Ovarian Transcriptome Profile from Egg-Laying Period to Incubation Period of Changshun Green-Shell Laying Hens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Sample Collection
2.3. RNA Isolation and Sequencing
2.4. Principal Component Analysis and Differential Expression Analysis
2.5. Enrichment Analysis and Protein–Protein Interaction
2.6. Gene Expression Analysis Using Quantitative Real-Time PCR
3. Results
3.1. RNA Sequencing Quality Assessment and Transcriptome Alignment
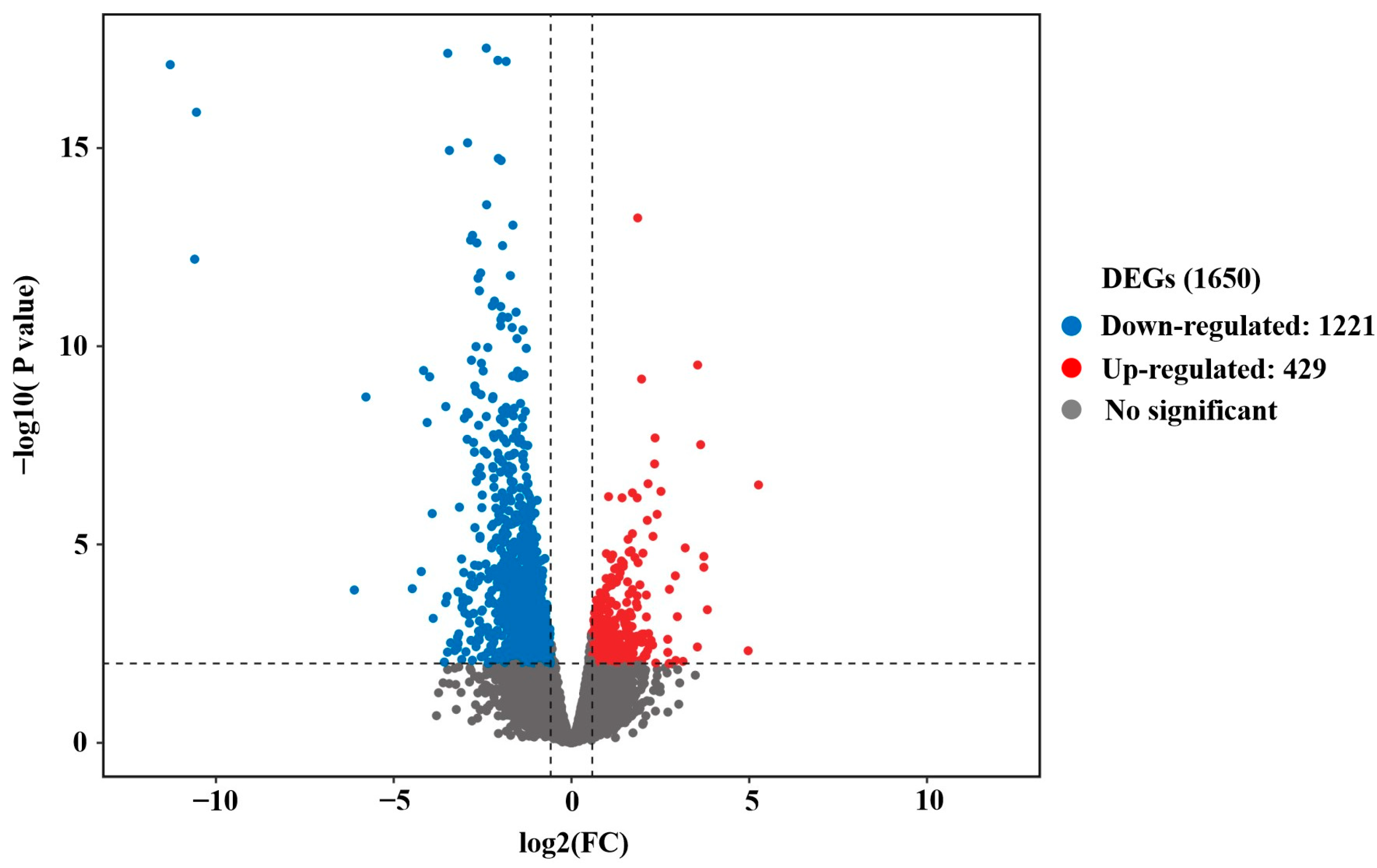
3.2. Differential Expressed Analysis
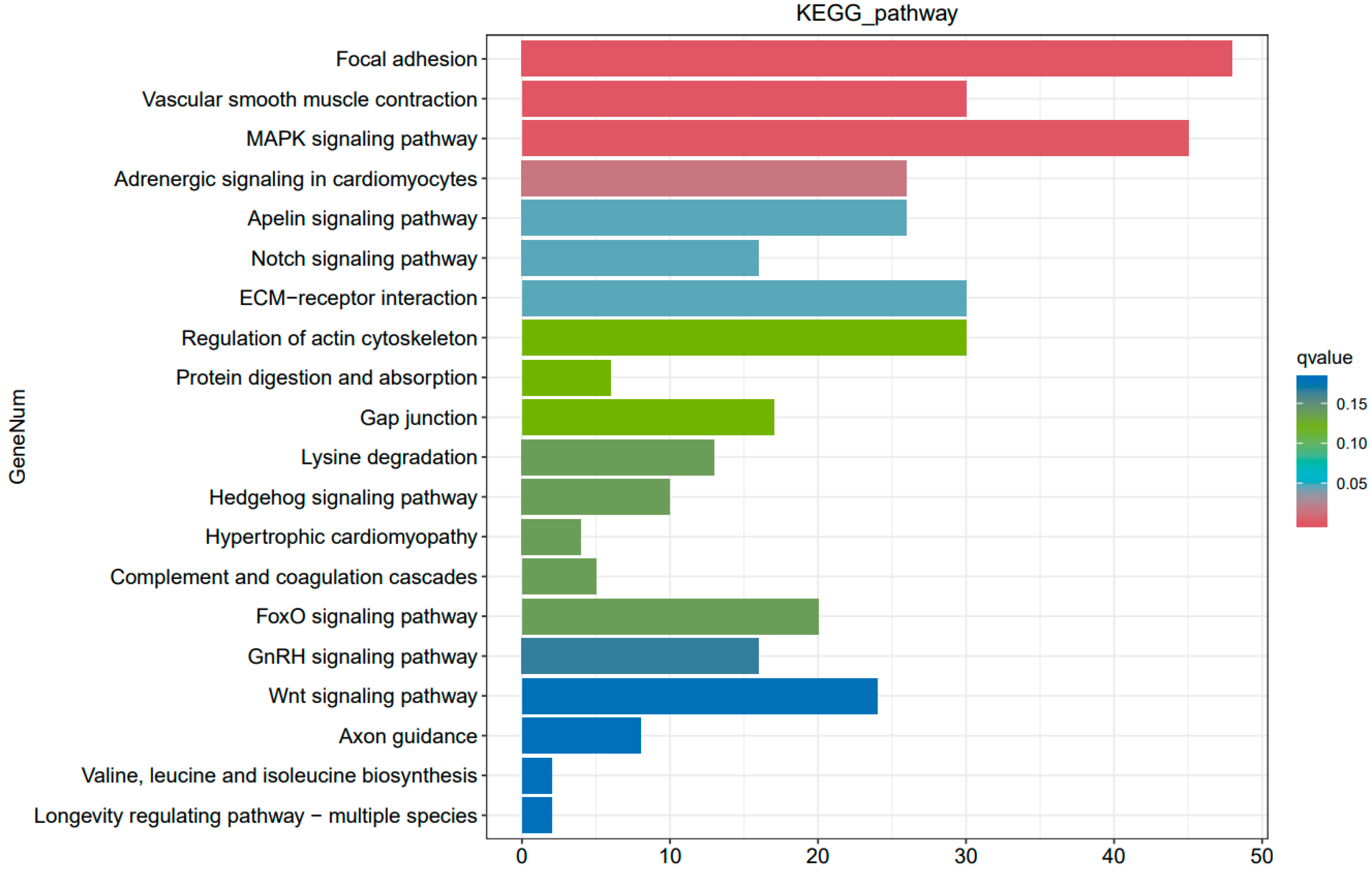
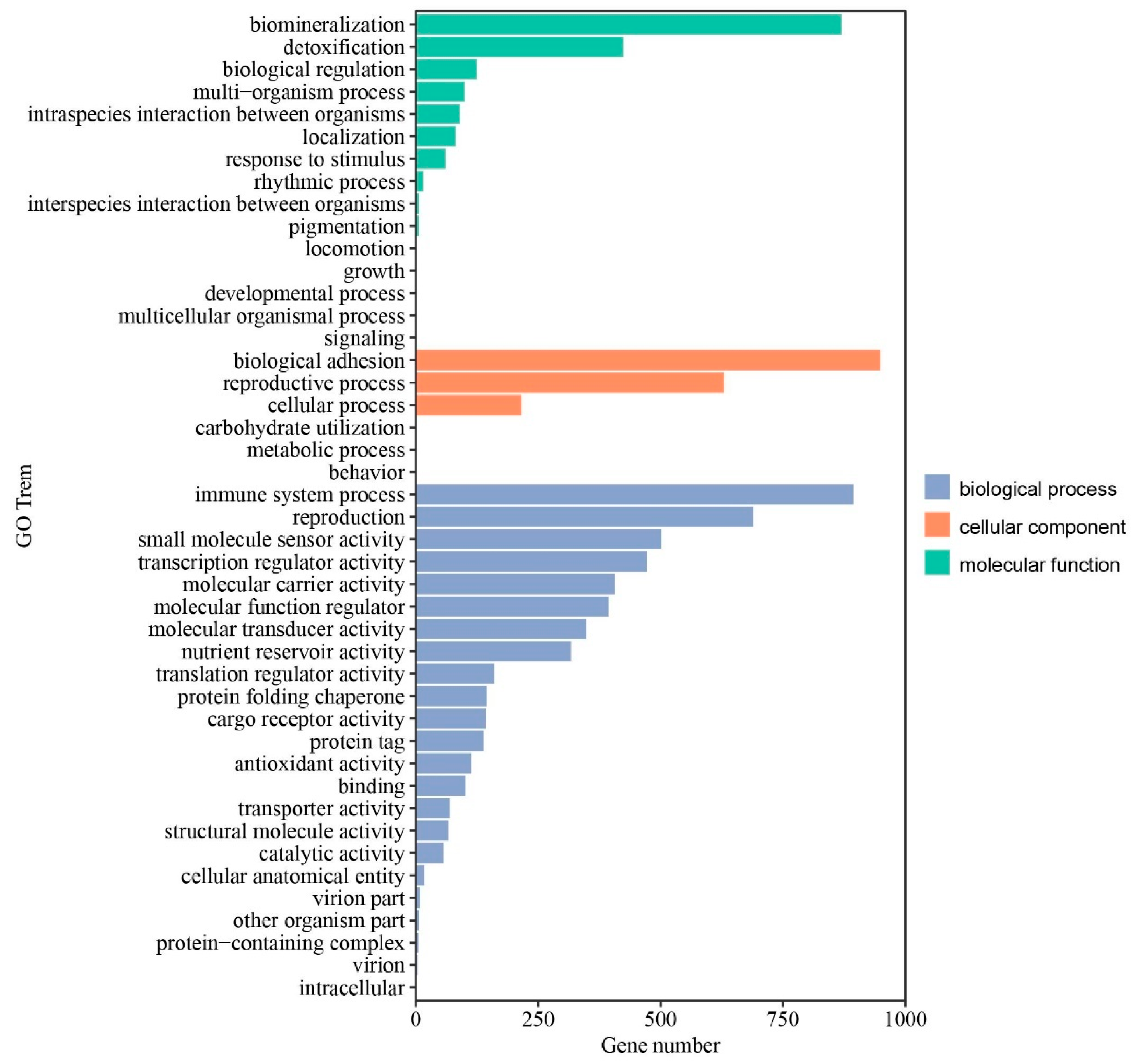
3.3. KEGG Pathway and GO Enrichment Analysis
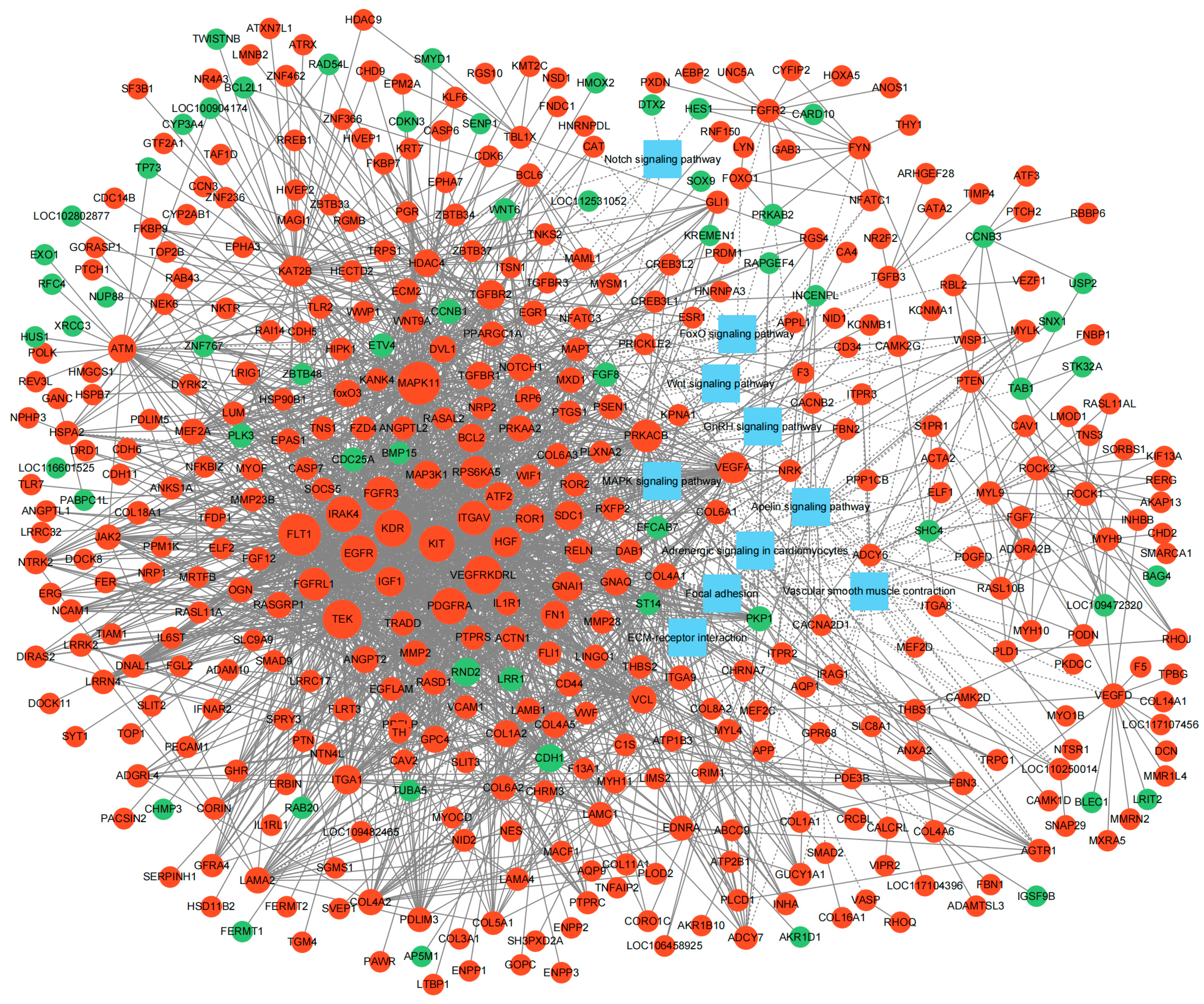
3.4. Interaction Network Construction of DEGs
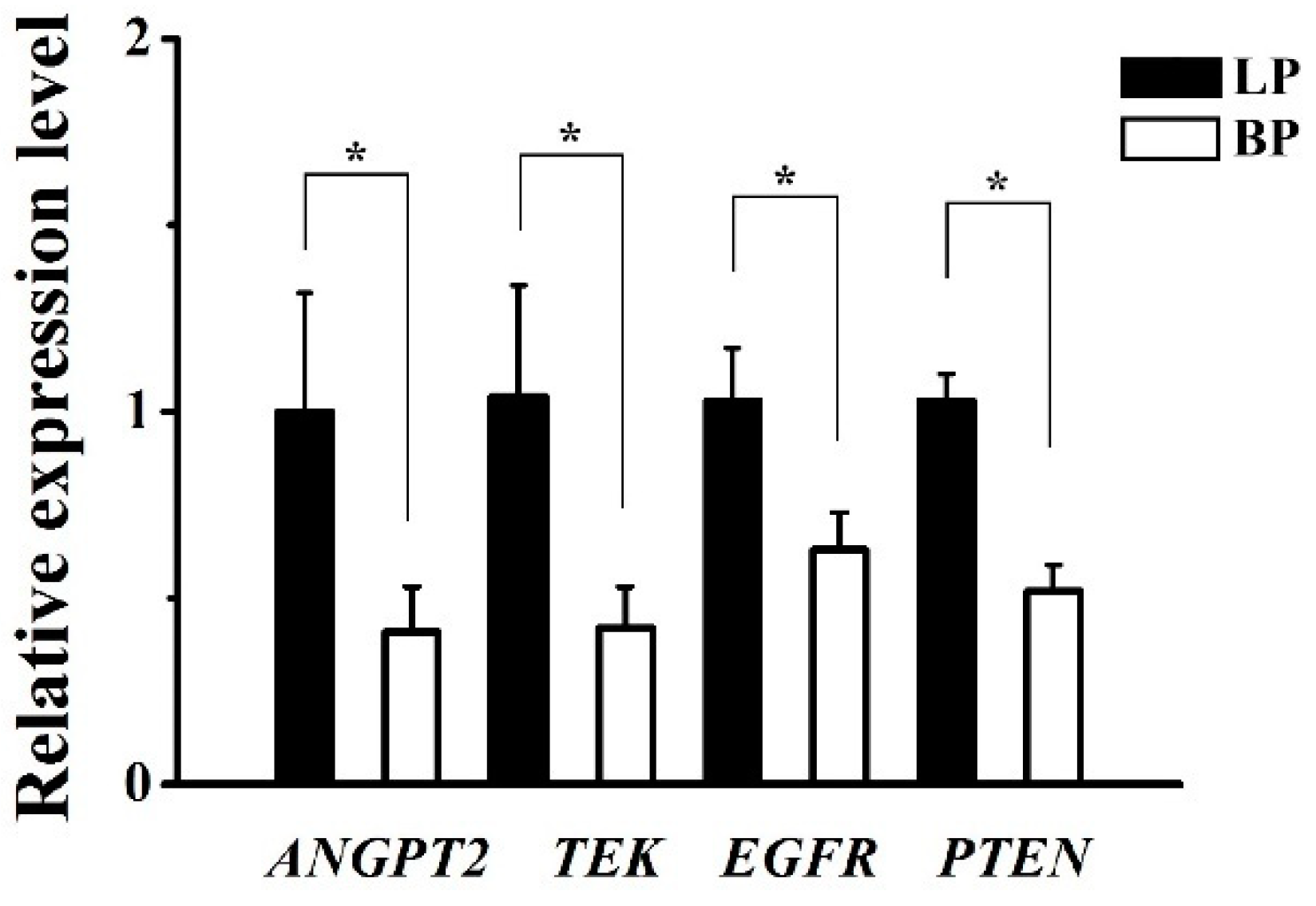
3.5. Validation of DEGs by qRT-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ohkubo, T. Neuroendocrine Control of Broodiness. Adv. Exp. Med. Biol. 2017, 1001, 151–171. [Google Scholar] [PubMed]
- Geng, A.L.; Xu, S.F.; Zhang, Y.; Zhang, J.; Chu, Q.; Liu, H.G. Effects of photoperiod on broodiness, egg-laying and endocrine responses in native laying hens. Br. Poult. Sci. 2014, 55, 264–269. [Google Scholar] [PubMed]
- Zhao, J.; Pan, H.; Liu, Y.; He, Y.; Shi, H.; Ge, C. Interacting Networks of the Hypothalamic-Pituitary-Ovarian Axis Regulate Layer Hens Performance. Genes 2023, 14, 141. [Google Scholar] [CrossRef]
- Sharp, P.J.; Scanes, C.G.; Williams, J.B.; Harvey, S.; Chadwick, A. Variations in concentrations of prolactin, luteinizing hormone, growth hormone and progesterone in the plasma of broody bantams (Gallus domesticus). J. Endocrinol. 1979, 80, 51–57. [Google Scholar]
- Sharp, P.J.; Macnamee, M.C.; Sterling, R.J.; Lea, R.W.; Pedersen, H.C. Relationships between prolactin, LH and broody behaviour in bantam hens. J. Endocrinol. 1988, 118, 279–286. [Google Scholar] [PubMed]
- Zadworny, D.; Shimada, K.; Ishida, H.; Sumi, C.; Sato, K. Changes in plasma levels of prolactin and estradiol, nutrient intake, and time spent nesting during the incubation phase of broodiness in the Chabo hen (Japanese bantam). Gen. Comp. Endocrinol. 1988, 71, 406–412. [Google Scholar] [PubMed]
- March, J.B.; Sharp, P.J.; Wilson, P.W.; Sang, H.M. Effect of active immunization against recombinant-derived chicken prolactin fusion protein on the onset of broodiness and photoinduced egg laying in bantam hens. J. Reprod. Fertil. 1994, 101, 227–233. [Google Scholar]
- Macnamee, M.C.; Sharp, P.J.; Lea, R.W.; Sterling, R.J.; Harvey, S. Evidence that vasoactive intestinal polypeptide is a physiological prolactin-releasing factor in the bantam hen. Gen. Comp. Endocrinol. 1986, 62, 470–478. [Google Scholar] [CrossRef]
- Opel, H.; Proudman, J.A. Stimulation of prolactin release in turkeys by vasoactive intestinal peptide. Proc. Soc. Exp. Biol. Med. 1988, 187, 455–460. [Google Scholar]
- El Halawani, M.E.; Silsby, J.L.; Mauro, L.J. Vasoactive intestinal peptide is a hypothalamic prolactin-releasing neuropeptide in the turkey (Meleagris gallopavo). Gen. Comp. Endocrinol. 1990, 78, 66–73. [Google Scholar]
- Rozenboim, I.; Silsby, J.L.; Tabibzadeh, C.; Pitts, G.R.; Youngren, O.M.; El Halawani, M.E. Hypothalamic and posterior pituitary content of vasoactive intestinal peptide and gonadotropin-releasing hormones I and II in the turkey hen. Biol. Reprod. 1993, 49, 622–626. [Google Scholar] [CrossRef]
- Youngren, O.M.; Pitts, G.R.; Phillips, R.E.; El Halawani, M.E. Dopaminergic control of prolactin secretion in the turkey. Gen. Comp. Endocrinol. 1996, 104, 225–230. [Google Scholar] [CrossRef] [PubMed]
- El Halawani, M.E.; Youngren, O.M.; Rozenboim, I.; Pitts, G.R.; Silsby, J.L.; Phillips, R.E. Phillips, Serotonergic stimulation of prolactin secretion is inhibited by vasoactive intestinal peptide immunoneutralization in the turkey. Gen. Comp. Endocrinol. 1995, 99, 69–74. [Google Scholar] [CrossRef]
- Youngren, O.M.; Silsby, J.L.; Phillips, R.E.; El Halawani, M.E. Dynorphin modulates prolactin secretion in the turkey. Gen. Comp. Endocrinol. 1993, 91, 224–231. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, L.; Xu, F.; Cao, S.; Chen, Y.; Zhang, Y.; He, W.; Yan, M.; Lian, S.; Li, A. Long noncoding RNAs profiling in ovary during laying and nesting in Muscovy ducks (Cairina moschata). Anim. Reprod. Sci. 2021, 230, 106762. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Huang, Y.; Xu, C.; Huang, X.; Li, S.; Yin, Z. Long noncoding RNAs and mRNAs profiling in ovary during laying and broodiness in Taihe Black-Bone Silky Fowls (Gallus gallus Domesticus Brisson). BMC Genom. 2024, 25, 357. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Pan, X.; Cao, S.; Xu, F.; Lan, L.; Zhang, Y.; Lian, S.; Yan, M.; Li, A. iTRAQ-based quantitative proteomic analysis provides insights into strong broodiness in Muscovy duck (Cairina moschata) combined with metabolomics analysis. J. Proteom. 2019, 204, 103401. [Google Scholar] [CrossRef]
- Xu, Q.; Zhao, W.; Chen, Y.; Tong, Y.; Rong, G.; Huang, Z.; Zhang, Y.; Chang, G.; Wu, X.; Chen, G. Transcriptome profiling of the goose (Anser cygnoides) ovaries identify laying and broodiness phenotypes. PLoS ONE 2013, 8, e55496. [Google Scholar] [CrossRef]
- Yu, J.; Lou, Y.; Zhao, A. Transcriptome analysis of follicles reveals the importance of autophagy and hormones in regulating broodiness of Zhedong white goose. Sci. Rep. 2016, 6, 36877. [Google Scholar] [CrossRef]
- Yu, J.; Lou, Y.; He, K.; Yang, S.; Yu, W.; Han, L.; Zhao, A. Goose broodiness is involved in granulosa cell autophagy and homeostatic imbalance of follicular hormones. Poult. Sci. 2016, 95, 1156–1164. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Li, L.; Han, C.; He, H.; Xu, H. Transcriptome analysis revealed the possible regulatory pathways initiating female geese broodiness within the hypothalamic-pituitary-gonadal axis. PLoS ONE 2018, 13, e0191213. [Google Scholar]
- Qin, H.; Li, X.; Wang, J.; Sun, G.; Mu, X.; Ji, R. Ovarian transcriptome profile from pre-laying period to broody period of Xupu goose. Poult. Sci. 2021, 100, 101403. [Google Scholar] [CrossRef]
- Chen, Z.; Wen, D.; Zhang, Y.; Chen, J.; Pan, F.; Zhang, W.; Zhou, S.; Wang, F.; Mu, R. Pituitary transcriptome profile from laying period to incubation period of Changshun green-shell laying hens. BMC Genom. 2024, 25, 309. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar]
- Gilbert, A.B.; Perry, M.M.; Waddington, D.; Hardie, M.A. Role of atresia in establishing the follicular hierarchy in the ovary of the domestic hen (Gallus domesticus). J. Reprod. Fertil. 1983, 69, 221–227. [Google Scholar]
- Hernandez, A.G.; Bahr, J.M. Role of FSH and epidermal growth factor (EGF) in the initiation of steroidogenesis in granulosa cells associated with follicular selection in chicken ovaries. Reproduction 2003, 125, 683–691. [Google Scholar]
- Yu, J.; He, K.; Ren, T.; Lou, Y.; Zhao, A. High-throughput sequencing reveals differential expression of miRNAs in prehierarchal follicles of laying and brooding geese. Physiol. Genom. 2016, 48, 455–463. [Google Scholar]
- Zhang, B.B.; Li, M.X.; Wang, H.N.; Liu, C.; Sun, Y.Y.; Ma, T.H. An integrative analysis of lncRNAs and mRNAs highlights the potential roles of lncRNAs in the process of follicle selection in Taihang chickens. Theriogenology 2023, 195, 122–130. [Google Scholar] [PubMed]
- Zhang, T.; Chen, L.; Han, K.; Zhang, X.; Zhang, G.; Dai, G.; Wang, J.; Xie, K. Transcriptome analysis of ovary in relatively greater and lesser egg producing Jinghai Yellow Chicken. Anim. Reprod. Sci. 2019, 208, 106114. [Google Scholar]
- Sun, T.; Xiao, C.; Deng, J.; Yang, Z.; Zou, L.; Du, W.; Li, S.; Huo, X.; Zeng, L.; Yang, X. Transcriptome analysis reveals key genes and pathways associated with egg production in Nandan-Yao domestic chicken. Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 40, 100889. [Google Scholar]
- Bello, S.F.; Xu, H.; Guo, L.; Li, K.; Zheng, M.; Xu, Y.; Zhang, S.; Bekele, E.J.; Bahareldin, A.A.; Zhu, W.; et al. Hypothalamic and ovarian transcriptome profiling reveals potential candidate genes in low and high egg production of white Muscovy ducks (Cairina moschata). Poult. Sci. 2021, 100, 101310. [Google Scholar] [PubMed]
- He, Z.; Ouyang, Q.; Chen, Q.; Song, Y.; Hu, J.; Hu, S.; He, H.; Li, L.; Liu, H.; Wang, J. Molecular mechanisms of hypothalamic-pituitary-ovarian/thyroid axis regulating age at first egg in geese. Poult. Sci. 2024, 103, 103478. [Google Scholar] [CrossRef]
- Tong, X.; Li, X.; Wang, Y.; Xie, F.; Li, R.; Ren, M.; Hu, Q.; Li, S. Comprehensive analysis of mRNA and miRNA differential expression profiles in the hypothalamus-pituitary-gonadal axis in laying and broodiness period of Wanxi white geese. Poult. Sci. 2025, 104, 104510. [Google Scholar]
- Shen, M.; Li, T.; Feng, Y.; Wu, P.; Serrano, B.R.; Barcenas, A.R.; Qu, L.; Zhao, W. Effects of quercetin on granulosa cells from prehierarchical follicles by modulating MAPK signaling pathway in chicken. Poult. Sci. 2023, 102, 102736. [Google Scholar] [CrossRef]
- Xu, R.; Qin, N.; Xu, X.; Sun, X.; Chen, X.; Zhao, J. Inhibitory effect of SLIT2 on granulosa cell proliferation mediated by the CDC42-PAKs-ERK1/2 MAPK pathway in the prehierarchical follicles of the chicken ovary. Sci. Rep. 2018, 8, 9168. [Google Scholar]
- Woods, D.C.; Haugen, M.J.; Johnson, A.L. Actions of epidermal growth factor receptor/mitogen-activated protein kinase and protein kinase C signaling in granulosa cells from Gallus gallus are dependent upon stage of differentiation. Biol. Reprod. 2007, 77, 61–70. [Google Scholar] [PubMed]
- Johnson, A.L.; Lee, J. Granulosa cell responsiveness to follicle stimulating hormone during early growth of hen ovarian follicles. Poult. Sci. 2016, 95, 108–114. [Google Scholar]
- Bothun, A.M.; Woods, D.C. Dynamics of WNT signaling components in the human ovary from development to adulthood. Histochem. Cell Biol. 2019, 151, 115–123. [Google Scholar] [CrossRef]
- Ren, J.; Du, X.; Zeng, T.; Chen, L.; Shen, J.; Lu, L.; Hu, J. Divergently expressed gene identification and interaction prediction of long noncoding RNA and mRNA involved in duck reproduction. Anim. Reprod. Sci. 2017, 185, 8–17. [Google Scholar] [PubMed]
- Brooke, M.H.; Kaiser, K.K. Muscle fiber types: How many and what kind? Arch. Neurol. 1970, 23, 369–379. [Google Scholar]
- Woods, A.; Azzout-Marniche, D.; Foretz, M.; Stein, S.C.; Lemarchand, P.; Ferré, P.; Foufelle, F.; Carling, D. Characterization of the role of AMP-activated protein kinase in the regulation ofglucose-activated gene expression using constitutively active anddominant negative forms of the kinase. Mol. Cell. Biol. 2000, 20, 6704–6711. [Google Scholar]
- Carling, D. AMP-activated protein kinase: Balancing thescales. Biochimie 2005, 87, 87–91. [Google Scholar] [CrossRef]
- Madsen, J.F.; Ernst, E.H.; Amoushahi, M.; Dueholm, M.; Ernst, E.; Lykke-Hartmann, K. Dorsomorphin inhibits AMPK, upregulates Wnt and Foxo genes and promotes the activation of dormant follicles. Commun. Biol. 2024, 7, 747. [Google Scholar] [CrossRef]
- Lin, J.; Guan, L.; Ge, L.; Liu, G.; Bai, Y.; Liu, X. Nanopore-based full-length transcriptome sequencing of Muscovy duck (Cairina moschata) ovary. Poult. Sci. 2021, 100, 101246. [Google Scholar]
- Onagbesan, O.M.; Vleugels, B.; Buys, N.; Bruggeman, V.; Safi, M.; Decuypere, E. Insulin-Like growth factors in the regulation of avian ovarian functions. Domest. Anim. Endocrinol. 1999, 17, 299–313. [Google Scholar] [PubMed]
- Tosca, L.; Chabrolle, C.; Crochet, S.; Tesseraud, S.; Dupont, J. IGF-1 receptor signaling pathways and effects of AMPK activation on IGF-1-induced progesterone secretion in hen granulosa cells. Domest. Anim. Endocrinol. 2008, 34, 204–216. [Google Scholar] [PubMed]
- Kim, D.; Lee, J.; Johnson, A.L. Vascular endothelial growth factor and angiopoietins during hen ovarian follicle development. Gen. Comp. Endocrinol. 2016, 232, 25–31. [Google Scholar]
- Terakawa, J.; Serna, V.A.; Taketo, M.M.; Daikoku, T.; Suarez, A.A.; Kurita, T. Ovarian insufficiency and CTNNB1 mutations drive malignant transformation of endometrial hyperplasia with altered PTEN/PI3K activities. Proc. Natl. Acad. Sci. USA 2019, 116, 4528–4537. [Google Scholar] [CrossRef]
- Wang, P.; Gong, Y.; Li, D.; Zhao, X.; Zhang, Y.; Zhang, J.; Geng, X.; Zhang, X.; Tian, Y.; Li, W.; et al. Effect of induced molting on ovarian function remodeling in laying hens. Poult. Sci. 2023, 102, 102820. [Google Scholar]
- Shen, M.; Wu, P.; Li, T.; Wu, P.; Chen, F.; Chen, L.; Xie, K.; Wang, J.; Zhang, G. Transcriptome Analysis of circRNA and mRNA in Theca Cells during Follicular Development in Chickens. Genes 2020, 11, 489. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Huang, X.; Wang, J.; Zhang, H.; Zhou, W.; Xu, C.; Huang, Y.; Tan, Y.; Yin, Z. Transcriptome Analysis of the Ovaries of Taihe Black-Bone Silky Fowls at Different Egg-Laying Stages. Genes 2022, 13, 2066. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, L.; Zeng, T.; Du, X.; Tao, Z.; Li, G.; Zhong, S.; Wen, J.; Zhou, C.; Xu, X. Transcriptome analyses of potential regulators of pre- and post-ovulatory follicles in the pigeon (Columba livia). Reprod. Fertil. Dev. 2022, 34, 689–697. [Google Scholar]
- Lei, M.; Chen, R.; Qin, Q.; Zhu, H.; Shi, Z. Transcriptome analysis to unravel the gene expression profile of ovarian follicular development in Magang goose. J. Reprod. Dev. 2020, 66, 331–340. [Google Scholar]
- Ma, Z.; Jiang, K.; Wang, D.; Wang, Z.; Gu, Z.; Li, G.; Jiang, R.; Tian, Y.; Kang, X.; Li, H.; et al. Comparative analysis of hypothalamus transcriptome between laying hens with different egg-laying rates. Poult. Sci. 2021, 100, 101110. [Google Scholar]
- Xie, J.; Kalwar, Q.; Yan, P.; Guo, X. Effect of Concentrate Supplementation on the Expression Profile of miRNA in the Ovaries of Yak during Non-Breeding Season. Animals 2020, 10, 1640. [Google Scholar] [CrossRef] [PubMed]
- Uengwetwanit, T.; Ponza, P.; Sangsrakru, D.; Wichadakul, D.; Ingsriswang, S.; Leelatanawit, R.; Klinbunga, S.; Tangphatsornruang, S.; Karoonuthaisiri, N. Transcriptome-based discovery of pathways and genes related to reproduction of the black tiger shrimp (Penaeus monodon). Mar. Genom. 2018, 37, 69–73. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, J.; Sun, Y.; Li, Y.; Wang, P.; Shi, L.; Ni, A.; Zong, Y.; Zhao, J.; Bian, S.; et al. Genetic Basis of Sexual Maturation Heterosis: Insights from Ovary lncRNA and mRNA Repertoire in Chicken. Front. Endocrinol. 2022, 13, 951534. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shen, C.; Zhang, K.; Niu, Z.; Liu, Z.; Zhang, S.; Wang, Y.; Lan, X. Polymorphic variants of bovine ADCY5 gene identified in GWAS analysis were significantly associated with ovarian morphological related traits. Gene 2021, 766, 145158. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, Q.; Gilbert, E.R.; Cui, Z.; Zhao, X.; Wang, Y.; Yin, H.; Li, D.; Zhang, H.; Zhu, Q. Whole-transcriptome analysis of atrophic ovaries in broody chickens reveals regulatory pathways associated with proliferation and apoptosis. Sci. Rep. 2018, 8, 7231. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.X.; Jia, Y.D.; Zhang, C.Q. Effect of epidermal growth factor on follicle-stimulating hormone-induced proliferation of granulosa cells from chicken prehierarchical follicles. J. Zhejiang Univ. Sci. B 2011, 12, 875–883. [Google Scholar] [CrossRef]
- Zhu, G.; Fang, C.; Mo, C.; Wang, Y.; Huang, Y.; Li, J. Transcriptomic analysis of granulosa cell populations proximal and distal to the germinal disc of chicken preovulatory follicles. Sci. Rep. 2021, 11, 4683. [Google Scholar] [CrossRef]
- Yao, H.H.; Bahr, J.M. Chicken granulosa cells show differential expression of epidermal growth factor (EGF) and luteinizing hormone (LH) receptor messenger RNA and differential responsiveness to EGF and LH dependent upon location of granulosa cells to the germinal disc. Biol. Reprod. 2001, 64, 1790–1796. [Google Scholar] [CrossRef]
- Volentine, K.K.; Yao, H.H.; Bahr, J.M. Epidermal growth factor in the germinal disc and its potential role in follicular development in the chicken. Biol. Reprod. 1998, 59, 522–526. [Google Scholar] [CrossRef]
- Olsson, A.K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. VEGF receptor signalling-in control of vascular function. Nat. Rev. Mol. Cell. Biol. 2006, 7, 359–371. [Google Scholar] [CrossRef]
- van Rooijen, E.; Voest, E.E.; Logister, I.; Bussmann, J.; Korving, J.; van Eeden, F.J.; Giles, R.H.; Schulte-Merker, S. von Hippel-Lindau tumor suppressor mutants faithfully model pathological hypoxia-driven angiogenesis and vascular retinopathies in zebrafish. Dis. Model Mech. 2010, 3, 343–353. [Google Scholar] [PubMed]
- Ferrara, N.; Houck, K.A.; Jakeman, L.B.; Winer, J.; Leung, D.W. The vascular endothelial growth factor family of polypeptides. J. Cell. Biochem. 1991, 47, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Cébe-Suarez, S.; Zehnder-Fjällman, A.; Ballmer-Hofer, K. The role of VEGF receptors in angiogenesis; complex partnerships. Cell. Mol. Life Sci. 2006, 63, 601–615. [Google Scholar]
- Chen, H.; Chen, X.; Ping, Z.; Fang, L.; Jiang, X.; Ge, M.; Ma, J.; Yu, W. Ligusticum chuanxiong promotes the angiogenesis of preovulatory follicles (F1–F3) in late-phase laying hens. Poult. Sci. 2023, 102, 102430. [Google Scholar]
- Ping, Z.; Chen, X.; Fang, L.; Wu, K.; Liu, C.; Chen, H.; Jiang, X.; Ma, J.; Yu, W. Effect of Angelica Sinensis extract on the angiogenesis of preovulatory follicles (F1–F3) in late-phase laying hens. Poult. Sci. 2023, 102, 102415. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Chen, H.H.; Winer, J.; Houck, K.A.; Ferrara, N. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J. Biol. Chem. 1994, 269, 25646–25654. [Google Scholar]
- Dreszer, T.R.; Karolchik, D.; Zweig, A.S.; Hinrichs, A.S.; Raney, B.J.; Kuhn, R.M.; Meyer, L.R.; Wong, M.; Sloan, C.A.; Rosenbloom, K.R.; et al. The UCSC Genome Browser database: Extensions and updates 2011. Nucleic Acids Res. 2012, 40, D918–D923. [Google Scholar] [CrossRef]
- Matsui, T.; Heidaran, M.; Miki, T.; Popescu, N.; La Rochelle, W.; Kraus, M.; Pierce, J.; Aaronson, S. Isolation of a novel receptor cDNA establishes the existence of two PDGF receptor genes. Science 1989, 243, 800–804. [Google Scholar] [CrossRef]
- Pinkas, H.; Fisch, B.; Rozansky, G.; Felz, C.; Kessler-Icekson, G.; Krissi, H.; Nitke, S.; Ao, A.; Abir, R. Platelet-Derived growth factors (PDGF-A and -B) and their receptors in human fetal and adult ovaries. Mol. Hum. Reprod. 2008, 14, 199–206. [Google Scholar]
- Okamura, Y.; Myoumoto, A.; Manabe, N.; Tanaka, N.; Okamura, H.; Fukumoto, M. Protein tyrosine kinase expression in the porcine ovary. Mol. Hum. Reprod. 2001, 7, 723–729. [Google Scholar]
- Yoon, S.J.; Kim, K.H.; Chung, H.M.; Choi, D.H.; Lee, W.S.; Cha, K.Y.; Lee, K.A. Gene expression profiling of early follicular development in primordial, primary, and secondary follicles. Fertil. Steril. 2006, 85, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Brito, I.R.; Lima, I.M.; Saraiva, M.V.; Silva, C.M.; Magalhães-Padilha, D.M.; Araújo, V.R.; Barreto Luz, V.; Barbalho Silva, A.W.; Campello, C.C.; Silva, J.R.; et al. Expression levels of mRNA-encoding PDGF receptors in goat ovaries and the influence of PDGF on the in vitro development of caprine pre-antral follicles. Reprod. Domest. Anim. 2012, 47, 695–703. [Google Scholar] [CrossRef]
- Schmahl, J.; Rizzolo, K.; Soriano, P. The PDGF signaling pathway controls multiple steroid-producing lineages. Genes Dev. 2008, 22, 3255–3267. [Google Scholar] [CrossRef] [PubMed]
- Downward, J. Ras signalling and apoptosis. Curr. Opin. Genet. Dev. 1998, 8, 49–54. [Google Scholar] [CrossRef]
- Lindholm, C.K.; Frantz, J.D.; Shoelson, S.E.; Welsh, M. Shf, a Shb-like adapter protein, is involved in PDGF-alpha-receptor regulation of apoptosis. Biochem. Biophys. Res. Commun. 2000, 278, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.G.; Acosta, T.J.; Tetsuka, M.; Berisha, B.; Matsui, M.; Schams, D.; Ohtani, M.; Miyamoto, A. Involvement of angiopoietin-tie system in bovine follicular development and atresia: Messenger RNA expression in theca interna and effect on steroid secretion. Biol. Reprod. 2003, 69, 2078–2084. [Google Scholar] [CrossRef]
- Maisonpierre, P.C.; Suri, C.; Jones, P.F.; Bartunkova, S.; Wiegand, S.J.; Radziejewski, C.; Compton, D.; McClain, J.; Aldrich, T.H.; Papadopoulos, N.; et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 1997, 277, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Miraie-Ashtiani, S.R.; Sadeghi, M.; Najafi, A.; Ranjbar, R. Dynamic modeling of folliculogenesis signaling pathways in the presence of miRNAs expression. J. Ovarian Res. 2017, 10, 76. [Google Scholar] [CrossRef]
- Hanahan, D. Signaling vascular morphogenesis and maintenance. Science 1997, 277, 48–50. [Google Scholar] [CrossRef]
- Hutt, K.J.; McLaughlin, E.A.; Holland, M.K. Kit ligand and c-Kit have diverse roles during mammalian oogenesis and folliculogenesis. Mol. Hum. Reprod. 2006, 12, 61–69. [Google Scholar] [CrossRef]
- Fleischman, R.A. From white spots to stem cells: The role of the Kit receptor in mammalian development. Trends Genet. 1993, 9, 285–290. [Google Scholar] [PubMed]
- Parrott, J.A.; Skinner, M.K. Direct actions of kit-ligand on theca cell growth and differentiation during follicle development. Endocrinology 1997, 138, 3819–3827. [Google Scholar]
- Clark, D.E.; Tisdall, D.J.; Fidler, A.E.; McNatty, K.P. Localization of mRNA encoding c-kit during the initiation of folliculogenesis in ovine fetal ovaries. J. Reprod. Fertil. 1996, 106, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Tanikawa, M.; Harada, T.; Mitsunari, M.; Onohara, Y.; Iwabe, T.; Terakawa, N. Expression of c-kit messenger ribonucleic acid in human oocyte and presence of soluble c-kit in follicular fluid. J. Clin. Endocrinol. Metab. 1998, 83, 1239–1242. [Google Scholar]
- Kundu, M.C.; Wojtusik, J.; Johnson, P.A. Expression and regulation of Kit ligand in the ovary of the hen. Gen. Comp. Endocrinol. 2012, 179, 47–52. [Google Scholar]
- Sasaki, E.; Okamura, H.; Chikamune, T.; Kanai, Y.; Watanabe, M.; Naito, M.; Sakurai, M. Cloning and expression of the chicken c-kit proto-oncogene. Gene 1993, 128, 257–261. [Google Scholar]
- Ben-Haroush, A.; Abir, R.; Ao, A.; Jin, S.; Kessler-Icekson, G.; Feldberg, D.; Fisch, B. Expression of basic fibroblast growth factor and its receptors in human ovarian follicles from adults and fetuses. Fertil. Steril. 2005, 84, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Drummond, A.E.; Tellbach, M.; Dyson, M.; Findlay, J.K. Fibroblast growth factor-9, a local regulator of ovarian function. Endocrinology 2007, 148, 3711–3721. [Google Scholar]
- Chitiashvili, T.; Hsu, F.M.; Dror, I.; Plath, K.; Clark, A. FGFR3 is expressed by human primordial germ cells and is repressed after meiotic initiation to form primordial oocytes. Stem Cell Rep. 2022, 17, 1268–1278. [Google Scholar] [CrossRef]
- Mishra, S.R.; Thakur, N.; Somal, A.; Parmar, M.S.; Reshma, R.; Rajesh, G.; Yadav, V.P.; Bharti, M.K.; Bharati, J.; Paul, A.; et al. Expression and localization of fibroblast growth factor (FGF) family in buffalo ovarian follicle during different stages of development and modulatory role of FGF2 on steroidogenesis and survival of cultured buffalo granulosa cells. Res. Vet. Sci. 2016, 108, 98–111. [Google Scholar]
- Cronauer, M.V.; Schulz, W.A.; Seifert, H.H.; Ackermann, R.; Burchardt, M. Fibroblast growth factors and their receptors in urological cancers: Basic research and clinical implications. Eur. Urol. 2003, 43, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Amsterdam, A.; Kannan, K.; Givol, D.; Yoshida, Y.; Tajima, K.; Dantes, A. Apoptosis of granulosa cells and female infertility in achondroplastic mice expressing mutant fibroblast growth factor receptor 3G374R. Mol. Endocrinol. 2001, 15, 1610–1623. [Google Scholar] [PubMed]
- Ayers, K.L.; Lambeth, L.S.; Davidson, N.M.; Sinclair, A.H.; Oshlack, A.; Smith, C.A. Identification of candidate gonadal sex differentiation genes in the chicken embryo using RNA-seq. BMC Genom. 2015, 16, 704. [Google Scholar] [CrossRef]
- El Halawani, M.E.; Burke, W.H.; Dennison, P.T. Effect of nest-deprivation on serum prolactin level in nesting female turkeys. Biol. Reprod. 1980, 23, 118–123. [Google Scholar] [PubMed]
- Huang, T.; Fei, M.; Zhou, X.; He, K.; Yang, S.; Zhao, A. Effects of Different Photoperiods on the Transcriptome of the Ovary and Small White Follicles in Zhedong White Geese. Animals 2024, 14, 2747. [Google Scholar] [CrossRef]
- Li, D.; Tong, Q.; Shi, Z.; Li, H.; Wang, Y.; Li, B.; Yan, G.; Chen, H.; Zheng, W. Effects of chronic heat stress and ammonia concentration on blood parameters of laying hens. Poult. Sci. 2020, 99, 3784–3792. [Google Scholar]
- Xin, Q.; Li, L.; Zhao, B.; Shi, W.; Hao, X.; Zhang, L.; Miao, Z.; Zhu, Z.; Huang, Q.; Zheng, N. The network regulation mechanism of the effects of heat stress on the production performance and egg quality of Jinding duck was analyzed by miRNA-mRNA. Poult. Sci. 2024, 103, 103255. [Google Scholar] [CrossRef]

| Sample | Clean Reads | Clean Bases | Q20 (%) | Q30 (%) | GC (%) | Total Mapped | Uniquely Mapped | Multiple Mapped |
|---|---|---|---|---|---|---|---|---|
| LP1 | 22,097,938 | 6,611,280,448 | 98.46 | 95.39 | 49.5 | 41,172,905 (93.16%) | 40,260,429 (91.10%) | 912,476 (2.06%) |
| LP2 | 19,277,102 | 5,768,363,046 | 98.54 | 95.51 | 48.36 | 36,607,544 (94.95%) | 35,884,081 (93.07%) | 723,463 (1.88%) |
| LP3 | 23,428,349 | 7,006,988,490 | 98.41 | 95.25 | 49.60 | 43,906,095 (93.70%) | 42,884,740 (91.52%) | 1,021,355 (2.18%) |
| BP1 | 20,509,236 | 6,134,357,836 | 98.45 | 95.56 | 51.22 | 38,159,435 (93.03%) | 37,161,046 (90.60%) | 998,389 (2.43%) |
| BP2 | 21,578,186 | 6,454,693,384 | 98.34 | 94.87 | 47.94 | 41,280,264 (95.65%) | 40,498,880 (93.84%) | 781,384 (1.81%) |
| BP3 | 20,424,383 | 6,112,551,828 | 98.46 | 95.21 | 47.89 | 38,963,103 (95.38%) | 38,206,570 (93.53%) | 756,533 (1.85%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Wen, D. Ovarian Transcriptome Profile from Egg-Laying Period to Incubation Period of Changshun Green-Shell Laying Hens. Genes 2025, 16, 394. https://doi.org/10.3390/genes16040394
Chen Z, Wen D. Ovarian Transcriptome Profile from Egg-Laying Period to Incubation Period of Changshun Green-Shell Laying Hens. Genes. 2025; 16(4):394. https://doi.org/10.3390/genes16040394
Chicago/Turabian StyleChen, Zhi, and Di Wen. 2025. "Ovarian Transcriptome Profile from Egg-Laying Period to Incubation Period of Changshun Green-Shell Laying Hens" Genes 16, no. 4: 394. https://doi.org/10.3390/genes16040394
APA StyleChen, Z., & Wen, D. (2025). Ovarian Transcriptome Profile from Egg-Laying Period to Incubation Period of Changshun Green-Shell Laying Hens. Genes, 16(4), 394. https://doi.org/10.3390/genes16040394




