Molecular Abnormalities and Carcinogenesis in Barrett’s Esophagus: Implications for Cancer Treatment and Prevention
Abstract
1. Introduction
2. Methods
2.1. Database Search
2.2. Study Selection
2.3. Data Extraction and Main Outcomes
2.4. Data Synthesis
3. Results
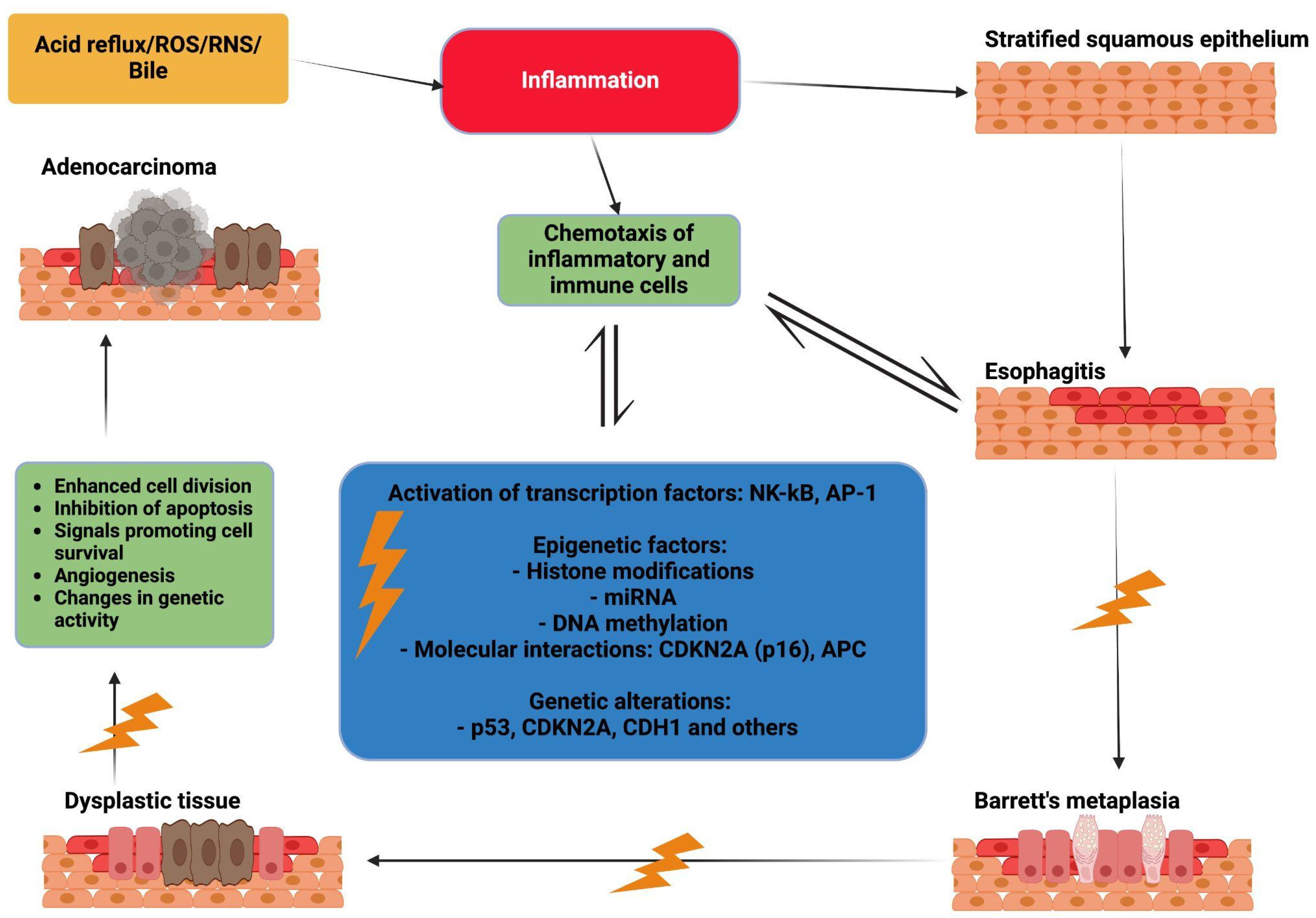
3.1. Chronic Inflammation
3.2. Genetic Alteration
3.2.1. Point Mutations
3.2.2. Deletion
3.2.3. Amplifications
3.3. Epigenetic Changes
3.3.1. Histone Modifications
3.3.2. MiRNA
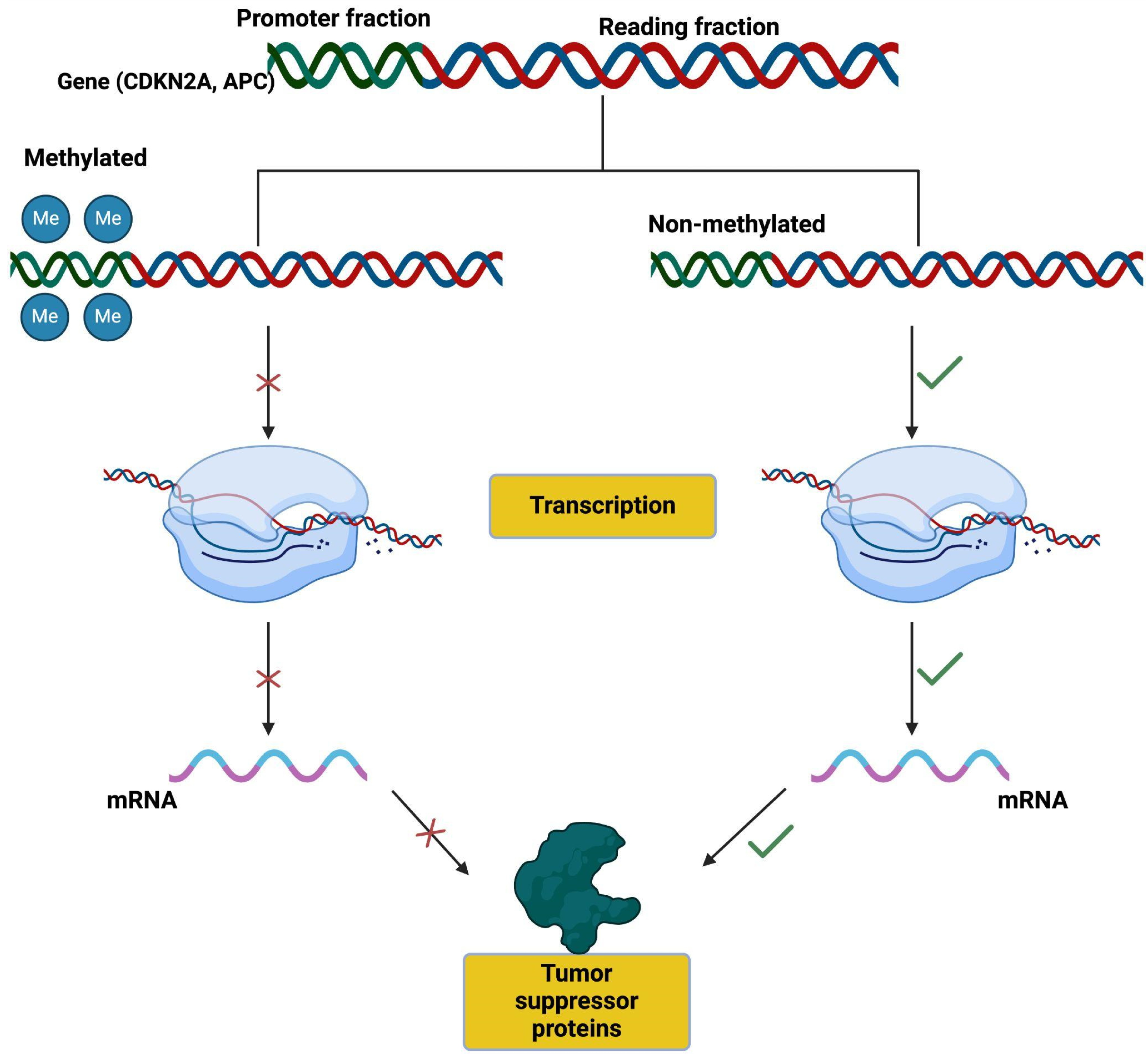
3.3.3. DNA Methylation
3.3.4. Main Molecular Interactions
CDKN2A (p16)
APC
3.3.5. Other Hypermethylated Genes in Barrett’s Carcinoma
3.4. Chromosomal Alterations
3.5. The Combination of Genetic, Epigenetic, and Chromosomal Alterations in Predictive Models
4. The Future of Research on Carcinogenesis in Barrett’s Esophagus: Prevention, Surveillance, and Precision Medicine
4.1. Prevention Strategies: The Role of Chemoprevention and Surgery
4.2. Individualized Surveillance: Considering Risk Factors
4.3. Precision Medicine: Identifying Molecular Targets
4.4. Artificial Intelligence (AI)
4.5. The Role of PPIs and Nutrition
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barrett, N.R. Chronic peptic ulcerz of the œophagus and ‘œsophagitis’. Br. J. Surg. 1950, 38, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, N.J.; Falk, G.W.; Iyer, P.G.; Souza, R.F.; Yadlapati, R.H.; Sauer, B.G.; Wani, S. Diagnosis and Management of Barrett’s Esophagus: An Updated ACG Guideline. Am. J. Gastroenterol. 2022, 117, 559–587. [Google Scholar] [CrossRef] [PubMed]
- Qumseya, B.J.; Bukannan, A.; Gendy, S.; Ahemd, Y.; Sultan, S.; Bain, P.; Gross, S.A.; Iyer, P.; Wani, S. Systematic review and meta-analysis of prevalence and risk factors for Barrett’s esophagus. Gastrointest. Endosc. 2019, 90, 707–717.e1. [Google Scholar] [CrossRef] [PubMed]
- Morales, C.P.; Spechler, S.J. Intestinal metaplasia at the gastroesophageal junction: Barrett’s, bacteria, and biomarkers. Am. J. Gastroenterol. 2003, 98, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Thrift, A.P. Global burden and epidemiology of Barrett oesophagus and oesophageal cancer. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Puli, S.; El-Serag, H.B.; Bansal, A.; Wani, S.; Sharma, P. Incidence of esophageal adenocarcinoma in patients with Barrett’s esophagus and high-grade dysplasia: A meta-analysis. Gastrointest. Endosc. 2008, 67, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.; Soerjomataram, I.; Rumgay, H.; Coleman, H.G.; Thrift, A.P.; Vignat, J.; Laversanne, M.; Ferlay, J.; Arnold, M. The Global Landscape of Esophageal Squamous Cell Carcinoma and Esophageal Adenocarcinoma Incidence and Mortality in 2020 and Projections to 2040: New Estimates from GLOBOCAN 2020. Gastroenterology 2022, 163, 649–658.e2. [Google Scholar] [CrossRef] [PubMed]
- Maslyonkina, K.S.; Konyukova, A.K.; Alexeeva, D.Y.; Sinelnikov, M.Y.; Mikhaleva, L.M. Barrett’s esophagus: The pathomorphological and molecular genetic keystones of neoplastic progression. Cancer Med. 2022, 11, 447–478. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peters, Y.; Al-Kaabi, A.; Shaheen, N.J.; Chak, A.; Blum, A.; Souza, R.F.; Di Pietro, M.; Iyer, P.G.; Pech, O.; Fitzgerald, R.C.; et al. Barrett oesophagus. Nat. Rev. Dis. Primers 2019, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.P.; Harris, C.C. Inflammation and cancer: An ancient link with novel potentials. Int. J. Cancer 2007, 121, 2373–2380. [Google Scholar] [CrossRef]
- Tustumi, F.; de Moura, D.T.H.; Waisberg, J.; Herbella, F.A.M. Editorial: Premalignant conditions in the esophagus and stomach. Front. Oncol. 2022, 12, 1091911. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Warren, J.R.; Marshall, B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1983, 1, 1273–1275. [Google Scholar] [PubMed]
- Chiba, T.; Marusawa, H.; Seno, H.; Watanabe, N. Mechanism for gastric cancer development by Helicobacter pylori infection. J. Gastroenterol. Hepatol. 2008, 23 Pt 1, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, N.J.; Richter, J.E. Barrett’s oesophagus. Lancet 2009, 373, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oberg, S.; Clark, G.W.; DeMeester, T.R. Barrett’s esophagus. Update of pathophysiology and management. Hepatogastroenterology 1998, 45, 1348–1356. [Google Scholar] [PubMed]
- Fitzgerald, R.C.; Omary, M.B.; Triadafilopoulos, G. Dynamic effects of acid on Barrett’s esophagus. An ex vivo proliferation and differentiation model. J. Clin. Investig. 1996, 98, 2120–2128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abdel-Latif, M.M.; Duggan, S.; Reynolds, J.V.; Kelleher, D. Inflammation and esophageal carcinogenesis. Curr. Opin. Pharmacol. 2009, 9, 396–404. [Google Scholar] [CrossRef] [PubMed]
- El-Omar, E.M.; Carrington, M.; Chow, W.H.; McColl, K.E.; Bream, J.H.; Young, H.A.; Herrera, J.; Lissowska, J.; Yuan, C.C.; Rothman, N.; et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 2000, 404, 398–402, Erratum in Nature 2001, 412, 99. [Google Scholar] [CrossRef] [PubMed]
- Ben-Neriah, Y.; Karin, M. Inflammation meets cancer, with NF-κB as the matchmaker. Nat. Immunol. 2011, 12, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Kuraishy, A.; Karin, M.; Grivennikov, S.I. Tumor promotion via injury- and death-induced inflammation. Immunity 2011, 35, 467–477. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Domazet-Loso, T.; Tautz, D. Phylostratigraphic tracking of cancer genes suggests a link to the emergence of multicellularity in metazoa. BMC Biol. 2010, 8, 66. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duckett, C.S. Apoptosis and NF-κB: The FADD connection. J. Clin. Investig. 2002, 109, 579–580. [Google Scholar] [CrossRef] [PubMed]
- Guttridge, D.C.; Albanese, C.; Reuther, J.Y.; Pestell, R.G.; Baldwin, A.S., Jr. NF-κB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol. Cell. Biol. 1999, 19, 5785–5799. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- La Rosa, F.A.; Pierce, J.W.; Sonenshein, G.E. Differential regulation of the c-myc oncogene promoter by the NF-kappa B rel family of transcription factors. Mol. Cell. Biol. 1994, 14, 1039–1044. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hussain, S.P.; Hofseth, L.J.; Harris, C.C. Radical causes of cancer. Nat. Rev. Cancer 2003, 3, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Dubois, R.N. Eicosanoids and cancer. Nat. Rev. Cancer 2010, 10, 181–193. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kanda, K.; Komekado, H.; Sawabu, T.; Ishizu, S.; Nakanishi, Y.; Nakatsuji, M.; Akitake-Kawano, R.; Ohno, M.; Hiraoka, Y.; Kawada, M.; et al. Nardilysin and ADAM proteases promote gastric cancer cell growth by activating intrinsic cytokine signalling via enhanced ectodomain shedding of TNF-α. EMBO Mol. Med. 2012, 4, 396–411. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Häcker, H.; Karin, M. Regulation and function of IKK and IKK-related kinases. Sci. STKE 2006, 2006, re13. [Google Scholar] [CrossRef] [PubMed]
- Pyo, C.W.; Yang, Y.L.; Yoo, N.K.; Choi, S.Y. Reactive oxygen species activate HIV long terminal repeat via post-translational control of NF-κB. Biochem. Biophys. Res. Commun. 2008, 376, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Soutto, M.; Belkhiri, A.; Piazuelo, M.B.; Schneider, B.G.; Peng, D.; Jiang, A.; Washington, M.K.; Kokoye, Y.; Crowe, S.E.; Zaika, A.; et al. Loss of TFF1 is associated with activation of NF-κB-mediated inflammation and gastric neoplasia in mice and humans. J. Clin. Investig. 2011, 121, 1753–1767. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karin, M. NF-κB and cancer: Mechanisms and targets. Mol. Carcinog. 2006, 45, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Tselepis, C.; Perry, I.; Dawson, C.; Hardy, R.; Darnton, S.J.; McConkey, C.; Stuart, R.C.; Wright, N.; Harrison, R.; Jankowski, J.A. Tumour necrosis factor-α in Barrett’s oesophagus: A potential novel mechanism of action. Oncogene 2002, 21, 6071–6081. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T. Interleukin 6 and its receptor: Ten years later. Int. Rev. Immunol. 1998, 16, 249–284. [Google Scholar] [CrossRef] [PubMed]
- Krueger, J.; Ray, A.; Tamm, I.; Sehgal, P.B. Expression and function of interleukin-6 in epithelial cells. J. Cell. Biochem. 1991, 45, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Cohen, T.; Nahari, D.; Cerem, L.W.; Neufeld, G.; Levi, B.Z. Interleukin 6 induces the expression of vascular endothelial growth factor. J. Biol. Chem. 1996, 271, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Chow, K.C.; Wu, C.W. Expression and up-regulation of interleukin-6 in oesophageal carcinoma cells by n-sodium butyrate. Br. J. Cancer 1999, 80, 1617–1622. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Dvorakova, K.; Payne, C.M.; Ramsey, L.; Holubec, H.; Sampliner, R.; Dominguez, J.; Dvorak, B.; Bernstein, H.; Bernstein, C.; Prasad, A.; et al. Increased expression and secretion of interleukin-6 in patients with Barrett’s esophagus. Clin. Cancer Res. 2004, 10, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Sekikawa, A.; Fukui, H.; Fujii, S.; Ichikawa, K.; Tomita, S.; Imura, J.; Chiba, T.; Fujimori, T. REG Iα protein mediates an anti-apoptotic effect of STAT3 signaling in gastric cancer cells. Carcinogenesis 2008, 29, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Fukui, H.; Franceschi, F.; Penland, R.L.; Sakai, T.; Sepulveda, A.R.; Fujimori, T.; Terano, A.; Chiba, T.; Genta, R.M. Effects of Helicobacter pylori infection on the link between regenerating gene expression and serum gastrin levels in Mongolian gerbils. Lab. Investig. 2003, 83, 1777–1786. [Google Scholar] [CrossRef] [PubMed]
- Sekikawa, A.; Fukui, H.; Fujii, S.; Takeda, J.; Nanakin, A.; Hisatsune, H.; Seno, H.; Takasawa, S.; Okamoto, H.; Fujimori, T.; et al. REG Iα protein may function as a trophic and/or anti-apoptotic factor in the development of gastric cancer. Gastroenterology 2005, 128, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Sekikawa, A.; Fukui, H.; Fujii, S.; Nanakin, A.; Kanda, N.; Uenoyama, Y.; Sawabu, T.; Hisatsune, H.; Kusaka, T.; Ueno, S.; et al. Possible role of REG Iα protein in ulcerative colitis and colitic cancer. Gut 2005, 54, 1437–1444. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fukui, H.; Fujii, S.; Takeda, J.; Kayahara, T.; Sekikawa, A.; Nanakin, A.; Suzuki, K.; Hisatsune, H.; Seno, H.; Sawada, M.; et al. Expression of reg Iα protein in human gastric cancers. Digestion 2004, 69, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Bromberg, J.; Darnell, J.E., Jr. The role of STATs in transcriptional control and their impact on cellular function. Oncogene 2000, 19, 2468–2473. [Google Scholar] [CrossRef] [PubMed]
- Buettner, R.; Mora, L.B.; Jove, R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin. Cancer Res. 2002, 8, 945–954. [Google Scholar] [PubMed]
- Levy, D.E.; Darnell, J.E., Jr. Stats: Transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 2002, 3, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Yao, Q.; Gu, X.; Shi, Q.; Yuan, X.; Chu, Q.; Bao, Z.; Lu, J.; Li, L. Evolving cognition of the JAK-STAT signaling pathway: Autoimmune disorders and cancer. Signal Transduct. Target. Ther. 2023, 8, 204. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, H.; Pardoll, D.; Jove, R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer 2009, 9, 798–809. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cardin, R.; Piciocchi, M.; Tieppo, C.; Maddalo, G.; Zaninotto, G.; Mescoli, C.; Rugge, M.; Farinati, F. Oxidative DNA Damage in Barrett Mucosa: Correlation with Telomeric Dysfunction and P53 Mutation. Ann. Surg. Oncol. 2013, 20 (Suppl. S3), S583–S589. [Google Scholar] [CrossRef]
- Altorki, N.K.; Oliveria, S.; Schrump, D.S. Epidemiology and Molecular Biology of Barrett’s Adenocarcinoma. Semin. Surg. Oncol. 1997, 13, 270–280. [Google Scholar]
- Wiseman, H.; Halliwell, B. Damage to DNA by reactive oxygen and nitrogen species: Role in inflammatory disease and progression to cancer. Biochem. J. 1996, 313, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, S.; Hiraku, Y. Oxidative and nitrative DNA damage as biomarker for carcinogenesis with special reference to inflammation. Antioxid. Redox Signal. 2006, 8, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Thanan, R.; Ma, N.; Kawanishi, S. Role of nitrative and oxidative DNA damage in inflammation-related carcinogenesis. J. Biomed. Biotechnol. 2012, 2012, 623019. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, S.; Ohnishi, S.; Ma, N.; Hiraku, Y.; Oikawa, S.; Murata, M. Nitrative and oxidative DNA damage in infection-related carcinogenesis in relation to cancer stem cells. Genes Environ. 2017, 38, 55. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Zhang, C. The Oxidative Damage and Inflammation Mechanisms in GERD-Induced Barrett’s Esophagus. Front. Cell Dev. Biol. 2022, 10, 885537. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thanan, R.; Ma, N.; Hiraku, Y.; Iijima, K.; Koike, T.; Shimosegawa, T.; Murata, M.; Kawanishi, S. DNA Damage in CD133-Positive Cells in Barrett’s Esophagus and Esophageal Adenocarcinoma. Mediat. Inflamm. 2016, 2016, 7937814. [Google Scholar] [CrossRef]
- Liu, C.; Yu, X. ADP-ribosyltransferases and Poly ADP-Ribosylation. Curr. Protein Pept. Sci. 2015, 16, 491–501. [Google Scholar] [CrossRef]
- Schreiber, V.; Dantzer, F.; Ame, J.-C.; de Murcia, G. Poly(ADP-ribose): Novel Functions for an Old Molecule. Nat. Rev. Mol. Cell Biol. 2006, 7, 517–528. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, T.; Luo, T.; Li, A.; Gao, X.; Wang, Z.G.; Li, F. Dysregulation of PARP1 Is Involved in Development of Barrett’s Esophagus. World J. Gastroenterol. 2018, 24, 982–991. [Google Scholar] [CrossRef]
- Hassa, P.O.; Hottiger, M.O. A Role of Poly (ADP-Ribose) Polymerase in NF-κB Transcriptional Activation. Biol. Chem. 1999, 380, 953–959. [Google Scholar] [CrossRef]
- Liu, L.; Ke, Y.; Jiang, X.; He, F.; Pan, L.; Xu, L.; Zeng, X.; Ba, X. Lipopolysaccharide Activates ERK-PARP-1-RelA Pathway and Promotes Nuclear Factor-Κb Transcription in Murine Macrophages. Hum. Immunol. 2012, 73, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Quesada, R.; Munoz-Gamez, J.A.; Martin-Oliva, D.; Peralta-Leal, A.; Quiles-Perez, R.; Rodriguez-Vargas, J.M.; Ruiz de Almodovar, M.; Conde, C.; Ruiz-Extremera, A.; Oliver, F.J. Modulation of Transcription by PARP-1: Consequences in Carcinogenesis and Inflammation. Curr. Med. Chem. 2007, 14, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Brunyánszki, A.; Hegedűs, C.; Szántó, M.; Erdélyi, K.; Kovács, K.; Schreiber, V.; Gergely, S.; Kiss, B.; Szabó, É.; Virág, L.; et al. Genetic Ablation of PARP-1 Protects against Oxazolone-Induced Contact Hypersensitivity by Modulating Oxidative Stress. J. Investig. Dermatol. 2010, 130, 2629–2637. [Google Scholar] [CrossRef] [PubMed]
- Tustumi, F.; Agareno, G.A.; Galletti, R.P.; da Silva, R.B.R.; Quintas, J.G.; Sesconetto, L.A.; Szor, D.J.; Wolosker, N. The Role of the Heat-Shock Proteins in Esophagogastric Cancer. Cells 2022, 11, 2664. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakamura, E.T.; Park, A.; Pereira, M.A.; Kikawa, D.; Tustumi, F. Prognosis value of heat-shock proteins in esophageal and esophagogastric cancer: A systematic review and meta-analysis. World J. Gastrointest. Oncol. 2024, 16, 1578–1595. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jolly, C.; Morimoto, R.I. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J. Natl. Cancer Inst. 2000, 92, 1564–1572. [Google Scholar] [CrossRef]
- Yun, C.W.; Kim, H.J.; Lim, J.H.; Lee, S.H. Heat Shock Proteins: Agents of Cancer Development and Therapeutic Targets in Anti-Cancer Therapy. Cells 2019, 9, 60. [Google Scholar] [CrossRef]
- Zheng, C.X.; Wang, Z.Q.; Lin, W.B.; Chu, Z.H.; Chen, L.H.; Ji, Z.Q. Expression of heat shock protein 27 in the esophageal tissue of rats with reflux esophagitis. Chin. Med. J. 2011, 124, 2347–2353. [Google Scholar]
- Dutta, S.M.; Mustafi, S.B.; Raha, S.; Chakraborty, S.K. Assessment of thermal stress adaptation by monitoring Hsp70 and MnSOD in the freshwater gastropod, Bellamya bengalensis (Lamark 1882). Environ. Monit. Assess. 2014, 186, 8961–8967. [Google Scholar] [CrossRef]
- Hamel, C.; Ahmadzai, N.; Beck, A.; Thuku, M.; Skidmore, B.; Pussegoda, K.; Bjerre, L.; Chatterjee, A.; Dennis, K.; Ferri, L.; et al. Screening for esophageal adenocarcinoma and precancerous conditions (dysplasia and Barrett’s esophagus) in patients with chronic gastroesophageal reflux disease with or without other risk factors: Two systematic reviews and one overview of reviews to inform a guideline of the Canadian Task Force on Preventive Health Care (CTFPHC). Syst. Rev. 2020, 9, 20. [Google Scholar] [CrossRef]
- Landry, J.; Chrétien, P.; Lambert, H.; Hickey, E.; Weber, L.A. Heat shock resistance conferred by expression of the human HSP27 gene in rodent cells. J. Cell Biol. 1989, 109, 7–15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mehlen, P.; Mehlen, A.; Guillet, D.; Preville, X.; Arrigo, A.P. Tumor necrosis factor-α induces changes in the phosphorylation, cellular localization, and oligomerization of human hsp27, a stress protein that confers cellular resistance to this cytokine. J. Cell. Biochem. 1995, 58, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Mehlen, P.; Preville, X.; Chareyron, P.; Briolay, J.; Klemenz, R.; Arrigo, A.P. Constitutive expression of human hsp27, Drosophila hsp27, or human α B-crystallin confers resistance to TNF- and oxidative stress-induced cytotoxicity in stably transfected murine L929 fibroblasts. J. Immunol. 1995, 154, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Huot, J.; Roy, G.; Lambert, H.; Chrétien, P.; Landry, J. Increased survival after treatments with anticancer agents of Chinese hamster cells expressing the human Mr 27,000 heat shock protein. Cancer Res. 1991, 51, 5245–5252. [Google Scholar] [PubMed]
- Richards, E.H.; Hickey, E.; Weber, L.; Master, J.R. Effect of overexpression of the small heat shock protein HSP27 on the heat and drug sensitivities of human testis tumor cells. Cancer Res. 1996, 56, 2446–2451. [Google Scholar] [PubMed]
- Wu, W.; Welsh, M.J. Expression of the 25-kDa heat-shock protein (HSP27) correlates with resistance to the toxicity of cadmium chloride, mercuric chloride, cis-platinum(II)-diammine dichloride, or sodium arsenite in mouse embryonic stem cells transfected with sense or antisense HSP27 cDNA. Toxicol. Appl. Pharmacol. 1996, 141, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Soldes, O.S.; Kuick, R.D.; Thompson, I.A., II; Hughes, S.J.; Orringer, M.B.; Iannettoni, M.D.; Hanash, S.M.; Beer, D.G. Differential expression of Hsp27 in normal oesophagus, Barrett’s metaplasia and oesophageal adenocarcinomas. Br. J. Cancer 1999, 79, 595–603. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stephanie, M.; Nour, H.; de Sá Inês, M.; Shanker, K.; Kevin, K.; Mario, D.R.; Prateek, S. Gender differences in Barrett’s esophagus and progression of disease: A systematic review and meta-analysis. Dis. Esophagus 2022, 35, doab075. [Google Scholar] [CrossRef] [PubMed]
- Fuqua, S.A.; Blum-Salingaros, M.; McGuire, W.L. Induction of the estrogen-regulated “24K” protein by heat shock. Cancer Res. 1989, 49, 4126–4129. [Google Scholar] [PubMed]
- Ciocca, D.R.; Adams, D.J.; Edwards, D.P.; Bjercke, R.J.; McGuire, W.L. Distribution of an estrogen-induced protein with a molecular weight of 24,000 in normal and malignant human tissues and cells. Cancer Res. 1983, 43, 1204–1210. [Google Scholar] [PubMed]
- Rafiee, P.; Theriot, M.E.; Nelson, V.M.; Heidemann, J.; Kanaa, Y.; Horowitz, S.A.; Rogaczewski, A.; Johnson, C.P.; Ali, I.; Shaker, R.; et al. Human esophageal microvascular endothelial cells respond to acidic pH stress by PI3K/AKT and p38 MAPK-regulated induction of Hsp70 and Hsp27. Am. J. Physiol. Physiol. 2006, 291, C931–C945. [Google Scholar] [CrossRef] [PubMed]
- Mauchley, D.; Meng, X.; Johnson, T.; Teitelbaum, J.; Babu, A.; Fullerton, D.A.; Weyant, M.J. Heat shock protein 27: Induction by gastroduodenal reflux in vivo and augmentation of human esophageal mucosal cell growth in vitro. J. Thorac. Cardiovasc. Surg. 2010, 139, 1019–1025. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Concannon, C.G.; Gorman, A.M.; Samali, A. On the role of Hsp27 in regulating apoptosis. Apoptosis 2003, 8, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Shehata, A.M.; Saadeldin, I.M.; Tukur, H.A.; Habashy, W.S. Modulation of Heat-Shock Proteins Mediates Chicken Cell Survival against Thermal Stress. Animals 2020, 10, 2407. [Google Scholar] [CrossRef]
- Zhang, R.-G.; Wang, C.-S.; Gao, C.-F. Prevalence and pathogenesis of Barrett’s esophagus in Luoyang, China. Asian Pac. J. Cancer Prev. 2012, 13, 2185–2191. [Google Scholar] [CrossRef]
- Hao, Y.; Gu, X.; Wang, X. Overexpression of heat shock protein 70 and its relationship to intestine under acute heat stress in broilers: 1. Intestinal structure and digestive function. Poult. Sci. 2012, 91, 781–789. [Google Scholar] [CrossRef]
- Kontomanolis, E.N.; Koutras, A.; Syllaios, A.; Schizas, D.; Mastoraki, A.; Garmpis, N.; Diakosavvas, M.; Angelou, K.; Tsatsaris, G.; Pagkalos, A.; et al. Role of Oncogenes and Tumor-suppressor Genes in Carcinogenesis: A Review. Anticancer Res. 2020, 40, 6009–6015. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Demple, B.; Harrison, L. Repair of oxidative damage to DNA: Enzymology and biology. Annu. Rev. Biochem. 1994, 63, 915–948. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Morgillo, F.; Tuccillo, C.; Ciardiello, F.; Loguercio, C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer 2007, 121, 2381–2386. [Google Scholar] [CrossRef] [PubMed]
- Chong, X.; Madeti, Y.; Cai, J.; Li, W.; Cong, L.; Lu, J.; Mo, L.; Liu, H.; He, S.; Yu, C.; et al. Recent developments in immunotherapy for gastrointestinal tract cancers. J. Hematol. Oncol. 2024, 17, 65. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chiba, T.; Marusawa, H.; Ushijima, T. Inflammation-associated cancer development in digestive organs: Mechanisms and roles for genetic and epigenetic modulation. Gastroenterology 2012, 143, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Halazonetis, T.D.; Gorgoulis, V.G.; Bartek, J. An oncogene-induced DNA damage model for cancer development. Science 2008, 319, 1352–1355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, J.; Long, Y.; Maimaitijiang, A.; Su, Z.; Li, W.; Li, J. Unraveling the Guardian: p53’s Multifaceted Role in the DNA Damage Response and Tumor Treatment Strategies. Int. J. Mol. Sci. 2024, 25, 12928. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bellini, M.F.; Cadamuro, A.C.; Succi, M.; Proença, M.A.; Silva, A.E. Alterations of the TP53 gene in gastric and esophageal carcinogenesis. J. Biomed. Biotechnol. 2012, 2012, 891961. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gleeson, C.M.; Sloan, J.M.; McManus, D.T.; Maxwell, P.; Arthur, K.; McGuigan, J.A.; Ritchie, A.J.; Russell, S.E. Comparison of p53 and DNA content abnormalities in adenocarcinoma of the oesophagus and gastric cardia. Br. J. Cancer 1998, 77, 277–286. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morgan, R.J.; Newcomb, P.V.; Bailey, M.; Hardwick, R.H.; Alderson, D. Loss of heterozygosity at microsatellite marker sites for tumour suppressor genes in oesophageal adenocarcinoma. Eur. J. Surg. Oncol. 1998, 24, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.T.; Watanabe, T.; Heitmiller, R.; Zahurak, M.; Forastiere, A.A.; Hamilton, S.R. Genetic alterations in Barrett esophagus and adenocarcinomas of the esophagus and esophagogastric junction region. Am. J. Pathol. 1998, 153, 287–294. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stachler, M.D.; Camarda, N.D.; Deitrick, C.; Kim, A.; Agoston, A.T.; Odze, R.D.; Hornick, J.L.; Nag, A.; Thorner, A.R.; Ducar, M.; et al. Detection of Mutations in Barrett’s Esophagus Before Progression to High-Grade Dysplasia or Adenocarcinoma. Gastroenterology 2018, 155, 156–167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hamelin, R.; Fléjou, J.F.; Muzeau, F.; Potet, F.; Laurent-Puig, P.; Fékété, F.; Thomas, G. TP53 gene mutations and p53 protein immunoreactivity in malignant and premalignant Barrett’s esophagus. Gastroenterology 1994, 107, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Doak, S.H.; Jenkins, G.J.; Parry, E.M.; Griffiths, A.P.; Shah, V.; Baxter, J.N.; Parry, J.M. Characterisation of p53 status at the gene, chromosomal and protein levels in oesophageal adenocarcinoma. Br. J. Cancer 2003, 89, 1729–1735. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Soslow, R.A.; Altorki, N.K.; Yang, G.; Xie, D.; Yang, C.S. mdm-2 expression correlates with wild-type p53 status in esophageal adenocarcinoma. Mod. Pathol. 1999, 12, 580–586. [Google Scholar] [PubMed]
- Ireland, A.P.; Shibata, D.K.; Chandrasoma, P.; Lord, R.V.; Peters, J.H.; DeMeester, T.R. Clinical significance of p53 mutations in adenocarcinoma of the esophagus and cardia. Ann. Surg. 2000, 231, 179–187. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schneider, P.M.; Stoeltzing, O.; Roth, J.A.; Hoelscher, A.H.; Wegerer, S.; Mizumoto, S.; Becker, K.; Dittler, H.J.; Fink, U.; Siewert, J.R. P53 mutational status improves estimation of prognosis in patients with curatively resected adenocarcinoma in Barrett’s esophagus. Clin. Cancer Res. 2000, 6, 3153–3158. [Google Scholar] [PubMed]
- Redston, M.; Noffsinger, A.; Kim, A.; Akarca, F.G.; Rara, M.; Stapleton, D.; Nowden, L.; Lash, R.; Bass, A.J.; Stachler, M.D. Abnormal TP53 Predicts Risk of Progression in Patients with Barrett’s Esophagus Regardless of a Diagnosis of Dysplasia. Gastroenterology 2022, 162, 468–481. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bian, Y.S.; Osterheld, M.C.; Fontolliet, C.; Bosman, F.T.; Benhattar, J. p16 Inactivation by methylation of the CDKN2A promoter occurs early during neoplastic progression in Barrett’s esophagus. Gastroenterology 2002, 122, 1113–1121. [Google Scholar] [CrossRef]
- Galipeau, P.C.; Prevo, L.J.; Sanchez, C.A.; Longton, G.M.; Reid, B.J. Clonal expansion and loss of heterozygosity at chromosomes 9p and 17p in premalignant esophageal (Barrett’s) tissue. J. Natl. Cancer Inst. 1999, 91, 2087–2095. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brankley, S.M.; Fritcher, E.G.B.; Smyrk, T.C.; Keeney, M.E.; Campion, M.B.; Voss, J.S.; Clayton, A.C.; Wang, K.K.; Lutzke, L.S.; Kipp, B.R.; et al. Fluorescence in situ hybridization mapping of esophagectomy specimens from patients with Barrett’s esophagus with high-grade dysplasia or adenocarcinoma. Hum. Pathol. 2012, 43, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, J.L.; Komissarova, E.V.; Kongkarnka, S.; Friedman, R.A.; Davison, J.M.; Levy, B.; Bryk, D.; Jobanputra, V.; Del Portillo, A.; Falk, G.W.; et al. High-resolution genomic alterations in Barrett’s metaplasia of patients who progress to esophageal dysplasia and adenocarcinoma. Int. J. Cancer 2019, 145, 2754–2766. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rygiel, A.M.; Milano, F.; Ten Kate, F.J.; Schaap, A.; Wang, K.K.; Peppelenbosch, M.P.; Bergman, J.J.; Krishnadath, K.K. Gains and amplifications of c-myc, EGFR, and 20.q13 loci in the no dysplasia-dysplasia-adenocarcinoma sequence of Barrett’s esophagus. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Venkitachalam, S.; Babu, D.; Ravillah, D.; Katabathula, R.M.; Joseph, P.; Singh, S.; Udhayakumar, B.; Miao, Y.; Martinez-Uribe, O.; Hogue, J.A.; et al. The Ephrin B2 Receptor Tyrosine Kinase Is a Regulator of Proto-oncogene MYC and Molecular Programs Central to Barrett’s Neoplasia. Gastroenterology 2022, 163, 1228–1241. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pretto, G.; Gurski, R.R.; Binato, M.; Navarini, D.; Aguiar, W.W.; Meurer, L. Increase of epidermal growth factor receptor expression in progression of GERD, Barrett, and adenocarcinoma of esophagus. Dig. Dis. Sci. 2013, 58, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Cronin, J.; McAdam, E.; Danikas, A.; Tselepis, C.; Griffiths, P.; Baxter, J.; Thomas, L.; Manson, J.; Jenkins, G. Epidermal Growth Factor Receptor (EGFR) Is Overexpressed in High-Grade Dysplasia and Adenocarcinoma of the Esophagus and May Represent a Biomarker of Histological Progression in Barrett’s Esophagus (BE). Am. J. Gastroenterol. 2011, 106, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Deissova, T.; Cvanova, M.; Kala, Z.; Jiraskova Zakostelska, Z.; Dolina, J.; Kunovsky, L.; Kroupa, R.; Pavlovsky, Z.; Lipovy, B.; Danek, Z.; et al. Lack of Association between Epidermal Growth Factor or Its Receptor and Reflux Esophagitis, Barrett’s Esophagus, and Esophageal Adenocarcinoma: A Case-Control Study. Dis. Markers 2022, 2022, 8790748. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peixoto, P.; Cartron, P.F.; Serandour, A.A.; Hervouet, E. From 1957 to Nowadays: A Brief History of Epigenetics. Int. J. Mol. Sci. 2020, 21, 7571. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ergun, P.; Kipcak, S.; Bor, S. Epigenetic Alterations from Barrett’s Esophagus to Esophageal Adenocarcinoma. Int. J. Mol. Sci. 2023, 24, 7817. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, B.; Carey, M.; Workman, J.L. The role of chromatin during transcription. Cell 2007, 128, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Jenuwein, T.; Allis, C.D. Translating the histone code. Science 2001, 293, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.G.; Baylin, S.B. Gene silencing in cancer in association with promoter hypermethylation. N. Engl. J. Med. 2003, 349, 2042–2054. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Shen, L.; Cheng, A.S.; Ahmed, S.; Boumber, Y.; Charo, C.; Yamochi, T.; Urano, T.; Furukawa, K.; Kwabi-Addo, B.; et al. Gene silencing in cancer by histone H3 lysine 27 trimethylation independent of promoter DNA methylation. Nat. Genet. 2008, 40, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Croce, C.M. MicroRNA signatures in human cancers. Nat. Rev. Cancer 2006, 6, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, A.P.; Tycko, B. The history of cancer epigenetics. Nat. Rev. Cancer 2004, 4, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Yamashita, S.; Takamura-Enya, T.; Niwa, T.; Ando, T.; Enomoto, S.; Maekita, T.; Nakazawa, K.; Tatematsu, M.; Ichinose, M.; et al. Alu and Satα hypomethylation in Helicobacter pylori-infected gastric mucosae. Int. J. Cancer 2011, 128, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Hosoya, K.; Gyobu, K.; Takeshima, H.; Ushijima, T. Development of a novel output value for quantitative assessment in methylated DNA immunoprecipitation-CpG island microarray analysis. DNA Res. 2009, 16, 275–286. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rauch, T.A.; Zhong, X.; Wu, X.; Wang, M.; Kernstine, K.H.; Wang, Z.; Riggs, A.D.; Pfeifer, G.P. High-resolution mapping of DNA hypermethylation and hypomethylation in lung cancer. Proc. Natl. Acad. Sci. USA 2008, 105, 252–257. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jones, P.A.; Baylin, S.B. The epigenomics of cancer. Cell 2007, 128, 683–692. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, R.Z.; Pettersson, U.; Beard, C.; Jackson-Grusby, L.; Jaenisch, R. DNA hypomethylation leads to elevated mutation rates. Nature 1998, 395, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Polineni, R.; Hussein, Z.; Vigoda, I.; Bhagat, T.D.; Bhattacharyya, S.; Maitra, A.; Verma, A. Role of epigenetic alterations in the pathogenesis of Barrett’s esophagus and esophageal adenocarcinoma. Int. J. Clin. Exp. Pathol. 2012, 5, 382–396. [Google Scholar] [PubMed] [PubMed Central]
- Alvarez, H.; Opalinska, J.; Zhou, L.; Sohal, D.; Fazzari, M.J.; Yu, Y.; Montagna, C.; Montgomery, E.A.; Canto, M.; Dunbar, K.B.; et al. Widespread hypomethylation occurs early and synergizes with gene amplification during esophageal carcinogenesis. PLoS Genet. 2011, 7, e1001356, Erratum in PLoS Genet. 2011, 7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hsieh, C.J.; Klump, B.; Holzmann, K.; Borchard, F.; Gregor, M.; Porschen, R. Hypermethylation of the p16INK4a promoter in colectomy specimens of patients with long-standing and extensive ulcerative colitis. Cancer Res. 1998, 58, 3942–3945. [Google Scholar] [PubMed]
- Issa, J.P.; Ahuja, N.; Toyota, M.; Bronner, M.P.; Brentnall, T.A. Accelerated age-related CpG island methylation in ulcerative colitis. Cancer Res. 2001, 61, 3573–3577. [Google Scholar] [PubMed]
- Ushijima, T.; Hattori, N. Molecular pathways: Involvement of Helicobacter pylori-triggered inflammation in the formation of an epigenetic field defect, and its usefulness as cancer risk and exposure markers. Clin. Cancer Res. 2012, 18, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Tang, J.C.; Gopalan, V.; Lam, A.K. Epigenetics: DNA Methylation Analysis in Esophageal Adenocarcinoma. Methods Mol. Biol. 2018, 1756, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; De Young, N.J.; Pavey, S.J.; Hayward, N.K.; Nancarrow, D.J.; Whiteman, D.C.; Smithers, B.M.; Ruszkiewicz, A.R.; Clouston, A.D.; Gotley, D.C.; et al. Similarity of aberrant DNA methylation in Barrett’s esophagus and esophageal adenocarcinoma. Mol. Cancer 2008, 7, 75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schulmann, K.; Sterian, A.; Berki, A.; Yin, J.; Sato, F.; Xu, Y.; Olaru, A.; Wang, S.; Mori, Y.; Deacu, E.; et al. Inactivation of p16, RUNX3, and HPP1 occurs early in Barrett’s-associated neoplastic progression and predicts progression risk. Oncogene 2005, 24, 4138–4148. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Mori, Y.; Yang, J.; Sato, F.; Ito, T.; Cheng, Y.; Paun, B.; Hamilton, J.P.; Kan, T.; Olaru, A.; et al. Hypermethylation of the nel-like 1 gene is a common and early event and is associated with poor prognosis in early-stage esophageal adenocarcinoma. Oncogene 2007, 26, 6332–6340. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Olaru, A.; Yang, J.; Sato, F.; Cheng, Y.; Kan, T.; Mori, Y.; Mantzur, C.; Paun, B.; Hamilton, J.P.; et al. Hypermethylation of tachykinin-1 is a potential biomarker in human esophageal cancer. Clin. Cancer Res. 2007, 13, 6293–6300. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Mori, Y.; Hamilton, J.P.; Olaru, A.; Sato, F.; Yang, J.; Ito, T.; Kan, T.; Agarwal, R.; Meltzer, S.J. Hypermethylation of the somatostatin promoter is a common, early event in human esophageal carcinogenesis. Cancer 2008, 112, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Hamilton, J.P.; Yang, J.; Mori, Y.; Olaru, A.; Sato, F.; Ito, T.; Kan, T.; Cheng, Y.; Paun, B.; et al. Hypermethylation of the AKAP12 promoter is a biomarker of Barrett’s-associated esophageal neoplastic progression. Cancer Epidemiol. Biomark. Prev. 2008, 17, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Cheng, Y.; Olaru, A.; Kan, T.; Yang, J.; Paun, B.; Ito, T.; Hamilton, J.P.; David, S.; Agarwal, R.; et al. Promoter hypermethylation of CDH13 is a common, early event in human esophageal adenocarcinogenesis and correlates with clinical risk factors. Int. J. Cancer 2008, 123, 2331–2336. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Cheng, Y.; Gu, W.; Zheng, Y.; Sato, F.; Mori, Y.; Olaru, A.V.; Paun, B.C.; Yang, J.; Kan, T.; et al. A multicenter, double-blinded validation study of methylation biomarkers for progression prediction in Barrett’s esophagus. Cancer Res. 2009, 69, 4112–4115. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moinova, H.; Leidner, R.S.; Ravi, L.; Lutterbaugh, J.; Barnholtz-Sloan, J.S.; Chen, Y.; Chak, A.; Markowitz, S.D.; Willis, J.E. Aberrant vimentin methylation is characteristic of upper gastrointestinal pathologies. Cancer Epidemiol. Biomark. Prev. 2012, 21, 594–600. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moinova, H.R.; LaFramboise, T.; Lutterbaugh, J.D.; Chandar, A.K.; Dumot, J.; Faulx, A.; Brock, W.; De la Cruz Cabrera, O.; Guda, K.; Barnholtz-Sloan, J.S.; et al. Identifying DNA methylation biomarkers for non-endoscopic detection of Barrett’s esophagus. Sci. Transl. Med. 2018, 10, eaao5848. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, M.; Moinova, H.R.; Willbanks, A.; Cannon, V.K.; Wang, T.; Carter, K.; Kaz, A.; Reddi, D.; Inadomi, J.; Luebeck, G.; et al. Novel DNA Methylation Biomarker Panel for Detection of Esophageal Adenocarcinoma and High-Grade Dysplasia. Clin. Cancer Res. 2022, 28, 3761–3769. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alvi, M.A.; Liu, X.; O’Donovan, M.; Newton, R.; Wernisch, L.; Shannon, N.B.; Shariff, K.; di Pietro, M.; Bergman, J.J.; Ragunath, K.; et al. DNA methylation as an adjunct to histopathology to detect prevalent, inconspicuous dysplasia and early-stage neoplasia in Barrett’s esophagus. Clin. Cancer Res. 2013, 19, 878–888. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kawakami, K.; Brabender, J.; Lord, R.V.; Groshen, S.; Greenwald, B.D.; Krasna, M.J.; Yin, J.; Fleisher, A.S.; Abraham, J.M.; Beer, D.G.; et al. Hypermethylated APC DNA in plasma and prognosis of patients with esophageal adenocarcinoma. J. Natl. Cancer Inst. 2000, 92, 1805–1811. [Google Scholar] [CrossRef] [PubMed]
- Ushijima, T.; Asada, K. Aberrant DNA methylation in contrast with mutations. Cancer Sci. 2010, 101, 300–305. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jammula, S.; Katz-Summercorn, A.C.; Li, X.; Linossi, C.; Smyth, E.; Killcoyne, S.; Biasci, D.; Subash, V.V.; Abbas, S.; Blasko, A.; et al. Oesophageal Cancer Clinical and Molecular Stratification (OCCAMS) consortium; Fitzgerald RC. Identification of Subtypes of Barrett’s Esophagus and Esophageal Adenocarcinoma Based on DNA Methylation Profiles and Integration of Transcriptome and Genome Data. Gastroenterology 2020, 158, 1682–1697.e1, Erratum in Gastroenterology 2021, 161, 1727.. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hong, J.; Resnick, M.; Behar, J.; Wang, L.J.; Wands, J.; DeLellis, R.A.; Souza, R.F.; Spechler, S.J.; Cao, W. Acid-Induced P16 Hypermethylation Contributes to Development of Esophageal Adenocarcinoma via Activation of NADPH Oxidase NOX5-S. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G697–G706. [Google Scholar] [CrossRef]
- Fu, X.; Beer, D.G.; Behar, J.; Wands, J.; Lambeth, D.; Cao, W. cAMP- response element-binding protein mediates acid-induced NADPH oxidase NOX5-S expression in Barrett esophageal adenocarcinoma cells. J. Biol. Chem. 2006, 281, 20368–20382. [Google Scholar] [CrossRef]
- Wang, J.S.; Guo, M.; Montgomery, E.A.; Thompson, R.E.; Cosby, H.; Hicks, L.; Wang, S.; Herman, J.G.; Canto, M.I. DNA promoter hypermethylation of p16 and APC predicts neoplastic progression in Barrett’s esophagus. Am. J. Gastroenterol. 2009, 104, 2153–2160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chueca, E.; Valero, A.; Hördnler, C.; Puertas, A.; Carrera, P.; García-González, M.A.; Strunk, M.; Lanas, A.; Piazuelo, E. Quantitative Analysis of P16 Methylation in Barrett’s Carcinogenesis. Ann. Diagn. Pathol. 2020, 47, 151554. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Song, H.; Jiang, H.; Fu, Y.; Ding, X.; Zhou, C. Early diagnostic potential of APC hypermethylation in esophageal cancer. Cancer Manag. Res. 2018, 10, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Fukui, S.; Watari, J.; Tomita, T.; Yamasaki, T.; Okugawa, T.; Kondo, T.; Kono, T.; Tozawa, K.; Ikehara, H.; Ohda, Y.; et al. Localization of specialized intestinal metaplasia and the molecular alterations in Barrett esophagus in a Japanese population: An analysis of biopsy samples based on the “Seattle” biopsy protocol. Hum. Pathol. 2016, 51, 32–40. [Google Scholar] [CrossRef]
- Galipeau, P.C.; Cowan, D.S.; Sanchez, C.A.; Barrett, M.T.; Emond, M.J.; Levine, D.S.; Rabinovitch, P.S.; Reid, B.J. 17p (p53) allelic losses, 4N (G2/tetraploid) populations, and progression to aneuploidy in Barrett’s esophagus. Proc. Natl. Acad. Sci. USA 1996, 93, 7081–7084. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reid, B.J.; Levine, D.S.; Longton, G.; Blount, P.L.; Rabinovitch, P.S. Predictors of progression to cancer in Barrett’s esophagus: Baseline histology and flow cytometry identify low- and high-risk patient subsets. Am. J. Gastroenterol. 2000, 95, 1669–1676. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chao, D.L.; Sanchez, C.A.; Galipeau, P.C.; Blount, P.L.; Paulson, T.G.; Cowan, D.S.; Ayub, K.; Odze, R.D.; Rabinovitch, P.S.; Reid, B.J. Cell proliferation, cell cycle abnormalities, and cancer outcome in patients with Barrett’s esophagus: A long-term prospective study. Clin. Cancer Res. 2008, 14, 6988–6995. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.; Galipeau, P.C.; Paulson, T.G.; Sanchez, C.A.; Arnaudo, J.; Liu, K.; Sather, C.L.; Kostadinov, R.L.; Odze, R.D.; Kuhner, M.K.; et al. Temporal and spatial evolution of somatic chromosomal alterations: A case-cohort study of Barrett’s esophagus. Cancer Prev. Res. 2014, 7, 114–127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoefnagel, S.J.M.; Mostafavi, N.; Timmer, M.R.; Lau, C.T.; Meijer, S.L.; Wang, K.K.; Krishnadath, K.K. A genomic biomarker-based model for cancer risk stratification of non-dysplastic Barrett’s esophagus patients after extended follow up; results from Dutch surveillance cohorts. PLoS ONE 2020, 15, e0231419. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- di Pietro, M.; Boerwinkel, D.F.; Shariff, M.K.; Liu, X.; Telakis, E.; Lao-Sirieix, P.; Walker, E.; Couch, G.; Mills, L.; Nuckcheddy-Grant, T.; et al. The combination of autofluorescence endoscopy and molecular biomarkers is a novel diagnostic tool for dysplasia in Barrett’s oesophagus. Gut 2015, 64, 49–56. [Google Scholar] [CrossRef]
- Timmer, M.R.; Martinez, P.; Lau, C.T.; Westra, W.M.; Calpe, S.; Rygiel, A.M.; Rosmolen, W.D.; Meijer, S.L.; Ten Kate, F.J.; Dijkgraaf, M.G.; et al. Derivation of genetic biomarkers for cancer risk stratification in Barrett’s oesophagus: A prospective cohort study. Gut 2016, 65, 1602–1610. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jankowski, J.A.Z.; de Caestecker, J.; Love, S.B.; Reilly, G.; Watson, P.; Sanders, S.; Ang, Y.; Morris, D.; Bhandari, P.; Brooks, C.; et al. Esomeprazole and aspirin in Barrett’s oesophagus (AspECT): A randomised factorial trial. Lancet 2018, 392, 400–408, Erratum in Lancet 2018, 392, 2552. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Szachnowicz, S.; Duarte, A.F.; Nasi, A.; da Rocha, J.R.M.; Seguro, F.B.; Bianchi, E.T.; Tustumi, F.; de Moura, E.G.H.; Sallum, R.A.A.; Cecconello, I. Laparoscopic total fundoplication is superior to medical treatment for reducing the cancer risk in Barrett’s esophagus: A long-term analysis. Dis. Esophagus 2022, 35, doac026. [Google Scholar] [CrossRef] [PubMed]
- Gallon, E.; Szachnowicz, S.; Duarte, A.F.; Tustumi, F.; Sallum, R.A.A.; Herman, P.; Ribeiro Junior, U. Adenocarcinoma and Dysplasia in Barrett Esophagus: Critical Analysis of Risk Factors and Surveillance Protocols. Arq. Bras. Cir. Dig. 2024, 37, e1826. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zaninotto, G.; Parente, P.; Salvador, R.; Farinati, F.; Tieppo, C.; Passuello, N.; Zanatta, L.; Fassan, M.; Cavallin, F.; Costantini, M.; et al. Long-term follow-up of Barrett’s epithelium: Medical versus antireflux surgical therapy. J. Gastrointest. Surg. 2012, 16, 7–14; discussion 14–15. [Google Scholar] [CrossRef] [PubMed]
- Neureiter, D.; Mayr, C.; Winkelmann, P.; Neumayer, B.; Klieser, E.; Wagner, A.; Hufnagl, C.; Emmanuel, K.; Holzinger, J.; Koch, O.; et al. Expression of the microRNA-200 Family, microRNA-205, and Markers of Epithelial-Mesenchymal Transition as Predictors for Endoscopic Submucosal Dissection over Esophagectomy in Esophageal Adenocarcinoma: A Single-Center Experience. Cells 2020, 9, 486. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Botros, M.; de Boer, O.J.; Cardenas, B.; Bekkers, E.J.; Jansen, M.; van der Wel, M.J.; Sánchez, C.I.; Meijer, S.L. Deep Learning for Histopathological Assessment of Esophageal Adenocarcinoma Precursor Lesions. Pathol. Mod. 2024, 37, 100531. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.; Malerba, S.; Nardone, V.; Salvestrini, V.; Calomino, N.; Testini, M.; Boccardi, V.; Desideri, I.; Gentili, C.; De Luca, R.; et al. Progress and Challenges in Integrating Nutritional Care into Oncology Practice: Results from a National Survey on Behalf of the NutriOnc Research Group. Nutrients 2025, 17, 188. [Google Scholar] [CrossRef]

| miRNA | Function in Cell | Role in Barrett’s Esophagus |
|---|---|---|
| miR-192 | Tumor suppressor inhibits cell proliferation and induces apoptosis | Downregulated in BE; reduced expression is linked to higher progression risk |
| miR-194 | Tumor suppressor or oncomir | Upregulated in BE and EAC; associated with metaplasia and neoplastic progression |
| miR-215 | Tumor suppressor or oncomir; regulates cell differentiation and apoptosis | Upregulated in BE but downregulated in EAC; potential biomarker for progression |
| miR-203 | Tumor suppressor regulates cellular growth and differentiation | Downregulated during progression from normal esophagus to EAC |
| miR-205 | Tumor suppressor; involved in epithelial–mesenchymal transition (EMT) | Lower expression in BE and EAC compared to normal tissue; regulates EMT |
| Gene | Protein | Effect on Cell |
|---|---|---|
| AKAP12 | A-kinase anchoring protein 12 | Regulates β2-adrenergic receptor signaling, involved in cell adhesion |
| GPS &GST | Glutathione-S-transferase superfamily (GST) and glutathione peroxidase (GPX) | Cellular antioxidants |
| CDH13 | Cadherin-13 (H-cadherin/T-cadherin) | Tumor suppressor regulates cell–cell adhesion |
| DAPK1 | Death-associated protein kinase 1 | Induces apoptosis, tumor suppressor |
| TAC1 | Tachykinin precursor 1 | Involved in neuropeptide signaling, anti-apoptotic effects |
| TIMP3 | Tissue inhibitor of metalloproteinases-3 | Inhibits tumor growth, angiogenesis, and promotes apoptosis |
| WIF1 | Wnt inhibitory factor 1 | Inhibits Wnt signaling, tumor suppressor |
| SFRP1 | Secreted frizzled-related protein 1 | Inhibits Wnt signaling, tumor suppressor |
| SFRP2 | Secreted frizzled-related protein 2 | Inhibits Wnt signaling, tumor suppressor |
| SFRP4 | Secreted frizzled-related protein 4 | Inhibits Wnt signaling, tumor suppressor |
| MGMT | O-methylguanine-DNA methyltransferase | DNA repair enzyme |
| NELL1 | Protein kinase C binding protein | Control cell differentiation and growth |
| SOCS | STAT-induced inhibitors | Cytokine-inducible negative regulators of cytokine signaling |
| SST | Somatostatin | Regulates exocrine and endocrine secretion and motor activity. It is the primary inhibitor of gastrin-stimulated gastric acid secretion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Melo Viana, T.C.; Nakamura, E.T.; Park, A.; Filardi, K.F.X.C.; de Almeida Leite, R.M.; Baltazar, L.F.S.R.; Usón Junior, P.L.S.; Tustumi, F. Molecular Abnormalities and Carcinogenesis in Barrett’s Esophagus: Implications for Cancer Treatment and Prevention. Genes 2025, 16, 270. https://doi.org/10.3390/genes16030270
de Melo Viana TC, Nakamura ET, Park A, Filardi KFXC, de Almeida Leite RM, Baltazar LFSR, Usón Junior PLS, Tustumi F. Molecular Abnormalities and Carcinogenesis in Barrett’s Esophagus: Implications for Cancer Treatment and Prevention. Genes. 2025; 16(3):270. https://doi.org/10.3390/genes16030270
Chicago/Turabian Stylede Melo Viana, Thaís Cabral, Eric Toshiyuki Nakamura, Amanda Park, Kaique Flávio Xavier Cardoso Filardi, Rodrigo Moisés de Almeida Leite, Luiz Fernando Sposito Ribeiro Baltazar, Pedro Luiz Serrano Usón Junior, and Francisco Tustumi. 2025. "Molecular Abnormalities and Carcinogenesis in Barrett’s Esophagus: Implications for Cancer Treatment and Prevention" Genes 16, no. 3: 270. https://doi.org/10.3390/genes16030270
APA Stylede Melo Viana, T. C., Nakamura, E. T., Park, A., Filardi, K. F. X. C., de Almeida Leite, R. M., Baltazar, L. F. S. R., Usón Junior, P. L. S., & Tustumi, F. (2025). Molecular Abnormalities and Carcinogenesis in Barrett’s Esophagus: Implications for Cancer Treatment and Prevention. Genes, 16(3), 270. https://doi.org/10.3390/genes16030270







