Neuromolecular Basis of Impaired Conditioned Taste Aversion Acquisition in Valproate-Induced Rat Model of Autism Spectrum Disorder
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sodium Valproate Exposure
2.3. Establishing Responsiveness to i.p. LiCl
2.4. Effect of LiCl on c-Fos Immunoreactivity in the Brain in VPA Versus Control Rats
2.5. Gene Expression Analyses
2.5.1. Experimental Groups and Microdissection
2.5.2. qRT-PCR Protocol and Data Analysis
2.6. Data Analysis
3. Results
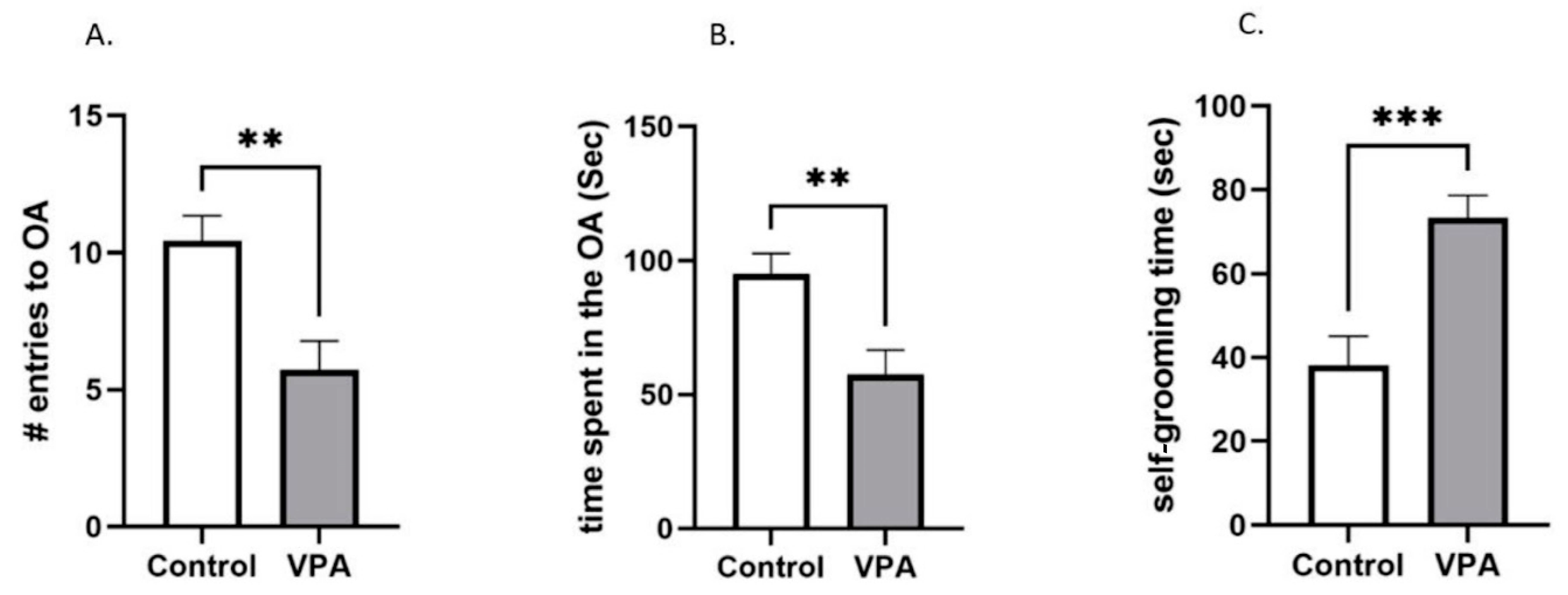
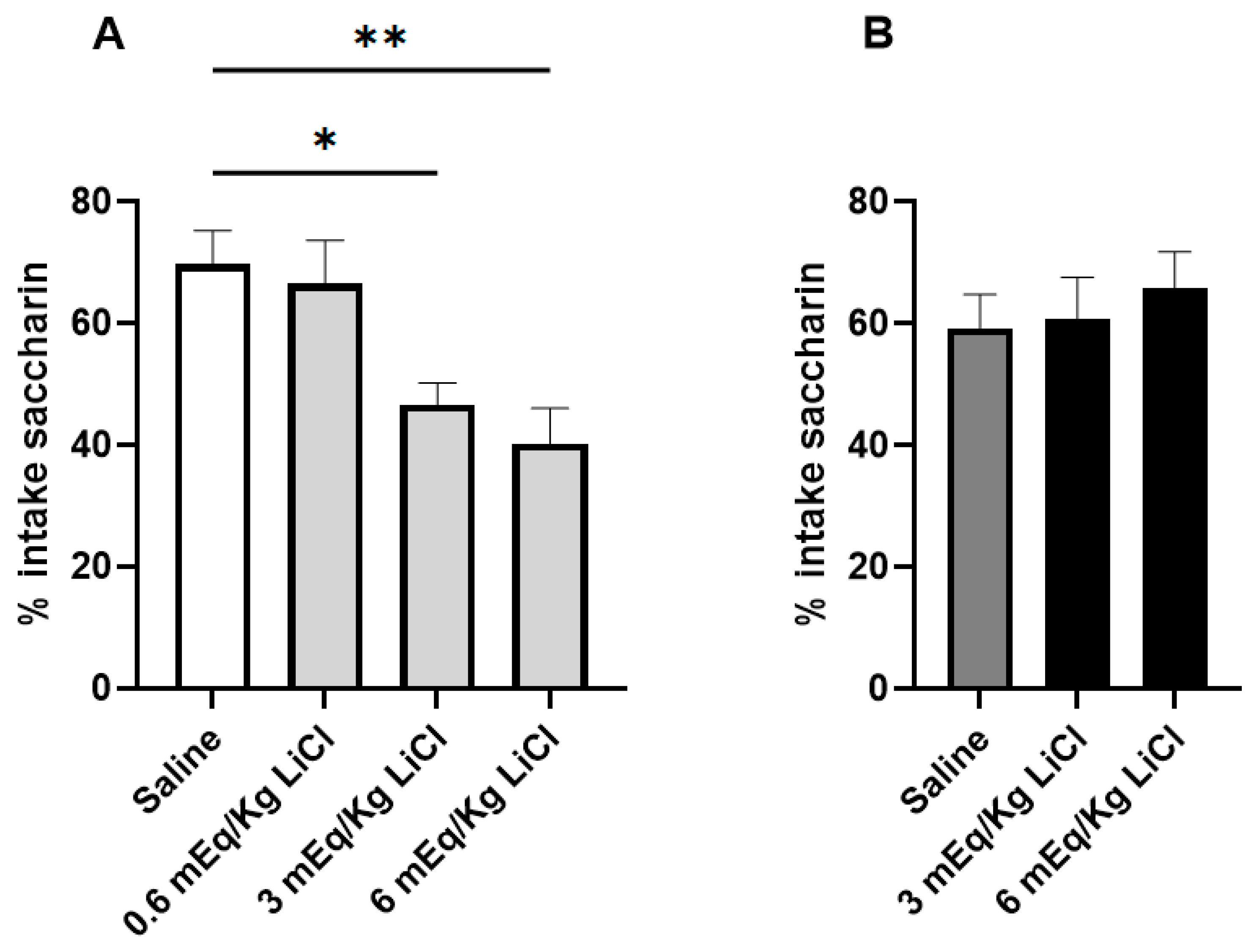
3.1. VPA Animals Are Resistant to CTA Acquisition
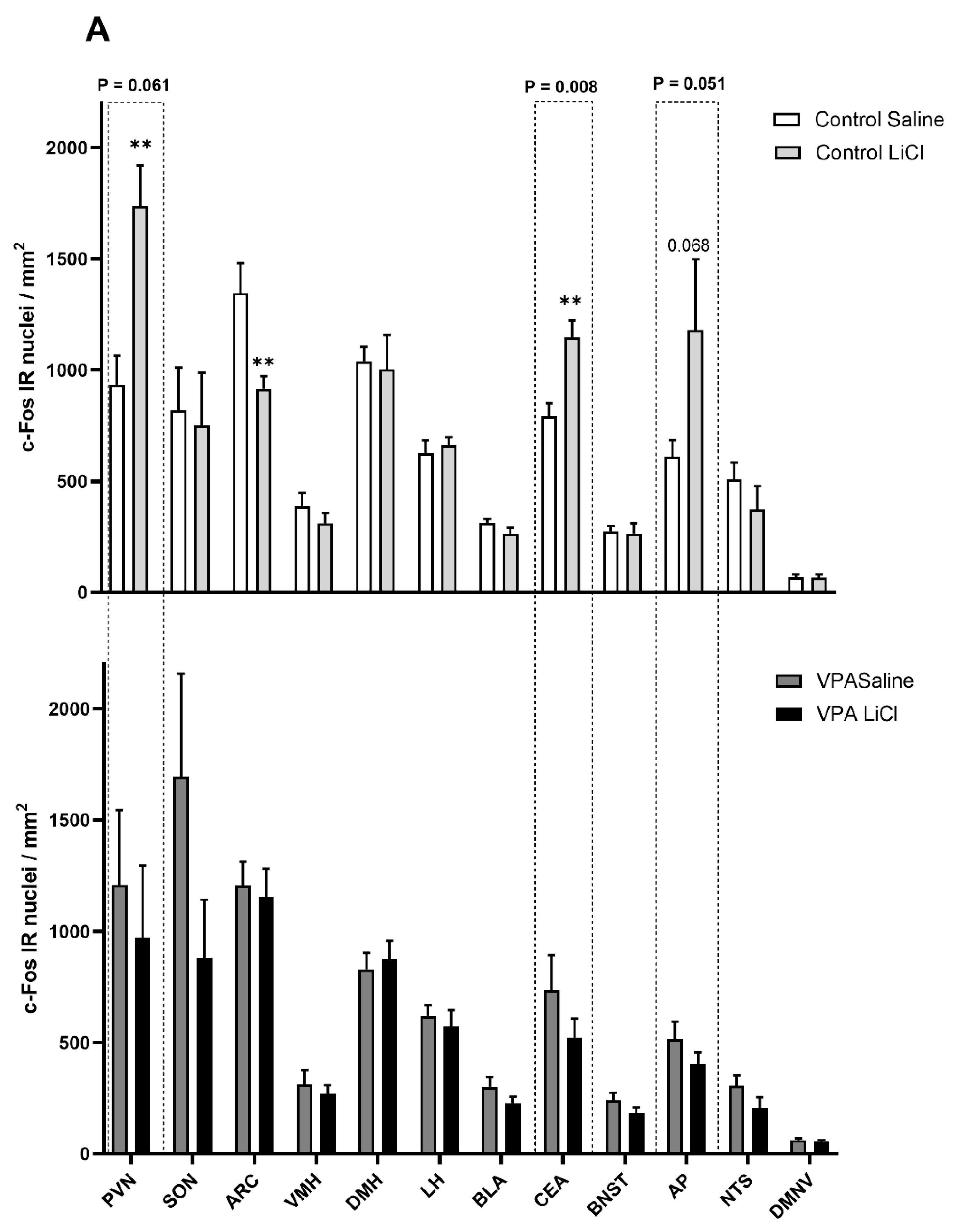
3.2. i.p. 3 mEq/kg LiCl Fails to Drive Changes in c-Fos Immunoreactivity in the VPA Rats
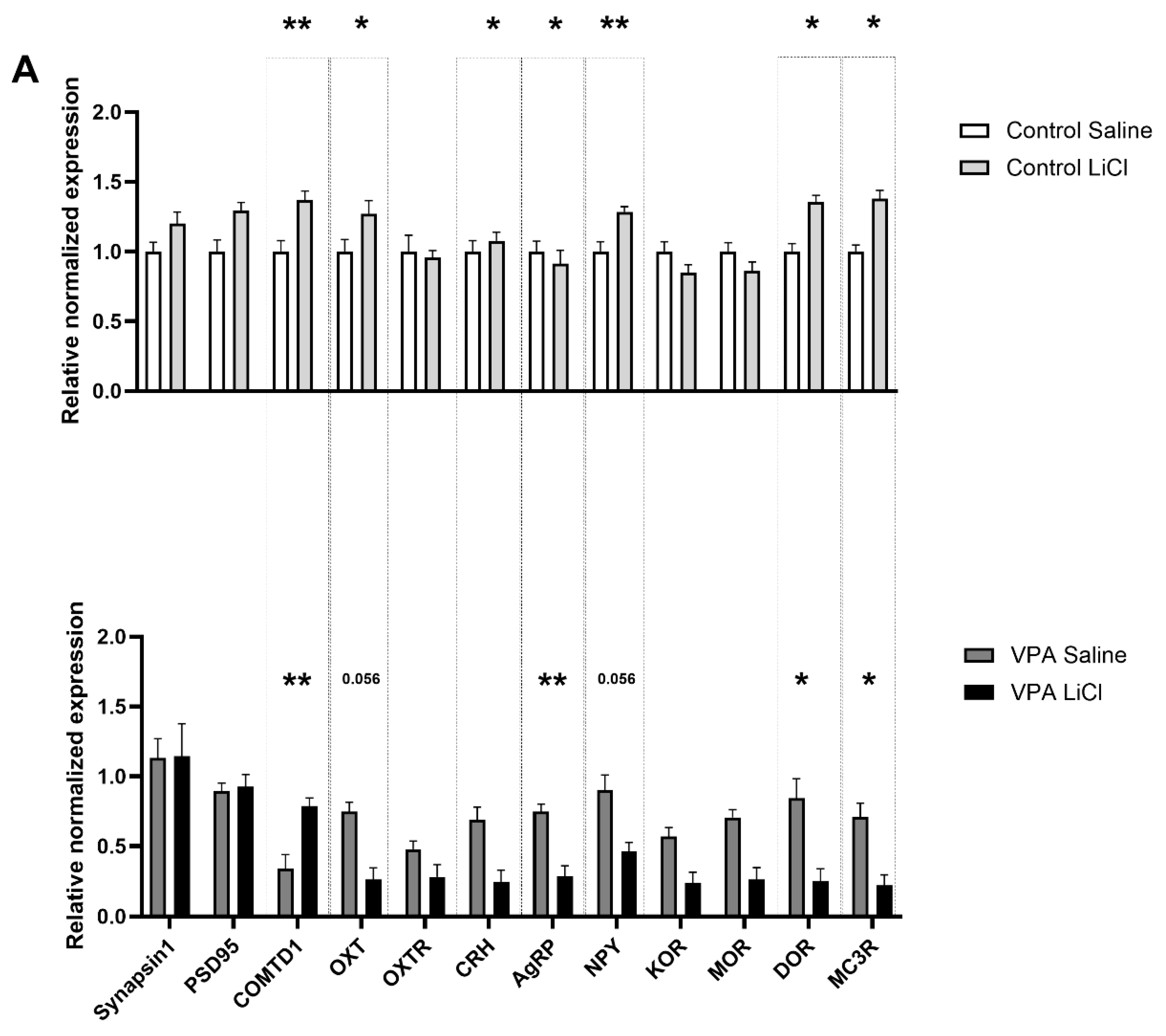
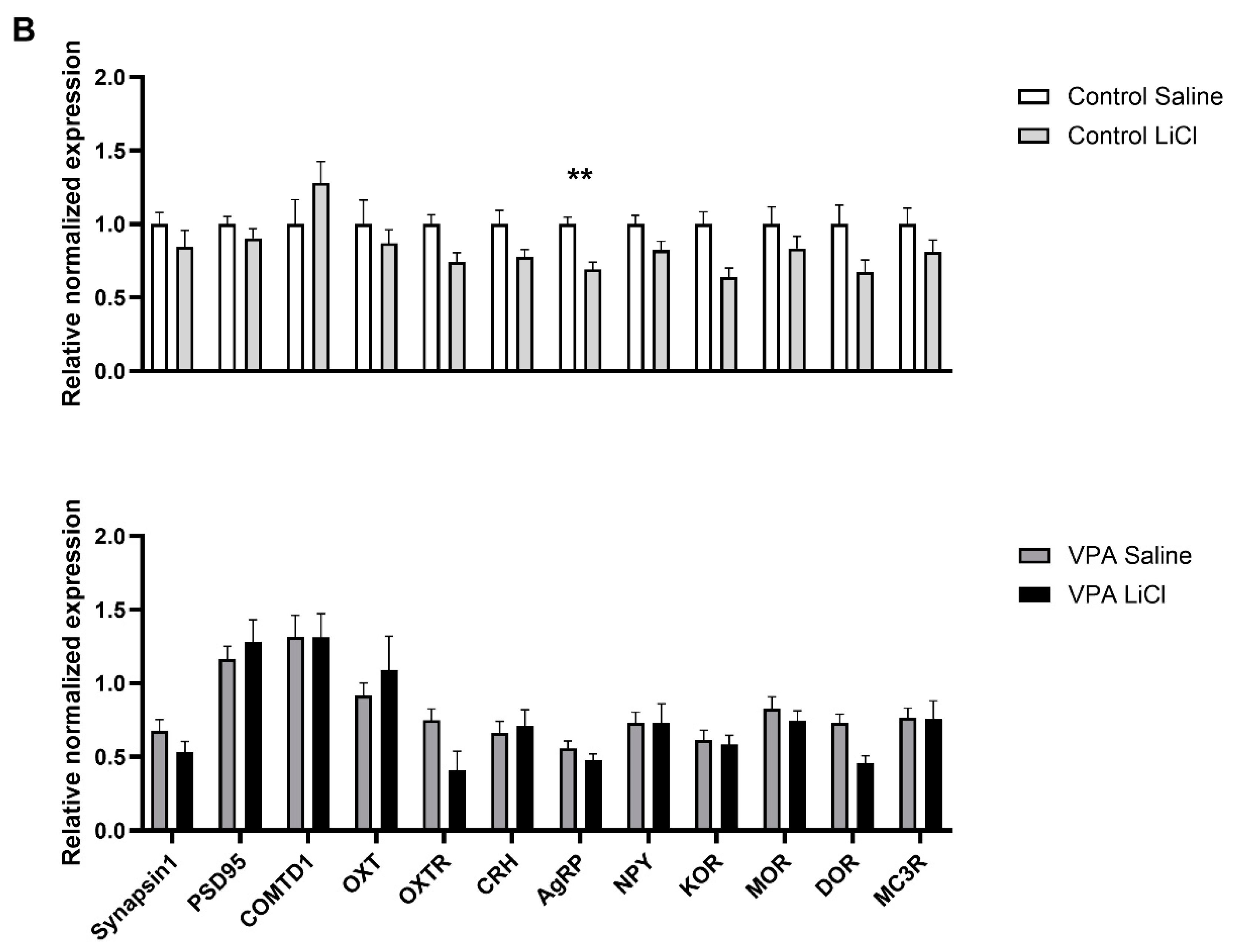
3.3. LiCl Injection Affects Expression of Different Transcripts in the VPA Rats than in Controls
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yarmolinsky, D.A.; Zuker, C.S.; Ryba, N.J. Common sense about taste: From mammals to insects. Cell 2009, 139, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Glendinning, J.I. Is chemosensory input essential for the rapid rejection of toxic foods? J. Exp. Biol. 1996, 199, 1523–1534. [Google Scholar] [CrossRef]
- Neath, K.N.; Limebeer, C.L.; Reilly, S.; Parker, L.A. Increased liking for a solution is not necessary for the attenuation of neophobia in rats. Behav. Neurosci. 2010, 124, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Welzl, H.; D’Adamo, P.; Lipp, H.P. Conditioned taste aversion as a learning and memory paradigm. Behav. Brain Res. 2001, 125, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Horn, C.C.; Kimball, B.A.; Wang, H.; Kaus, J.; Dienel, S.; Nagy, A.; Gathright, G.R.; Yates, B.J.; Andrews, P.L. Why can’t rodents vomit? A comparative behavioral, anatomical, and physiological study. PLoS ONE 2013, 8, e60537. [Google Scholar] [CrossRef]
- Liu, L.S.; Winston, J.H.; Shenoy, M.M.; Song, G.Q.; Chen, J.D.; Pasricha, P.J. A rat model of chronic gastric sensorimotor dysfunction resulting from transient neonatal gastric irritation. Gastroenterology 2008, 134, 2070–2079. [Google Scholar] [CrossRef]
- Wang, J.; Gu, C.; Al-Chaer, E.D. Altered behavior and digestive outcomes in adult male rats primed with minimal colon pain as neonates. Behav. Brain Funct. 2008, 4, 28. [Google Scholar] [CrossRef]
- Rollins, B.L.; Stines, S.G.; McGuire, H.B.; King, B.M. Effects of amygdala lesions on body weight, conditioned taste aversion, and neophobia. Physiol. Behav. 2001, 72, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Panguluri, S.K.; Kuwabara, N.; Kang, Y.; Cooper, N.; Lundy, R.F. Conditioned taste aversion dependent regulation of amygdala gene expression. Physiol. Behav. 2012, 105, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Haubensak, W.; Anthony, T.E.; Anderson, D.J. Central amygdala PKC-δ+ neurons mediate the influence of multiple anorexigenic signals. Nat. Neurosci. 2014, 17, 1240–1248. [Google Scholar] [CrossRef]
- Ding, W.; Weltzien, H.; Peters, C.; Klein, R. Nausea-induced suppression of feeding is mediated by central amygdala Dlk1-expressing neurons. Cell Rep. 2024, 43, 113990. [Google Scholar] [CrossRef]
- Weissberg, O.; Elliott, E. The Mechanisms of CHD8 in Neurodevelopment and Autism Spectrum Disorders. Genes 2021, 12, 1133. [Google Scholar] [CrossRef] [PubMed]
- Weiss, L.A.; Escayg, A.; Kearney, J.A.; Trudeau, M.; MacDonald, B.T.; Mori, M.; Reichert, J.; Buxbaum, J.D.; Meisler, M.H. Sodium channels SCN1A, SCN2A and SCN3A in familial autism. Mol. Psychiatry 2003, 8, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Szafranski, P.; Golla, S.; Jin, W.; Fang, P.; Hixson, P.; Matalon, R.; Kinney, D.; Bock, H.G.; Craigen, W.; Smith, J.L.; et al. Neurodevelopmental and neurobehavioral characteristics in males and females with CDKL5 duplications. Eur. J. Hum. Genet. 2015, 23, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Amllal, N.; Lyahyai, J.; Elalaoui, S.C.; El Kadiri, Y.; Sefiani, A. Novel Splice Site Pathogenic Variant in STXBP1 Gene in a Child with Intellectual Disability, Epilepsy, and Autism Spectrum Disorder: A Case Report. Mol. Syndr. Syndromol. 2024, 15, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Goss, J.R.; Prosser, B.; Helbig, I.; Son Rigby, C. STXBP1: Fast-forward to a brighter future—A patient organization perspective. Ther. Adv. Rare Dis. 2024, 5, 26330040241257221. [Google Scholar] [CrossRef]
- Luppe, J.; Sticht, H.; Lecoquierre, F.; Goldenberg, A.; Gorman, K.M.; Molloy, B.; Agolini, E.; Novelli, A.; Briuglia, S.; Kuismin, O.; et al. Heterozygous and homozygous variants in STX1A cause a neurodevelopmental disorder with or without epilepsy. Eur. J. Hum. Genet. 2023, 31, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.L. SNAREs and developmental disorders. J. Cell Physiol. 2021, 236, 2482–2504. [Google Scholar] [CrossRef]
- Neul, J.L. The relationship of Rett syndrome and MECP2 disorders to autism. Dialogues Clin. Neurosci. 2012, 14, 253–262. [Google Scholar] [CrossRef]
- Wang, J.; Frohlich, H.; Torres, F.B.; Silva, R.L.; Poschet, G.; Agarwal, A.; Rappold, G.A. Mitochondrial dysfunction and oxidative stress contribute to cognitive and motor impairment in FOXP1 syndrome. Proc. Natl. Acad. Sci. USA 2022, 119, e2112852119. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Dharmadhikari, A.V.; Varland, S.; Ma, N.; Domingo, D.; Kleyner, R.; Rope, A.F.; Yoon, M.; Stray-Pedersen, A.; Posey, J.E.; et al. Truncating Variants in NAA15 Are Associated with Variable Levels of Intellectual Disability, Autism Spectrum Disorder, and Congenital Anomalies. Am. J. Hum. Genet. 2018, 102, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, S.; Eickholt, B.J. PTEN in Autism and Neurodevelopmental Disorders. Cold Spring Harb. Perspect. Med. 2019, 9, a036780. [Google Scholar] [CrossRef] [PubMed]
- Chiurazzi, P.; Kiani, A.K.; Miertus, J.; Paolacci, S.; Barati, S.; Manara, E.; Stuppia, L.; Gurrieri, F.; Bertelli, M. Genetic analysis of intellectual disability and autism. Acta Biomed. 2020, 91, e2020003. [Google Scholar] [CrossRef]
- Vinci, M.; Treccarichi, S.; Galati Rando, R.; Musumeci, A.; Todaro, V.; Federico, C.; Saccone, S.; Elia, M.; Calì, F. A de novo ARIH2 gene mutation was detected in a patient with autism spectrum disorders and intellectual disability. Sci. Rep. 2024, 14, 15848. [Google Scholar] [CrossRef]
- Huxham, L.; Marais, M.; van Niekerk, E. Idiosyncratic food preferences of children with autism spectrum disorder in England. S. Afr. J. Clin. Nutr. 2021, 34, 90–96. [Google Scholar] [CrossRef]
- Schreck, K.A.; Williams, K. Food preferences and factors influencing food selectivity for children with autism spectrum disorders. Res. Dev. Disabil. 2006, 27, 353–363. [Google Scholar] [CrossRef]
- Pliner, P. Development of measures of food neophobia in children. Appetite 1994, 23, 147–163. [Google Scholar] [CrossRef]
- Ahearn, W.H.; Castine, T.; Nault, K.; Green, G. An assessment of food acceptance in children with autism or pervasive developmental disorder-not otherwise specified. J. Autism Dev. Disord. 2001, 31, 505–511. [Google Scholar] [CrossRef]
- Fields, V.L.; Soke, G.N.; Reynolds, A.; Tian, L.H.; Wiggins, L.; Maenner, M.; DiGuiseppi, C.; Kral, T.V.E.; Hightshoe, K.; Schieve, L.A. Pica, Autism, and Other Disabilities. Pediatrics 2021, 147, e20200462. [Google Scholar] [CrossRef]
- Schnitzler, E. The Neurology and Psychopathology of Pica. Curr. Neurol. Neurosci. Rep. 2022, 22, 531–536. [Google Scholar] [CrossRef]
- Takeda, N.; Hasegawa, S.; Morita, M.; Matsunaga, T. Pica in rats is analogous to emesis: An animal model in emesis research. Pharmacol. Biochem. Behav. 1993, 45, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Kosaki, Y.; Watanabe, S. Impaired Pavlovian predictive learning between temporally phasic but not static events in autism-model strain mice. Neurobiol. Learn. Mem. 2016, 134 Pt B, 304–316. [Google Scholar] [CrossRef]
- Strekalova, T.; Svirin, E.; Veniaminova, E.; Kopeikina, E.; Veremeyko, T.; Yung, A.W.Y.; Proshin, A.; Walitza, S.; Anthony, D.C.; Lim, L.W.; et al. ASD-like behaviors, a dysregulated inflammatory response and decreased expression of PLP1 characterize mice deficient for sialyltransferase ST3GAL5. Brain Behav. Immun.—Health 2021, 16, 100306. [Google Scholar] [CrossRef] [PubMed]
- Klockars, A.; Pal, T.; Levine, A.S.; Olszewski, P.K. Neural Basis of Dysregulation of Palatability-Driven Appetite in Autism. Curr. Nutr. Rep. 2021, 10, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Harvey, S.; Liyanagamage, D.; Pal, T.; Klockars, A.; Levine, A.S.; Olszewski, P.K. Palatable solution overconsumption in the Cntnap2-/- murine model of autism: A link with oxytocin. Neuroreport 2024, 35, 980–986. [Google Scholar] [CrossRef]
- Kuo, H.-Y.; Liu, F.-C. Pathophysiological Studies of Monoaminergic Neurotransmission Systems in Valproic Acid-Induced Model of Autism Spectrum Disorder. Biomedicines 2022, 10, 560. [Google Scholar] [CrossRef]
- Chomiak, T.; Turner, N.; Hu, B. What We Have Learned about Autism Spectrum Disorder from Valproic Acid. Pathol. Res. Int. 2013, 2013, 712758. [Google Scholar] [CrossRef]
- Mabunga, D.F.; Gonzales, E.L.; Kim, J.W.; Kim, K.C.; Shin, C.Y. Exploring the Validity of Valproic Acid Animal Model of Autism. Exp. Neurobiol. 2015, 24, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.; Roth, A.; Kyzar, E.J.; Poudel, M.K.; Wong, K.; Stewart, A.M.; Kalueff, A.V. Decoding the contribution of dopaminergic genes and pathways to autism spectrum disorder (ASD). Neurochem. Int. 2014, 66, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Pal, T.; Laloli, K.J.; Moscrip, C.A.; Olszewski, P.K.; Klockars, A. Mild Hypophagia and Associated Changes in Feeding-Related Gene Expression and c-Fos Immunoreactivity in Adult Male Rats with Sodium Valproate-Induced Autism. Genes 2022, 13, 259. [Google Scholar] [CrossRef]
- Yamasue, H.; Domes, G. Oxytocin and Autism Spectrum Disorders. In Behavioral Pharmacology of Neuropeptides: Oxytocin; Hurlemann, R., Grinevich, V., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 449–465. [Google Scholar]
- Jung, S.; Park, M. Shank postsynaptic scaffolding proteins in autism spectrum disorder: Mouse models and their dysfunctions in behaviors, synapses, and molecules. Pharmacol. Res. 2022, 182, 106340. [Google Scholar] [CrossRef]
- Makris, G.; Agorastos, A.; Chrousos, G.P.; Pervanidou, P. Stress System Activation in Children and Adolescents with Autism Spectrum Disorder. Front. Neurosci. 2022, 15, 756628. [Google Scholar] [CrossRef] [PubMed]
- Klockars, A.; Wood, E.L.; Gartner, S.N.; McColl, L.K.; Levine, A.S.; Carpenter, E.A.; Prosser, C.G.; Olszewski, P.K. Palatability of Goat’s versus Cow’s Milk: Insights from the Analysis of Eating Behavior and Gene Expression in the Appetite-Relevant Brain Circuit in Laboratory Animal Models. Nutrients 2019, 11, 720. [Google Scholar] [CrossRef]
- Williams, P.G.; Dalrymple, N.; Neal, J. Eating habits of children with autism. Pediatr. Nurs. 2000, 26, 259–264. [Google Scholar] [PubMed]
- Wang, T.; Feng, J.; Xue, Y.; Shan, L.; Jia, F.; Yue, X. Feeding problems, age of introduction of complementary food and autism symptom in children with autism spectrum disorder. Front. Pediatr. 2022, 10, 860947. [Google Scholar] [CrossRef]
- Keen, D.V. Childhood autism, feeding problems and failure to thrive in early infancy. Seven case studies. Eur. Child. Adolesc. Psychiatry 2008, 17, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Kalat, J.W. Taste salience depends on novelty, not concentration, in taste-aversion learning in the rat. J. Comp. Physiol. Psychol. 1974, 86, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.D.; Daly, E.M.; Bullmore, E.T.; Williams, S.C.; Van Amelsvoort, T.; Robertson, D.M.; Rowe, A.; Phillips, M.; McAlonan, G.; Howlin, P.; et al. The functional neuroanatomy of social behaviour: Changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain 2000, 123 Pt 11, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Hall, G.B.; Szechtman, H.; Nahmias, C. Enhanced salience and emotion recognition in Autism: A PET study. Am. J. Psychiatry 2003, 160, 1439–1441. [Google Scholar] [CrossRef]
- Hubl, D.; Bölte, S.; Feineis-Matthews, S.; Lanfermann, H.; Federspiel, A.; Strik, W.; Poustka, F.; Dierks, T. Functional imbalance of visual pathways indicates alternative face processing strategies in autism. Neurology 2003, 61, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.T.; Dapretto, M.; Hariri, A.R.; Sigman, M.; Bookheimer, S.Y. Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. J. Am. Acad. Child. Adolesc. Psychiatry 2004, 43, 481–490. [Google Scholar] [CrossRef]
- Aja, S.; Sisouvong, S.; Barrett, J.A.; Gietzen, D.W. Basolateral and central amygdaloid lesions leave aversion to dietary amino acid imbalance intact. Physiol. Behav. 2000, 71, 533–541. [Google Scholar] [CrossRef]
- Lasiter, P.S.; Glanzman, D.L. Cortical substrates of taste aversion learning: Involvement of dorsolateral amygdaloid nuclei and temporal neocortex in taste aversion learning. Behav. Neurosci. 1985, 99, 257–276. [Google Scholar] [CrossRef]
- Yamamoto, T. Brain regions responsible for the expression of conditioned taste aversion in rats. Chem. Senses 2007, 32, 105–109. [Google Scholar] [CrossRef]
- Agüera, A.D.; Puerto, A. Lesions of the central nucleus of the amygdala only impair flavor aversion learning in the absence of olfactory information. Acta Neurobiol. Exp. 2015, 75, 381–390. [Google Scholar] [CrossRef]
- Ferretti, V.; Maltese, F.; Contarini, G.; Nigro, M.; Bonavia, A.; Huang, H.; Gigliucci, V.; Morelli, G.; Scheggia, D.; Managò, F.; et al. Oxytocin Signaling in the Central Amygdala Modulates Emotion Discrimination in Mice. Curr. Biol. 2019, 29, 1938–1953.e1936. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, P.K.; Waas, J.R.; Brooks, L.L.; Herisson, F.; Levine, A.S. Oxytocin receptor blockade reduces acquisition but not retrieval of taste aversion and blunts responsiveness of amygdala neurons to an aversive stimulus. Peptides 2013, 50, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Spencer, C.M.; Eckel, L.A.; Nardos, R.; Houpt, T.A. Area postrema lesions attenuate LiCl-induced c-Fos expression correlated with conditioned taste aversion learning. Physiol. Behav. 2012, 105, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Rinaman, L.; Dzmura, V. Experimental dissociation of neural circuits underlying conditioned avoidance and hypophagic responses to lithium chloride. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1495–R1503. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.M.; Bureš, J. Unit activity changes elicited in amygdala and neocortex of anaesthetized rats by intraperitoneal injection of lithium chloride. Neurosci. Lett. 1981, 22, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Sako, N.; Sakai, N.; Iwafune, A. Gustatory and visceral inputs to the amygdala of the rat: Conditioned taste aversion and induction of c-fos-like immunoreactivity. Neurosci. Lett. 1997, 226, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Spray, K.J.; Cubero, I.; Thiele, T.E.; Bernstein, I.L. cFos induction during conditioned taste aversion expression varies with aversion strength. Brain Res. 2000, 887, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Klockars, O.A.; Klockars, A.; Levine, A.S.; Olszewski, P.K. Oxytocin administration in the basolateral and central nuclei of amygdala moderately suppresses food intake. Neuroreport 2018, 29, 504–510. [Google Scholar] [CrossRef]
- Lamprecht, R.; Hazvi, S.; Dudai, Y. cAMP response element-binding protein in the amygdala is required for long- but not short-term conditioned taste aversion memory. J. Neurosci. 1997, 17, 8443–8450. [Google Scholar] [CrossRef] [PubMed]
- Wirth, M.M.; Olszewski, P.K.; Levine, A.S.; Giraudo, S.Q. Effect of Agouti-related protein on development of conditioned taste aversion and oxytocin neuronal activation. NeuroReport 2002, 13, 1355–1358. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Li, Y.; Meng, X.; Liu, A.; Mao, Y.; Zhu, X.; Meng, Q.; Jin, Y.; Zhang, Z.; Tao, W. Switching of delta opioid receptor subtypes in central amygdala microcircuits is associated with anxiety states in pain. J. Biol. Chem. 2021, 296, 100277. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, P.; Bedenbaugh, M.N.; Maldonado, J.; Pan, P.; Fowler, K.; Williams, S.Y.; Gimenez, L.E.; Ghamari-Langroudi, M.; Downing, G.; Gui, Y.; et al. The melanocortin-3 receptor is a pharmacological target for the regulation of anorexia. Sci. Transl. Med. 2021, 13, eabd6434. [Google Scholar] [CrossRef]
- Yamamoto, T.; Shimura, T.; Sako, N.; Azuma, S.; Bai, W.Z.; Wakisaka, S. C-fos expression in the rat brain after intraperitoneal injection of lithium chloride. Neuroreport 1992, 3, 1049–1052. [Google Scholar] [CrossRef]
- Portillo, F.; Carrasco, M.; Juan Vallo, J. Separate populations of neurons within the paraventricular hypothalamic nucleus of the rat project to vagal and thoracic autonomic preganglionic levels and express c-Fos protein induced by lithium chloride. J. Chem. Neuroanat. 1998, 14, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, P.K.; Grace, M.K.; Fard, S.S.; Le Grevès, M.; Klockars, A.; Massi, M.; Schiöth, H.B.; Levine, A.S. Central nociceptin/orphanin FQ system elevates food consumption by both increasing energy intake and reducing aversive responsiveness. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R655–R663. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Gonzalez, M.F.; Chin, D.Y.; Deutsch, J.A. Expression of c-fos in brain subcortical structures in response to nauseant lithium chloride and osmotic pressure in rats. Neurosci. Lett. 1993, 157, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Jiang, S.; Wang, J.; Bai, Y.; Kim, C.S.; Blake, D.; Weintraub, N.L.; Lei, Y.; Lu, X.-Y. Chronic unpredictable stress induces depression-related behaviors by suppressing AgRP neuron activity. Mol. Psychiatry 2021, 26, 2299–2315. [Google Scholar] [CrossRef] [PubMed]
- Essner, R.A.; Smith, A.G.; Jamnik, A.A.; Ryba, A.R.; Trutner, Z.D.; Carter, M.E. AgRP Neurons Can Increase Food Intake during Conditions of Appetite Suppression and Inhibit Anorexigenic Parabrachial Neurons. J. Neurosci. 2017, 37, 8678–8687. [Google Scholar] [CrossRef]
- Adachi, A.; Kobashi, M.; Miyoshi, N.; Tsukamoto, G. Chemosensitive neurons in the area postrema of the rat and their possible functions. Brain Res. Bull. 1991, 26, 137–140. [Google Scholar] [CrossRef]

| Housekeeping Genes | ||
|---|---|---|
| Gene | Forward | Reverse |
| Actin b | 5′-AGTGTGACGTTGACATCC GT-3′ | 5′-TGCTAGGAGCCAGAGCAGTA-3′ |
| TBP | 5′-AGAACAATCCAGACTAGCAGA-3′ | 5′-GGGAACTTCACATCACAGCTC-3′ |
| Genes of Interest | ||
| Gene | Forward | Reverse |
| MC3R | 5′-AGCAACCGGAGTGGCAGT-3′ | 5′-GGCCACGATCAAGGAGAG-3′ |
| AgRP | 5′-CAGAGTTCTCAGGTCTAAGTC-3′ | 5′-TTGAAGAAGCGGCAGTAGCAC-3′ |
| NPY | 5′-AGGTAACAAACGAATGGGGCT-3′ | 5′-TGATGTAGTGTCGCAGAGCG-3′ |
| COMTD1 | 5′-TGTGTGCGGAACCTAAACGA-3′ | 5′-GAAGGTCGCGTGTTCCAGTA-3′ |
| CRH | 5′-TGGATCTCACCTTCCACCTT-3′ | 5′-TTCATTTCCCGATAATCTCCA-3′ |
| MOR | 5′-CGGACTCGGTAGGCTGTAAC-3′ | 5′-CCTGCCGCTCTTCTCTGG-3′ |
| KOR | 5′-AGACCGCAACCAACATCTACAT-3′ | 5′-GCACAGAACATCTCCAAAAGG-3′ |
| DOR | 5′-GCRACATTGCGGTCTGCCAC-3′ | 5′-CGAAGGCGAAGAGGAACACG-3′ |
| OXT | 5′-GACGGTGGATCTCGGACTGAA-3′ | 5′-CGCCCCTAAAGGTATCATCACAAA-3′ |
| OXTR | 5′-GATCACGCTCGCCGTCTA-3′ | 5′-CCGTCTTGAGTCGCAGATTC-3′ |
| Synapsin1 | 5′-CACCAGGATGAAGACAAGCA-3′ | 5′-GTCGTTGTTGAGCAGGAGGT-3′ |
| PSD95 | 5′-CTTCTCAGCCATCGTAGAGG-3′ | 5′-GAGAGGTCTTCAATGACACG-3′ |
| Gene | F (DFn, DFd) | p Value |
|---|---|---|
| COMTD1 | F (1, 33) = 9.304 | p = 0.004 |
| OXT | F (1,31) = 6.904 | p = 0.013 |
| CRH | F (1, 33) = 4.496 | p = 0.042 |
| AgRP | F (1, 30) = 5.544 | p = 0.025 |
| NPY | F (1, 34) = 9.861 | p = 0.003 |
| DOR | F (1, 31) = 5.996 | p = 0.020 |
| MC3R | F (1, 32) = 7.068 | p = 0.012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pal, T.; Harvey, S.; Levine, A.S.; Olszewski, P.K.; Klockars, A. Neuromolecular Basis of Impaired Conditioned Taste Aversion Acquisition in Valproate-Induced Rat Model of Autism Spectrum Disorder. Genes 2025, 16, 203. https://doi.org/10.3390/genes16020203
Pal T, Harvey S, Levine AS, Olszewski PK, Klockars A. Neuromolecular Basis of Impaired Conditioned Taste Aversion Acquisition in Valproate-Induced Rat Model of Autism Spectrum Disorder. Genes. 2025; 16(2):203. https://doi.org/10.3390/genes16020203
Chicago/Turabian StylePal, Tapasya, Savannah Harvey, Allen S. Levine, Pawel K. Olszewski, and Anica Klockars. 2025. "Neuromolecular Basis of Impaired Conditioned Taste Aversion Acquisition in Valproate-Induced Rat Model of Autism Spectrum Disorder" Genes 16, no. 2: 203. https://doi.org/10.3390/genes16020203
APA StylePal, T., Harvey, S., Levine, A. S., Olszewski, P. K., & Klockars, A. (2025). Neuromolecular Basis of Impaired Conditioned Taste Aversion Acquisition in Valproate-Induced Rat Model of Autism Spectrum Disorder. Genes, 16(2), 203. https://doi.org/10.3390/genes16020203







