Transcriptomic and Metabolomic Analysis of the Uterine Tissue of Yaoshan Chicken and Its Crossbreeds to Reveal the Molecular Mechanism Influencing Eggshell Quality
Abstract
:1. Introduction
2. Materials and Methods
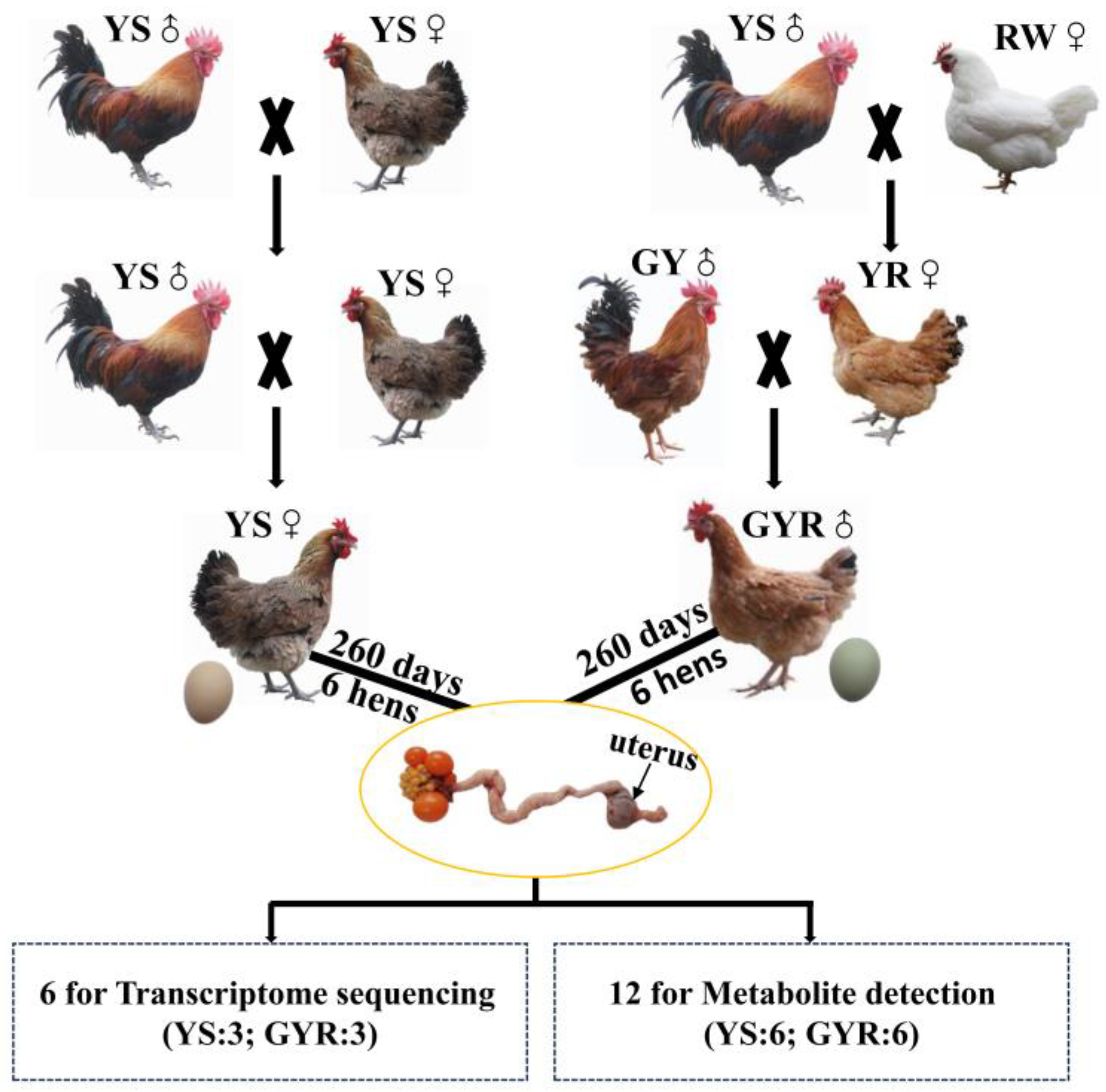
2.1. Experimental Animals and Tissue Collection
2.2. Transcriptome Sequencing and Data Analysis
2.3. Metabolites Detection and Analysis
2.4. Integrated Analysis of Transcriptome and Metabolome
2.5. Statistical Analysis
3. Results
3.1. Differences in Eggshell Quality Between the YS and GYR
3.2. DEGs Analysis Between YS and GYR
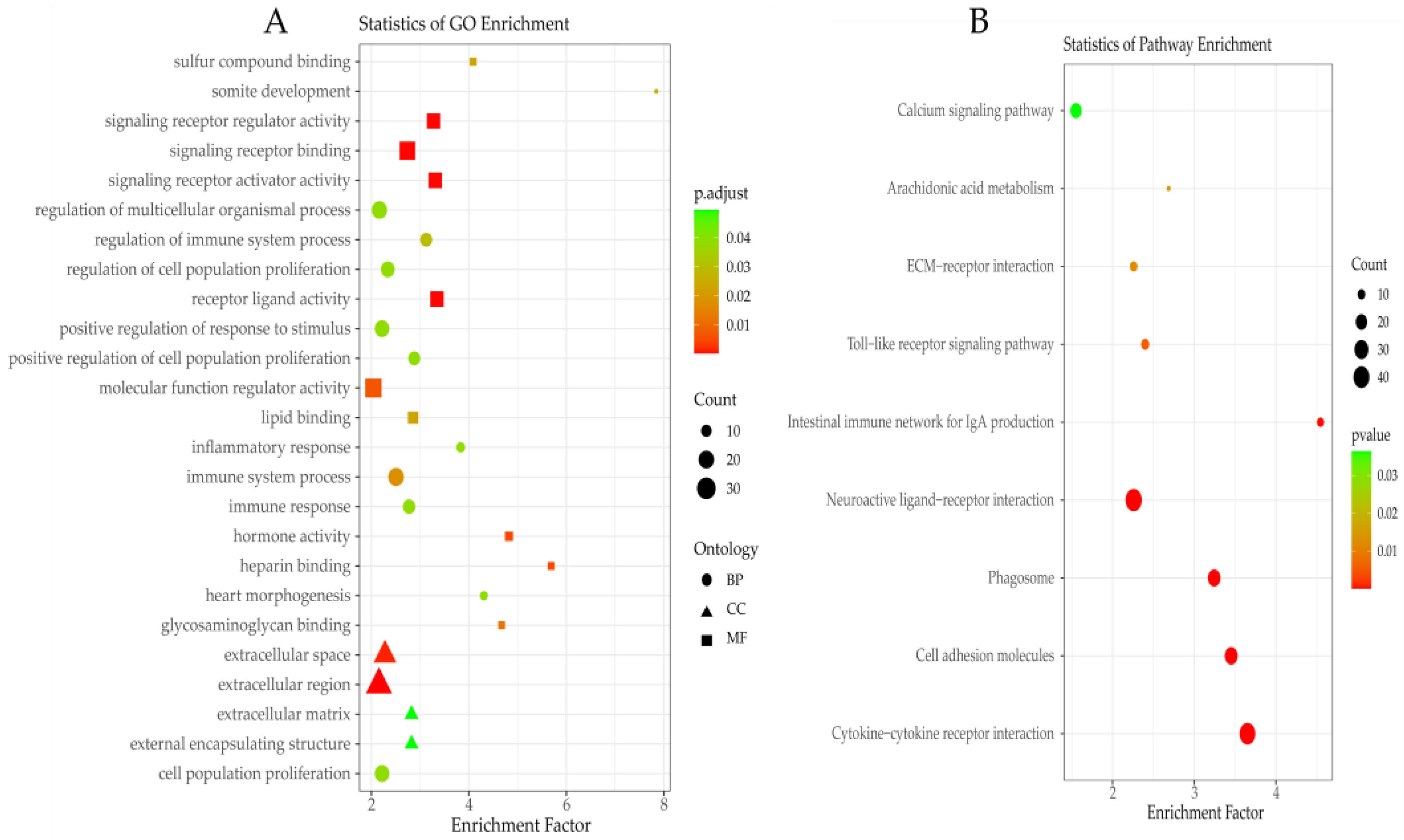
3.3. Functional Enrichment Analysis of DEGs
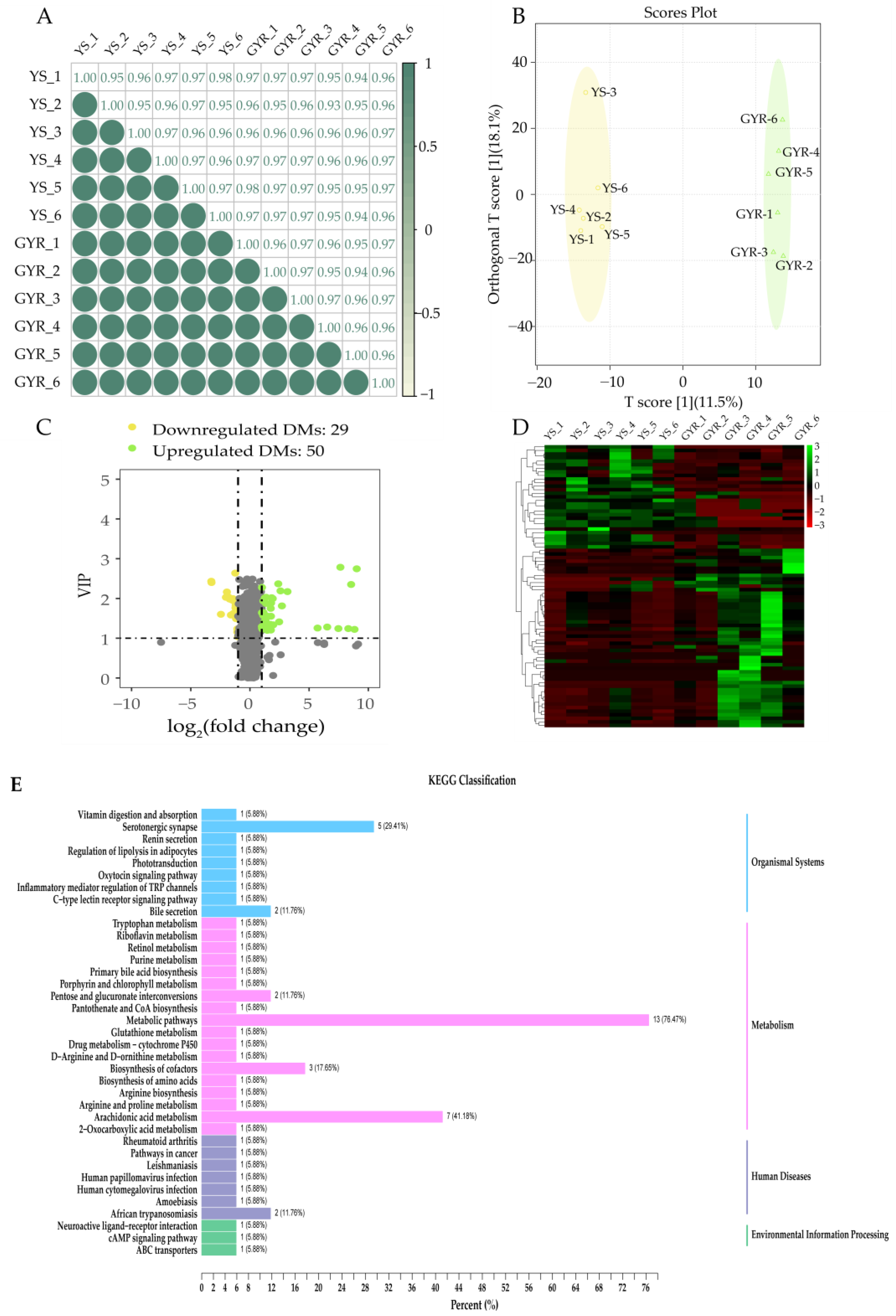
3.4. DMs Analysis Between YS and GYR
3.5. Integrated Analysis of Transcriptome and Metabolome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| YS | Yaoshan chicken |
| RW | recessive white plumage layers |
| YR | offspring of YS (♂) and RW (♀) |
| LD | linear dichroism |
| GY | green-shelled chicken with yellow plumage |
| GYR | offspring of GY(♂) and YR (♀) |
| DEGs | differentially expressed genes |
| DMs | differential metabolites |
References
- Chen, X.; Li, T.; He, K.; Geng, Z.; Wan, X. Dietary green tea powder supplementation enriched egg nutrients and physicochemical property in an indigenous chicken breed. Poult. Sci. 2021, 100, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Samiullah, S.; Omar, A.S.; Roberts, J.; Chousalkar, K. Effect of production system and flock age on eggshell and egg internal quality measurements. Poult. Sci. 2017, 96, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Liu, L.; He, Y.; Dou, T.; Jia, J.; Ge, C. Endocrine and genetic factors affecting egg laying performance in chickens: A review. Br. Poult. Sci. 2020, 61, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wu, Q.; Pan, J.; Li, T.; Liu, L.; Chen, D.; Zhang, X.; Chen, H.; Li, Y.; Lin, R. Identification of differentially expressed genes and signalling pathways in the ovary of higher and lower laying ducks. Br. Poult. Sci. 2020, 61, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Song, W.; Zhu, C.; Xu, W.; Liu, H.; Zhang, S.; Huifang, L. Comparative transcriptomic analysis of high and low egg-producing duck ovaries. Poult. Sci. 2017, 96, 4378–4388. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, P.; Cong, G.; Liu, M.; Shi, S.; Shao, D.; Tan, B. Comparative transcriptomic analysis of ovaries from high and low egg-laying Lingyun black-bone chickens. Vet. Med. Sci. 2021, 7, 1867–1880. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, L.; Han, K.; Zhang, X.; Zhang, G.; Dai, G.; Wang, J.; Xie, K. Transcriptome analysis of ovary in relatively greater and lesser egg producing Jinghai Yellow Chicken. Anim. Reprod. Sci. 2019, 208, 106114. [Google Scholar] [CrossRef]
- Fathi, M.M.; Galal, A.; Ali, U.M.; Abou-Emera, O.K. Physical and mechanical properties of eggshell as affected by chicken breed and flock age. Br. Poult. Sci. 2019, 60, 506–512. [Google Scholar] [CrossRef]
- Lu, M.Y.; Xu, L.; Qi, G.H.; Zhang, H.J.; Qiu, K.; Wang, J.; Wu, S.G. Mechanisms associated with the depigmentation of brown eggshells: A review. Poult. Sci. 2021, 100, 101273. [Google Scholar] [CrossRef]
- Tang, J.; Liu, J.; Miao, X.; Li, H.; Han, X.; Li, L.; Wang, X.; Liao, Z. Analysis and evaluation of muscle nutrient composition in Yaoshan chicken and Changshun green-shelled egg-laying chicken. Chin. J. Anim. Sci. 2021, 57, 235–238+255. (In Chinese) [Google Scholar]
- Wu, S.; Zhu, L.; Tang, J.; Wu, G. TGFβ3 gene polymorphism and correlations between TGFβ3 gene and reproduction traits in Liboyaoshan chicken. Southwest. China J. Agric. Sci. 2017, 30, 1923–1928. (In Chinese) [Google Scholar]
- Guizhou Institute of Animal Husbandry and Veterinary Medicine. Preliminary breeding success of new high-quality local chicken lines in Guizhou. Guizhou J. Anim. Husb. Vet. 2023, 47, 5. (In Chinese) [Google Scholar]
- Miao, X.; Wu, T.; Pan, H.; Zhang, Y.; Liu, J.; Fan, Y.; Du, L.; Gong, Y.; Li, L.; Huang, T.; et al. Integrative analysis of the ovarian metabolome and transcriptome of the Yaoshan chicken and its improved hybrids. Front. Genet. 2024, 15, 1416283. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Wu, S.B.; Roberts, J. RNA-sequencing analysis of shell gland shows differences in gene expression profile at two time-points of eggshell formation in laying chickens. BMC Genom. 2019, 20, 89. [Google Scholar] [CrossRef]
- Brionne, A.; Nys, Y.; Hennequet-Antier, C.; Gautron, J. Hen uterine gene expression profiling during eggshell formation reveals putative proteins involved in the supply of minerals or in the shell mineralization process. BMC Genom. 2014, 15, 220. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.W.; Qi, G.H.; Cui, C.F.; Wu, S.G.; Zhang, H.J.; Xu, L.; Wang, J. Effects of dietary Bacillus subtilis supplementation and calcium levels on performance and eggshell quality of laying hens in the late phase of production. Poult. Sci. 2021, 100, 100970. [Google Scholar] [CrossRef]
- Nys, Y.; Zawadzki, J.; Gautron, J.; Mills, A.D. Whitening of brown-shelled eggs: Mineral composition of uterine fluid and rate of protoporphyrin deposition. Poult. Sci. 1991, 70, 1236–1245. [Google Scholar] [CrossRef]
- Zheng, C.; Li, Z.; Yang, N.; Ning, Z. Quantitative expression of candidate genes affecting eggshell color. Anim. Sci. J. 2014, 85, 506–510. [Google Scholar] [CrossRef]
- Zhao, R.; Xu, G.Y.; Liu, Z.Z.; Li, J.Y.; Yang, N. A study on eggshell pigmentation: Biliverdin in blue-shelled chickens. Poult. Sci. 2006, 85, 546–549. [Google Scholar] [CrossRef]
- Wang, T.; Yang, M.; Shi, X.; Tian, S.; Li, Y.; Xie, W.; Zou, Z.; Leng, D.; Zhang, M.; Zheng, C.; et al. Multiomics analysis provides insights into musk secretion in muskrat and musk deer. Gigascience 2025, 14, giaf006. [Google Scholar] [CrossRef]
- Pan, H.; Huang, T.; Yu, L.; Wang, P.; Su, S.; Wu, T.; Bai, Y.; Teng, Y.; Wei, Y.; Zhou, L.; et al. Transcriptome Analysis of the Adipose Tissue of Luchuan and Duroc Pigs. Animals 2022, 12, 2258. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Leng, D.; Cai, Z.; Chen, B.; Li, J.; Kui, H.; Li, D.; Li, Z. Insights into left-right asymmetric development of chicken ovary at the single-cell level. J. Genet. Genom. 2024, 51, 1265–1277. [Google Scholar] [CrossRef] [PubMed]
- Leng, D.; Zeng, B.; Wang, T.; Chen, B.; Li, D.; Li, Z. Single nucleus/cell RNA-seq of the chicken hypothalamic-pituitary-ovarian axis offers new insights into the molecular regulatory mechanisms of ovarian development. Zool. Res. 2024, 45, 1088–1107. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; Dai, Z.; Xi, Y.; Li, M.; Yan, J.; Yu, J.; Chen, Z.; Shi, Z. Metabolomic evaluation of serum metabolites of geese reared at different stocking densities. Br. Poult. Sci. 2021, 62, 304–309. [Google Scholar] [CrossRef]
- Zhan, H.; Xiong, Y.; Wang, Z.; Dong, W.; Zhou, Q.; Xie, S.; Li, X.; Zhao, S.; Ma, Y. Integrative analysis of transcriptomic and metabolomic profiles reveal the complex molecular regulatory network of meat quality in Enshi black pigs. Meat. Sci. 2022, 183, 108642. [Google Scholar] [CrossRef]
- Wang, T.; Li, P.; Bai, X.; Tian, S.; Yang, M.; Leng, D.; Kui, H.; Zhang, S.; Yan, X.; Zheng, Q.; et al. Vaginal microbiota are associated with in vitro fertilization during female infertility. Imeta 2024, 3, e185. [Google Scholar] [CrossRef]
- Cheng, X.; Wei, Y.; Liu, Y.; Ma, Y.; Zhang, Y.; Li, W.; Luo, Y.; Yan, W.; Qu, L.; Ning, Z. Research Note: Correlation between the reproductive tract microbiota and speckled eggs in laying hens. Poult. Sci. 2024, 103, 104181. [Google Scholar] [CrossRef]
- Khogali, M.K.; Wen, K.; Jauregui, D.; Liu, L.; Zhao, M.; Gong, D.; Geng, T. Uterine structure and function contributes to the formation of the sandpaper-shelled eggs in laying hens. Anim. Reprod. Sci. 2021, 232, 106826. [Google Scholar] [CrossRef]
- Sah, N.; Kuehu, D.L.; Khadka, V.S.; Deng, Y.; Peplowska, K.; Jha, R.; Mishra, B. RNA sequencing-based analysis of the laying hen uterus revealed the novel genes and biological pathways involved in the eggshell biomineralization. Sci. Rep. 2018, 8, 16853. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, Q.; Zhang, X.; Lu, L. Microarray analysis of genes involved with shell strength in layer shell gland at the early stage of active calcification. Asian-Australas. J. Anim. Sci. 2013, 26, 609–624. [Google Scholar] [CrossRef]
- Chen, S.; Chen, K.; Xu, J.; Li, F.; Ding, J.; Ma, Z.; Li, G.; Li, H. Insights Into mRNA and Long Non-coding RNA Profiling RNA Sequencing in Uterus of Chickens With Pink and Blue Eggshell Colors. Front. Vet. Sci. 2021, 8, 736387. [Google Scholar] [CrossRef]
- Wang, Z.; Meng, G.; Bai, Y.; Liu, R.; Du, Y.; Su, L. Comparative transcriptome analysis provides clues to molecular mechanisms underlying blue-green eggshell color in the Jinding duck (Anas platyrhynchos). BMC Genom. 2017, 18, 725. [Google Scholar] [CrossRef]
- Roberts, J.R.; Chousalkar, K.; Samiullah. Egg quality and age of laying hens: Implications for product safety. Anim. Prod. Sci. 2013, 53, 1291–1297. [Google Scholar] [CrossRef]
- Zi, X.; Ge, X.; Zhu, Y.; Liu, Y.; Sun, D.; Li, Z.; Liu, M.; You, Z.; Wang, B.; Kang, J.; et al. Transcriptome Profile Analysis Identifies Candidate Genes for the Melanin Pigmentation of Skin in Tengchong Snow Chickens. Vet. Sci. 2023, 10, 341. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, J.; Wang, H.; Li, H.; Qu, C.; Wen, J.; Zhang, X.; Zhu, T.; Nie, C.; Li, X.; et al. Genomic and transcriptomic analyses reveal genetic adaptation to cold conditions in the chickens. Genomics 2022, 114, 110485. [Google Scholar] [CrossRef]
- Ouyang, Q.; Hu, S.; Tang, B.; Hu, B.; Hu, J.; He, H.; Li, L.; Wang, J. Comparative Transcriptome Analysis Provides Novel Insights into the Effect of Lipid Metabolism on Laying of Geese. Animals 2022, 12, 1775. [Google Scholar] [CrossRef]
- Xiang, X.; Huang, X.; Wang, J.; Zhang, H.; Zhou, W.; Xu, C.; Huang, Y.; Tan, Y.; Yin, Z. Transcriptomic and metabolomic analyses of the ovaries of Taihe black-bone silky fowls at the peak egg-laying and nesting period. Front. Genet. 2023, 14, 1222087. [Google Scholar] [CrossRef]
- Kim, H.; Choo, H.; Cha, J.; Jang, M.; Son, J.; Jeong, T.; Choi, B.H.; Lim, Y.; Chai, H.H.; Lee, J.; et al. Blood transcriptome comparison between sexes and their function in 4-week Rhode Island red chickens. Anim. Cells. Syst 2022, 26, 358–368. [Google Scholar] [CrossRef]
- Sun, T.; Xiao, C.; Yang, Z.; Deng, J.; Yang, X. Grade follicles transcriptional profiling analysis in different laying stages in chicken. BMC Genom. 2022, 23, 492. [Google Scholar] [CrossRef]
- Chen, X.; Cao, J.; Chang, C.; Geng, A.; Wang, H.; Chu, Q.; Yan, Z.; Zhang, X.; Zhang, Y.; Liu, H.; et al. Effects of Age on Compounds, Metabolites and Meat Quality in Beijing-You Chicken Breast Meat. Animals 2023, 13, 3419. [Google Scholar] [CrossRef]
- Huang, Z.; Cai, Z.; Zhang, J.; Gu, Y.; Wang, J.; Yang, J.; Lv, G.; Yang, C.; Zhang, Y.; Ji, C.; et al. Integrating proteomics and metabolomics to elucidate the molecular network regulating of inosine monophosphate-specific deposition in Jingyuan chicken. Poult. Sci. 2023, 102, 103118. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liu, H.; Wang, L.; Wei, B.; Wu, Q.; Xu, Q.; Tang, Q.; Qi, J.; Li, J.; Wang, J.; et al. Untargeted metabolomics study on the effects of rearing ducks in cages on bone quality. Poult. Sci. 2022, 101, 101604. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xiao, H.; Zhang, H.; Pan, A.; Shen, J.; Sun, J.; Liang, Z.; Pi, J. Quasi-Targeted Metabolomics Approach Reveal the Metabolite Differences of Three Poultry Eggs. Foods 2023, 12, 2765. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Qu, F.; Zhang, C.; Chen, Z.; Li, Y.; Wen, L. Multi-Omics Analyses Unravel Metabolic and Transcriptional Differences in Tender Shoots from Two Sechium edule Varieties. Curr. Issues Mol. Biol. 2023, 45, 9060–9075. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Mu, R.; Gegen, T.; Luo, J.; Xiao, Y.; Ou, S.; Wu, Q.; Zuo, Y.; Chen, Z.; Li, F. Comparative analysis of hepatic transcriptomes and metabolomes of Changshun green-shell laying hens based on different green eggshell color intensities. Poult. Sci. 2024, 103, 103220. [Google Scholar] [CrossRef]
- Wilson, P.B. Recent advances in avian egg science: A review. Poult. Sci. 2017, 96, 3747–3754. [Google Scholar] [CrossRef]
- Bagh, J.; Panigrahi, B.; Panda, N.; Pradhan, C.R.; Mallik, B.K.; Majhi, B.; Rout, S.S. Body weight, egg production, and egg quality traits of gray, brown, and white varieties of Japanese quail (Coturnix coturnix japonica) in coastal climatic condition of Odisha. Vet. World 2016, 9, 832–836. [Google Scholar] [CrossRef]
- Lordelo, M.; Cid, J.; Cordovil, C.; Alves, S.P.; Bessa, R.J.B.; Carolino, I. A comparison between the quality of eggs from indigenous chicken breeds and that from commercial layers. Poult. Sci. 2020, 99, 1768–1776. [Google Scholar] [CrossRef]
- Sun, T.; Xiao, C.; Yang, Z.; Deng, J.; Yang, X. Transcriptome profiling analysis of uterus during chicken laying periods. BMC Genom. 2023, 24, 433. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Q.; Wang, Y.; Wang, Y.; Liu, R. Refine localizations of functional variants affecting eggshell color of Lueyang black-boned chicken in the SLCO1B3. Poult. Sci. 2024, 103, 103212. [Google Scholar] [CrossRef]
- Hagenbuch, B.; Gui, C. Xenobiotic transporters of the human organic anion transporting polypeptides (OATP) family. Xenobiotica 2008, 38, 778–801. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Wang, C.X.; Jia, F.J.; Jahejo, A.R.; Li, X.; Ning, G.B.; Zhang, D.; Ma, H.L.; Hao, W.F.; Gao, W.W.; et al. The expression of prostaglandins-related genes in erythrocytes of broiler chicken responds to thiram-induced tibial dyschondroplasia and recombinant glutathione-S-transferase A3 protein. Res. Vet. Sci. 2019, 124, 112–117. [Google Scholar] [CrossRef] [PubMed]
- King, J.S.; Wan, M.; Wagley, Y.; Stestiv, M.; Kalajzic, I.; Hankenson, K.D.; Sanjay, A. Signaling pathways associated with Lgr6 to regulate osteogenesis. Bone 2024, 187, 117207. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Wang, M.; Xiong, Y.; Wen, X.; Zhang, M.; Ma, L.; Zhang, Y. MELTF Might Regulate Ferroptosis, Pyroptosis, and Autophagy in Platelet-Rich Plasma-Mediated Endometrial Epithelium Regeneration. Reprod. Sci. 2023, 30, 1506–1520. [Google Scholar] [CrossRef]
- Lim, S.; Kierzek, M.; O’Connor, A.E.; Brenker, C.; Merriner, D.J.; Okuda, H.; Volpert, M.; Gaikwad, A.; Bianco, D.; Potter, D.; et al. CRISP2 Is a Regulator of Multiple Aspects of Sperm Function and Male Fertility. Endocrinology 2019, 160, 915–924. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, M.; Guo, F.; Song, Y.; Fan, X.; Qin, G. Identification of Tumor Microenvironment-Related Prognostic Biomarkers in Luminal Breast Cancer. Front. Genet. 2020, 11, 555865. [Google Scholar] [CrossRef]
- Zeng, L.; Shi, X.; Xuan, L.; Zheng, J. Comparative N-Glycoproteomic Investigation of Eggshell Cuticle and Mineralized Layer Proteins. J. Agric. Food. Chem. 2023, 71, 10448–10458. [Google Scholar] [CrossRef]
- Wang, Y.; Lupiani, B.; Reddy, S.M.; Lamont, S.J.; Zhou, H. RNA-seq analysis revealed novel genes and signaling pathway associated with disease resistance to avian influenza virus infection in chickens. Poult. Sci. 2014, 93, 485–493. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, F.; Liu, L.; Zheng, C.W.; Wang, D.H.; Hou, Z.C.; Ning, Z.H. Integrating transcriptome and genome re-sequencing data to identify key genes and mutations affecting chicken eggshell qualities. PLoS ONE 2015, 10, e0125890. [Google Scholar] [CrossRef]
- Song, X.; Li, S.; He, S.; Zheng, H.; Li, R.; Liu, L.; Geng, T.; Zhao, M.; Gong, D. Integration of Whole-Genome Resequencing and Transcriptome Sequencing Reveals Candidate Genes in High Glossiness of Eggshell. Animals 2024, 14, 1141. [Google Scholar] [CrossRef]
- Ma, P.; Xue, F.; Chen, J.; Zhang, X.; Xu, X.; Ma, Z.; Zhang, H.; Wu, Y.; Li, L.; Qu, Y.; et al. Transcriptomic insight into the underlying mechanism of induced molting on reproductive remodeling, performance and egg quality in laying hen. Poult. Sci. 2024, 104, 104692. [Google Scholar] [CrossRef] [PubMed]
- Elhamouly, M.; Isobe, N.; Yoshimura, Y. Expression and localization of cyclooxygenases in the oviduct of laying hens during the ovulatory cycle. Theriogenology 2017, 101, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Tajima, H.; Nakagawa-Mizuyachi, K.; Nakayama, H.; Kawashima, M. Changes in prostaglandin F2alpha receptor bindings in the hen oviduct uterus before and after oviposition. Poult. Sci. 2011, 90, 1767–1773. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, Y.; Zhou, P.; Lu, S.; Lin, J.; Wen, G.; Shi, X.; Guo, Y. Effects of dietary glucosamine sulfate sodium on early laying performance and eggshell quality of laying hens. Poult. Sci. 2024, 103, 103982. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, H.; Wang, J.; Li, L.; Han, C.; Mustafa, A.; Xiong, X. Evidences in duck (Anas platyrhynchos) by transcriptome data for supporting the biliverdin was mainly synthesized by shell gland. Poult. Sci. 2019, 98, 2260–2271. [Google Scholar] [CrossRef]
- Zi, Q.; Li, P.; Lei, J.; Qu, X.; He, C.; Yao, Y.; Zou, X.; Guo, S. Identification of Genes Affected Blue Eggshell Coloration in Xuefeng Black-Bone Chickens. Braz. J. Poult. Sci. 2024, 26, eRBCA-2022-1729. [Google Scholar] [CrossRef]
- Li, Z.; Ren, T.; Li, W.; Zhou, Y.; Han, R.; Li, H.; Jiang, R.; Yan, F.; Sun, G.; Liu, X.; et al. Association Between the Methylation Statuses at CpG Sites in the Promoter Region of the SLCO1B3, RNA Expression and Color Change in Blue Eggshells in Lushi Chickens. Front. Genet. 2019, 10, 161. [Google Scholar] [CrossRef]
- Nakazawa, F.; Alev, C.; Jakt, L.M.; Sheng, G. Yolk sac endoderm is the major source of serum proteins and lipids and is involved in the regulation of vascular integrity in early chick development. Dev. Dyn. 2011, 240, 2002–2010. [Google Scholar] [CrossRef]
- Yamamoto, K.; Hirowatari, A. Investigation of the Substrate-Binding Site of a Prostaglandin E Synthase in Bombyx mori. Protein. J. 2021, 40, 63–67. [Google Scholar] [CrossRef]
- Hales, D.B.; Zhuge, Y.; Lagman, J.A.; Ansenberger, K.; Mahon, C.; Barua, A.; Luborsky, J.L.; Bahr, J.M. Cyclooxygenases expression and distribution in the normal ovary and their role in ovarian cancer in the domestic hen (Gallus domesticus). Endocrine 2008, 33, 235–244. [Google Scholar] [CrossRef]



| Items | Phase I 1 (%) | Phase II 1 (%) | Phase III 1 (%) |
|---|---|---|---|
| Ingredients | |||
| Maize | 65.0 | 64.0 | 60.0 |
| Soybean meal | 31.0 | 25.0 | 28.0 |
| Soybean oil | – | – | 1 |
| Wheat bran | – | 7.0 | – |
| Limestone | – | – | 7 |
| Premix 2 | 4.0 | 4.0 | 4.0 |
| Total | 100 | 100 | 100 |
| Nutrient levels | |||
| ME/(MJ/kg) | 11.92 | 11.30 | 11.51 |
| Crude protein/% | 18.0 | 14.0 | 16.5 |
| Crude fat/% | 3.0 | 3.0 | 3.0 |
| Crude fiber/% | 3.0 | 3.0 | 3.0 |
| Calcium/% | 0.90 | 1.00 | 3.20 |
| Available P/% | 0.45 | 0.40 | 0.40 |
| NaCl/% | 0.36 | 0.36 | 0.32 |
| Met+Cys/% Lys/% | 0.71 0.85 | 0.56 0.55 | 0.64 0.69 |
| Sample | Raw Reads | Clean Reads | Mapping (%) | Q20 (%) | Q30 (%) | GC (%) |
|---|---|---|---|---|---|---|
| YS_1 | 48,666,990 | 47,833,650 | 88.8 | 96.98 | 91.94 | 51.97 |
| YS_2 | 49,079,746 | 48,281,330 | 91.0 | 97.16 | 92.32 | 51.10 |
| YS_3 | 44,958,804 | 44,288,324 | 91.0 | 96.92 | 91.71 | 51.77 |
| GYR_1 | 45,735,116 | 45,072,044 | 90.9 | 97.18 | 92.36 | 51.79 |
| GYR_2 | 45,695,862 | 45,033,400 | 91.0 | 97.13 | 92.18 | 51.42 |
| GYR_3 | 47,173,102 | 46,403,390 | 90.2 | 97.26 | 92.62 | 51.59 |
| Gene | log2 (Fold Change) | p-Value | p-Adjust | Regulated |
|---|---|---|---|---|
| SLCO1B3 | 9.4677 | 1.41 × 10−24 | 3.17 × 10−20 | up |
| SLCO1B1 | 3.2076 | 1.48 × 10−09 | 1.67 × 10−05 | up |
| PTGR1 | 1.8471 | 7.93 × 10−08 | 6.00 × 10−04 | up |
| LGR6 | 2.6386 | 1.68 × 10−07 | 9.00 × 10−04 | up |
| MELTF | 2.2275 | 3.87 × 10−07 | 1.50 × 10−03 | up |
| PSAT1 | 2.1938 | 5.79 × 10−07 | 1.90 × 10−03 | up |
| AVD | 4.2607 | 1.05 × 10−06 | 2.60 × 10−03 | up |
| AGBL3 | 4.4962 | 5.75 × 10−06 | 1.08 × 10−02 | up |
| CRMP1 | 1.3833 | 1.64 × 10−05 | 1.69 × 10−02 | up |
| LOC419409 | 1.9462 | 2.01 × 10−05 | 1.86 × 10−02 | up |
| ZFPM1 | 1.4061 | 2.09 × 10−05 | 1.86 × 10−02 | up |
| GALC | 1.2642 | 6.43 × 10−05 | 4.11 × 10−02 | up |
| ATP6V0A4 | 1.5728 | 6.50 × 10−05 | 4.11 × 10−02 | up |
| SLC28A3 | 2.1652 | 8.48 × 10−05 | 4.60 × 10−02 | up |
| USP43 | 1.3754 | 1.00 × 10−04 | 5.84 × 10−02 | up |
| CRISP2 | −9.1557 | 3.86 × 10−07 | 1.50 × 10−03 | down |
| GVINP1 | −4.1667 | 8.31 × 10−07 | 2.30 × 10−03 | down |
| LOC112532804 | −5.7186 | 1.85 × 10−06 | 4.10 × 10−03 | down |
| OVSTL | −1.6217 | 7.81 × 10−06 | 1.20 × 10−02 | down |
| LOC121106625 | −3.2059 | 1.47 × 10−05 | 1.59 × 10−02 | down |
| LOC100857858 | −1.1501 | 1.84 × 10−05 | 1.80 × 10−02 | down |
| CCDC141 | −6.4296 | 2.14 × 10−05 | 1.86 × 10−02 | down |
| CCL19 | −5.4059 | 2.46 × 10−05 | 2.06 × 10−02 | down |
| CSMD1 | −9.1551 | 2.79 × 10−05 | 2.23 × 10−02 | down |
| LOC100857191 | −4.6780 | 3.23 × 10−05 | 2.43 × 10−02 | down |
| LOC121106469 | −2.9620 | 4.98 × 10−05 | 3.63 × 10−02 | down |
| SCIN | −1.9398 | 5.23 × 10−05 | 3.69 × 10−02 | down |
| RHOBTB1 | −4.1245 | 5.59 × 10−05 | 3.83 × 10−02 | down |
| C10H15ORF48 | −3.6282 | 8.13 × 10−05 | 4.60 × 10−02 | down |
| LOC121112483 | −5.3421 | 8.36 × 10−05 | 4.60 × 10−02 | down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, X.; Liu, J.; Gong, Q.; Li, F.; Zhang, Y.; Liang, Q.; Li, D.; Ning, Z. Transcriptomic and Metabolomic Analysis of the Uterine Tissue of Yaoshan Chicken and Its Crossbreeds to Reveal the Molecular Mechanism Influencing Eggshell Quality. Genes 2025, 16, 383. https://doi.org/10.3390/genes16040383
Miao X, Liu J, Gong Q, Li F, Zhang Y, Liang Q, Li D, Ning Z. Transcriptomic and Metabolomic Analysis of the Uterine Tissue of Yaoshan Chicken and Its Crossbreeds to Reveal the Molecular Mechanism Influencing Eggshell Quality. Genes. 2025; 16(4):383. https://doi.org/10.3390/genes16040383
Chicago/Turabian StyleMiao, Xiaomeng, Jia Liu, Qian Gong, Fugui Li, Yalan Zhang, Qiyue Liang, Diyan Li, and Zhonghua Ning. 2025. "Transcriptomic and Metabolomic Analysis of the Uterine Tissue of Yaoshan Chicken and Its Crossbreeds to Reveal the Molecular Mechanism Influencing Eggshell Quality" Genes 16, no. 4: 383. https://doi.org/10.3390/genes16040383
APA StyleMiao, X., Liu, J., Gong, Q., Li, F., Zhang, Y., Liang, Q., Li, D., & Ning, Z. (2025). Transcriptomic and Metabolomic Analysis of the Uterine Tissue of Yaoshan Chicken and Its Crossbreeds to Reveal the Molecular Mechanism Influencing Eggshell Quality. Genes, 16(4), 383. https://doi.org/10.3390/genes16040383






