Clinical Significance of Fragile X Syndrome 2 (FXR2) in Breast Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. FXR2 Protein Analysis Study Cohort
- Luminal A: ER-positive and/or PR-positive, HER2-negative, low proliferation (Ki67% < 20%).
- Luminal B: ER-positive and/or PR-positive, HER-2-negative or -positive, high proliferation (Ki67% ≥ 20%).
- HER2: ER-negative and/or PR-negative, HER2-positive.
- TNBC: ER-negative and/or PR-negative, HER2-negative.
2.2. A Comprehensive Transcriptomics Analysis of FXR2 in BC
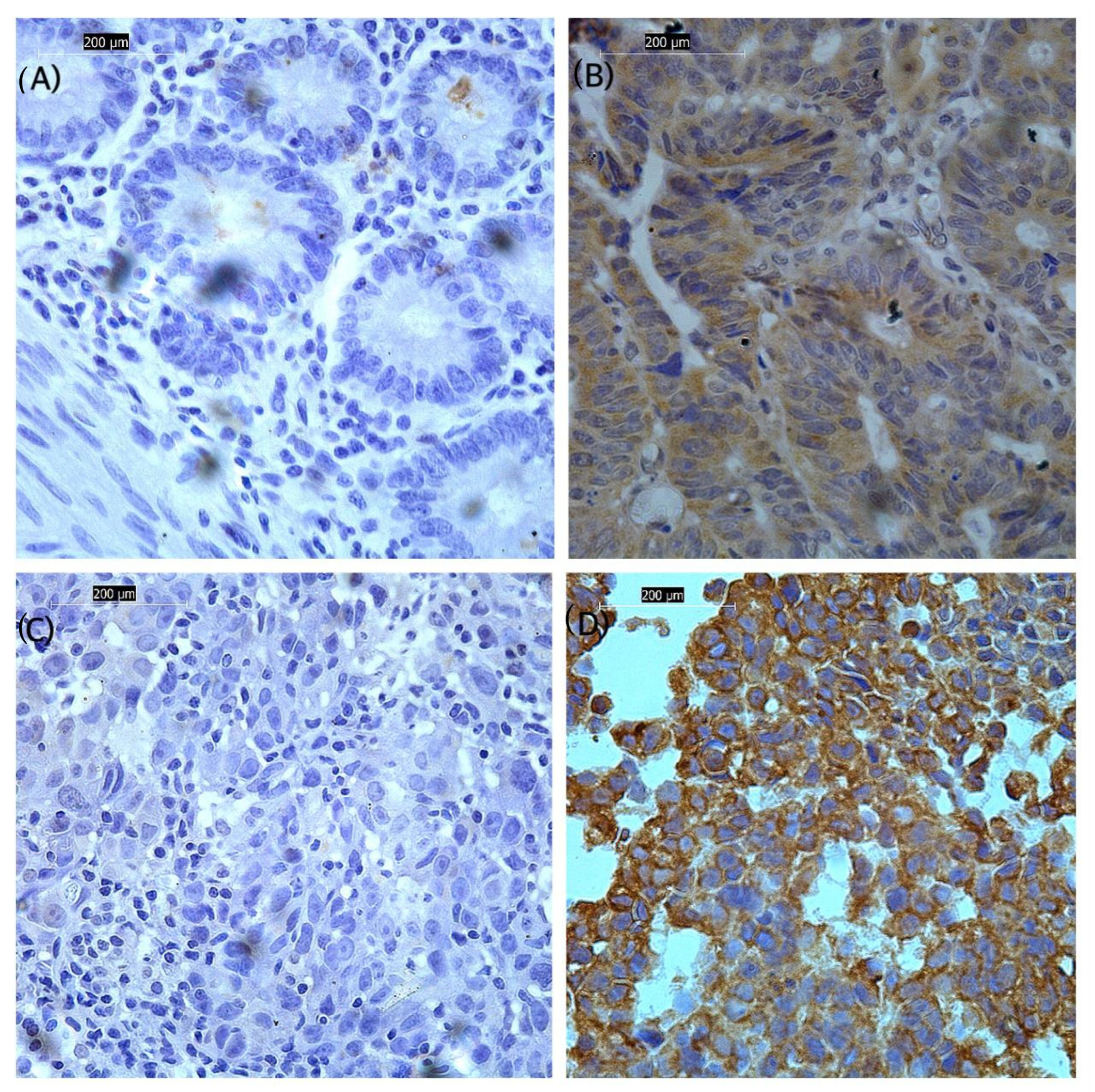
2.3. Validation of FXR2 Transcriptomics Analysis: Immunohistochemical Staining for FXR2 in FFPE Tissue Sections
2.4. Scoring
2.5. Statistical Analysis
3. Results
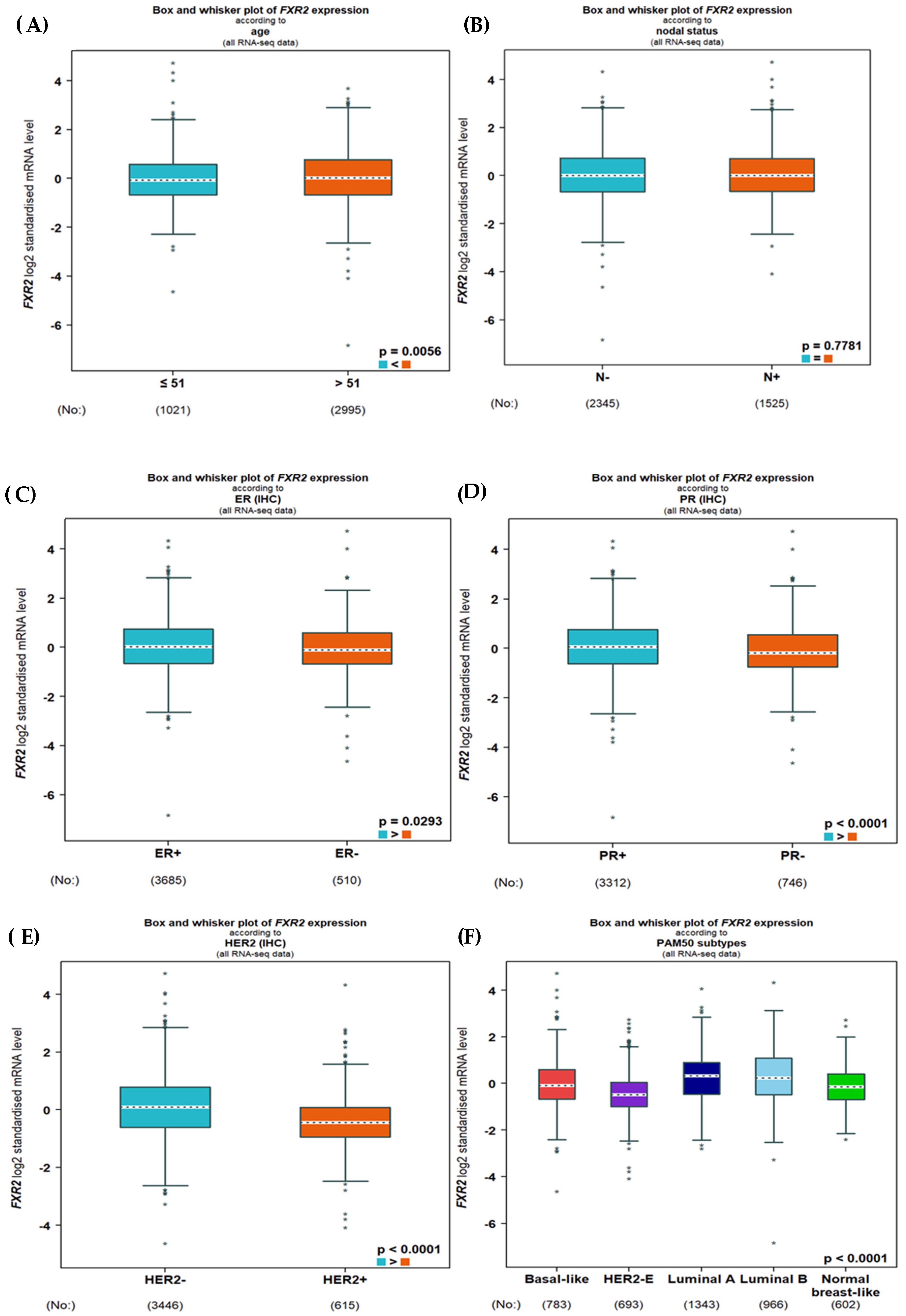
3.1. Expression of FXR2 mRNA
3.2. Association of FXR2 Protein Expression Level with Clinicopathological Parameters
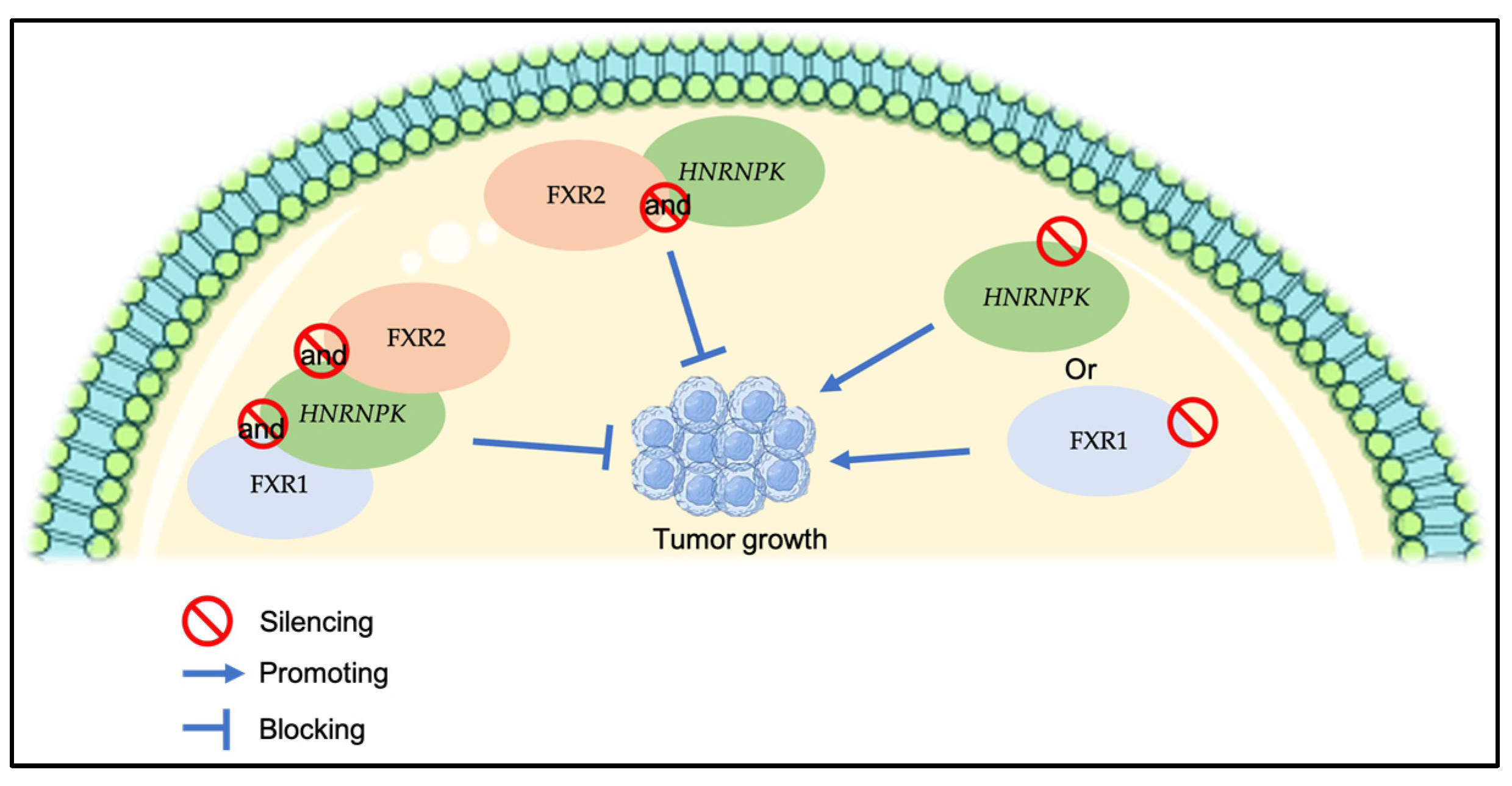
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer-Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies—An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef] [PubMed]
- Lüönd, F.; Tiede, S.; Christofori, G. Breast cancer as an example of tumour heterogeneity and tumour cell plasticity during malignant progression. Br. J. Cancer 2021, 125, 164–175. [Google Scholar] [CrossRef] [PubMed]
- McAndrew, N.; Finn, R. Clinical Review on the Management of Hormone Receptor-Positive Metastatic Breast Cancer. JCO Oncol. Pract. 2022, 18, 319–327. [Google Scholar] [CrossRef]
- Carey, L.A.; Perou, C.M.; Livasy, C.A.; Dressler, L.G.; Cowan, D.; Conway, K.; Karaca, G.; Troester, M.A.; Tse, C.K.; Edmiston, S.; et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006, 295, 2492–2502. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.O.; Hsu, F.D.; Jensen, K.; Cheang, M.; Karaca, G.; Hu, Z.; Hernandez-Boussard, T.; Livasy, C.; Cowan, D.; Dressler, L.; et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin. Cancer Res. 2004, 10, 5367–5374. [Google Scholar] [CrossRef]
- Krystel-Whittemore, M.; Tan, P.H.; Wen, H.Y. Predictive and prognostic biomarkers in breast tumours. Pathology 2024, 56, 186–191. [Google Scholar] [CrossRef]
- Simms, L.; Barraclough, H.; Govindan, R. Biostatistics primer: What a clinician ought to know--prognostic and predictive factors. J. Thorac. Oncol. 2013, 8, 808–813. [Google Scholar] [CrossRef]
- Majumder, M.; Johnson, R.H.; Palanisamy, V. Fragile X-related protein family: A double-edged sword in neurodevelopmental disorders and cancer. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 409–424. [Google Scholar] [CrossRef]
- Ramos, A.; Hollingworth, D.; Adinolfi, S.; Castets, M.; Kelly, G.; Frenkiel, T.A.; Bardoni, B.; Pastore, A. The structure of the N-terminal domain of the fragile X mental retardation protein: A platform for protein-protein interaction. Structure 2006, 14, 21–31. [Google Scholar] [CrossRef]
- Hoogeveen, A.T.; Willemsen, R.; Oostra, B.A. Fragile X syndrome, the Fragile X related proteins, and animal models. Microsc. Res. Tech. 2002, 57, 148–155. [Google Scholar] [CrossRef]
- Chen, E.; Sharma, M.R.; Shi, X.; Agrawal, R.K.; Joseph, S. Fragile X mental retardation protein regulates translation by binding directly to the ribosome. Mol. Cell 2014, 54, 407–417. [Google Scholar] [CrossRef]
- Uhlén, M.; Zhang, C.; Lee, S.; Sjöstedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357, eaan2507. [Google Scholar] [CrossRef] [PubMed]
- Cizkova, K.; Foltynkova, T.; Gachechiladze, M.; Tauber, Z. Comparative Analysis of Immunohistochemical Staining Intensity Determined by Light Microscopy, ImageJ and QuPath in Placental Hofbauer Cells. Acta Histochem. Cytochem. 2021, 54, 21–29. [Google Scholar] [CrossRef]
- Kunheri, B.; Raj, R.V.; Vijaykumar, D.; Pavithran, K. Impact of St. Gallen surrogate classification for intrinsic breast cancer sub-types on disease features, recurrence, and survival in South Indian patients. Indian J. Cancer 2020, 57, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Jézéquel, P.; Gouraud, W.; Ben Azzouz, F.; Guérin-Charbonnel, C.; Juin, P.P.; Lasla, H.; Campone, M. bc-GenExMiner 4.5: New mining module computes breast cancer differential gene expression analyses. Database 2021, 2021, baab007. [Google Scholar] [CrossRef] [PubMed]
- Alanyalı, S.D. Prognostic and Predictive Factors. In Principles and Practice of Modern Radiotherapy Techniques in Breast Cancer; Haydaroglu, A., Ozyigit, G., Eds.; Springer: New York, NY, USA, 2013; pp. 35–47. [Google Scholar]
- Fumagalli, C.; Barberis, M. Breast Cancer Heterogeneity. Diagnostics 2021, 11, 1555. [Google Scholar] [CrossRef]
- Majumder, M.; House, R.; Palanisamy, N.; Qie, S.; Day, T.A.; Neskey, D.; Diehl, J.A.; Palanisamy, V. RNA-Binding Protein FXR1 Regulates p21 and TERC RNA to Bypass p53-Mediated Cellular Senescence in OSCC. PLoS Genet. 2016, 12, e1006306. [Google Scholar]
- Cao, H.; Gao, R.; Yu, C.; Chen, L.; Feng, Y. The RNA-binding protein FXR1 modulates prostate cancer progression by regulating FBXO4. Funct. Integr. Genom. 2019, 19, 487–496. [Google Scholar] [CrossRef]
- Harbeck, N.; Gnant, M. Breast cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef]
- Gamrani, S.; Akhouayri, L.; Boukansa, S.; Karkouri, M.; El Fatemi, H. The Clinicopathological Features and Prognostic Significance of HER2-Low in Early Breast Tumors Patients Prognostic Comparison of HER-Low and HER2-Negative Breast Cancer Stratified by Hormone Receptor Status. Breast J. 2023, 2023, 6621409. [Google Scholar] [CrossRef]
- Davey, M.G.; Hynes, S.O.; Kerin, M.J.; Miller, N.; Lowery, A.J. Ki-67 as a Prognostic Biomarker in Invasive Breast Cancer. Cancers 2021, 13, 4455. [Google Scholar] [CrossRef] [PubMed]
- Gumireddy, K.; Li, A.; Yan, J.; Setoyama, T.; Johannes, G.J.; A Ørom, U.; Tchou, J.; Liu, Q.; Zhang, L.; Speicher, D.W.; et al. Identification of a long non-coding RNA-associated RNP complex regulating metastasis at the translational step. EMBO J. 2013, 32, 2672–2684. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.D.; Smeds, J.; George, J.; Vega, V.B.; Vergara, L.; Ploner, A.; Pawitan, Y.; Hall, P.; Klaar, S.; Liu, E.T.; et al. An expression signature for p53 status in human breast cancer predicts mutation status, transcriptional effects, and patient survival. Proc. Natl. Acad. Sci. USA 2005, 102, 13550–13555. [Google Scholar] [CrossRef] [PubMed]
- Monti, P.; Campomenosi, P.; Ciribilli, Y.; Iannone, R.; Inga, A.; Abbondandolo, A.; Resnick, M.A.; Fronza, G. Tumour p53 mutations exhibit promoter selective dominance over wild type p53. Oncogene 2002, 21, 1641–1648. [Google Scholar] [CrossRef]
- Fan, Y.; Yue, J.; Xiao, M.; Han-Zhang, H.; Wang, Y.V.; Ma, C.; Deng, Z.; Li, Y.; Yu, Y.; Wang, X.; et al. FXR1 regulates transcription and is required for growth of human cancer cells with TP53/FXR2 homozygous deletion. Elife 2017, 6, e26129. [Google Scholar] [CrossRef]
- So, J.Y.; Ohm, J.; Lipkowitz, S.; Yang, L. Triple negative breast cancer (TNBC): Non-genetic tumor heterogeneity and immune microenvironment: Emerging treatment options. Pharmacol. Ther. 2022, 237, 108253. [Google Scholar] [CrossRef]

| Clinicopathological Parameters | FXR2 Protein Expression | p-Value | |
|---|---|---|---|
| Low (n = 44) n (%) | High (n = 56) n (%) | ||
| Age (years) | |||
| <50 | 25 (53.2) | 22 (46.8) | 0.548 |
| ≥50 | 25 (47.2) | 28 (52.8) | |
| Tumor size | |||
| <10 mm | 17 (54.8) | 14 (45.2) | 0.141 |
| ≥10 mm | 10 (35.7) | 18 (64.3) | |
| Tumor grade | |||
| I | 3 (37.5) | 5 (62.5) | 0.271 |
| II | 26 (46.4) | 30 (53.6) | |
| III | 19 (63.3) | 11 (36.7) | |
| TNM stages | |||
| Stage I | 4 (44.4) | 5 (55.6) | 0.117 |
| Stage IIA | 10 (50) | 10 (50) | |
| Stage IIB | 1 (9.1) | 10 (90.9) | |
| Stage IIIA | 4 (80) | 1 (20) | |
| Stage IIIB | 2 (50) | 2 (50) | |
| Stage IIIC | 0 (0) | 1 (100) | |
| Stage IV | 6 (60) | 4 (40) | |
| Lymph nodal status | |||
| Negative | 9 (36) | 16 (64) | 0.200 |
| Positive | 14 (53.8) | 12 (46.2) | |
| Estrogen receptor (ER), IHC | |||
| Negative | 2 (9.1) | 20 (90.9) | 0.369 |
| Positive | 13(16.9) | 64 (83.1) | |
| Progesterone receptor (PR), IHC | |||
| Negative | 3 (12) | 22 (88) | 0.611 |
| Positive | 12 (16.2) | 62 (83.8) | |
| Human Epidermal Growth Factor Receptor 2 (HER2), IHC | |||
| Negative | 30 (41.1) | 43 (58.9) | 0.008 |
| Positive | 18 (72) | 7 (28) | |
| Ki67 (IHC) | |||
| Negative (<20) | 10 (26.3) | 28 (73.7) | <0.001 |
| Positive (>20) | 37 (64.9) | 20 (35.1) | |
| Immunohistochemistry subtype | |||
| Luminal A | 10 (25.6) | 29 (74.4) | <0.001 |
| Luminal B | 34 (94.4) | 2 (5.6) | |
| HER2-positive | 1 (12.5) | 7 (87.5) | |
| Triple negative | 2 (14.3) | 12 (85.7) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsalmi, O.A.; Aljohani, A.I.; Almutairi, S.M.; Alsufyani, R.O.; Alrubayee, A.R.; Alzahrani, K.J.; Alkhammash, G.E.; Aljuaid, H.M.; Alghamdi, H.S.; Alsaeedi, F.A. Clinical Significance of Fragile X Syndrome 2 (FXR2) in Breast Cancer. Genes 2025, 16, 302. https://doi.org/10.3390/genes16030302
Alsalmi OA, Aljohani AI, Almutairi SM, Alsufyani RO, Alrubayee AR, Alzahrani KJ, Alkhammash GE, Aljuaid HM, Alghamdi HS, Alsaeedi FA. Clinical Significance of Fragile X Syndrome 2 (FXR2) in Breast Cancer. Genes. 2025; 16(3):302. https://doi.org/10.3390/genes16030302
Chicago/Turabian StyleAlsalmi, Ohud A., Abrar I. Aljohani, Shahad M. Almutairi, Rana O. Alsufyani, Abdulrahman R. Alrubayee, Khalid J. Alzahrani, Ghaida E. Alkhammash, Hessa M. Aljuaid, Hanan S. Alghamdi, and Fouzeyyah A. Alsaeedi. 2025. "Clinical Significance of Fragile X Syndrome 2 (FXR2) in Breast Cancer" Genes 16, no. 3: 302. https://doi.org/10.3390/genes16030302
APA StyleAlsalmi, O. A., Aljohani, A. I., Almutairi, S. M., Alsufyani, R. O., Alrubayee, A. R., Alzahrani, K. J., Alkhammash, G. E., Aljuaid, H. M., Alghamdi, H. S., & Alsaeedi, F. A. (2025). Clinical Significance of Fragile X Syndrome 2 (FXR2) in Breast Cancer. Genes, 16(3), 302. https://doi.org/10.3390/genes16030302






