Phenology and Spatial Genetic Structure of Anadenanthera colubrina (Vell.), a Resilient Species Amid Territorial Transformation in an Urban Deciduous Forest of Southeastern Brazil
Abstract
1. Introduction
2. Materials and Methods
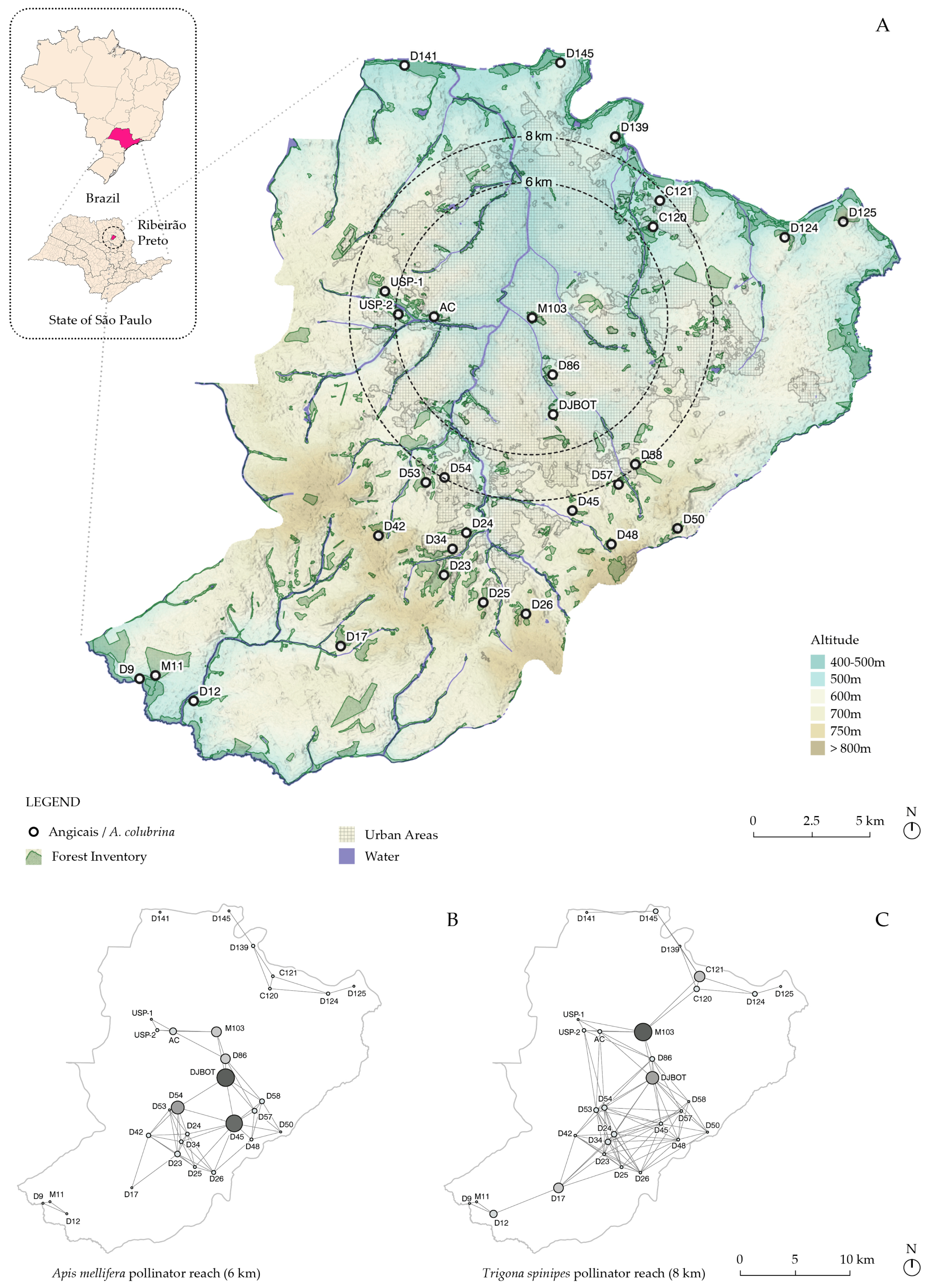
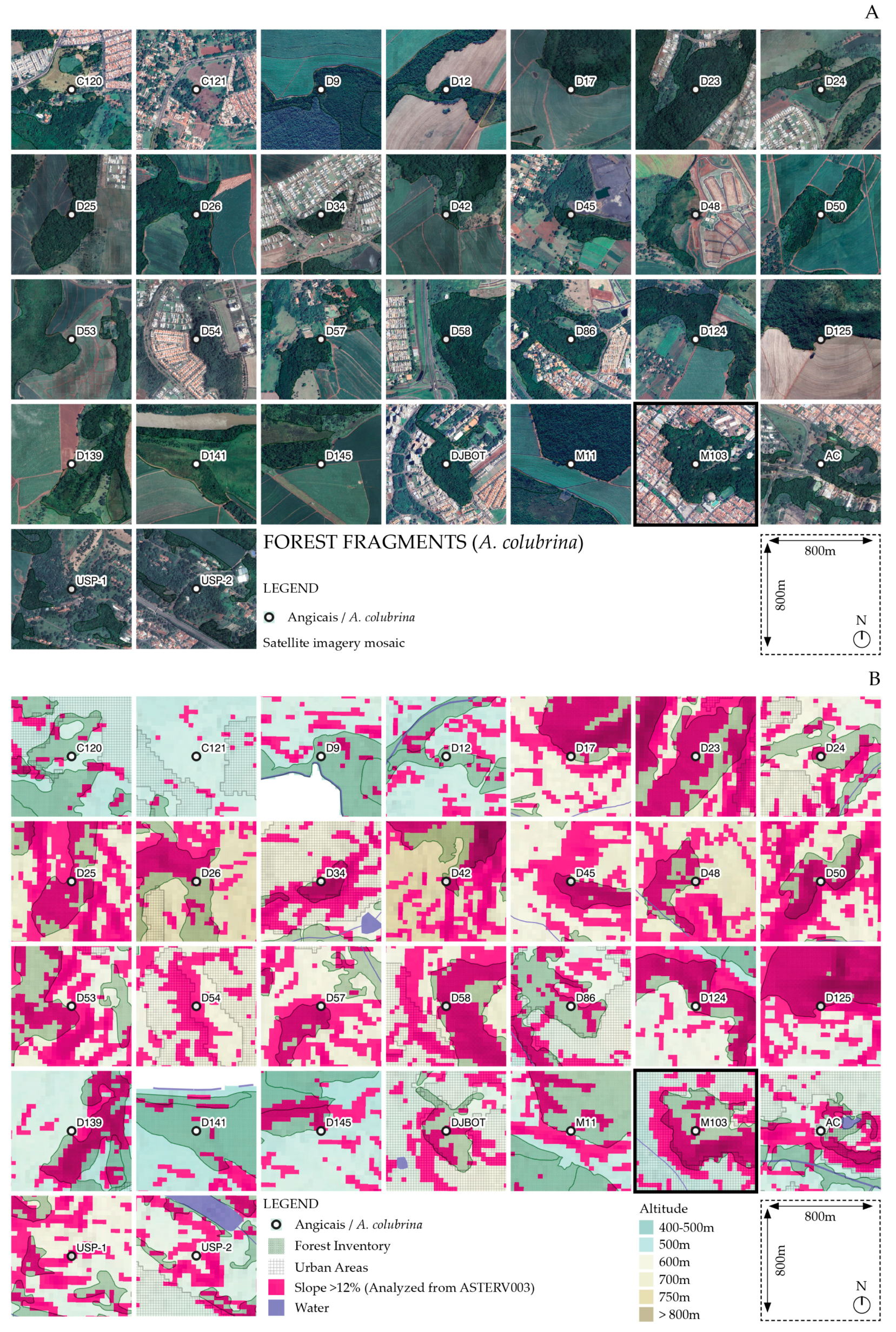
2.1. Analysis of Fragments with A. colubrina Populations in Ribeirão Preto, SP, Brazil
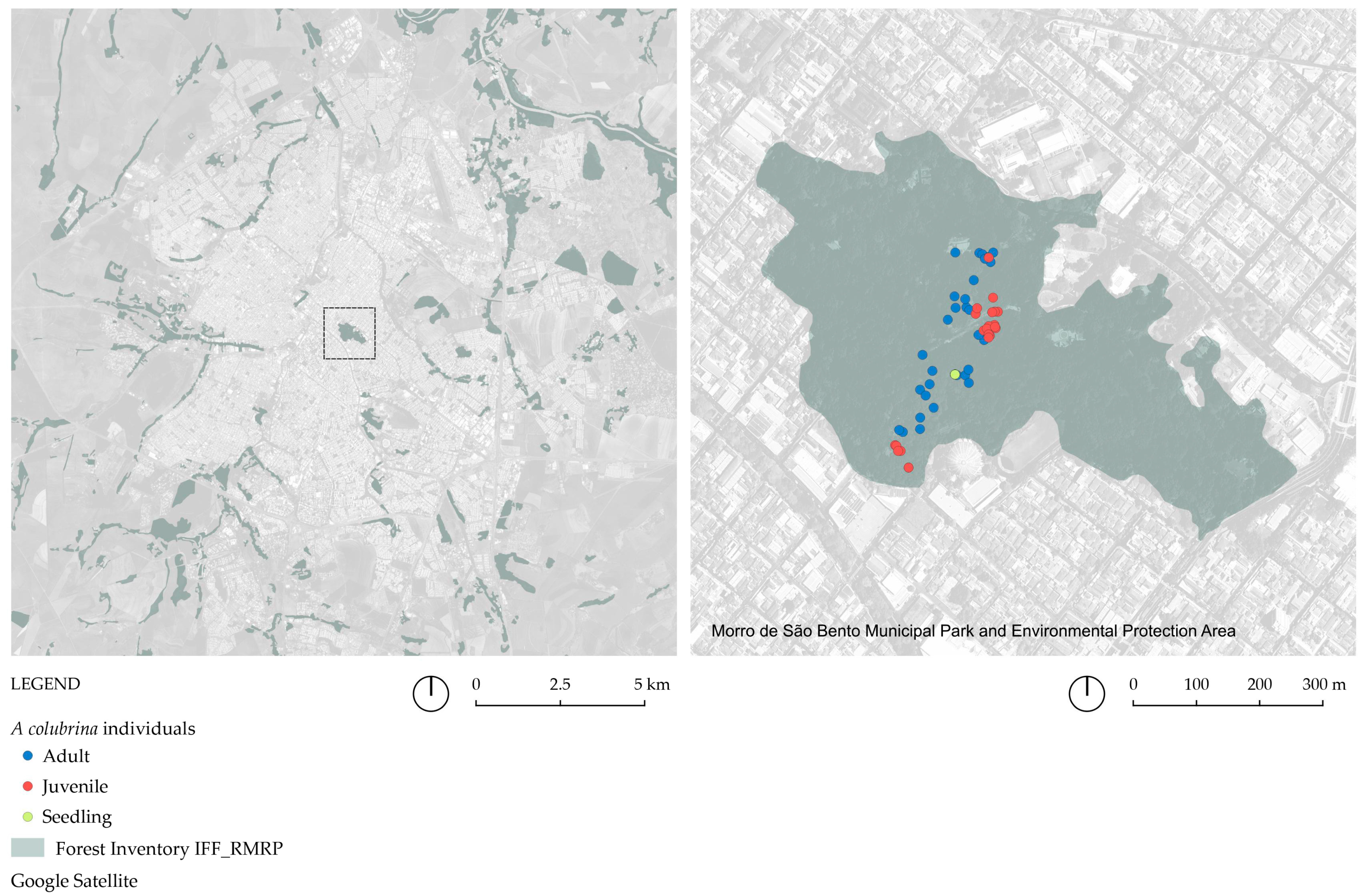
2.2. Identification and Sampling of A. colubrina Populations
2.3. Phenological Observations
2.4. DNA Extraction, Amplification, and Electrophoresis
2.5. Statistical Analyses
3. Results
3.1. Mapping the Regional Fragments with A. colubrina Occurrence
3.2. Phenological Events
3.3. Genetic Characterization of AcolPM
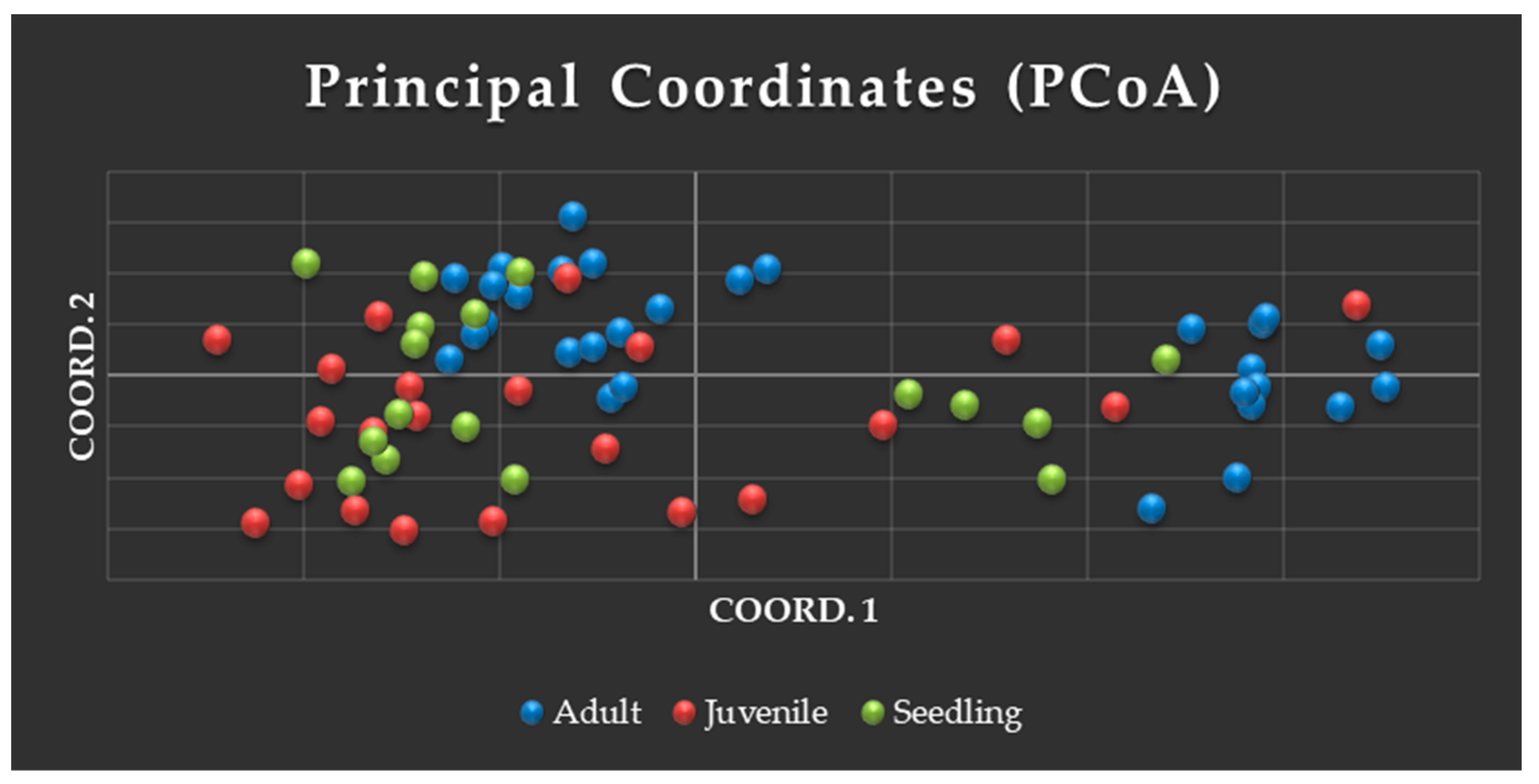
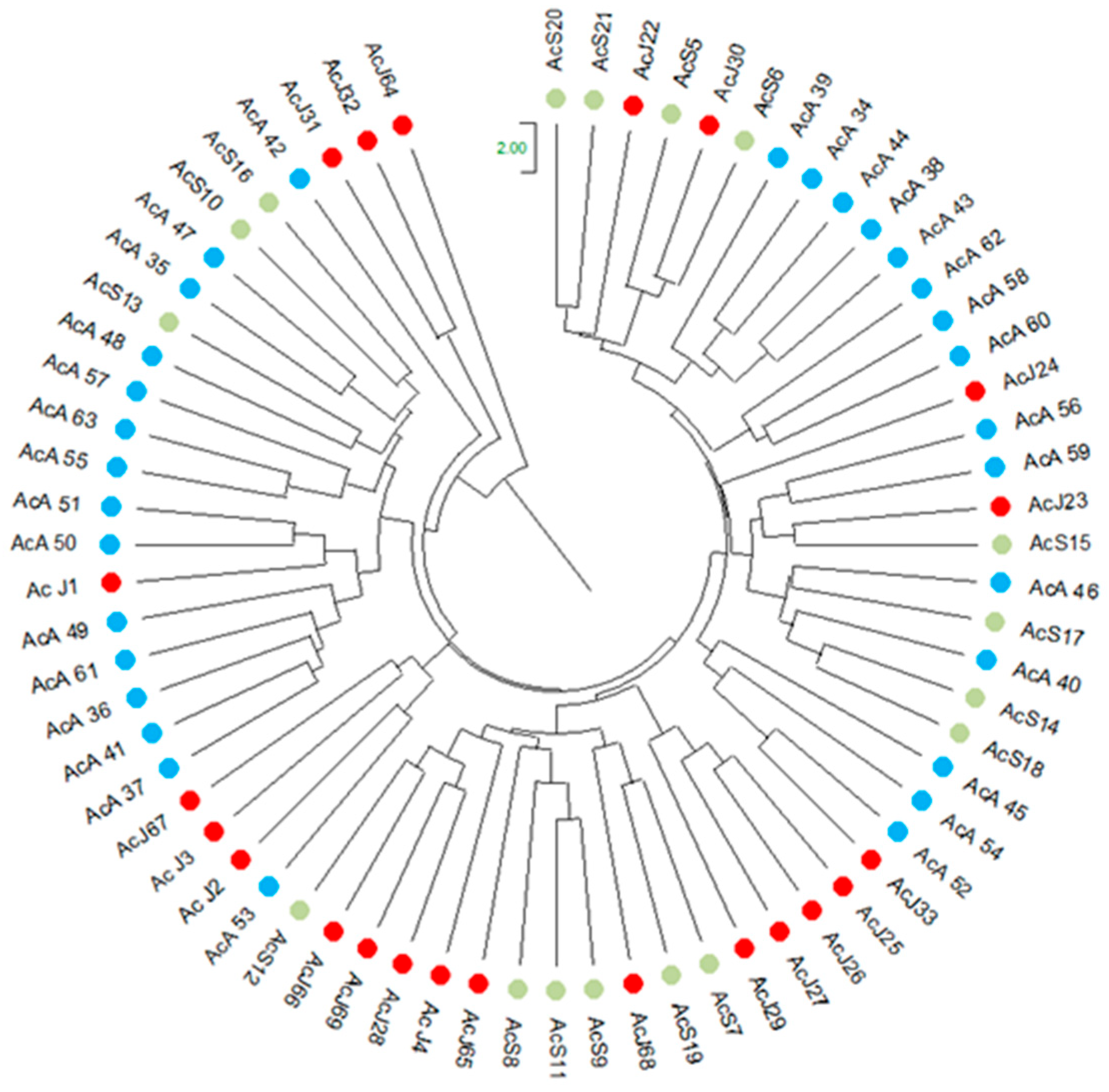
3.3.1. Analysis of PCoA and UPGMA
3.3.2. Genetic Diversity Among Generations of A. colubrina
3.3.3. Inbreeding, Genetic Structure, and Genetic Distances
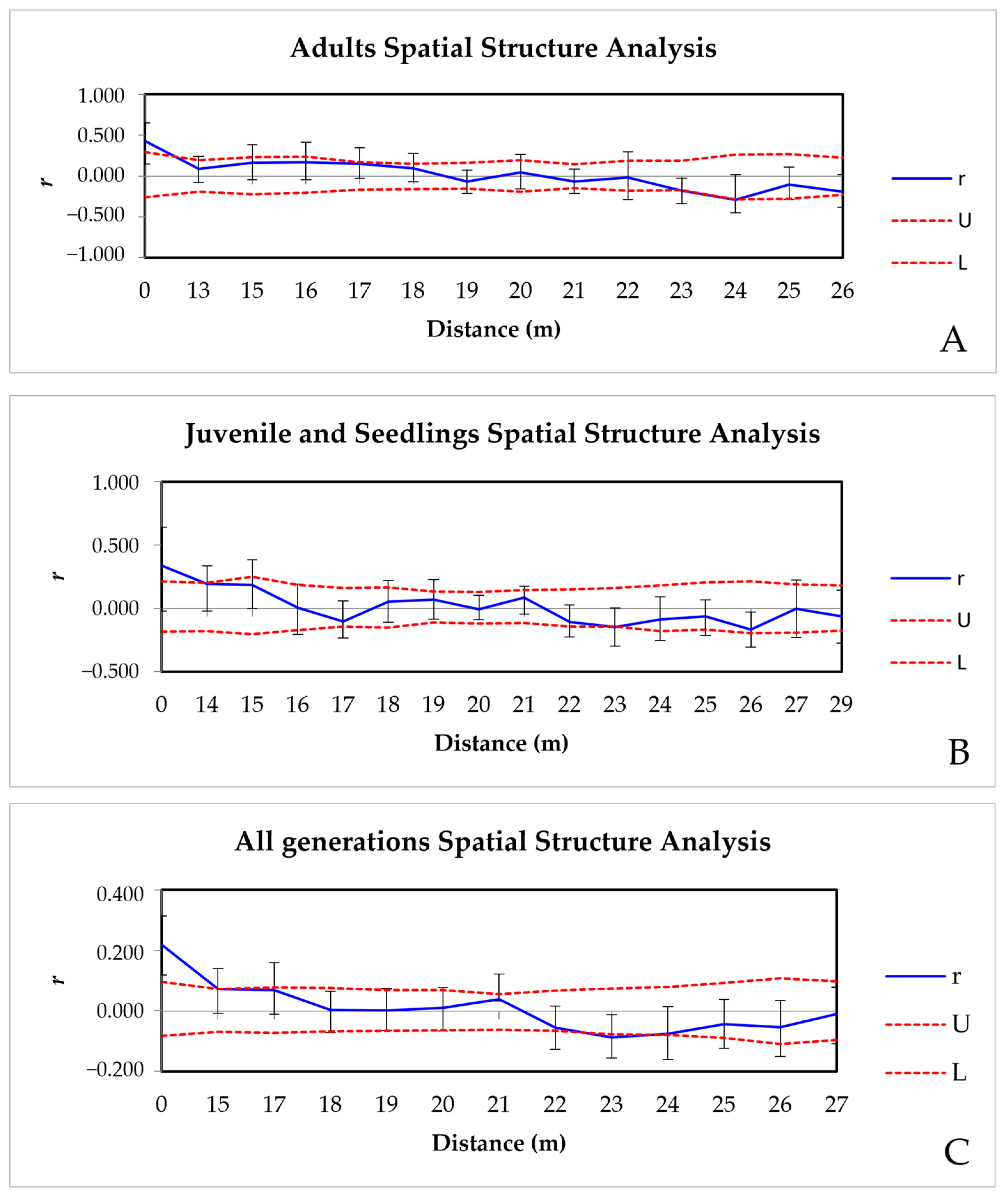
3.3.4. Spatial Genetic Structure (SGS)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AcolPM | Anadenanthera colubrina population from Parque Municipal Morro de São Bento (same as AcolBM in [21]). |
| AcolUSP | A. colubrina population from São Paulo University (USP), Ribeirão Preto Campus, Ribeirão Preto, SP, Brazil |
| AcolBP | A. colubrina population from Bomfim Paulista, a district of Ribeirão Preto, SP, Brazil |
| AMOVA | Analysis of Molecular Variance |
| PCoA | Principal Coordinate Analysis |
| SGS | Spatial genetic structure |
| SSR | Simple sequence repeats |
References
- Young, A.; Boyle, T.; Brown, T. The Population Genetic Consequences of Habitat Fragmentation for Plants. Trends Ecol. Evol. 1996, 11, 413–418. [Google Scholar] [PubMed]
- Epperson, B.K. Spatial Structure of Genetic Variation within Populations of Forest Trees. In Proceedings of the Population Genetics of Forest Trees: Proceedings of the International Symposium on Population Genetics of Forest Trees, Corvallis, OR, USA, 31 July–2 August 1990; Springer: Berlin/Heidelberg, Germany, 1992; pp. 257–278. [Google Scholar]
- Loveless, M.D.; Hamrick, J.L. Ecological Determinants of Genetic Structure in Plant Populations. Annu. Rev. Ecol. Syst. 1984, 15, 65–95. [Google Scholar] [CrossRef]
- Von Reis Altschul, S. A Taxonomic Study of the Genus Anadenanthera. Contrib. Gray Herb. Harv. Univ. 1964, 193, 3–65. [Google Scholar]
- Vieira, D.L.M.; Scariot, A. Principles of Natural Regeneration of Tropical Dry Forests for Restoration. Restor. Ecol. 2006, 14, 11–20. [Google Scholar]
- Pennington, R.T.; Lewis, G.P.; Ratter, J.A. An Overview of the Plant Diversity, Biogeography and Conservation of Neotropical Savannas and Seasonally Dry Forests. In Neotropical Savannas and Seasonally Dry Forests; CRC Press: Boca Raton, FL, USA, 2006; pp. 1–29. [Google Scholar]
- Lorenzi, H. Árvores Brasileiras: Manual de Identificação e Cultivo de Espécies Arbóreas Nativas Do Brasil; Instituto Plantarum: Nova Odessa, Brazil, 2002; 384p. [Google Scholar]
- Carvalho, P.E.R. Espécies Florestais Brasileiras: Recomendações Silviculturais, Potencialidades e Uso Da Madeira. 1994. Available online: https://www.cnpf.embrapa.br/pesquisa/efb/temp/index_especies (accessed on 13 January 2025).
- Feres, J.M.; Nazareno, A.G.; Borges, L.M.; Guidugli, M.C.; Bonifacio-Anacleto, F.; Alzate-Marin, A.L. Depicting the Mating System and Patterns of Contemporary Pollen Flow in Trees of the Genus Anadenanthera (Fabaceae). PeerJ 2021, 9, e10579. [Google Scholar]
- Borges, L.A.; Machado, I.C.; Lopes, A.V. Bee Pollination and Evidence of Substitutive Nectary in Anadenanthera colubrina (Leguminosae-Mimosoideae). Arthropod-Plant Interact. 2017, 11, 263–271. [Google Scholar]
- Araújo, E.D.; Costa, M.; Chaud-Netto, J.; Fowler, H.G. Body Size and Flight Distance in Stingless Bees (Hymenoptera: Meliponini): Inference of Flight Range and Possible Ecological Implications. Braz. J. Biol. 2004, 64, 563–568. [Google Scholar]
- Alzate-Marin, A.L.; Rivas, P.M.S.; Galaschi-Teixeira, J.S.; Bonifácio-Anacleto, F.; Silva, C.C.; Schuster, I.; Nazareno, A.G.; Giuliatti, S.; da Rocha Filho, L.C.; Garófalo, C.A. Warming and Elevated CO2 Induces Changes in the Reproductive Dynamics of a Tropical Plant Species. Sci. Total Environ. 2021, 768, 144899. [Google Scholar]
- Moreti, A.C.d.C.C.; Marchini, L.C. Altura de Voo Das Abelhas Africanizadas (Apis mellifera L.) Para Coleta de Alimentos. Sci. Agrícola 1998, 55, 260–264. [Google Scholar]
- Kerr, W.E. Bionomy of Meliponids—VI—Aspects of Food Gathering and Processing in Some Stingless Bees. In Symposium on Food Gathering Behavior of Hymenoptera; Cornell University: Ithaca, NY, USA, 1959; pp. 2–4. [Google Scholar]
- Jaffé, R.; Castilla, A.; Pope, N.; Imperatriz-Fonseca, V.L.; Metzger, J.P.; Arias, M.C.; Jha, S. Landscape Genetics of a Tropical Rescue Pollinator. Conserv. Genet. 2016, 17, 267–278. [Google Scholar]
- Abou-Shaara, H.F. The Foraging Behaviour of Honey Bees, Apis mellifera: A Review. Vet. Med. 2014, 59, 1–10. [Google Scholar] [CrossRef]
- Barbosa, L.M. Lista de Espécies Indicadas Para Restauração Ecológica Para Diversas Regiões Do Estado de São Paulo; Instituto de Botânica: São Paulo, Brazil, 2017; 344p. Available online: https://www.infraestruturameioambiente.sp.gov.br/institutodebotanica/wp-content/uploads/sites/235/2019/10/lista-especies-rad-2019.pdf (accessed on 25 February 2025).
- Kotchetkoff-Henriques, O. Characterization of the Natural Vegetation in Ribeirão Preto, SP: Bases for Conservation. Ph.D. Thesis, Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, Brazil, 2003. [Google Scholar]
- Kotchetkoff-Henriques, O.; Joly, C.A.; Bernacci, L.C. Soil and Floristic Composition of Native Vegetation Remnants Relationship in the Municipality of Ribeirão Preto, SP. Braz. J. Bot. 2005, 28, 541–562. [Google Scholar] [CrossRef]
- Feres, J.M. Diversidade Genética, Fluxo Génico e Sistema de Cruzamento de Anadenanthera colubrina (Vell.) Brenan e Anadenanthera peregrina (L.) Speg: Duas Espécies Que Ocorrem Em Alta Densidade No Interior Do Estado de São Paulo. Ph.D. Thesis, Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, Brazil, 2014. [Google Scholar]
- Bonifácio-Anacleto, F.; Moraes Filho, R.M.; Borges, L.M.; Martinez, C.A.; Alzate-Marin, A.L. Molecular Discrimination for Two Anadenanthera Species of Seasonally Dry Tropical Forest Remnants in Brazil. Taxonomy 2024, 4, 150–162. [Google Scholar] [CrossRef]
- Inventário Florestal IFF RMRP. Plano de Desenvolvimento Urbano Integrado, Região Metropolitana de Ribeirão Preto. Dataset. 2022. AT-5 Patrimônio Ambiental e Recursos Hídricos. Available online: https://rmrp.pdui.sp.gov.br/?page_id=1125 (accessed on 22 February 2025).
- Projeto MapBiomas. Coleção [Cobertura—Area Urbanizada] Da Série Anual de Mapas de Cobertura e Uso Da Terra Do Brasil. 2017. Available online: https://doi.org/10.58053/MapBiomas/XXUKA8 (accessed on 22 February 2025).
- Agência Nacional de Águas—ANA. Base Hidrográfica Ottocodificada—Trecho de Drenagem. 2017. Available online: https://dadosabertos.ana.gov.br/datasets/5b97dc790ebc4307938d8a5b089c1aab_0/about (accessed on 22 February 2025).
- Malha Municipal Digital e Áreas Territoriais 2023—Informações Técnicas e Legais para a Utilização dos Dados Publicados—IBGE (Instituto Brasileiro de Geografia e Estatística). 2023. Available online: https://www.ibge.gov.br/geociencias/organizacao-do-territorio/malhas-territoriais/15774-malhas.html (accessed on 24 March 2025).
- Agência Nacional de Águas—ANA Massas d’Água. 2020. Available online: https://dadosabertos.ana.gov.br/datasets/4c606c38ee534b84bffe70ca6c8552c6_0/about (accessed on 22 February 2025).
- NASA/METI/AIST/Japan Spacesystems; U.S./Japan ASTER Science Team. ASTER Global Digital Elevation Model V003. NASA EOSDIS Land Processes Distributed Active Archive Center, ASTGTM.003. 2019. Available online: https://lpdaac.usgs.gov/products/astgtmv003/ (accessed on 26 February 2025).
- CIIAGRO. Portal Agrometeorológico e Hidrológico Do Estado de São Paulo. 2025. Available online: http://www.ciiagro.org.br/mensal/cmensal (accessed on 15 February 2025).
- Alzate-Marin, A.L.; Guidugli, M.C.; Soriani, H.H.; Martinez, C.A.; Mestriner, M.A. An Efficient and Rapid DNA Minipreparation Procedure Suitable for PCR/SSR and RAPD Analyses in Tropical Forest Tree Species. Braz. Arch. Biol. Technol. 2009, 52, 1217–1224. [Google Scholar] [CrossRef]
- Feres, J.M.; Monteiro, M.; Zucchi, M.I.; Pinheiro, J.B.; Mestriner, M.A.; Alzate-Marin, A.L. Development of microsatellite markers for Anadenanthera colubrina (Leguminosae), a neotropical tree species. Am. J. Bot. 2012, 99, e154–e156. [Google Scholar] [CrossRef]
- Sanguinetti, C.J.; Simpson, A.J. Rapid Silver Staining and Recovery of PCR Products Separated on Polyacrylamide Gels. Biotechniques 1994, 17, 914–921. [Google Scholar]
- Smouse, P.E.; Peakall, R.O.D. Spatial Autocorrelation Analysis of Individual Multiallele and Multilocus Genetic Structure. Heredity 1999, 82, 561–573. [Google Scholar] [CrossRef]
- Peakall, R.O.D.; Smouse, P.E. GenAlEx 6.5: Genetic Analysis in Excel. Population Genetic Software for Teaching and Research—An Update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Goudet, J. FSTAT (Version 1.2): A Computer Program to Calculate F-Statistics. J. Hered. 1995, 86, 485–486. [Google Scholar] [CrossRef]
- Weir, B.S.; Cockerham, C.C. Estimating F-Statistics for the Analysis of Population Structure. Evolution 1984, 38, 1358–1370. [Google Scholar] [CrossRef]
- Sneath, P.H.A. The Principles and Practice of Numerical Classification. In Numerical Taxonomy; WF Freeman & Co.: San Francisco, CA, USA, 1973; 573p. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Hammer, O. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- SÃO PAULO. Decreto No 47.700, de 11 de Março de 2003: Regulamenta a Lei No 11.241, de 19 de Setembro de 2002, Que Dispõe Sobre a Eliminação Gradativa Da Queima Da Palha Da Cana-de-Açúcar e Dá Providências Correlatas. 2003. Available online: https://www.al.sp.gov.br/repositorio/legislacao/decreto/2003/decreto-47700-11.03.2003.html (accessed on 26 February 2025).
- Borchert, R. Soil and Stem Water Storage Determine Phenology and Distribution of Tropical Dry Forest Trees. Ecology 1994, 75, 1437–1449. [Google Scholar] [CrossRef]
- Hamrick, J.L.; Godt, M.J.W.; Sherman-Broyles, S.L. Factors Influencing Levels of Genetic Diversity in Woody Plant Species. In Proceedings of the Population Genetics of Forest Trees: Proceedings of the International Symposium on Population Genetics of Forest Trees, Corvallis, OR, USA, 31 July–2 August 1990; Springer: Berlin/Heidelberg, Germany, 1992; pp. 95–124. [Google Scholar]
- Barrandeguy, M.E.; Prinz, K.; García, M.V.; Finkeldey, R. Development of Microsatellite Markers for Anadenanthera Colubrina Var. Cebil (Fabaceae), a Native Tree from South America. Am. J. Bot. 2012, 99, e372–e374. [Google Scholar] [CrossRef]
- Alzate-Marin, A.L.; Ferreira-Ramos, R.; Guidugli, M.; Martinez, C.A.; Mestriner, M.A. Genetic Diversity Assessed in Individuals of Aspidosperma polyneuron and Cariniana estrellensis Used as Seed Donors in a Forest Gene Bank. BMC Proc. 2011, 5, 8. [Google Scholar] [CrossRef]
- Alzate-Marin, A.L.; Bonifacio-Anacleto, F.; de Moraes Filho, R.M.; Machado, G.P.; Nazareno, A.G. Genetic Analysis across Life Stages of Metrodorea nigra (Rutaceae) in a Population Located in an Urban Landscape of Southeastern Brazil Using a New Set of Microsatellite Markers. Braz. J. Bot. 2016, 39, 795–799. [Google Scholar] [CrossRef]
- Goncalves, A.L.; Barrandeguy, M.E.; García, M.V. Spatio-Temporal Influence of Gene Dispersal on Genetic Variability Distribution: Anadenanthera colubrina Var. Cebil (Leguminosae) as Case of Study. In Agricultural Research Updates; Gorawala, P., Mandhatri, S., Eds.; Nova Science Publishers: New York, NY, USA, 2021; Volume 33, pp. 189–209. [Google Scholar]
- Moraes Filho, R.M.d.; Bonifácio-Anacleto, F.; Alzate-Martinez, F.A.; Martinez, C.A.; Alzate-Marin, A.L. A Spatial Structure of Key Tree Species Metrodorea nigra St. Hill. (Rutaceae) Is Associated with Historical Disturbance and Isolation in Southeastern Brazil. Plants 2025, 14, 702. [Google Scholar] [CrossRef]
- Williams, R.J.; Myers, B.A.; Eamus, D.; Duff, G.A. Reproductive Phenology of Woody Species in a North Australian Tropical Savanna 1. Biotropica 1999, 31, 626–636. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, J.; Traveset, A. Effects of Flowering Phenology and Synchrony on the Reproductive Success of a Long-Flowering Shrub. AoB Plants 2016, 8, plw007. [Google Scholar] [CrossRef]
- Arroyo, M.T.K.; Tamburrino, I.; Pliscoff, P.; Robles, V.; Colldecarrera, M.; Guerrero, P. Flowering Phenology Adjustment and Flower Longevity in a South American Alpine Species. Plants 2021, 10, 461. [Google Scholar] [CrossRef]
- Morellato, L.P.C.; Rodrigues, R.R.; Leitao Filho, H.F.d.; Joly, C.A. Estudo Comparativo Da Fenologia de Espécies Arbóreas de Floresta de Altitude e Floresta Mesófila Semidecídua Na Serra Do Japi, Jundiaí, São Paulo. Rev. Bras. Bot. 1989, 12, 85–98. [Google Scholar]
- Justiniano, M.J.; Fredericksen, T.S. Phenology of Tree Species in Bolivian Dry Forests. Biotropica 2000, 32, 276–281. [Google Scholar] [CrossRef]
- Ragusa-Netto, J.; Silva, R.R. Canopy Phenology of a Dry Forest in Western Brazil. Braz. J. Biol. 2007, 67, 569–575. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morellato, L.P.C.; Altomare, M.; Gressler, E. Altomare, M.; Gressler, E. A Review of Reproductive Plant Phenology in South and Central America: New Perspectives. In BT—Phenology: An Integrative Environmental Science; Schwartz, M.D., Ed.; Springer Nature: Cham, Switzerland, 2024; pp. 107–138. ISBN 978-3-031-75027-4. [Google Scholar]
- De Barros Ruas, R.; Costa, L.M.S.; Bered, F. Urbanization Driving Changes in Plant Species and Communities—A Global View. Glob. Ecol. Conserv. 2022, 38, e02243. [Google Scholar]
- Suijkerbuijk, H.A.C.; Ramos, S.E.; Poelman, E.H. Plasticity in Plant Mating Systems. Trends Plant Sci. 2024. [Google Scholar] [CrossRef]
- Bonifácio-Anacleto, F.; San Martin, J.A.B.; Reutemann, A.G.; Habermann, E.; Pozner, R.E.; Nazareno, A.G.; Nogueira, F.M.; Martinez, C.A.; Alzate-Marin, A.L. Warming and Water Deficit Impact the Reproductive Features of the Tropical Forage Species Stylosanthes Capitata. Environ. Exp. Bot. 2024, 226, 105899. [Google Scholar] [CrossRef]
- Rodrigues, R.R.; Lima, R.A.F.; Gandolfi, S.; Nave, A.G. On the Restoration of High Diversity Forests: 30 Years of Experience in the Brazilian Atlantic Forest. Biol. Conserv. 2009, 142, 1242–1251. [Google Scholar] [CrossRef]
- Rother, D.C.; Liboni, A.P.; Magnago, L.F.S.; Chao, A.; Chazdon, R.L.; Rodrigues, R.R. Ecological Restoration Increases Conservation of Taxonomic and Functional β Diversity of Woody Plants in a Tropical Fragmented Landscape. For. Ecol. Manag. 2019, 451, 117538. [Google Scholar] [CrossRef]
- Lima, R.A.F.; Oliveira, A.A.; Pitta, G.R.; Pitta, G.; Gasper, A.L.; Vibrans, A.C.; Chave, J.; Steege, H.T.; Prado, P.I. The Erosion of Biodiversity and Biomass in the Atlantic Forest Biodiversity Hotspot. Nat. Commun. 2020, 11, 6347. [Google Scholar] [CrossRef]
- Prefeitura RP, 2021. Available online: https://www.ribeiraopreto.sp.gov.br/portal/noticia/prefeitura-assina-carta-de-compromisso-com-programa-ribeirao--3c) (accessed on 25 February 2025).
- Valverde, M.C.; Rosa, M.B. Heat Waves in São Paulo State, Brazil: Intensity, Duration, Spatial Scope, and Atmospheric Characteristics. Int. J. Climatol. 2023, 43, 3782–3798. [Google Scholar] [CrossRef]


| Pop | %P | N | Na | Ne | Ho | He | F | |
|---|---|---|---|---|---|---|---|---|
| Adults | Mean | 93 | 29.93 | 6.857 | 4.271 | 0.530 NS | 0.619 NS | 0.125 NS |
| SE | - | 0.071 | 1.217 | 0.745 | 0.073 | 0.079 | 0.062 | |
| Juveniles | Mean | 100 | 21.93 | 6.357 | 4.152 | 0.521 NS | 0.635 NS | 0.145 NS |
| SE | - | 0.071 | 1.020 | 0.734 | 0.062 | 0.065 | 0.071 | |
| Seedlings | Mean | 93 | 17.00 | 5.857 | 3.888 | 0.563 NS | 0.616 NS | 0.102 NS |
| SE | - | 0.000 | 0.937 | 0.580 | 0.093 | 0.076 | 0.100 | |
| Grand Mean | Mean | 95 | 22.95 | 6.357 | 4.104 | 0.538 | 0.623 | 0.125 |
| SE | 2.4 | 0.833 | 0.603 | 0.390 | 0.043 | 0.041 | 0.045 |
| All Pops. | Fis (f) | Fit (F) | Fst (Ɵ) | Nm |
|---|---|---|---|---|
| Mean | 0.164 ** | 0.194 ** | 0.036 ** | 6.7 |
| SE | 0.056 | 0.058 | 0.009 | |
| 99% CI | 0.019 | 0.042 | 0.016 | |
| 0.305 | 0.339 | 0.060 |
| Adults | Juveniles | Seedlings | |
|---|---|---|---|
| 0.000 | Adults | ||
| 0.090 | 0.000 | Juveniles | |
| 0.076 | 0.048 | 0.000 | Seedlings |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzate-Marin, A.L.; Bomfim Rodrigues, P.A.; Alzate-Martinez, F.A.; Pinheiro Machado, G.; Martinez, C.A.; Bonifácio-Anacleto, F. Phenology and Spatial Genetic Structure of Anadenanthera colubrina (Vell.), a Resilient Species Amid Territorial Transformation in an Urban Deciduous Forest of Southeastern Brazil. Genes 2025, 16, 388. https://doi.org/10.3390/genes16040388
Alzate-Marin AL, Bomfim Rodrigues PA, Alzate-Martinez FA, Pinheiro Machado G, Martinez CA, Bonifácio-Anacleto F. Phenology and Spatial Genetic Structure of Anadenanthera colubrina (Vell.), a Resilient Species Amid Territorial Transformation in an Urban Deciduous Forest of Southeastern Brazil. Genes. 2025; 16(4):388. https://doi.org/10.3390/genes16040388
Chicago/Turabian StyleAlzate-Marin, Ana Lilia, Paulo Augusto Bomfim Rodrigues, Fabio Alberto Alzate-Martinez, Gabriel Pinheiro Machado, Carlos Alberto Martinez, and Fernando Bonifácio-Anacleto. 2025. "Phenology and Spatial Genetic Structure of Anadenanthera colubrina (Vell.), a Resilient Species Amid Territorial Transformation in an Urban Deciduous Forest of Southeastern Brazil" Genes 16, no. 4: 388. https://doi.org/10.3390/genes16040388
APA StyleAlzate-Marin, A. L., Bomfim Rodrigues, P. A., Alzate-Martinez, F. A., Pinheiro Machado, G., Martinez, C. A., & Bonifácio-Anacleto, F. (2025). Phenology and Spatial Genetic Structure of Anadenanthera colubrina (Vell.), a Resilient Species Amid Territorial Transformation in an Urban Deciduous Forest of Southeastern Brazil. Genes, 16(4), 388. https://doi.org/10.3390/genes16040388









