013, 288, 30509–30514. [Google Scholar] [CrossRef]
- Brutlag, D.; Kornberg, A. Enzymatic synthesis of deoxyribonucleic acid. 36. A proofreading function for the 3′ leads to 5′ exonuclease activity in deoxyribonucleic acid polymerases. J. Biol. Chem. 1972, 247, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Muzyczka, N.; Poland, R.L.; Bessman, M.J. Studies on the biochemical basis of spontaneous mutation. I. A comparison of the deoxyribonucleic acid polymerases of mutator, antimutator, and wild type strains of bacteriophage T4. J. Biol. Chem. 1972, 247, 7116–7122. [Google Scholar] [CrossRef]
- Reha-Krantz, L.J. DNA polymerase proofreading: Multiple roles maintain genome stability. Biochim. Biophys. Acta 2010, 1804, 1049–1063. [Google Scholar] [CrossRef] [PubMed]
- Weissbach, A. Eukaryotic DNA polymerases. Annu. Rev. Biochem. 1977, 46, 25–47. [Google Scholar] [CrossRef]
- Byrnes, J.J.; Downey, K.M.; Black, V.L.; So, A.G. A new mammalian DNA polymerase with 3′ to 5′ exonuclease activity: DNA polymerase δ. Biochemistry 1976, 15, 2817–2823. [Google Scholar] [CrossRef]
- Byrnes, J.J.; Downey, K.M.; Que, B.G.; Lee, M.Y.; Black, V.L.; So, A.G. Selective inhibition of the 3′ to 5′ exonuclease activity associated with DNA polymerases: A mechanism of mutagenesis. Biochemistry 1977, 16, 3740–3746. [Google Scholar] [CrossRef]
- Lee, M.Y.; Byrnes, J.J.; Downey, K.M.; So, A.G. Mechanism of inhibition of deoxyribonucleic acid synthesis by 1-β-D-arabinofuranosyladenosine triphosphate and its potentiation by 6-mercaptopurine ribonucleoside 5′-monophosphate. Biochemistry 1980, 19, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Tan, C.K.; So, A.G.; Downey, K.M. Purification of deoxyribonucleic acid polymerase δ from calf thymus: Partial characterization of physical properties. Biochemistry 1980, 19, 2096–2101. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Tan, C.K.; Downey, K.M.; So, A.G. Structural and functional properties of calf thymus DNA polymerase δ. Prog. Nucleic Acid. Res. Mol. Biol. 1981, 26, 83–96. [Google Scholar]
- Lee, M.Y.; Tan, C.K.; Downey, K.M.; So, A.G. Further studies on calf thymus DNA polymerase δ purified to homogeneity by a new procedure. Biochemistry 1984, 23, 1906–1913. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Toomey, N.L.; Wright, G.E. Differential inhibition of human placental DNA polymerases δ and α by BuPdGTP and BuAdATP. Nucleic Acids Res. 1985, 13, 8623–8630. [Google Scholar] [CrossRef]
- Lee, M.Y.; Toomey, N.L. Human placental DNA polymerase δ: Identification of a 170-kilodalton polypeptide by activity staining and immunoblotting. Biochemistry 1987, 26, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y. Isolation of multiple forms of DNA polymerase δ: Evidence of proteolytic modification during isolation. Biochemistry 1988, 27, 5188–5193. [Google Scholar] [CrossRef]
- Lee, M.Y.; Alejandro, R.; Toomey, N.L. Immunochemical studies of DNA polymerase δ: Relationships with DNA polymerase α. Arch. Biochem. Biophys. 1989, 272, 1–9. [Google Scholar] [CrossRef]
- Lee, M.Y.; Jiang, Y.Q.; Zhang, S.J.; Toomey, N.L. Characterization of human DNA polymerase δ and its immunochemical relationships with DNA polymerase α and epsilon. J. Biol. Chem. 1991, 266, 2423–2429. [Google Scholar] [CrossRef] [PubMed]
- Syvaoja, J.; Linn, S. Characterization of a large form of DNA polymerase δ from HeLa cells that is insensitive to proliferating cell nuclear antigen. J. Biol. Chem. 1989, 264, 2489–2497. [Google Scholar] [CrossRef]
- Crute, J.J.; Wahl, A.F.; Bambara, R.A. Purification and characterization of two new high molecular weight forms of DNA polymerase δ. Biochemistry 1986, 25, 26–36. [Google Scholar] [CrossRef]
- Burgers, P.M.; Bambara, R.A.; Campbell, J.L.; Chang, L.M.; Downey, K.M.; Hubscher, U.; Lee, M.Y.; Linn, S.M.; So, A.G.; Spadari, S. Revised nomenclature for eukaryotic DNA polymerases. Eur. J. Biochem. 1990, 191, 617–618. [Google Scholar] [CrossRef] [PubMed]
- Pospiech, H.; Syvaoja, J.E. DNA polymerase epsilon—More than a polymerase. Sci. World J. 2003, 3, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chung, D.W.; Tan, C.K.; Downey, K.M.; Davie, E.W.; So, A.G. Primary structure of the catalytic subunit of calf thymus DNA polymerase δ: Sequence similarities with other DNA polymerases. Biochemistry 1991, 30, 11742–11750. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Jiang, Y.; Zhang, S.J.; Zhang, P.; Zeng, R.X.; Lee, M.Y. Structural and functional relationships of human DNA polymerases. Chromosoma 1992, 102, S121–S127. [Google Scholar] [CrossRef]
- Yang, C.L.; Chang, L.S.; Zhang, P.; Hao, H.; Zhu, L.; Toomey, N.L.; Lee, M.Y. Molecular cloning of the cDNA for the catalytic subunit of human DNA polymerase δ. Nucleic Acids Res. 1992, 20, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Liu, L.; Leon, A.; Mazloum, N.; Lee, M.Y. Evidence that DNA polymerase δ isolated by immunoaffinity chromatography exhibits high-molecular weight characteristics and is associated with the KIAA0039 protein and RPA. Biochemistry 2000, 39, 7245–7254. [Google Scholar] [CrossRef]
- Hughes, P.; Tratner, I.; Ducoux, M.; Piard, K.; Baldacci, G. Isolation and identification of the third subunit of mammalian DNA polymerase δ by PCNA-affinity chromatography of mouse FM3A cell extracts. Nucleic Acids Res. 1999, 27, 2108–2114. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Mo, J.; Rodriguez-Belmonte, E.M.; Lee, M.Y. Identification of a fourth subunit of mammalian DNA polymerase δ. J. Biol. Chem. 2000, 275, 18739–18744. [Google Scholar] [CrossRef] [PubMed]
- Podust, V.N.; Chang, L.S.; Ott, R.; Dianov, G.L.; Fanning, E. Reconstitution of human DNA polymerase δ using recombinant baculoviruses: The p12 subunit potentiates DNA polymerizing activity of the four-subunit enzyme. J. Biol. Chem. 2002, 277, 3894–3901. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Mazloum, N.; Liu, L.; Rahmeh, A.; Li, H.; Lee, M.Y. Reconstitution and characterization of the human DNA polymerase δ four-subunit holoenzyme. Biochemistry 2002, 41, 13133–13142. [Google Scholar] [CrossRef]
- Li, H.; Xie, B.; Zhou, Y.; Rahmeh, A.; Trusa, S.; Zhang, S.; Gao, Y.; Lee, E.Y.; Lee, M.Y. Functional roles of p12, the fourth subunit of human DNA polymerase δ. J. Biol. Chem. 2006, 281, 14748–14755. [Google Scholar] [CrossRef]
- Gerik, K.J.; Li, X.; Pautz, A.; Burgers, P.M. Characterization of the two small subunits of Saccharomyces cerevisiae DNA polymerase δ. J. Biol. Chem. 1998, 273, 19747–19755. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Burgers, P.M. DNA polymerases that propagate the eukaryotic DNA replication fork. Crit. Rev. Biochem. Mol. Biol. 2005, 40, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Burgers, P.M. Polymerase dynamics at the eukaryotic DNA replication fork. J. Biol. Chem. 2009, 284, 4041–4045. [Google Scholar] [CrossRef]
- Burgers, P.M.J.; Kunkel, T.A. Eukaryotic DNA Replication Fork. Annu. Rev. Biochem. 2017, 86, 417–438. [Google Scholar] [CrossRef] [PubMed]
- Guilliam, T.A.; Yeeles, J.T.P. An updated perspective on the polymerase division of labor during eukaryotic DNA replication. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Nick McElhinny, S.A.; Gordenin, D.A.; Stith, C.M.; Burgers, P.M.; Kunkel, T.A. Division of labor at the eukaryotic replication fork. Mol. Cell 2008, 30, 137–144. [Google Scholar] [CrossRef]
- Zuo, S.; Gibbs, E.; Kelman, Z.; Wang, T.S.; O’Donnell, M.; MacNeill, S.A.; Hurwitz, J. DNA polymerase δ isolated from Schizosaccharomyces pombe contains five subunits. Proc. Natl. Acad. Sci. USA 1997, 94, 11244–11249. [Google Scholar] [CrossRef] [PubMed]
- Prelich, G.; Tan, C.K.; Kostura, M.; Mathews, M.B.; So, A.G.; Downey, K.M.; Stillman, B. Functional identity of proliferating cell nuclear antigen and a DNA polymerase-δ auxiliary protein. Nature 1987, 326, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, G.L.; Pfander, B.; Jentsch, S. PCNA, the maestro of the replication fork. Cell 2007, 129, 665–679. [Google Scholar] [CrossRef] [PubMed]
- MacNeill, S.A.; Moreno, S.; Reynolds, N.; Nurse, P.; Fantes, P.A. The fission yeast Cdc1 protein, a homologue of the small subunit of DNA polymerase δ, binds to Pol3 and Cdc27. EMBO J. 1996, 15, 4613–4628. [Google Scholar] [CrossRef]
- Bermudez, V.P.; MacNeill, S.A.; Tappin, I.; Hurwitz, J. The influence of the Cdc27 subunit on the properties of the Schizosaccharomyces pombe DNA polymerase δ. J. Biol. Chem. 2002, 277, 36853–36862. [Google Scholar] [CrossRef]
- Johansson, E.; Majka, J.; Burgers, P.M. Structure of DNA polymerase δ from Saccharomyces cerevisiae. J. Biol. Chem. 2001, 276, 43824–43828. [Google Scholar] [CrossRef]
- Loeillet, S.; Nicolas, A. DNA polymerase δ: A single Pol31 polymorphism suppresses the strain background-specific lethality of Pol32 inactivation in Saccharomyces cerevisiae. DNA Repair 2023, 127, 103514. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Tan, C.K.; Zhou, J.Q.; You, M.; Carastro, L.M.; Downey, K.M.; So, A.G. Direct interaction of proliferating cell nuclear antigen with the small subunit of DNA polymerase δ. J. Biol. Chem. 2002, 277, 24340–24345. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Chen, H.; Li, X.; Mai, W.; Chen, K.; Zhang, S.; Lee, E.Y.; Lee, M.Y.; Zhou, Y. P50, the small subunit of DNA polymerase δ, is required for mediation of the interaction of polymerase δ subassemblies with PCNA. PLoS ONE 2011, 6, e27092. [Google Scholar] [CrossRef] [PubMed]
- Rahmeh, A.A.; Zhou, Y.; Xie, B.; Li, H.; Lee, E.Y.; Lee, M.Y. Phosphorylation of the p68 subunit of Pol δ acts as a molecular switch to regulate its interaction with PCNA. Biochemistry 2012, 51, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Lancey, C.; Tehseen, M.; Raducanu, V.S.; Rashid, F.; Merino, N.; Ragan, T.J.; Savva, C.G.; Zaher, M.S.; Shirbini, A.; Blanco, F.J.; et al. Structure of the processive human Pol δ holoenzyme. Nat. Commun. 2020, 11, 1109. [Google Scholar] [CrossRef]
- Jozwiakowski, S.K.; Kummer, S.; Gari, K. Human DNA polymerase δ requires an iron-sulfur cluster for high-fidelity DNA synthesis. Life Sci. Alliance 2019, 2, e201900321. [Google Scholar] [CrossRef]
- Netz, D.J.; Stith, C.M.; Stumpfig, M.; Kopf, G.; Vogel, D.; Genau, H.M.; Stodola, J.L.; Lill, R.; Burgers, P.M.; Pierik, A.J. Eukaryotic DNA polymerases require an iron-sulfur cluster for the formation of active complexes. Nat. Chem. Biol. 2011, 8, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Meng, X.; Zhang, S.; Lee, E.Y.; Lee, M.Y. Characterization of human DNA polymerase δ and its subassemblies reconstituted by expression in the MultiBac system. PLoS ONE 2012, 7, e39156. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Suzuki, M.; Piao, J.; Gu, Y.; Tsurimoto, T.; Kamiya, K. Dynamics of human replication factors in the elongation phase of DNA replication. Nucleic Acids Res. 2007, 35, 6904–6916. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, S.J.; Wu, S.M.; Lee, M.Y. Immunoaffinity purification of DNA polymerase δ. Arch. Biochem. Biophys. 1995, 320, 297–304. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, S.; Zhang, Z.; Mazloum, N.A.; Lee, E.Y.C.; Lee, M.Y.W. The DHX9 helicase interacts with human DNA polymerase δ4 and stimulates its activity in D-loop extension synthesis. DNA Repair 2023, 128, 103513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chao, H.H.; Wang, X.; Zhang, Z.; Lee, E.Y.C.; Lee, M. Loss of the p12 subunit of DNA polymerase δ leads to a defect in HR and sensitization to PARP inhibitors. DNA Repair 2019, 73, 64–70. [Google Scholar] [CrossRef]
- Mailand, N.; Bekker-Jensen, S.; Faustrup, H.; Melander, F.; Bartek, J.; Lukas, C.; Lukas, J. RNF8 ubiquitylates histones at DNA double-strand breaks and promotes assembly of repair proteins. Cell 2007, 131, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Dutta, A. CRL4Cdt2: Master coordinator of cell cycle progression and genome stability. Cell Cycle 2011, 10, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Havens, C.G.; Walter, J.C. Mechanism of CRL4Cdt2, a PCNA-dependent E3 ubiquitin ligase. Genes Dev. 2011, 25, 1568–1582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhou, Y.; Trusa, S.; Meng, X.; Lee, E.Y.; Lee, M.Y. A novel DNA damage response: Rapid degradation of the p12 subunit of DNA polymerase δ. J. Biol. Chem. 2007, 282, 15330–15340. [Google Scholar] [CrossRef]
- Williams, R.M.; Zhang, X. Roles of ATM and ATR in DNA double strand breaks and replication stress. Prog. Biophys. Mol. Biol. 2021, 163, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Blackford, A.N.; Jackson, S.P. ATM, ATR, and DNA-PK: The Trinity at the Heart of the DNA Damage Response. Mol. Cell 2017, 66, 801–817. [Google Scholar] [CrossRef]
- Fan, L.; You, H.; Jiang, X.; Niu, Y.; Chen, Z.; Wang, H.; Xu, Y.; Zhou, P.; Wei, L.; Jiang, T.; et al. UCHL3 induces radiation resistance and acquisition of mesenchymal phenotypes by deubiquitinating POLD4 in glioma stem cells. Cell. Mol. Life Sci. 2024, 81, 247. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, Q.; You, C.; Qian, Y.; Gao, J.; Liu, P.; Chen, H.; Song, H.; Chen, Y.; Chen, K.; et al. Proteolysis of the human DNA polymerase δ smallest subunit p12 by mu-calpain in calcium-triggered apoptotic HeLa cells. PLoS ONE 2014, 9, e93642. [Google Scholar]
- Meng, X.; Zhou, Y.; Zhang, S.; Lee, E.Y.; Frick, D.N.; Lee, M.Y. DNA damage alters DNA polymerase δ to a form that exhibits increased discrimination against modified template bases and mismatched primers. Nucleic Acids Res. 2009, 37, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.H.; Wang, X.; Zhang, S.; Zhang, Z.; Lee, E.Y.; Lee, M.Y. Dynamics of enzymatic interactions during short flap human Okazaki fragment processing by two forms of human DNA polymerase δ. DNA Repair 2013, 12, 922–935. [Google Scholar] [CrossRef]
- Meng, X.; Zhou, Y.; Lee, E.Y.; Lee, M.Y.; Frick, D.N. The p12 subunit of human polymerase δ modulates the rate and fidelity of DNA synthesis. Biochemistry 2010, 49, 3545–3554. [Google Scholar] [CrossRef]
- Koc, K.N.; Stodola, J.L.; Burgers, P.M.; Galletto, R. Regulation of yeast DNA polymerase δ-mediated strand displacement synthesis by 5′-flaps. Nucleic Acids Res. 2015, 43, 4179–4190. [Google Scholar] [CrossRef]
- Barnes, R.P.; Thosar, S.A.; Opresko, P.L. Telomere Fragility and MiDAS: Managing the Gaps at the End of the Road. Genes 2023, 14, 348. [Google Scholar] [CrossRef] [PubMed]
- Dilley, R.L.; Verma, P.; Cho, N.W.; Winters, H.D.; Wondisford, A.R.; Greenberg, R.A. Break-induced telomere synthesis underlies alternative telomere maintenance. Nature 2016, 539, 54–58. [Google Scholar] [CrossRef]
- Minocherhomji, S.; Ying, S.; Bjerregaard, V.A.; Bursomanno, S.; Aleliunaite, A.; Wu, W.; Mankouri, H.W.; Shen, H.; Liu, Y.; Hickson, I.D. Replication stress activates DNA repair synthesis in mitosis. Nature 2015, 528, 286–290. [Google Scholar] [CrossRef]
- McVey, M.; Khodaverdian, V.Y.; Meyer, D.; Cerqueira, P.G.; Heyer, W.D. Eukaryotic DNA Polymerases in Homologous Recombination. Annu. Rev. Genet. 2016, 50, 393–421. [Google Scholar] [CrossRef] [PubMed]
- Sebesta, M.; Burkovics, P.; Juhasz, S.; Zhang, S.; Szabo, J.E.; Lee, M.Y.; Haracska, L.; Krejci, L. Role of PCNA and TLS polymerases in D-loop extension during homologous recombination in humans. DNA Repair 2013, 12, 691–698. [Google Scholar] [CrossRef]
- Sneeden, J.L.; Grossi, S.M.; Tappin, I.; Hurwitz, J.; Heyer, W.D. Reconstitution of recombination-associated DNA synthesis with human proteins. Nucleic Acids Res. 2013, 41, 4913–4925. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Dai, Q.; Du, P.; Li, N.; Li, J.; Zeng, S.; Peng, S.; Tang, S.; Wang, L.; Zhou, Z. Pold4 is dispensable for mouse development, DNA replication and DNA repair. Gene 2023, 851, 147029. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Malkova, A. Break-induced replication mechanisms in yeast and mammals. Curr. Opin. Genet. Dev. 2021, 71, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.A.; Kwon, Y.; Xu, Y.; Chung, W.H.; Chi, P.; Niu, H.; Mayle, R.; Chen, X.; Malkova, A.; Sung, P.; et al. Pif1 helicase and Poldelta promote recombination-coupled DNA synthesis via bubble migration. Nature 2013, 502, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Buzovetsky, O.; Kwon, Y.; Pham, N.T.; Kim, C.; Ira, G.; Sung, P.; Xiong, Y. Role of the Pif1-PCNA Complex in Pol δ-Dependent Strand Displacement DNA Synthesis and Break-Induced Replication. Cell Rep. 2017, 21, 1707–1714. [Google Scholar] [CrossRef]
- Li, S.; Wang, H.; Jehi, S.; Li, J.; Liu, S.; Wang, Z.; Truong, L.; Chiba, T.; Wang, Z.; Wu, X. PIF1 helicase promotes break-induced replication in mammalian cells. EMBO J. 2021, 40, e104509. [Google Scholar] [CrossRef]
- Wu, W.; Barwacz, S.A.; Bhowmick, R.; Lundgaard, K.; Goncalves Dinis, M.M.; Clausen, M.; Kanemaki, M.T.; Liu, Y. Mitotic DNA synthesis in response to replication stress requires the sequential action of DNA polymerases zeta and δ in human cells. Nat. Commun. 2023, 14, 706. [Google Scholar] [CrossRef] [PubMed]
- Chea, J.; Zhang, S.; Zhao, H.; Zhang, Z.; Lee, E.Y.; Darzynkiewicz, Z.; Lee, M.Y. Spatiotemporal recruitment of human DNA polymerase δ to sites of UV damage. Cell Cycle 2012, 11, 2885–2895. [Google Scholar] [CrossRef] [PubMed]
- Darzynkiewicz, Z.; Zhao, H.; Zhang, S.; Lee, M.Y.; Lee, E.Y.; Zhang, Z. Initiation and termination of DNA replication during S phase in relation to cyclins D1, E and A, p21WAF1, Cdt1 and the p12 subunit of DNA polymerase δ revealed in individual cells by cytometry. Oncotarget 2015, 6, 11735–11750. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, S.; Xu, D.; Lee, M.Y.; Zhang, Z.; Lee, E.Y.; Darzynkiewicz, Z. Expression of the p12 subunit of human DNA polymerase delta (Pol delta), CDK inhibitor p21WAF1, Cdt1, cyclin A, PCNA and Ki-67 in relation to DNA replication in individual cells. Cell Cycle 2014, 13, 3529–3540. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, S.; Zheng, R.; Yue, F.; Lin, S.H.; Rahmeh, A.A.; Lee, E.Y.; Zhang, Z.; Lee, M.Y. PDIP46 (DNA polymerase δ interacting protein 46) is an activating factor for human DNA polymerase δ. Oncotarget 2016, 7, 6294–6313. [Google Scholar] [CrossRef] [PubMed]
- Silver, D.P.; Livingston, D.M. Mechanisms of BRCA1 tumor suppression. Cancer Discov. 2012, 2, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Borresen-Dale, A.L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.M.; Tomida, S.; Masuda, Y.; Arima, C.; Cao, K.; Kasahara, T.A.; Osada, H.; Yatabe, Y.; Akashi, T.; Kamiya, K.; et al. Regulation of DNA polymerase POLD4 influences genomic instability in lung cancer. Cancer Res. 2010, 70, 8407–8416. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.T.; Ali, M.S.; Goel, H.; Singh, J.; Chatterjee, B.; Bose, S.; Hadda, V.; Chopra, A. Expression Profile, Molecular Association, and Clinical Significance of POLD4 in Glioblastoma. Cell. Mol. Neurobiol. 2023, 43, 3753–3765. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Fan, F.; Xu, W.; Jiang, X. POLD4 Promotes Glioma Cell Proliferation and Suppressive Immune Microenvironment: A Pan-Cancer Analysis Integrated with Experimental Validation. Int. J. Mol. Sci. 2023, 24, 13919. [Google Scholar] [CrossRef]
- Sahu, J.K.; Thakur, S.; Subhadarsini, I.; Acharya, N. p12 isoform-2 is a regulatory subunit of human DNA polymerase δ and is dysregulated in various cancers. FEBS Lett. 2024, 598, 3087–3104. [Google Scholar] [CrossRef] [PubMed]
- Groelly, F.J.; Fawkes, M.; Dagg, R.A.; Blackford, A.N.; Tarsounas, M. Targeting DNA damage response pathways in cancer. Nat. Rev. Cancer 2023, 23, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Brownlie, J.; Kulkarni, S.; Algethami, M.; Jeyapalan, J.N.; Mongan, N.P.; Rakha, E.A.; Madhusudan, S. Targeting DNA damage repair precision medicine strategies in cancer. Curr. Opin. Pharmacol. 2023, 70, 102381. [Google Scholar] [CrossRef] [PubMed]
- Kojima, K.; Ohkubo, H.; Kawasumi, R.; Hirota, K. Pold4 subunit of replicative polymerase δ promotes fork slowing at broken templates. DNA Repair 2024, 139, 103688. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.; Ayyamperumal, S.; Zhang, S.; Chen, J.; Lee, E.Y.C.; Lee, M.Y.W.T. The p12 Subunit Choreographs the Regulation and Functions of Two Forms of DNA Polymerase δ in Mammalian Cells. Genes 2025, 16, 188. https://doi.org/10.3390/genes16020188
Xu D, Ayyamperumal S, Zhang S, Chen J, Lee EYC, Lee MYWT. The p12 Subunit Choreographs the Regulation and Functions of Two Forms of DNA Polymerase δ in Mammalian Cells. Genes. 2025; 16(2):188. https://doi.org/10.3390/genes16020188
Chicago/Turabian StyleXu, Dazhong, Selvaraj Ayyamperumal, Sufang Zhang, Jinjin Chen, Ernest Y. C. Lee, and Marietta Y. W. T. Lee. 2025. "The p12 Subunit Choreographs the Regulation and Functions of Two Forms of DNA Polymerase δ in Mammalian Cells" Genes 16, no. 2: 188. https://doi.org/10.3390/genes16020188
APA StyleXu, D., Ayyamperumal, S., Zhang, S., Chen, J., Lee, E. Y. C., & Lee, M. Y. W. T. (2025). The p12 Subunit Choreographs the Regulation and Functions of Two Forms of DNA Polymerase δ in Mammalian Cells. Genes, 16(2), 188. https://doi.org/10.3390/genes16020188





