Aberrant Short Tandem Repeats: Pathogenicity, Mechanisms, Detection, and Roles in Neuropsychiatric Disorders
Abstract
1. Introduction of Short Tandem Repeats and Tandem Repeat Expansion Disorders
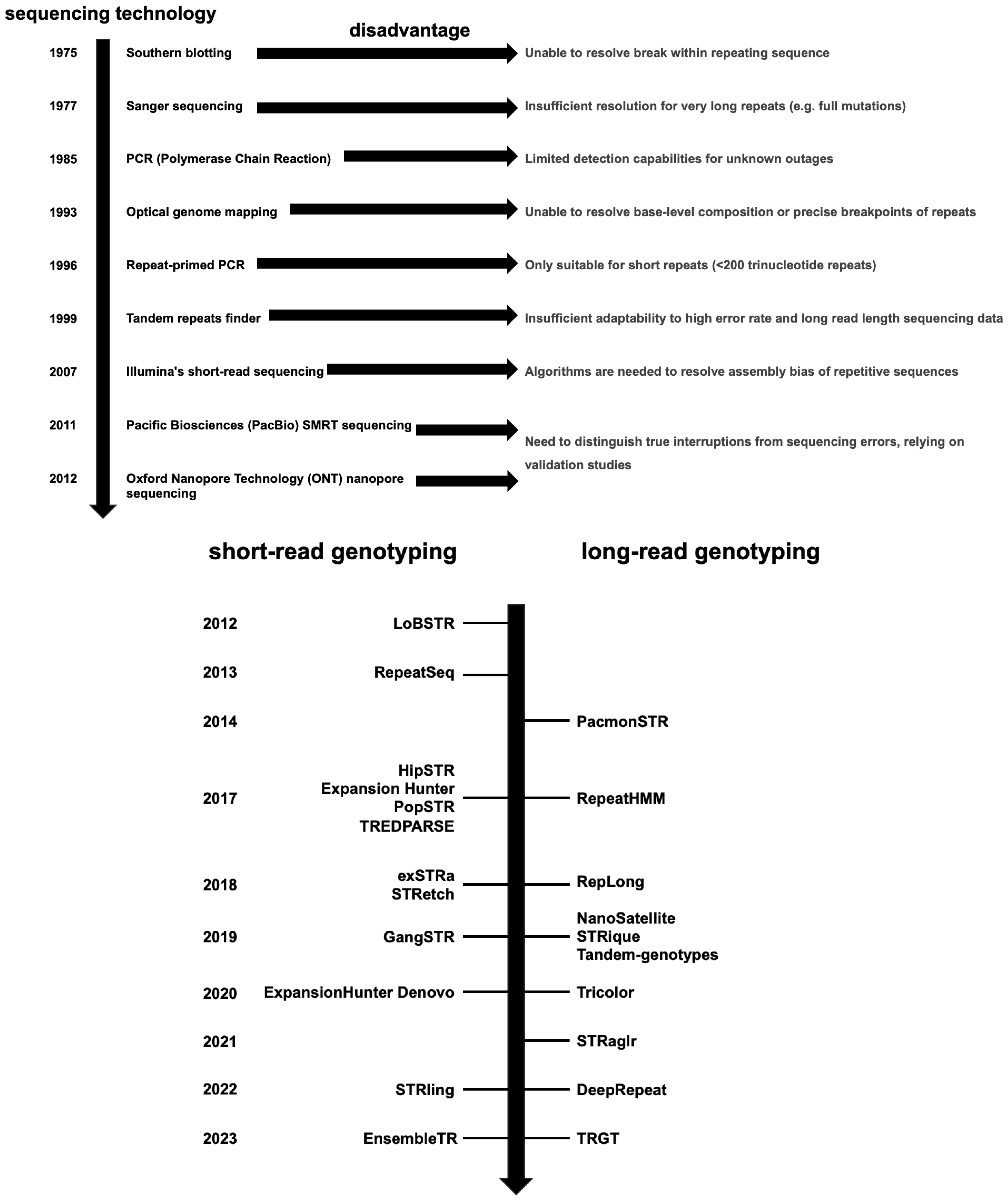
2. Methods for Detecting Variations in STR Sequence Composition
3. The Genetic Characteristics of Neurological Disorders Caused by Repeat Expansion Disorders
4. The Genetic Characteristics of Mental Disorders Associated with Tandem Repeats
5. The Genetic Characteristics of Repeat Expansion Disorders
6. The Pathogenic Mechanisms of STRs
7. The Common Animal Models Used in the Study of STR Variations in Neuropsychiatric Disorders
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Horton, C.A.; Alexandari, A.M.; Hayes, M.G.B.; Marklund, E.; Schaepe, J.M.; Aditham, A.K.; Shah, N.; Suzuki, P.H.; Shrikumar, A.; Afek, A.; et al. Short tandem repeats bind transcription factors to tune eukaryotic gene expression. Science 2023, 381, eadd1250. [Google Scholar] [CrossRef]
- Ziaei Jam, H.; Li, Y.; DeVito, R.; Mousavi, N.; Ma, N.; Lujumba, I.; Adam, Y.; Maksimov, M.; Huang, B.; Dolzhenko, E.; et al. A deep population reference panel of tandem repeat variation. Nat. Commun. 2023, 14, 6711. [Google Scholar] [CrossRef]
- Tanudisastro, H.A.; Deveson, I.W.; Dashnow, H.; MacArthur, D.G. Sequencing and characterizing short tandem repeats in the human genome. Nat. Rev. Genet. 2024, 25, 460–475. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Mishra, R.K.; Singh, L. Genome-wide analysis of microsatellite repeats in humans: Their abundance and density in specific genomic regions. Genome Biol. 2003, 4, R13. [Google Scholar] [CrossRef] [PubMed]
- Tóth, G.; Gáspári, Z.; Jurka, J. Microsatellites in different eukaryotic genomes: Survey and analysis. Genome. Res. 2000, 10, 967–981. [Google Scholar] [CrossRef]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [PubMed]
- Gymrek, M.; Golan, D.; Rosset, S.; Erlich, Y. lobSTR: A short tandem repeat profiler for personal genomes. Genome. Res. 2012, 22, 1154–1162. [Google Scholar] [CrossRef]
- Thornton, C.A. Myotonic dystrophy. Neurol. Clin. 2014, 32, 705–719. [Google Scholar] [CrossRef]
- Orr, H.T.; Zoghbi, H.Y. Trinucleotide repeat disorders. Annu. Rev. Neurosci. 2007, 30, 575–621. [Google Scholar] [CrossRef]
- Ross, C.A.; Tabrizi, S.J. Huntington’s disease: From molecular pathogenesis to clinical treatment. Lancet Neurol. 2011, 10, 83–98. [Google Scholar] [CrossRef]
- Fondon, J.W., 3rd; Garner, H.R. Molecular origins of rapid and continuous morphological evolution. Proc. Natl. Acad. Sci. USA 2004, 101, 18058–18063. [Google Scholar] [CrossRef] [PubMed]
- Gemayel, R.; Cho, J.; Boeynaems, S.; Verstrepen, K.J. Beyond junk-variable tandem repeats as facilitators of rapid evolution of regulatory and coding sequences. Genes 2012, 3, 461–480. [Google Scholar] [CrossRef] [PubMed]
- Kelkar, Y.D.; Strubczewski, N.; Hile, S.E.; Chiaromonte, F.; Eckert, K.A.; Makova, K.D. What is a microsatellite: A computational and experimental definition based upon repeat mutational behavior at A/T and GT/AC repeats. Genome Biol. Evol. 2010, 2, 620–635. [Google Scholar] [PubMed]
- Hannan, A.J. Repeating themes of plastic genes and therapeutic schemes targeting the ‘tandem repeatome’. Brain. Commun. 2024, 6, fcae047. [Google Scholar] [CrossRef]
- Sfera, A.; Sfera, D.O.; Sasannia, S.; Hazan, S. The MSH3 Gene From A Neuropsychiatrist’s Perspective. Adv. Clin. Med. Res. 2022, 3, 1–6. [Google Scholar] [CrossRef]
- Zarouchlioti, C.; Efthymiou, S.; Facchini, S.; Dominik, N.; Bhattacharyya, N.; Liu, S.; Costa, M.A.; Szabo, A.; Sadan, A.N.; Jun, A.S.; et al. Tissue-specific TCF4 triplet repeat instability revealed by optical genome mapping. EBioMedicine 2024, 108, 105328. [Google Scholar]
- La Rosa, P.; Petrillo, S.; Bertini, E.S.; Piemonte, F. Oxidative Stress in DNA Repeat Expansion Disorders: A Focus on NRF2 Signaling Involvement. Biomolecules 2020, 10, 702. [Google Scholar] [CrossRef]
- Zoghbi, H.Y.; Orr, H.T. Glutamine repeats and neurodegeneration. Annu. Rev. Neurosci. 2000, 23, 217–247. [Google Scholar]
- Chintalaphani, S.R.; Pineda, S.S.; Deveson, I.W.; Kumar, K.R. An update on the neurological short tandem repeat expansion disorders and the emergence of long-read sequencing diagnostics. Acta Neuropathol. Commun. 2021, 9, 98. [Google Scholar]
- Depienne, C.; Mandel, J.L. 30 years of repeat expansion disorders: What have we learned and what are the remaining challenges? Am. J. Hum. Genet. 2021, 108, 764–785. [Google Scholar]
- Malik, I.; Kelley, C.P.; Wang, E.T.; Todd, P.K. Molecular mechanisms underlying nucleotide repeat expansion disorders. Nat. Rev. Mol. Cell. Biol. 2021, 22, 589–607. [Google Scholar] [CrossRef] [PubMed]
- Inserra, A.; De Gregorio, D.; Gobbi, G. Psychedelics in Psychiatry: Neuroplastic, Immunomodulatory, and Neurotransmitter Mechanisms. Pharmacol. Rev. 2021, 73, 202–277. [Google Scholar] [PubMed]
- Magwai, T.; Shangase, K.B.; Oginga, F.O.; Chiliza, B.; Mpofana, T.; Xulu, K.R. DNA Methylation and Schizophrenia: Current Literature and Future Perspective. Cells 2021, 10, 2890. [Google Scholar]
- Stevanovski, I.; Chintalaphani, S.R.; Gamaarachchi, H.; Ferguson, J.M.; Pineda, S.S.; Scriba, C.K.; Tchan, M.; Fung, V.; Ng, K.; Cortese, A.; et al. Comprehensive genetic diagnosis of tandem repeat expansion disorders with programmable targeted nanopore sequencing. Sci. Adv. 2022, 8, eabm5386. [Google Scholar] [CrossRef]
- Xi, J.; Wang, X.; Yue, D.; Dou, T.; Wu, Q.; Lu, J.; Liu, Y.; Yu, W.; Qiao, K.; Lin, J.; et al. 5’ UTR CGG repeat expansion in GIPC1 is associated with oculopharyngodistal myopathy. Brain 2021, 144, 601–614. [Google Scholar]
- Firdaus, Z.; Li, X. Unraveling the Genetic Landscape of Neurological Disorders: Insights into Pathogenesis, Techniques for Variant Identification, and Therapeutic Approaches. Int. J. Mol. Sci. 2024, 25, 2320. [Google Scholar] [CrossRef]
- Höijer, I.; Tsai, Y.C.; Clark, T.A.; Kotturi, P.; Dahl, N.; Stattin, E.L.; Bondeson, M.L.; Feuk, L.; Gyllensten, U.; Ameur, A. Detailed analysis of HTT repeat elements in human blood using targeted amplification-free long-read sequencing. Hum. Mutat. 2018, 39, 1262–1272. [Google Scholar] [PubMed]
- Mitra, I.; Huang, B.; Mousavi, N.; Ma, N.; Lamkin, M.; Yanicky, R.; Shleizer-Burko, S.; Lohmueller, K.E.; Gymrek, M. Patterns of de novo tandem repeat mutations and their role in autism. Nature 2021, 589, 246–250. [Google Scholar]
- Trost, B.; Engchuan, W.; Nguyen, C.M.; Thiruvahindrapuram, B.; Dolzhenko, E.; Backstrom, I.; Mirceta, M.; Mojarad, B.A.; Yin, Y.; Dov, A.; et al. Genome-wide detection of tandem DNA repeats that are expanded in autism. Nature 2020, 586, 80–86. [Google Scholar]
- Fotsing, S.F.; Margoliash, J.; Wang, C.; Saini, S.; Yanicky, R.; Shleizer-Burko, S.; Goren, A.; Gymrek, M. The impact of short tandem repeat variation on gene expression. Nat. Genet. 2019, 51, 1652–1659. [Google Scholar] [CrossRef]
- Rajan-Babu, I.S.; Dolzhenko, E.; Eberle, M.A.; Friedman, J.M. Sequence composition changes in short tandem repeats: Heterogeneity, detection, mechanisms and clinical implications. Nat. Rev. Genet. 2024, 25, 476–499. [Google Scholar]
- Uguen, K.; Michaud, J.L.; Génin, E. Short Tandem Repeats in the era of next-generation sequencing: From historical loci to population databases. Eur. J. Hum. Genet. 2024, 32, 1037–1044. [Google Scholar] [PubMed]
- Xiao, X.; Zhang, C.Y.; Zhang, Z.; Hu, Z.; Li, M.; Li, T. Revisiting tandem repeats in psychiatric disorders from perspectives of genetics, physiology, and brain evolution. Mol. Psychiatry 2022, 27, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.D.; Jankovic, J.; Ashizawa, T.; Tan, E.K. Neurodegenerative diseases associated with non-coding CGG tandem repeat expansions. Nat. Rev. Neurol. 2022, 18, 145–157. [Google Scholar] [CrossRef]
- Verkerk, A.J.; Pieretti, M.; Sutcliffe, J.S.; Fu, Y.H.; Kuhl, D.P.; Pizzuti, A.; Reiner, O.; Richards, S.; Victoria, M.F.; Zhang, F.P.; et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 1991, 65, 905–914. [Google Scholar]
- Mor-Shaked, H.; Eiges, R. Reevaluation of FMR1 Hypermethylation Timing in Fragile X Syndrome. Front. Mol. Neurosci. 2018, 11, 31. [Google Scholar]
- Walker, F.O. Huntington’s disease. Lancet 2007, 369, 218–228. [Google Scholar] [PubMed]
- The Huntington’s Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 1993, 72, 971–983. [Google Scholar]
- Paulson, H. Repeat expansions in leukoencephalopathy. Ann. Neurol. 2019, 86, 809–811. [Google Scholar]
- Hsu, P.D.; Zhang, F. Dissecting neural function using targeted genome engineering technologies. ACS Chem. Neurosci. 2012, 3, 603–610. [Google Scholar] [CrossRef]
- Orack, J.C.; Deleidi, M.; Pitt, D.; Mahajan, K.; Nicholas, J.A.; Boster, A.L.; Racke, M.K.; Comabella, M.; Watanabe, F.; Imitola, J. Concise review: Modeling multiple sclerosis with stem cell biological platforms: Toward functional validation of cellular and molecular phenotypes in inflammation-induced neurodegeneration. Stem. Cells. Transl. Med. 2015, 4, 252–260. [Google Scholar] [CrossRef]
- Johnstone, S.E.; Baylin, S.B. Stress and the epigenetic landscape: A link to the pathobiology of human diseases? Nat. Rev. Genet. 2010, 11, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, R.I.; Niculescu, A.G.; Roza, E.; Vladâcenco, O.; Grumezescu, A.M.; Teleanu, D.M. Neurotransmitters-Key Factors in Neurological and Neurodegenerative Disorders of the Central Nervous System. Int. J. Mol. Sci. 2022, 23, 5954. [Google Scholar] [CrossRef] [PubMed]
- Boivin, M.; Deng, J.; Pfister, V.; Grandgirard, E.; Oulad-Abdelghani, M.; Morlet, B.; Ruffenach, F.; Negroni, L.; Koebel, P.; Jacob, H.; et al. Translation of GGC repeat expansions into a toxic polyglycine protein in NIID defines a novel class of human genetic disorders: The polyG diseases. Neuron 2021, 109, 1825–1835.e1825. [Google Scholar] [CrossRef]
- Napierała, M.; Krzyzosiak, W.J. CUG repeats present in myotonin kinase RNA form metastable “slippery” hairpins. J. Biol. Chem. 1997, 272, 31079–31085. [Google Scholar] [CrossRef]
- Mankodi, A.; Takahashi, M.P.; Jiang, H.; Beck, C.L.; Bowers, W.J.; Moxley, R.T.; Cannon, S.C.; Thornton, C.A. Expanded CUG repeats trigger aberrant splicing of ClC-1 chloride channel pre-mRNA and hyperexcitability of skeletal muscle in myotonic dystrophy. Mol. Cell. 2002, 10, 35–44. [Google Scholar] [CrossRef]
- Kanadia, R.N.; Johnstone, K.A.; Mankodi, A.; Lungu, C.; Thornton, C.A.; Esson, D.; Timmers, A.M.; Hauswirth, W.W.; Swanson, M.S. A muscleblind knockout model for myotonic dystrophy. Science 2003, 302, 1978–1980. [Google Scholar] [CrossRef] [PubMed]
- Prashad, S.; Gopal, P.P. RNA-binding proteins in neurological development and disease. RNA Biol. 2021, 18, 972–987. [Google Scholar] [CrossRef]
- Hannan, A.J. Tandem repeats mediating genetic plasticity in health and disease. Nat. Rev. Genet. 2018, 19, 286–298. [Google Scholar] [CrossRef]
- Subramanian, M.; Timmerman, C.K.; Schwartz, J.L.; Pham, D.L.; Meffert, M.K. Characterizing autism spectrum disorders by key biochemical pathways. Front. Neurosci. 2015, 9, 313. [Google Scholar] [CrossRef]
- Sullivan, P.F.; Daly, M.J.; O’Donovan, M. Genetic architectures of psychiatric disorders: The emerging picture and its implications. Nat. Rev. Genet. 2012, 13, 537–551. [Google Scholar] [PubMed]
- Nakamori, M.; Thornton, C. Epigenetic changes and non-coding expanded repeats. Neurobiol. Dis. 2010, 39, 21–27. [Google Scholar] [PubMed]
- Kim, J.H.; Koh, I.G.; Lee, H.; Lee, G.H.; Song, D.Y.; Kim, S.W.; Kim, Y.; Han, J.H.; Bong, G.; Lee, J.; et al. Short tandem repeat expansions in cortical layer-specific genes implicate in phenotypic severity and adaptability of autism spectrum disorder. Psychiatry. Clin. Neurosci. 2024, 78, 405–415. [Google Scholar] [PubMed]
- Mirkin, S.M. Expandable DNA repeats and human disease. Nature 2007, 447, 932–940. [Google Scholar]
- Lin, Z.B.; Chen, Z.J.; Yang, H.; Ding, X.R.; Li, J.; Pan, A.P.; Sun, H.S.; Yu, A.Y.; Chen, S.H. Expanded phenotypic spectrum of FOXL2 Variant c.672_701dup revealed by whole-exome sequencing in a rare blepharophimosis, ptosis, and epicanthus inversus syndrome family. BMC Ophthalmol. 2023, 23, 446. [Google Scholar] [CrossRef]
- Bachetti, T.; Ceccherini, I. Causative and common PHOX2B variants define a broad phenotypic spectrum. Clin. Genet. 2020, 97, 103–113. [Google Scholar]
- Mundlos, S. Cleidocranial dysplasia: Clinical and molecular genetics. J. Med. Genet. 1999, 36, 177–182. [Google Scholar]
- Spector, E.; Behlmann, A.; Kronquist, K.; Rose, N.C.; Lyon, E.; Reddi, H.V. Laboratory testing for fragile X, 2021 revision: A technical standard of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2021, 23, 799–812. [Google Scholar]
- Sequeiros, J.; Seneca, S.; Martindale, J. Consensus and controversies in best practices for molecular genetic testing of spinocerebellar ataxias. Eur. J. Hum. Genet. 2010, 18, 1188–1195. [Google Scholar]
- Krey, I.; Platzer, K.; Esterhuizen, A.; Berkovic, S.F.; Helbig, I.; Hildebrand, M.S.; Lerche, H.; Lowenstein, D.; Møller, R.S.; Poduri, A.; et al. Current practice in diagnostic genetic testing of the epilepsies. Epileptic. Disord. 2022, 24, 765–786. [Google Scholar] [CrossRef]
- Sequeiros, J.; Martindale, J.; Seneca, S.; Giunti, P.; Kämäräinen, O.; Volpini, V.; Weirich, H.; Christodoulou, K.; Bazak, N.; Sinke, R.; et al. EMQN Best Practice Guidelines for molecular genetic testing of SCAs. Eur. J. Hum. Genet. 2010, 18, 1173–1176. [Google Scholar] [CrossRef] [PubMed]
- Leferink, M.; Wong, D.P.W.; Cai, S.; Yeo, M.; Ho, J.; Lian, M.; Kamsteeg, E.J.; Chong, S.S.; Haer-Wigman, L.; Guan, M. Robust and accurate detection and sizing of repeats within the DMPK gene using a novel TP-PCR test. Sci. Rep. 2019, 9, 8280. [Google Scholar]
- Akimoto, C.; Volk, A.E.; van Blitterswijk, M.; Van den Broeck, M.; Leblond, C.S.; Lumbroso, S.; Camu, W.; Neitzel, B.; Onodera, O.; van Rheenen, W.; et al. A blinded international study on the reliability of genetic testing for GGGGCC-repeat expansions in C9orf72 reveals marked differences in results among 14 laboratories. J. Med. Genet. 2014, 51, 419–424. [Google Scholar] [CrossRef]
- Tomé, S.; Gourdon, G. Fast Assays to Detect Interruptions in CTG.CAG Repeat Expansions. Methods. Mol. Biol. 2020, 2056, 11–23. [Google Scholar] [PubMed]
- Jang, J.H.; Yoon, S.J.; Kim, S.K.; Cho, J.W.; Kim, J.W. Detection Methods and Status of CAT Interruption of ATXN1 in Korean Patients With Spinocerebellar Ataxia Type 1. Ann. Lab. Med. 2022, 42, 274–277. [Google Scholar] [CrossRef]
- Loomis, E.W.; Eid, J.S.; Peluso, P.; Yin, J.; Hickey, L.; Rank, D.; McCalmon, S.; Hagerman, R.J.; Tassone, F.; Hagerman, P.J. Sequencing the unsequenceable: Expanded CGG-repeat alleles of the fragile X gene. Genome. Res. 2013, 23, 121–128. [Google Scholar] [CrossRef]
- Rajan-Babu, I.S.; Law, H.Y.; Yoon, C.S.; Lee, C.G.; Chong, S.S. Simplified strategy for rapid first-line screening of fragile X syndrome: Closed-tube triplet-primed PCR and amplicon melt peak analysis. Expert. Rev. Mol. Med. 2015, 17, e7. [Google Scholar]
- Nethisinghe, S.; Kesavan, M.; Ging, H.; Labrum, R.; Polke, J.M.; Islam, S.; Garcia-Moreno, H.; Callaghan, M.F.; Cavalcanti, F.; Pook, M.A.; et al. Interruptions of the FXN GAA Repeat Tract Delay the Age at Onset of Friedreich’s Ataxia in a Location Dependent Manner. Int. J. Mol. Sci. 2021, 22, 7507. [Google Scholar] [CrossRef]
- Botta, A.; Rossi, G.; Marcaurelio, M.; Fontana, L.; D’Apice, M.R.; Brancati, F.; Massa, R.; G Monckton, D.; Sangiuolo, F.; Novelli, G. Identification and characterization of 5′ CCG interruptions in complex DMPK expanded alleles. Eur. J. Hum. Genet. 2017, 25, 257–261. [Google Scholar]
- Lian, M.; Law, H.Y.; Lee, C.G.; Chong, S.S. Defining the performance parameters of a rapid screening tool for myotonic dystrophy type 1 based on triplet-primed PCR and melt curve analysis. Expert. Rev. Mol. Diagn. 2016, 16, 1221–1232. [Google Scholar]
- Rajan-Babu, I.-S.; Chong, S.S. Molecular Correlates and Recent Advancements in the Diagnosis and Screening of FMR1-Related Disorders. Genes 2016, 7, 87. [Google Scholar] [CrossRef] [PubMed]
- Mantere, T.; Neveling, K.; Pebrel-Richard, C.; Benoist, M.; van der Zande, G.; Kater-Baats, E.; Baatout, I.; van Beek, R.; Yammine, T.; Oorsprong, M.; et al. Optical genome mapping enables constitutional chromosomal aberration detection. Am. J. Hum. Genet. 2021, 108, 1409–1422. [Google Scholar] [CrossRef]
- Yuan, Y.; Chung, C.Y.; Chan, T.F. Advances in optical mapping for genomic research. Comput. Struct. Biotechnol. J. 2020, 18, 2051–2062. [Google Scholar] [CrossRef]
- Corbett, M.A.; Depienne, C.; Veneziano, L.; Klein, K.M.; Brancati, F.; Guerrini, R.; Zara, F.; Tsuji, S.; Gecz, J. Genetics of familial adult myoclonus epilepsy: From linkage studies to noncoding repeat expansions. Epilepsia 2023, 64 (Suppl. S1), S14–S21. [Google Scholar] [CrossRef]
- Dolzhenko, E.; Bennett, M.F.; Richmond, P.A.; Trost, B.; Chen, S.; van Vugt, J.; Nguyen, C.; Narzisi, G.; Gainullin, V.G.; Gross, A.M.; et al. ExpansionHunter Denovo: A computational method for locating known and novel repeat expansions in short-read sequencing data. Genome Biol. 2020, 21, 102. [Google Scholar] [CrossRef]
- Dashnow, H.; Pedersen, B.S.; Hiatt, L.; Brown, J.; Beecroft, S.J.; Ravenscroft, G.; LaCroix, A.J.; Lamont, P.; Roxburgh, R.H.; Rodrigues, M.J.; et al. STRling: A k-mer counting approach that detects short tandem repeat expansions at known and novel loci. Genome Biol. 2022, 23, 257. [Google Scholar] [CrossRef] [PubMed]
- Dolzhenko, E.; Weisburd, B.; Ibañez, K.; Rajan-Babu, I.S.; Anyansi, C.; Bennett, M.F.; Billingsley, K.; Carroll, A.; Clamons, S.; Danzi, M.C.; et al. REViewer: Haplotype-resolved visualization of read alignments in and around tandem repeats. Genome Med. 2022, 14, 84. [Google Scholar] [CrossRef]
- Su, Y.; Fan, L.; Shi, C.; Wang, T.; Zheng, H.; Luo, H.; Zhang, S.; Hu, Z.; Fan, Y.; Dong, Y.; et al. Deciphering Neurodegenerative Diseases Using Long-Read Sequencing. Neurology 2021, 97, 423–433. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, E.L.; Naquin, D.; Gorrichon, K.; Jaszczyszyn, Y.; Ouazahrou, R.; Thermes, C.; Hernandez, C. Genomics in the long-read sequencing era. Trends Genet. 2023, 39, 649–671. [Google Scholar] [CrossRef]
- Chiara, M.; Zambelli, F.; Picardi, E.; Horner, D.S.; Pesole, G. Critical assessment of bioinformatics methods for the characterization of pathological repeat expansions with single-molecule sequencing data. Brief. Bioinform. 2020, 21, 1971–1986. [Google Scholar] [CrossRef]
- Chiu, R.; Rajan-Babu, I.S.; Friedman, J.M.; Birol, I. Straglr: Discovering and genotyping tandem repeat expansions using whole genome long-read sequences. Genome Biol. 2021, 22, 224. [Google Scholar]
- Mitsuhashi, S.; Frith, M.C.; Mizuguchi, T.; Miyatake, S.; Toyota, T.; Adachi, H.; Oma, Y.; Kino, Y.; Mitsuhashi, H.; Matsumoto, N. Tandem-genotypes: Robust detection of tandem repeat expansions from long DNA reads. Genome Biol. 2019, 20, 58. [Google Scholar]
- Liu, Q.; Zhang, P.; Wang, D.; Gu, W.; Wang, K. Interrogating the "unsequenceable" genomic trinucleotide repeat disorders by long-read sequencing. Genome Med. 2017, 9, 65. [Google Scholar] [PubMed]
- English, A.C.; Dolzhenko, E.; Ziaei Jam, H.; McKenzie, S.K.; Olson, N.D.; De Coster, W.; Park, J.; Gu, B.; Wagner, J.; Eberle, M.A.; et al. Analysis and benchmarking of small and large genomic variants across tandem repeats. Nat. Biotechnol. 2024, 43, 431–442. [Google Scholar]
- Ren, J.; Gu, B.; Chaisson, M.J.P. Vamos: Variable-number tandem repeats annotation using efficient motif sets. Genome Biol. 2023, 24, 175. [Google Scholar]
- Jam, H.Z.; Zook, J.M.; Javadzadeh, S.; Park, J.; Sehgal, A.; Gymrek, M. Genome-wide profiling of genetic variation at tandem repeat from long reads. bioRxiv 2024. [Google Scholar] [CrossRef]
- Uppili, B.; Sharma, P.; Ahmad, I.; Sahni, S.; Asokachandran, V.; Nagaraja, A.B.; Srivastava, A.K.; Faruq, M. Sequencing through hyperexpanded Friedreich’s ataxia-GAA repeats by nanopore technology: Implications in genotype-phenotype correlation. Brain Commun. 2023, 5, fcad020. [Google Scholar] [CrossRef]
- Marx, V. Method of the year: Long-read sequencing. Nat. Methods. 2023, 20, 6–11. [Google Scholar]
- Weber, J.L.; May, P.E. Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am. J. Hum. Genet. 1989, 44, 388–396. [Google Scholar]
- Ott, J. Analysis of Human Genetic Linkage; Johns Hopkins University Press: Baltimore, MD, USA, 1999. [Google Scholar]
- Strachan, T.; Read, A. Human Molecular Genetics; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Morton, N.E. Sequential tests for the detection of linkage. Am. J. Hum. Genet. 1955, 7, 277–318. [Google Scholar]
- Risch, N.J. Searching for genetic determinants in the new millennium. Nature 2000, 405, 847–856. [Google Scholar] [CrossRef]
- Elston, R.C.; Stewart, J. A general model for the genetic analysis of pedigree data. Hum. Hered. 1971, 21, 523–542. [Google Scholar] [CrossRef] [PubMed]
- Sproviero, W.; Ghose, U.; Winchester, L.M.; Fernandes, M.; Newby, D.; Sproviero, D.; Amin, N.; Smets, B.; He, K.Y.; Khramtsova, E.A.; et al. The impact of Short Tandem Repeats on grey matter brain imaging derived phenotypes in UK Biobank. medRxiv 2023. [Google Scholar] [CrossRef]
- Mattick, J.; Amaral, P. A new understanding of molecular biology. In RNA, the Epicenter of Genetic Information; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Guo, M.H.; Lee, W.P.; Vardarajan, B.; Schellenberg, G.D.; Phillips-Cremins, J.E. Polygenic burden of short tandem repeat expansions promotes risk for Alzheimer’s disease. Nat. Commun. 2025, 16, 1126. [Google Scholar] [CrossRef]
- Giesselmann, P.; Brändl, B.; Raimondeau, E.; Bowen, R.; Rohrandt, C.; Tandon, R.; Kretzmer, H.; Assum, G.; Galonska, C.; Siebert, R.; et al. Analysis of short tandem repeat expansions and their methylation state with nanopore sequencing. Nat. Biotechnol. 2019, 37, 1478–1481. [Google Scholar] [CrossRef]
- Ciobanu, C.G.; Nucă, I.; Popescu, R.; Antoci, L.M.; Caba, L.; Ivanov, A.V.; Cojocaru, K.A.; Rusu, C.; Mihai, C.T.; Pânzaru, M.C. Narrative Review: Update on the Molecular Diagnosis of Fragile X Syndrome. Int. J. Mol. Sci. 2023, 24, 9206. [Google Scholar] [CrossRef] [PubMed]
- Jeck, W.R.; Parker, J.; Carson, C.C.; Shields, J.M.; Sambade, M.J.; Peters, E.C.; Burd, C.E.; Thomas, N.E.; Chiang, D.Y.; Liu, W.; et al. Targeted next generation sequencing identifies clinically actionable mutations in patients with melanoma. Pigment. Cell. Melanoma. Res. 2014, 27, 653–663. [Google Scholar] [CrossRef]
- Willems, T.; Zielinski, D.; Yuan, J.; Gordon, A.; Gymrek, M.; Erlich, Y. Genome-wide profiling of heritable and de novo STR variations. Nat. Methods 2017, 14, 590–592. [Google Scholar] [CrossRef]
- Mousavi, N.; Shleizer-Burko, S.; Yanicky, R.; Gymrek, M. Profiling the genome-wide landscape of tandem repeat expansions. Nucleic. Acids. Res. 2019, 47, e90. [Google Scholar] [CrossRef]
- Jakubosky, D.; D’Antonio, M.; Bonder, M.J.; Smail, C.; Donovan, M.K.R.; Young Greenwald, W.W.; Matsui, H.; D’Antonio-Chronowska, A.; Stegle, O.; Smith, E.N.; et al. Properties of structural variants and short tandem repeats associated with gene expression and complex traits. Nat. Commun. 2020, 11, 2927. [Google Scholar] [CrossRef]
- Dashnow, H.; Lek, M.; Phipson, B.; Halman, A.; Sadedin, S.; Lonsdale, A.; Davis, M.; Lamont, P.; Clayton, J.S.; Laing, N.G.; et al. STRetch: Detecting and discovering pathogenic short tandem repeat expansions. Genome Biol. 2018, 19, 121. [Google Scholar]
- Ciaccio, C.; Fontana, L.; Milani, D.; Tabano, S.; Miozzo, M.; Esposito, S. Fragile X syndrome: A review of clinical and molecular diagnoses. Ital. J. Pediatr. 2017, 43, 39. [Google Scholar] [CrossRef]
- Hagerman, R.J.; Berry-Kravis, E.; Hazlett, H.C.; Bailey, D.B., Jr.; Moine, H.; Kooy, R.F.; Tassone, F.; Gantois, I.; Sonenberg, N.; Mandel, J.L.; et al. Fragile X syndrome. Nat. Rev. Dis. Primers 2017, 3, 17065. [Google Scholar] [PubMed]
- Richter, J.D.; Zhao, X. The molecular biology of FMRP: New insights into fragile X syndrome. Nat. Rev. Neurosci. 2021, 22, 209–222. [Google Scholar] [PubMed]
- Leitão, E.; Schröder, C.; Depienne, C. Identification and characterization of repeat expansions in neurological disorders: Methodologies, tools, and strategies. Rev. Neurol. 2024, 180, 383–392. [Google Scholar]
- Zheng, Z.; Li, A.; Holmes, B.B.; Marasa, J.C.; Diamond, M.I. An N-terminal nuclear export signal regulates trafficking and aggregation of Huntingtin (Htt) protein exon 1. J. Biol. Chem. 2013, 288, 6063–6071. [Google Scholar]
- Harding, R.J.; Loppnau, P.; Ackloo, S.; Lemak, A.; Hutchinson, A.; Hunt, B.; Holehouse, A.S.; Ho, J.C.; Fan, L.; Toledo-Sherman, L.; et al. Design and characterization of mutant and wildtype huntingtin proteins produced from a toolkit of scalable eukaryotic expression systems. J. Biol. Chem. 2019, 294, 6986–7001. [Google Scholar]
- Ruz, C.; Alcantud, J.L.; Vives Montero, F.; Duran, R.; Bandres-Ciga, S. Proteotoxicity and Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 5646. [Google Scholar] [CrossRef]
- Yang, H.; Yang, S.; Jing, L.; Huang, L.; Chen, L.; Zhao, X.; Yang, W.; Pan, Y.; Yin, P.; Qin, Z.S.; et al. Truncation of mutant huntingtin in knock-in mice demonstrates exon1 huntingtin is a key pathogenic form. Nat. Commun. 2020, 11, 2582. [Google Scholar]
- Emery, A.E. The muscular dystrophies. Lancet 2002, 359, 687–695. [Google Scholar]
- Mercuri, E.; Bönnemann, C.G.; Muntoni, F. Muscular dystrophies. Lancet 2019, 394, 2025–2038. [Google Scholar] [CrossRef]
- Gymrek, M.; Willems, T.; Guilmatre, A.; Zeng, H.; Markus, B.; Georgiev, S.; Daly, M.J.; Price, A.L.; Pritchard, J.K.; Sharp, A.J.; et al. Abundant contribution of short tandem repeats to gene expression variation in humans. Nat. Genet. 2016, 48, 22–29. [Google Scholar] [CrossRef]
- Oketch, J.W.; Wain, L.V.; Hollox, E.J. A comparison of software for analysis of rare and common short tandem repeat (STR) variation using human genome sequences from clinical and population-based samples. PLoS ONE 2024, 19, e0300545. [Google Scholar] [CrossRef] [PubMed]
- Hannan, A.J. Tandem repeat polymorphisms: Modulators of disease susceptibility and candidates for ‘missing heritability’. Trends Genet. 2010, 26, 59–65. [Google Scholar] [CrossRef]
- Dolzhenko, E.; Deshpande, V.; Schlesinger, F.; Krusche, P.; Petrovski, R.; Chen, S.; Emig-Agius, D.; Gross, A.; Narzisi, G.; Bowman, B.; et al. ExpansionHunter: A sequence-graph-based tool to analyze variation in short tandem repeat regions. Bioinformatics 2019, 35, 4754–4756. [Google Scholar] [CrossRef]
- Dolzhenko, E.; van Vugt, J.; Shaw, R.J.; Bekritsky, M.A.; van Blitterswijk, M.; Narzisi, G.; Ajay, S.S.; Rajan, V.; Lajoie, B.R.; Johnson, N.H.; et al. Detection of long repeat expansions from PCR-free whole-genome sequence data. Genome Res. 2017, 27, 1895–1903. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.O.; Civitello, A.; Hammond, H.; Caskey, C.T. DNA typing and genetic mapping with trimeric and tetrameric tandem repeats. Am. J. Hum. Genet. 1991, 49, 746–756. [Google Scholar] [PubMed]
- Press, M.O.; Carlson, K.D.; Queitsch, C. The overdue promise of short tandem repeat variation for heritability. bioRxiv 2014. [Google Scholar] [CrossRef]
- McMurray, C.T. Mechanisms of trinucleotide repeat instability during human development. Nat. Rev. Genet. 2010, 11, 786–799. [Google Scholar] [CrossRef]
- Henden, L.; Fearnley, L.G.; Grima, N.; McCann, E.P.; Dobson-Stone, C.; Fitzpatrick, L.; Friend, K.; Hobson, L.; Chan Moi Fat, S.; Rowe, D.B.; et al. Short tandem repeat expansions in sporadic amyotrophic lateral sclerosis and frontotemporal dementia. Sci. Adv. 2023, 9, eade2044. [Google Scholar]
- Fang, L.; Liu, Q.; Monteys, A.M.; Gonzalez-Alegre, P.; Davidson, B.L.; Wang, K. DeepRepeat: Direct quantification of short tandem repeats on signal data from nanopore sequencing. Genome Biol. 2022, 23, 108. [Google Scholar]
- Hannan, A.J. Repeat DNA expands our understanding of autism spectrum disorder. Nature 2021, 589, 200–202. [Google Scholar]
- RK, C.Y.; Merico, D.; Bookman, M.; Howe, J.L.; Thiruvahindrapuram, B.; Patel, R.V.; Whitney, J.; Deflaux, N.; Bingham, J.; Wang, Z.; et al. Whole genome sequencing resource identifies 18 new candidate genes for autism spectrum disorder. Nat. Neurosci. 2017, 20, 602–611. [Google Scholar]
- Fischbach, G.D.; Lord, C. The Simons Simplex Collection: A resource for identification of autism genetic risk factors. Neuron 2010, 68, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Auton, A.; Brooks, L.D.; Durbin, R.M.; Garrison, E.P.; Kang, H.M.; Korbel, J.O.; Marchini, J.L.; McCarthy, S.; McVean, G.A.; Abecasis, G.R. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar]
- Annear, D.J.; Vandeweyer, G.; Sanchis-Juan, A.; Raymond, F.L.; Kooy, R.F. Non-Mendelian inheritance patterns and extreme deviation rates of CGG repeats in autism. Genome Res. 2022, 32, 1967–1980. [Google Scholar] [PubMed]
- Halvorsen, M.; Huh, R.; Oskolkov, N.; Wen, J.; Netotea, S.; Giusti-Rodriguez, P.; Karlsson, R.; Bryois, J.; Nystedt, B.; Ameur, A.; et al. Increased burden of ultra-rare structural variants localizing to boundaries of topologically associated domains in schizophrenia. Nat. Commun. 2020, 11, 1842. [Google Scholar]
- Song, J.H.T.; Lowe, C.B.; Kingsley, D.M. Characterization of a Human-Specific Tandem Repeat Associated with Bipolar Disorder and Schizophrenia. Am. J. Hum. Genet. 2018, 103, 421–430. [Google Scholar] [PubMed]
- Šerý, O.; Paclt, I.; Drtílková, I.; Theiner, P.; Kopečková, M.; Zvolský, P.; Balcar, V.J. A 40-bp VNTR polymorphism in the 3′-untranslated region of DAT1/SLC6A3 is associated with ADHD but not with alcoholism. Behav. Brain Funct. 2015, 11, 21. [Google Scholar]
- Cichon, S.; Craddock, N.; Daly, M.; Faraone, S.V.; Gejman, P.V.; Kelsoe, J.; Lehner, T.; Levinson, D.F.; Moran, A.; Sklar, P.; et al. Genomewide association studies: History, rationale, and prospects for psychiatric disorders. Am. J. Psychiatry 2009, 166, 540–556. [Google Scholar]
- Wen, J.; Trost, B.; Engchuan, W.; Halvorsen, M.; Pallotto, L.M.; Mitina, A.; Ancalade, N.; Farrell, M.; Backstrom, I.; Guo, K.; et al. Rare tandem repeat expansions associate with genes involved in synaptic and neuronal signaling functions in schizophrenia. Mol. Psychiatry 2023, 28, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Pearson, C.E.; Nichol Edamura, K.; Cleary, J.D. Repeat instability: Mechanisms of dynamic mutations. Nat. Rev. Genet. 2005, 6, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Burgunder, J.M. Mechanisms underlying phenotypic variation in neurogenetic disorders. Nat. Rev. Neurol. 2023, 19, 363–370. [Google Scholar] [CrossRef]
- Paulson, H.; Shakkottai, V. Spinocerebellar Ataxia Type 3. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Cleary, J.D.; Subramony, S.H.; Ranum, L.P.W. Spinocerebellar Ataxia Type 8. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Bram, E.; Javanmardi, K.; Nicholson, K.; Culp, K.; Thibert, J.R.; Kemppainen, J.; Le, V.; Schlageter, A.; Hadd, A.; Latham, G.J. Comprehensive genotyping of the C9orf72 hexanucleotide repeat region in 2095 ALS samples from the NINDS collection using a two-mode, long-read PCR assay. Amyotroph. Lateral. Scler. Frontotemporal. Degener. 2019, 20, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Gall-Duncan, T.; Sato, N.; Yuen, R.K.C.; Pearson, C.E. Advancing genomic technologies and clinical awareness accelerates discovery of disease-associated tandem repeat sequences. Genome Res. 2022, 32, 1–27. [Google Scholar] [CrossRef]
- Lanni, S.; Pearson, C.E. Molecular genetics of congenital myotonic dystrophy. Neurobiol. Dis. 2019, 132, 104533. [Google Scholar]
- Morales, F.; Vásquez, M.; Corrales, E.; Vindas-Smith, R.; Santamaría-Ulloa, C.; Zhang, B.; Sirito, M.; Estecio, M.R.; Krahe, R.; Monckton, D.G. Longitudinal increases in somatic mosaicism of the expanded CTG repeat in myotonic dystrophy type 1 are associated with variation in age-at-onset. Hum. Mol. Genet. 2020, 29, 2496–2507. [Google Scholar] [CrossRef]
- Ishiura, H.; Doi, K.; Mitsui, J.; Yoshimura, J.; Matsukawa, M.K.; Fujiyama, A.; Toyoshima, Y.; Kakita, A.; Takahashi, H.; Suzuki, Y.; et al. Expansions of intronic TTTCA and TTTTA repeats in benign adult familial myoclonic epilepsy. Nat. Genet. 2018, 50, 581–590. [Google Scholar] [CrossRef]
- Flavell, J.; Franklin, C.; Nestor, P.J. A Systematic Review of Fragile X-Associated Neuropsychiatric Disorders. J. Neuropsychiatry Clin. Neurosci. 2023, 35, 110–120. [Google Scholar] [CrossRef]
- Nolin, S.L.; Sah, S.; Glicksman, A.; Sherman, S.L.; Allen, E.; Berry-Kravis, E.; Tassone, F.; Yrigollen, C.; Cronister, A.; Jodah, M.; et al. Fragile X AGG analysis provides new risk predictions for 45-69 repeat alleles. Am. J. Med. Genet. A 2013, 161, 771–778. [Google Scholar] [CrossRef]
- Alonso, I.; Jardim, L.B.; Artigalas, O.; Saraiva-Pereira, M.L.; Matsuura, T.; Ashizawa, T.; Sequeiros, J.; Silveira, I. Reduced penetrance of intermediate size alleles in spinocerebellar ataxia type 10. Neurology 2006, 66, 1602–1604. [Google Scholar] [CrossRef] [PubMed]
- Yum, K.; Wang, E.T.; Kalsotra, A. Myotonic dystrophy: Disease repeat range, penetrance, age of onset, and relationship between repeat size and phenotypes. Curr. Opin. Genet. Dev. 2017, 44, 30–37. [Google Scholar] [CrossRef]
- Musova, Z.; Mazanec, R.; Krepelova, A.; Ehler, E.; Vales, J.; Jaklova, R.; Prochazka, T.; Koukal, P.; Marikova, T.; Kraus, J.; et al. Highly unstable sequence interruptions of the CTG repeat in the myotonic dystrophy gene. Am. J. Med. Genet. A. 2009, 149, 1365–1374. [Google Scholar] [CrossRef]
- Hunter, J.E.; Berry-Kravis, E.; Hipp, H.; Todd, P.K. FMR1 Disorders. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Kandola, T.; Venkatesan, S.; Zhang, J.; Lerbakken, B.T.; Von Schulze, A.; Blanck, J.F.; Wu, J.; Unruh, J.R.; Berry, P.; Lange, J.J.; et al. Pathologic polyglutamine aggregation begins with a self-poisoning polymer crystal. eLife 2023, 12, RP86939. [Google Scholar] [CrossRef]
- Tassone, F.; Iong, K.P.; Tong, T.H.; Lo, J.; Gane, L.W.; Berry-Kravis, E.; Nguyen, D.; Mu, L.Y.; Laffin, J.; Bailey, D.B.; et al. FMR1 CGG allele size and prevalence ascertained through newborn screening in the United States. Genome Med. 2012, 4, 100. [Google Scholar] [CrossRef]
- Peprah, E. Fragile X syndrome: The FMR1 CGG repeat distribution among world populations. Ann. Hum. Genet. 2012, 76, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Bassell, G.J.; Warren, S.T. Fragile X syndrome: Loss of local mRNA regulation alters synaptic development and function. Neuron 2008, 60, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.; Zamiri, B.; Stanley, S.Y.R.; Macgregor, R.B., Jr.; Pearson, C.E. The disease-associated r(GGGGCC)n repeat from the C9orf72 gene forms tract length-dependent uni- and multimolecular RNA G-quadruplex structures. J. Biol. Chem. 2013, 288, 9860–9866. [Google Scholar] [CrossRef]
- Haeusler, A.R.; Donnelly, C.J.; Periz, G.; Simko, E.A.; Shaw, P.G.; Kim, M.S.; Maragakis, N.J.; Troncoso, J.C.; Pandey, A.; Sattler, R.; et al. C9orf72 nucleotide repeat structures initiate molecular cascades of disease. Nature 2014, 507, 195–200. [Google Scholar] [CrossRef]
- Rizzo, A.; Salvati, E.; Porru, M.; D’Angelo, C.; Stevens, M.F.; D’Incalci, M.; Leonetti, C.; Gilson, E.; Zupi, G.; Biroccio, A. Stabilization of quadruplex DNA perturbs telomere replication leading to the activation of an ATR-dependent ATM signaling pathway. Nucleic Acids Res. 2009, 37, 5353–5364. [Google Scholar] [CrossRef]
- Sarkies, P.; Reams, C.; Simpson, L.J.; Sale, J.E. Epigenetic instability due to defective replication of structured DNA. Mol. Cell. 2010, 40, 703–713. [Google Scholar]
- Fratta, P.; Mizielinska, S.; Nicoll, A.J.; Zloh, M.; Fisher, E.M.; Parkinson, G.; Isaacs, A.M. C9orf72 hexanucleotide repeat associated with amyotrophic lateral sclerosis and frontotemporal dementia forms RNA G-quadruplexes. Sci. Rep. 2012, 2, 1016. [Google Scholar] [CrossRef] [PubMed]
- Nolin, S.L.; Glicksman, A.; Tortora, N.; Allen, E.; Macpherson, J.; Mila, M.; Vianna-Morgante, A.M.; Sherman, S.L.; Dobkin, C.; Latham, G.J.; et al. Expansions and contractions of the FMR1 CGG repeat in 5,508 transmissions of normal, intermediate, and premutation alleles. Am. J. Med. Genet. A 2019, 179, 1148–1156. [Google Scholar]
- Nolin, S.L.; Brown, W.T.; Glicksman, A.; Houck, G.E., Jr.; Gargano, A.D.; Sullivan, A.; Biancalana, V.; Bröndum-Nielsen, K.; Hjalgrim, H.; Holinski-Feder, E.; et al. Expansion of the fragile X CGG repeat in females with premutation or intermediate alleles. Am. J. Hum. Genet. 2003, 72, 454–464. [Google Scholar]
- Nolin, S.L.; Glicksman, A.; Ersalesi, N.; Dobkin, C.; Brown, W.T.; Cao, R.; Blatt, E.; Sah, S.; Latham, G.J.; Hadd, A.G. Fragile X full mutation expansions are inhibited by one or more AGG interruptions in premutation carriers. Genet. Med. 2015, 17, 358–364. [Google Scholar] [PubMed]
- Annear, D.J.; Kooy, R.F. Unravelling the link between neurodevelopmental disorders and short tandem CGG-repeat expansions. Emerg. Top Life Sci. 2023, 7, 265–275. [Google Scholar]
- Acton, R.T.; Rivers, C.A.; Watson, B.; Oh, S.J. DMPK-associated myotonic dystrophy and CTG repeats in Alabama African Americans. Clin. Genet. 2007, 72, 448–453. [Google Scholar] [PubMed]
- Cossée, M.; Schmitt, M.; Campuzano, V.; Reutenauer, L.; Moutou, C.; Mandel, J.L.; Koenig, M. Evolution of the Friedreich’s ataxia trinucleotide repeat expansion: Founder effect and premutations. Proc. Natl. Acad. Sci. USA 1997, 94, 7452–7457. [Google Scholar]
- Vijayaraghavan, P.; Batalov, S.; Ding, Y.; Sanford, E.; Kingsmore, S.F.; Dimmock, D.; Hobbs, C.; Bainbridge, M. The Genomic landscape of short tandem repeats across multiple ancestries. PLoS ONE 2023, 18, e0279430. [Google Scholar]
- Pieretti, M.; Zhang, F.P.; Fu, Y.H.; Warren, S.T.; Oostra, B.A.; Caskey, C.T.; Nelson, D.L. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell 1991, 66, 817–822. [Google Scholar] [CrossRef]
- Sutcliffe, J.S.; Nelson, D.L.; Zhang, F.; Pieretti, M.; Caskey, C.T.; Saxe, D.; Warren, S.T. DNA methylation represses FMR-1 transcription in fragile X syndrome. Hum. Mol. Genet. 1992, 1, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Ranum, L.P.; Cooper, T.A. RNA-mediated neuromuscular disorders. Annu. Rev. Neurosci. 2006, 29, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Ash, P.E.; Bieniek, K.F.; Gendron, T.F.; Caulfield, T.; Lin, W.L.; Dejesus-Hernandez, M.; van Blitterswijk, M.M.; Jansen-West, K.; Paul, J.W., 3rd; Rademakers, R.; et al. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron 2013, 77, 639–646. [Google Scholar] [CrossRef]
- Miller, J.W.; Urbinati, C.R.; Teng-Umnuay, P.; Stenberg, M.G.; Byrne, B.J.; Thornton, C.A.; Swanson, M.S. Recruitment of human muscleblind proteins to (CUG)(n) expansions associated with myotonic dystrophy. Embo. J. 2000, 19, 4439–4448. [Google Scholar] [CrossRef]
- Warf, M.B.; Berglund, J.A. MBNL binds similar RNA structures in the CUG repeats of myotonic dystrophy and its pre-mRNA substrate cardiac troponin T. RNA 2007, 13, 2238–2251. [Google Scholar] [CrossRef] [PubMed]
- García-López, A.; Llamusí, B.; Orzáez, M.; Pérez-Payá, E.; Artero, R.D. In vivo discovery of a peptide that prevents CUG-RNA hairpin formation and reverses RNA toxicity in myotonic dystrophy models. Proc. Natl. Acad. Sci. USA 2011, 108, 11866–11871. [Google Scholar] [CrossRef]
- Ross, C.A.; Poirier, M.A. Protein aggregation and neurodegenerative disease. Nat. Med. 2004, 10, S10–S17. [Google Scholar]
- Zu, T.; Liu, Y.; Bañez-Coronel, M.; Reid, T.; Pletnikova, O.; Lewis, J.; Miller, T.M.; Harms, M.B.; Falchook, A.E.; Subramony, S.H.; et al. RAN proteins and RNA foci from antisense transcripts in C9ORF72 ALS and frontotemporal dementia. Proc. Natl. Acad. Sci. USA 2013, 110, E4968–E4977. [Google Scholar] [CrossRef]
- Gendron, T.F.; Bieniek, K.F.; Zhang, Y.J.; Jansen-West, K.; Ash, P.E.; Caulfield, T.; Daughrity, L.; Dunmore, J.H.; Castanedes-Casey, M.; Chew, J.; et al. Antisense transcripts of the expanded C9ORF72 hexanucleotide repeat form nuclear RNA foci and undergo repeat-associated non-ATG translation in c9FTD/ALS. Acta Neuropathol. 2013, 126, 829–844. [Google Scholar]
- Mori, K.; Weng, S.M.; Arzberger, T.; May, S.; Rentzsch, K.; Kremmer, E.; Schmid, B.; Kretzschmar, H.A.; Cruts, M.; Van Broeckhoven, C.; et al. The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science 2013, 339, 1335–1338. [Google Scholar] [CrossRef]
- DeJesus-Hernandez, M.; Mackenzie, I.R.; Boeve, B.F.; Boxer, A.L.; Baker, M.; Rutherford, N.J.; Nicholson, A.M.; Finch, N.A.; Flynn, H.; Adamson, J.; et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 2011, 72, 245–256. [Google Scholar] [CrossRef]
- Renton, A.E.; Majounie, E.; Waite, A.; Simón-Sánchez, J.; Rollinson, S.; Gibbs, J.R.; Schymick, J.C.; Laaksovirta, H.; van Swieten, J.C.; Myllykangas, L.; et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 2011, 72, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Gitler, A.D.; Tsuiji, H. There has been an awakening: Emerging mechanisms of C9orf72 mutations in FTD/ALS. Brain Res. 2016, 1647, 19–29. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, X.; He, J.; Wang, S.; Shen, X.; Liu, Q.; Wang, S. Advances in the Structure of GGGGCC Repeat RNA Sequence and Its Interaction with Small Molecules and Protein Partners. Molecules 2023, 28, 5801. [Google Scholar] [CrossRef] [PubMed]
- Balendra, R.; Isaacs, A.M. C9orf72-mediated ALS and FTD: Multiple pathways to disease. Nat. Rev. Neurol. 2018, 14, 544–558. [Google Scholar] [CrossRef]
- Shi, Y.; Lin, S.; Staats, K.A.; Li, Y.; Chang, W.H.; Hung, S.T.; Hendricks, E.; Linares, G.R.; Wang, Y.; Son, E.Y.; et al. Haploinsufficiency leads to neurodegeneration in C9ORF72 ALS/FTD human induced motor neurons. Nat. Med. 2018, 24, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Liang, C.; Chang, Q.; Zhang, W.; Yang, M.; Chen, J.F. C9orf72 deficiency promotes motor deficits of a C9ALS/FTD mouse model in a dose-dependent manner. Acta Neuropathol. Commun. 2019, 7, 32. [Google Scholar] [CrossRef]
- Neueder, A.; Landles, C.; Ghosh, R.; Howland, D.; Myers, R.H.; Faull, R.L.M.; Tabrizi, S.J.; Bates, G.P. The pathogenic exon 1 HTT protein is produced by incomplete splicing in Huntington’s disease patients. Sci. Rep. 2017, 7, 1307. [Google Scholar] [CrossRef]
- Lieberman, A.P.; Shakkottai, V.G.; Albin, R.L. Polyglutamine Repeats in Neurodegenerative Diseases. Annu. Rev. Pathol. 2019, 14, 1–27. [Google Scholar] [CrossRef]
- Zhang, N.; Ashizawa, T. RNA toxicity and foci formation in microsatellite expansion diseases. Curr. Opin. Genet. Dev. 2017, 44, 17–29. [Google Scholar] [CrossRef]
- Coffee, B.; Zhang, F.; Ceman, S.; Warren, S.T.; Reines, D. Histone modifications depict an aberrantly heterochromatinized FMR1 gene in fragile x syndrome. Am. J. Hum. Genet. 2002, 71, 923–932. [Google Scholar]
- Michaelson, J.J. Genetic Approaches to Understanding Psychiatric Disease. Neurotherapeutics 2017, 14, 564–581. [Google Scholar]
- Puccio, H.; Simon, D.; Cossée, M.; Criqui-Filipe, P.; Tiziano, F.; Melki, J.; Hindelang, C.; Matyas, R.; Rustin, P.; Koenig, M. Mouse models for Friedreich ataxia exhibit cardiomyopathy, sensory nerve defect and Fe-S enzyme deficiency followed by intramitochondrial iron deposits. Nat. Genet. 2001, 27, 181–186. [Google Scholar]
- Yang, N.; MacArthur, D.G.; Gulbin, J.P.; Hahn, A.G.; Beggs, A.H.; Easteal, S.; North, K. ACTN3 genotype is associated with human elite athletic performance. Am. J. Hum. Genet. 2003, 73, 627–631. [Google Scholar] [CrossRef]
- Silver, L.M. Mouse Genetics Concepts and Applications; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Ellenbroek, B.; Youn, J. Rodent models in neuroscience research: Is it a rat race? Dis. Model. Mech. 2016, 9, 1079–1087. [Google Scholar] [CrossRef]
- Nestler, E.J.; Hyman, S.E. Animal models of neuropsychiatric disorders. Nat. Neurosci. 2010, 13, 1161–1169. [Google Scholar] [PubMed]
- Kalueff, A.V.; Wheaton, M.; Murphy, D.L. What’s wrong with my mouse model? Advances and strategies in animal modeling of anxiety and depression. Behav. Brain. Res. 2007, 179, 1–18. [Google Scholar] [PubMed]
- Mulholland, M.M.; Navabpour, S.V.; Mareno, M.C.; Schapiro, S.J.; Young, L.J.; Hopkins, W.D. AVPR1A variation is linked to gray matter covariation in the social brain network of chimpanzees. Genes. Brain. Behav. 2020, 19, e12631. [Google Scholar] [PubMed]
- Bennett, B.T.; Abee, C.R.; Henrickson, R. Nonhuman Primates in Biomedical Research: Diseases; Academic Press: New York, NY, USA, 1998. [Google Scholar]
- Nelson, E.E.; Winslow, J.T. Non-human primates: Model animals for developmental psychopathology. Neuropsychopharmacology 2009, 34, 90–105. [Google Scholar]
- Capitanio, J.P.; Emborg, M.E. Contributions of non-human primates to neuroscience research. Lancet 2008, 371, 1126–1135. [Google Scholar]
- Liu, C.X.; Li, C.Y.; Hu, C.C.; Wang, Y.; Lin, J.; Jiang, Y.H.; Li, Q.; Xu, X. CRISPR/Cas9-induced shank3b mutant zebrafish display autism-like behaviors. Mol. Autism. 2018, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an emerging model for studying complex brain disorders. Trends. Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef]
- Lieschke, G.J.; Currie, P.D. Animal models of human disease: Zebrafish swim into view. Nat. Rev. Genet. 2007, 8, 353–367. [Google Scholar] [CrossRef] [PubMed]
| Gene | Diseases | OMIM ID | Repeat Motif | Mode of Inheritance | Location on Gene | Mechanism | Age of Onset | Repeat Arrangement in GRCh38 |
|---|---|---|---|---|---|---|---|---|
| HTT | HD | 143100 | CAG | AD | Exon 1 | GoF (polyQ) GoF (RNA) RAN translation | Pediatric/Adult (Mean: 35–44) | (CAG)19 CAA CAG CCG CCA (CCG)7 |
| FMR1 | FXTAS/ FXPOI | 300623 | CGG | XLD | 5′ UTR | GoF (RNA) RAN translation | Adult (FXTAS: 60–65. FXPOI < 40)19 | (CGG)10 AGG (CGG)9 |
| FMR1 | FXAND | - | CGG | XLD | 5′ UTR | GoF (RNA) RAN translation | Pediatric/Adult | (CGG)10 AGG (CGG)9 |
| FMR1 | FXS | 300624 | CGG | XLD | 5′ UTR | LoF | Pediatric | (CGG)10 AGG (CGG)9 |
| C9ORF72 | FTD and/ or ALS1 | 105550 | GGGGCC | AD | Intron 1 | LoF GoF (RNA) RAN translation | Adult (Typical: 50–64) | (GGGGCC)3 |
| ATXN1 | SCA1 | 164400 | CAG | AD | Exon 8 | GoF (polyQ) LoF | Adult (Typical: 30–40) | (CAG)12 CAT CAG CAT (CAG)14 |
| ATXN2 | SCA2/ ALS13 | 183090 | CAG | AD | Exon 1 | GoF (polyQ) GoF (RNA) RAN translation | Adult (Typical: 40) | (CAG)13 CAA (CAG)9 |
| ATXN3 | SCA3/ MJD | 109150 | CAG | AD | Exon 10 | GoF (polyQ) GoF (RNA) RAN translation | Pediatric/Adult (Typical: 20–50) | (CAG)2 CAA AAG CAG CAA (CAG)8 |
| ATXN7 | SCA7 | 164500 | CAG | AD | Exon 3 | GoF (polyQ) GoF (RNA) | Pediatric/Adult (Typical: >40) | (CAG)10 |
| ATXN8OS/ ATXN8 | SCA8 | 608768 | CTG/CAG | AD | 3′ UTR exon 5 | GoF (polyQ) GoF (RNA) RAN translation | Pediatric/Adult (Typical: 30–50) | (CTG/CAG)15 |
| ATXN10 | SCA10 | 603516 | ATTCT | AD | Intron 9 | GoF (RNA) | Pediatric/Adult (Range: 12–48) | (ATTCT)14 |
| TCF4 | FECD3 | 613267 | CTG | AD | Intron 3 | GoF (RNA) RAN translation | Adult | (CTG)24 |
| PRNP | CJD | 123400 | CCTCATGGTGGTGGCTGGGGGCAG | AD | Exon 2 | LoF? | Adult | (CCTCATGGTGGTGGCTGGGGGCAG)2 CCCCATGGTGGTGGCTGGGGGCAG CCTCATGGTGGTGGCTGGGGTCAA |
| ARX_1 | DEE1/ XLID29 | 308350/300419 | NGC | XLR | Exon 2 | LoF | Pediatric | (NGC)16 |
| ARX_2 | DEE1/ PRTS/ XLID29 | 308350/309510/300419 | NGC | XLR | Exon 2 | LoF | Pediatric | (NGC)12 |
| TBP | SCA17 | 607136 | CAG | AD | Exon 3 | LoF GoF (polyQ) | Pediatric/Adult (Mean: 34.6) | (CAG)3 CAA CAA CAA (CAG)8 CAA CAG CAA (CAG)19 |
| ZIC2 | HPE5 | 609637 | GCN | AD | Exon 3 | LoF | Pediatric | (GCN)15 |
| ZIC3 | VACTERLX | 314390 | GCC | XLR | Exon 1 | Unknown | Pediatric | (GCC)8 GCT GCC |
| NOTCH2NLC | NIID/ETM6/PD | 603472/618866/618600 | GGC | AD | 5′ UTR | GoF (RNA) GoF (polyG) | Adult (Mean: 60.5) | (GGC)9 GGA GGA(GGC)2 |
| DMPK | DM1 | 160900 | CTG | AD | 3′ UTR | GoF (RNA) RAN translation | Pediatric/Adult (Range: birth–70) | (CTG)20 |
| Disease | Associated Genes/Locations | Functional Impact | Genetic Characteristics | Key Findings | Year of Study |
|---|---|---|---|---|---|
| ASD | DMPK, FXN, FGF14, CACNB1, genes related to cortical development | Abnormalities in neurodevelopment, cardiovascular/muscular system gene functions; disruption of regulatory regions (enhancer/promoter) | Maternal mutations tend to be more substantial; higher frequency of new TR mutations (compared to unaffected siblings) | Rare tandem repeats (TREs) contribute to 2.6% of ASD risk Mutations are enriched near exons/splice sites and regulatory regions of fetal brain development Carriers of STR expansions exhibit more severe phenotypes and lower adaptive capacity | 2020, 2021, 2023 |
| Schizophrenia | Postsynaptic genes, brain-expressed genes (e.g., intronic region of CACNA1C) | Abnormal synaptic signaling, neuronal excitability, and calcium signaling pathways | Higher burden of rare TREs in patients; reduced enhancer activity of risk alleles | TREs are enriched in gene regions (e.g., postsynaptic genes) Length of intronic STR in CACNA1C influences enhancer activity, correlating with disease risk | 2021, 2023 |
| Bipolar Disorder | Non-coding region of the third intron of CACNA1C gene (30 bp repeat unit) | Variations in enhancer activity lead to abnormalities in neuronal excitability and synaptic plasticity | Significantly reduced enhancer activity of risk alleles | Long STR alleles are associated with increased risk of BD/SCZ Repeat lengths can reach thousands of base pairs, impacting calcium signaling pathways | 2018 |
| Attention Deficit Hyperactivity Disorder (ADHD) | VNTR of the DAT1 gene (10-repeat allele) | Altered dopamine reuptake efficiency | Variations in dopamine transporter-encoding gene | The 10-repeat allele is associated with an increased risk of ADHD | 2015 |
| Depression | Tandem repeats in the BDNF gene | Abnormal expression of neurotrophic factors | Mechanism not fully understood | Specific STR variations may contribute to pathogenesis by influencing BDNF expression | 2009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Xia, K. Aberrant Short Tandem Repeats: Pathogenicity, Mechanisms, Detection, and Roles in Neuropsychiatric Disorders. Genes 2025, 16, 406. https://doi.org/10.3390/genes16040406
Liu Y, Xia K. Aberrant Short Tandem Repeats: Pathogenicity, Mechanisms, Detection, and Roles in Neuropsychiatric Disorders. Genes. 2025; 16(4):406. https://doi.org/10.3390/genes16040406
Chicago/Turabian StyleLiu, Yuzhong, and Kun Xia. 2025. "Aberrant Short Tandem Repeats: Pathogenicity, Mechanisms, Detection, and Roles in Neuropsychiatric Disorders" Genes 16, no. 4: 406. https://doi.org/10.3390/genes16040406
APA StyleLiu, Y., & Xia, K. (2025). Aberrant Short Tandem Repeats: Pathogenicity, Mechanisms, Detection, and Roles in Neuropsychiatric Disorders. Genes, 16(4), 406. https://doi.org/10.3390/genes16040406






