Targeting Optimal Bone Regions: Correlations Between Bone Density and DNA Quality in Small Skeletal Elements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Selection, CT Imaging, and Region of Interest Selection
2.2. Sample Preparation
2.3. DNA Extraction, Purification, and Quantification
2.4. STR Typing
2.5. Contamination Control Measures and Authenticity Criteria
2.6. Statistical Analysis
3. Results
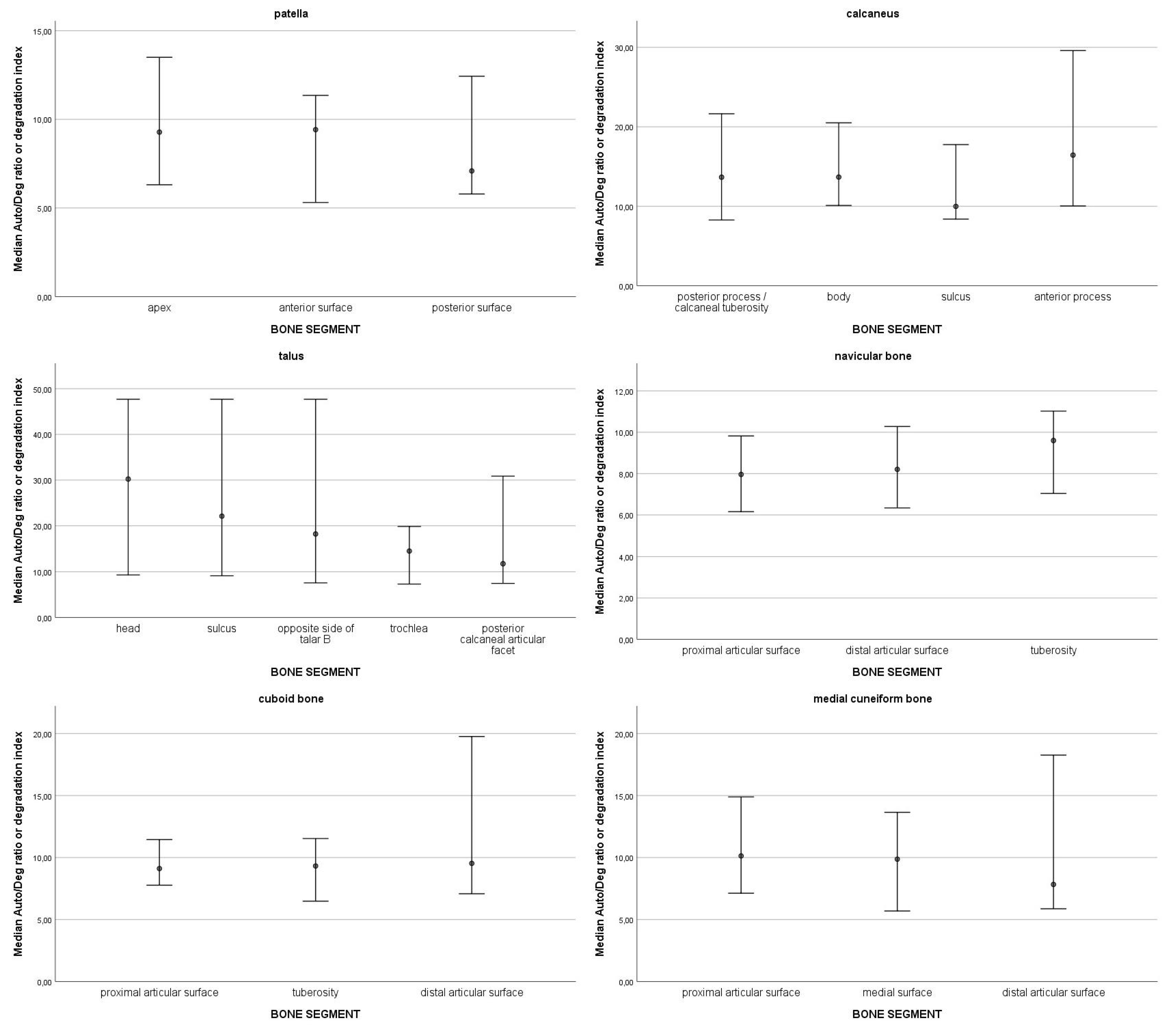
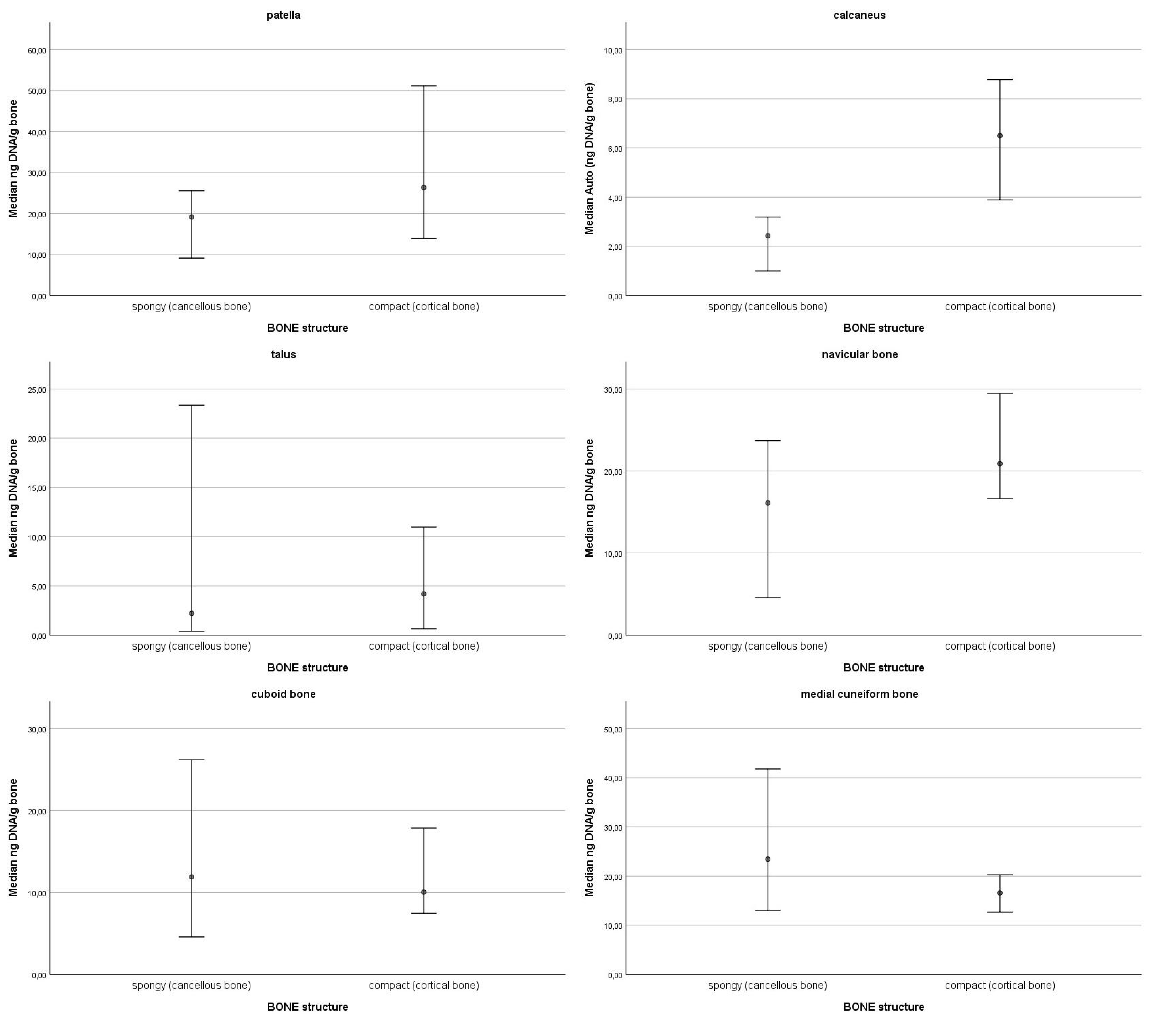
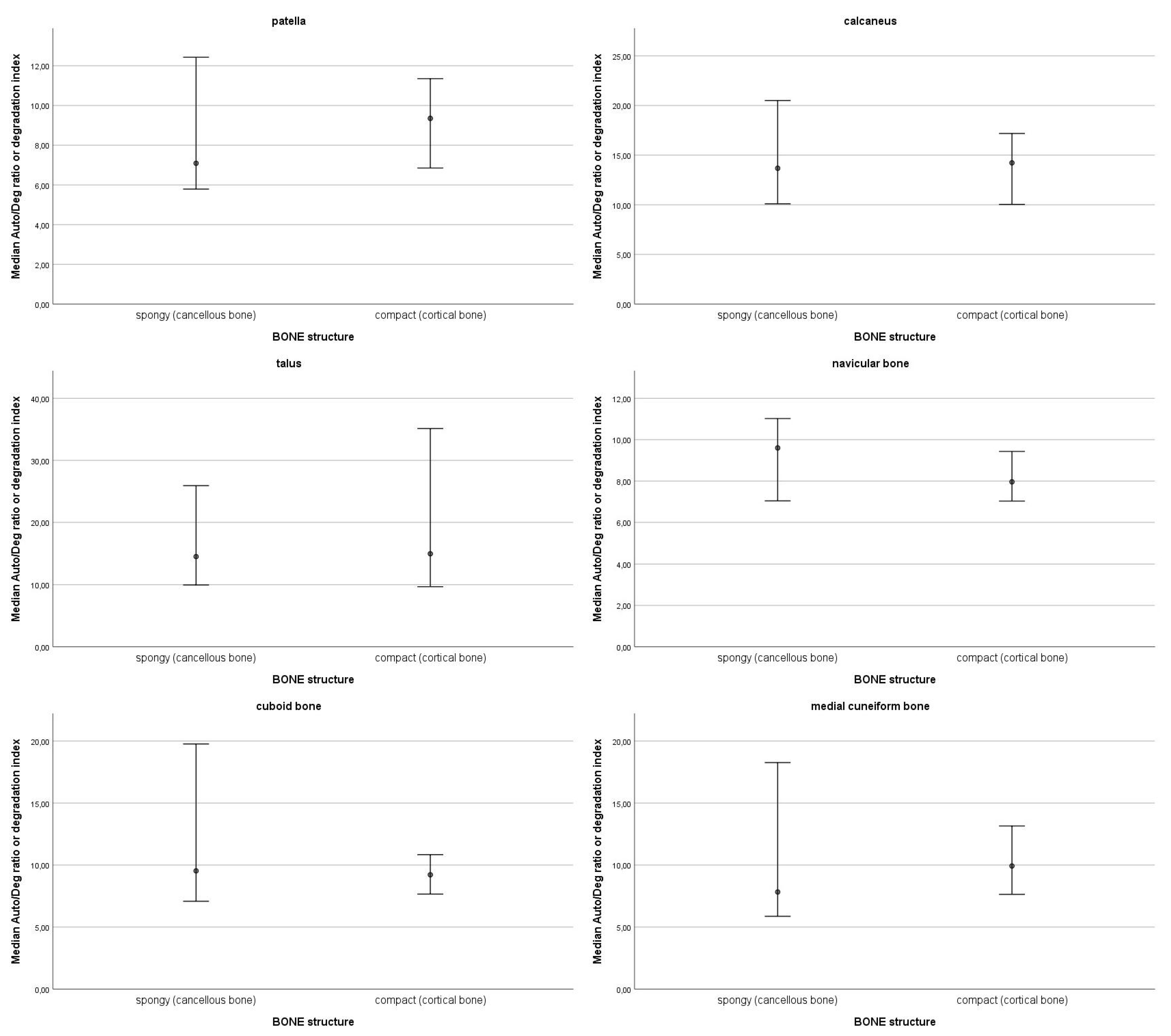
3.1. DNA Quantity and Degradation
3.2. Correlations Between DNA Quantity and Bone Density
3.3. STR Typing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Emmons, A.L.; Mundorff, A.Z.; Hoeland, K.M.; Davoren, J.; Keenan, S.W.; Carter, D.O.; Campagna, S.R.; DeBruyn, J.M. Postmortem Skeletal Microbial Community Composition and Function in Buried Human Remains. mSystems 2022, 7, e00041-22. [Google Scholar] [CrossRef]
- Finaughty, C.; Heathfield, L.J.; Kemp, V.; Márquez-Grant, N. Forensic DNA Extraction Methods for Human Hard Tissue: A Systematic Literature Review and Meta-Analysis of Technologies and Sample Type. Forensic Sci. Int. Genet. 2023, 63, 102818. [Google Scholar] [CrossRef]
- Emery, M.V.; Bolhofner, K.; Winingear, S.; Oldt, R.; Montes, M.; Kanthaswamy, S.; Buikstra, J.E.; Fulginiti, L.C.; Stone, A.C. Reconstructing Full and Partial STR Profiles from Severely Burned Human Remains Using Comparative Ancient and Forensic DNA Extraction Techniques. Forensic Sci. Int. Genet. 2020, 46, 102272. [Google Scholar] [CrossRef] [PubMed]
- Allentoft, M.E.; Collins, M.; Harker, D.; Haile, J.; Oskam, C.L.; Hale, M.L.; Campos, P.F.; Samaniego, J.A.; Gilbert, M.T.P.; Willerslev, E.; et al. The Half-Life of DNA in Bone: Measuring Decay Kinetics in 158 Dated Fossils. Proc. R. Soc. B Biol. Sci. 2012, 279, 4724–4733. [Google Scholar] [CrossRef] [PubMed]
- Rohland, N.; Hofreiter, M. Ancient DNA Extraction from Bones and Teeth. Nat. Protoc. 2007, 2, 1756–1762. [Google Scholar] [CrossRef] [PubMed]
- Raffone, C.; Baeta, M.; Lambacher, N.; Granizo-Rodríguez, E.; Etxeberria, F.; de Pancorbo, M.M. Intrinsic and Extrinsic Factors That May Influence DNA Preservation in Skeletal Remains: A Review. Forensic Sci. Int. 2021, 325, 110859. [Google Scholar] [CrossRef]
- Leskovar, T.; Zupanič Pajnič, I.; Geršak, Ž.M.; Jerman, I.; Črešnar, M. ATR-FTIR Spectroscopy Combined with Data Manipulation as a Pre-Screening Method to Assess DNA Preservation in Skeletal Remains. Forensic Sci. Int. Genet. 2020, 44, 102196. [Google Scholar] [CrossRef] [PubMed]
- Waldron, T. The Relative Survival of the Human Skeleton: Implications for Palaeopathology. In Death, Decay, and Reconstruction: Approaches to Archaeology and Forensic Science; Boddington, A., Garland, A.N., Janaway, R.C., Eds.; Manchester University Press: Manchester, UK, 1987; pp. 55–64. [Google Scholar]
- Von Endt, D.W.; Ortner, D.J. Experimental Effects of Bone Size and Temperature on Bone Diagenesis. J. Archaeol. Sci. 1984, 11, 247–253. [Google Scholar] [CrossRef]
- Christina, N.-M.; Angela, G.; Gordon, T.-W.; Robert, H.; Alistair, P.; Matthew, C. The Chemical Degradation of Bones. In Human Osteology in Archaeology and Forensic Science; Cox, M., Mays, S., Eds.; Greenwich Medical Media: London, UK, 2000; pp. 439–451. [Google Scholar]
- Pääbo, S. Ancient DNA: Extraction, Characterization, Molecular Cloning, and Enzymatic Amplification. Proc. Natl. Acad. Sci. USA 1989, 86, 1939–1943. [Google Scholar] [CrossRef] [PubMed]
- Zupanič Pajnič, I. Extraction of DNA from Human Skeletal Material. In Forensic DNA Typing Protocols; William, G., Ed.; Humana Press: New York, NY, USA, 2016; pp. 89–108. [Google Scholar]
- Loreille, O.M.; Diegoli, T.M.; Irwin, J.A.; Coble, M.D.; Parsons, T.J. High Efficiency DNA Extraction from Bone by Total Demineralization. Forensic Sci. Int. Genet. 2007, 1, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Amory, S.; Huel, R.; Bilić, A.; Loreille, O.; Parsons, T.J. Automatable Full Demineralization DNA Extraction Procedure from Degraded Skeletal Remains. Forensic Sci. Int. Genet. 2012, 6, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.B.; Zhang, A.; Kim, H.Y.; Yi, J.A.; Lee, H.Y.; Shin, D.H.; Lee, S.D. Technical Note: Efficiency of Total Demineralization and Ion-exchange Column for DNA Extraction from Bone. Am. J. Phys. Anthropol. 2010, 141, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Caputo, M.; Irisarri, M.; Alechine, E.; Corach, D. A DNA Extraction Method of Small Quantities of Bone for High-Quality Genotyping. Forensic Sci. Int. Genet. 2013, 7, 488–493. [Google Scholar] [CrossRef]
- Zupanič Pajnič, I.; Leskovar, T.; Zupanc, T.; Podovšovnik, E. A Fast and Highly Efficient Automated DNA Extraction Method from Small Quantities of Bone Powder from Aged Bone Samples. Forensic Sci. Int. Genet. 2023, 65, 102882. [Google Scholar] [CrossRef]
- Gonzalez, A.; Cannet, C.; Zvénigorosky, V.; Geraut, A.; Koch, G.; Delabarde, T.; Ludes, B.; Raul, J.-S.; Keyser, C. The Petrous Bone: Ideal Substrate in Legal Medicine? Forensic Sci. Int. Genet. 2020, 47, 102305. [Google Scholar] [CrossRef]
- Hansen, H.B.; Damgaard, P.B.; Margaryan, A.; Stenderup, J.; Lynnerup, N.; Willerslev, E.; Allentoft, M.E. Comparing Ancient DNA Preservation in Petrous Bone and Tooth Cementum. PLoS ONE 2017, 12, e0170940. [Google Scholar] [CrossRef] [PubMed]
- Pilli, E.; Vai, S.; Caruso, M.G.; D’Errico, G.; Berti, A.; Caramelli, D. Neither Femur nor Tooth: Petrous Bone for Identifying Archaeological Bone Samples via Forensic Approach. Forensic Sci. Int. 2018, 283, 144–149. [Google Scholar] [CrossRef]
- Pinhasi, R.; Fernandes, D.; Sirak, K.; Novak, M.; Connell, S.; Alpaslan-Roodenberg, S.; Gerritsen, F.; Moiseyev, V.; Gromov, A.; Raczky, P.; et al. Optimal Ancient DNA Yields from the Inner Ear Part of the Human Petrous Bone. PLoS ONE 2015, 10, e0129102. [Google Scholar] [CrossRef] [PubMed]
- Zupanič Pajnič, I.; Inkret, J.; Zupanc, T.; Podovšovnik, E. Comparison of Nuclear DNA Yield and STR Typing Success in Second World War Petrous Bones and Metacarpals III. Forensic Sci. Int. Genet. 2021, 55, 102578. [Google Scholar] [CrossRef]
- Ibrahim, J.; Brumfeld, V.; Addadi, Y.; Rubin, S.; Weiner, S.; Boaretto, E. The Petrous Bone Contains High Concentrations of Osteocytes: One Possible Reason Why Ancient DNA Is Better Preserved in This Bone. PLoS ONE 2022, 17, e0269348. [Google Scholar] [CrossRef] [PubMed]
- Krause, S.; Lemke, A.-J. Morphometric Analysis of the Petrous Bone—A Preliminary Study of Intra- and Inter-Observer Variation of Basic Measurements. Anthropol. Anz. 2021, 78, 103–113. [Google Scholar] [CrossRef]
- Inkret, J.; Podovšovnik, E.; Zupanc, T.; Haring, G.; Pajnič, I.Z. Intra-Bone Nuclear DNA Variability in Second World War Metatarsal and Metacarpal Bones. Int. J. Leg. Med. 2021, 135, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Božič, L.; Benedik Bevc, T.; Podovšovnik, E.; Zupanc, T.; Zupanič Pajnič, I. Comparison of DNA Preservation between Ribs and Vertebrae. Int. J. Leg. Med. 2022, 136, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Benedik Bevc, T.; Božič, L.; Podovšovnik, E.; Zupanc, T.; Zupanič Pajnič, I. Intra-Bone Nuclear DNA Variability and STR Typing Success in Second World War 12th Thoracic Vertebrae. Forensic Sci. Int. Genet. 2021, 55, 102587. [Google Scholar] [CrossRef] [PubMed]
- Božič, L.; Benedik Bevc, T.; Podovšovnik, E.; Zupanc, T.; Zupanič Pajnič, I. Intra-Bone Nuclear DNA Variability and STR Typing Success in Second World War First Ribs. Int. J. Leg. Med. 2021, 135, 2199–2208. [Google Scholar] [CrossRef]
- Zupanič Pajnič, I.; Kovačič, N. DNA Preservation in Compact and Trabecular Bone. Forensic Sci. Int. Genet. 2024, 71, 103067. [Google Scholar] [CrossRef] [PubMed]
- Geršak, Ž.M.; Golob, A.; Kravanja, P.; Concato, M.; Leskovar, T.; Pajnič, I.Z. Patellae as a Source of DNA in Forensic and Archaeological Analysis. Int. J. Leg. Med. 2024, 139, 473–482. [Google Scholar] [CrossRef]
- Mundorff, A.; Davoren, J.M. Examination of DNA Yield Rates for Different Skeletal Elements at Increasing Post Mortem Intervals. Forensic Sci. Int. Genet. 2014, 8, 55–63. [Google Scholar] [CrossRef]
- Emmons, A.L.; Davoren, J.; DeBruyn, J.M.; Mundorff, A.Z. Inter and Intra-Individual Variation in Skeletal DNA Preservation in Buried Remains. Forensic Sci. Int. Genet. 2020, 44, 102193. [Google Scholar] [CrossRef] [PubMed]
- Antinick, T.C.; Foran, D.R. Intra- and Inter-Element Variability in Mitochondrial and Nuclear <scp>DNA</Scp> from Fresh and Environmentally Exposed Skeletal Remains. J. Forensic Sci. 2019, 64, 88–97. [Google Scholar] [CrossRef]
- Šuligoj, A.; Mesesnel, S.; Leskovar, T.; Podovšovnik, E.; Zupanič Pajnič, I. Comparison of DNA Preservation between Adult and Non-Adult Ancient Skeletons. Int. J. Leg. Med. 2022, 136, 1521–1539. [Google Scholar] [CrossRef] [PubMed]
- Andronowski, J.M.; Mundorff, A.Z.; Pratt, I.V.; Davoren, J.M.; Cooper, D.M.L. Evaluating Differential Nuclear DNA Yield Rates and Osteocyte Numbers among Human Bone Tissue Types: A Synchrotron Radiation Micro-CT Approach. Forensic Sci. Int. Genet. 2017, 28, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Alberti, F.; Gonzalez, J.; Paijmans, J.L.A.; Basler, N.; Preick, M.; Henneberger, K.; Trinks, A.; Rabeder, G.; Conard, N.J.; Münzel, S.C.; et al. Optimized DNA Sampling of Ancient Bones Using Computed Tomography Scans. Mol. Ecol. Resour. 2018, 18, 1196–1208. [Google Scholar] [CrossRef] [PubMed]
- McCollough, C.H.; Leng, S.; Yu, L.; Fletcher, J.G. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology 2015, 276, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Rajiah, P.; Sundaram, M.; Subhas, N. Dual-Energy CT in Musculoskeletal Imaging: What Is the Role Beyond Gout? Am. J. Roentgenol. 2019, 213, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y.; Lee, S.-W.; In, Y.; Kim, M.S.; Kim, Y.D.; Lee, S.; Lee, J.-W.; Koh, I.J. Dual-Energy CT-Based Bone Mineral Density Has Practical Value for Osteoporosis Screening around the Knee. Medicina 2022, 58, 1085. [Google Scholar] [CrossRef]
- Brant William, E. Diagnostic Imaging Methods. In Brant and Helm’s Fundamentals of Diagnostic Radiology; Klein Jeffrey, S., Brant William, E., Helms Clyde, A., Vinson Emily, N., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2019; pp. 2–26. [Google Scholar]
- Lindahl, T. Instability and Decay of the Primary Structure of DNA. Nature 1993, 362, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Hoss, M.; Jaruga, P.; Zastawny, T.H.; Dizdaroglu, M.; Paabo, S. DNA Damage and DNA Sequence Retrieval from Ancient Tissues. Nucleic Acids Res. 1996, 24, 1304–1307. [Google Scholar] [CrossRef] [PubMed]
- Poinar, H.N.; Höss, M.; Bada, J.L.; Pääbo, S. Amino Acid Racemization and the Preservation of Ancient DNA. Science 1996, 272, 864–866. [Google Scholar] [CrossRef]
- Tuross, N. The Biochemistry of Ancient DNA in Bone. Experientia 1994, 50, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.I.; Chamberlain, A.T.; Riley, M.S.; Stringer, C.; Collins, M.J. The Thermal History of Human Fossils and the Likelihood of Successful DNA Amplification. J. Hum. Evol. 2003, 45, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Pruvost, M.; Schwarz, R.; Correia, V.B.; Champlot, S.; Braguier, S.; Morel, N.; Fernandez-Jalvo, Y.; Grange, T.; Geigl, E.-M. Freshly Excavated Fossil Bones Are Best for Amplification of Ancient DNA. Proc. Natl. Acad. Sci. USA 2007, 104, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Miriam, G.Ž.; Irena, Z.-P.; Eva, P.; Vladka, S. Computed Tomography Differentiation of Compact and Cancellous Bone Tissue in Short and Sesamoid Bones. Radiol. Oncol. 2025, 59. in press. [Google Scholar]
- Promega Corporation. PowerQuant System Technical Manual; Promega Corporation: Madison, WI, USA, 2022. [Google Scholar]
- Zupanič Pajnič, I. Identification of a Slovenian Prewar Elite Couple Killed in the Second World War. Forensic Sci. Int. 2021, 327, 110994. [Google Scholar] [CrossRef] [PubMed]
- Zupanič Pajnič, I.; Leskovar, T.; Črešnar, M. Improving Kinship Probability in Analysis of Ancient Skeletons Using Identity SNPs and MPS Technology. Int. J. Leg. Med. 2023, 137, 1007–1015. [Google Scholar] [CrossRef]
- DiCiccio, T.J.; Efron, B. Bootstrap Confidence Intervals. Stat. Sci. 1996, 11, 189–228. [Google Scholar] [CrossRef]
- Carpenter, J.; Bithell, J. Bootstrap Confidence Intervals: When, Which, What? A Practical Guide for Medical Statisticians. Stat. Med. 2000, 19, 1141–1164. [Google Scholar] [CrossRef]
- Edson, S.M.; Christensen, A.F.; Barritt, S.M.; Meehan, A.; Leney, M.D.; Finelli, L.N. Sampling of the Cranium for Mitochondrial DNA Analysis of Human Skeletal Remains. Forensic Sci. Int. Genet. Suppl. Ser. 2009, 2, 269–270. [Google Scholar] [CrossRef]
- Gamba, C.; Jones, E.R.; Teasdale, M.D.; McLaughlin, R.L.; Gonzalez-Fortes, G.; Mattiangeli, V.; Domboróczki, L.; Kővári, I.; Pap, I.; Anders, A.; et al. Genome Flux and Stasis in a Five Millennium Transect of European Prehistory. Nat. Commun. 2014, 5, 5257. [Google Scholar] [CrossRef]
- Kulstein, G.; Hadrys, T.; Wiegand, P. As Solid as a Rock—Comparison of CE- and MPS-Based Analyses of the Petrosal Bone as a Source of DNA for Forensic Identification of Challenging Cranial Bones. Int. J. Leg. Med. 2018, 132, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Rohrlach, A.B.; Friederich, S.; Nagel, S.; Meyer, M.; Krause, J.; Bos, K.I.; Haak, W. A Systematic Investigation of Human DNA Preservation in Medieval Skeletons. Sci. Rep. 2020, 10, 18225. [Google Scholar] [CrossRef]
- Haber, M.; Doumet-Serhal, C.; Scheib, C.; Xue, Y.; Danecek, P.; Mezzavilla, M.; Youhanna, S.; Martiniano, R.; Prado-Martinez, J.; Szpak, M.; et al. Continuity and Admixture in the Last Five Millennia of Levantine History from Ancient Canaanite and Present-Day Lebanese Genome Sequences. Am. J. Human. Genet. 2017, 101, 274–282. [Google Scholar] [CrossRef]
- Geigl, E.; Grange, T. Ancient DNA: The Quest for the Best. Mol. Ecol. Resour. 2018, 18, 1185–1187. [Google Scholar] [CrossRef] [PubMed]
- Furtwängler, A.; Reiter, E.; Neumann, G.U.; Siebke, I.; Steuri, N.; Hafner, A.; Lösch, S.; Anthes, N.; Schuenemann, V.J.; Krause, J. Ratio of Mitochondrial to Nuclear DNA Affects Contamination Estimates in Ancient DNA Analysis. Sci. Rep. 2018, 8, 14075. [Google Scholar] [CrossRef] [PubMed]
- Campos, P.F.; Craig, O.E.; Turner-Walker, G.; Peacock, E.; Willerslev, E.; Gilbert, M.T.P. DNA in Ancient Bone—Where Is It Located and How Should We Extract It? Ann. Anat.—Anat. Anz. 2012, 194, 7–16. [Google Scholar] [CrossRef]
- Sirak, K.; Fernandes, D.; Cheronet, O.; Harney, E.; Mah, M.; Mallick, S.; Rohland, N.; Adamski, N.; Broomandkhoshbacht, N.; Callan, K.; et al. Human Auditory Ossicles as an Alternative Optimal Source of Ancient DNA. Genome Res. 2020, 30, 427–436. [Google Scholar] [CrossRef]
- Golob, A.; Kravanja, P.; Concato, M.; Leskovar, T.; Zupanič Pajnič, I. Searching for Alternative High DNA-Yielding Bone Types for DNA Analysis of Aged Skeletal Remains. Forensic Sci. Int. 2024, 362, 112184. [Google Scholar] [CrossRef] [PubMed]
- Gaudio, D.; Fernandes, D.M.; Schmidt, R.; Cheronet, O.; Mazzarelli, D.; Mattia, M.; O’Keeffe, T.; Feeney, R.N.M.; Cattaneo, C.; Pinhasi, R. Genome-Wide DNA from Degraded Petrous Bones and the Assessment of Sex and Probable Geographic Origins of Forensic Cases. Sci. Rep. 2019, 9, 8226. [Google Scholar] [CrossRef]
- Schwark, T.; Heinrich, A.; Preuße-Prange, A.; von Wurmb-Schwark, N. Reliable Genetic Identification of Burnt Human Remains. Forensic Sci. Int. Genet. 2011, 5, 393–399. [Google Scholar] [CrossRef]
- Frank, E.M.; Mundorff, A.Z.; Davoren, J.M. The Effect of Common Imaging and Hot Water Maceration on DNA Recovery from Skeletal Remains. Forensic Sci. Int. 2015, 257, 189–195. [Google Scholar] [CrossRef]
- Pestle, W.J. Chemical, Elemental, and Isotopic Effects of Acid Concentration and Treatment Duration on Ancient Bone Collagen: An Exploratory Study. J. Archaeol. Sci. 2010, 37, 3124–3128. [Google Scholar] [CrossRef]
- Turner-Walker, G. The Chemical and Microbial Degradation of Bones and Teeth. In Advances in Human Palaeopathology; Wiley: Hoboken, NJ, USA, 2007; pp. 3–29. [Google Scholar]
- Jans, M.M.E.; Nielsen-Marsh, C.M.; Smith, C.I.; Collins, M.J.; Kars, H. Characterisation of Microbial Attack on Archaeological Bone. J. Archaeol. Sci. 2004, 31, 87–95. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Zhang, S.; Wang, W.; Li, X.; Gao, S.; Li, K.; Chen, J.; Wang, H.; Chen, L.; et al. Microstructure of the Hyoid Bone Based on Micro-Computed Tomography Findings. Medicine 2020, 99, e22246. [Google Scholar] [CrossRef] [PubMed]
- Kozerska, M.; Szczepanek, A.; Tarasiuk, J.; Wroński, S. Micro-CT Analysis of the Internal Acoustic Meatus Angles as a Method of Sex Estimation in Skeletal Remains. HOMO 2020, 71, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Duffett Carlson, K.S.; Mandl, K.; McCall, A.; Brönnimann, D.; Teschler-Nicola, M.; Weiss-Krejci, E.; Metscher, B. 3D Visualization of Bioerosion in Archaeological Bone. J. Archaeol. Sci. 2022, 145, 105646. [Google Scholar] [CrossRef]
- Nicklisch, N.; Schierz, O.; Enzmann, F.; Knipper, C.; Held, P.; Vach, W.; Dresely, V.; Meller, H.; Friederich, S.; Alt, K.W. Dental Pulp Calcifications in Prehistoric and Historical Skeletal Remains. Ann. Anat.—Anat. Anz. 2021, 235, 151675. [Google Scholar] [CrossRef]
- Akbulut, N.; Çetin, S.; Bilecenoğlu, B.; Altan, A.; Ocak, M.; Şen, E.; Atakan, C.; Orhan, K. Evaluation of the Detectability of Early Mandible Fracture Healing Findings in Terms of Vitality Aspect by Using Micro-CT Technology in Postmortem Interval. Leg. Med. 2021, 52, 101914. [Google Scholar] [CrossRef] [PubMed]
- Viero, A.; Biehler-Gomez, L.; Messina, C.; Cappella, A.; Giannoukos, K.; Viel, G.; Tagliaro, F.; Cattaneo, C. Utility of Micro-CT for Dating Post-Cranial Fractures of Known Post-Traumatic Ages through 3D Measurements of the Trabecular Inner Morphology. Sci. Rep. 2022, 12, 10543. [Google Scholar] [CrossRef] [PubMed]
- Thurzo, A.; Jančovičová, V.; Hain, M.; Thurzo, M.; Novák, B.; Kosnáčová, H.; Lehotská, V.; Varga, I.; Kováč, P.; Moravanský, N. Human Remains Identification Using Micro-CT, Chemometric and AI Methods in Forensic Experimental Reconstruction of Dental Patterns after Concentrated Sulphuric Acid Significant Impact. Molecules 2022, 27, 4035. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geršak, Ž.M.; Salapura, V.; Podovšovnik, E.; Zupanič-Pajnič, I. Targeting Optimal Bone Regions: Correlations Between Bone Density and DNA Quality in Small Skeletal Elements. Genes 2025, 16, 291. https://doi.org/10.3390/genes16030291
Geršak ŽM, Salapura V, Podovšovnik E, Zupanič-Pajnič I. Targeting Optimal Bone Regions: Correlations Between Bone Density and DNA Quality in Small Skeletal Elements. Genes. 2025; 16(3):291. https://doi.org/10.3390/genes16030291
Chicago/Turabian StyleGeršak, Živa Miriam, Vladka Salapura, Eva Podovšovnik, and Irena Zupanič-Pajnič. 2025. "Targeting Optimal Bone Regions: Correlations Between Bone Density and DNA Quality in Small Skeletal Elements" Genes 16, no. 3: 291. https://doi.org/10.3390/genes16030291
APA StyleGeršak, Ž. M., Salapura, V., Podovšovnik, E., & Zupanič-Pajnič, I. (2025). Targeting Optimal Bone Regions: Correlations Between Bone Density and DNA Quality in Small Skeletal Elements. Genes, 16(3), 291. https://doi.org/10.3390/genes16030291





