A Genome-Wide Association Study of First-Episode Psychosis: A Genetic Exploration in an Italian Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. DNA Extraction and Genotyping
2.3. Genomic Analysis
2.4. In Silico Functional Analyses
3. Results
3.1. The PICOS Participants
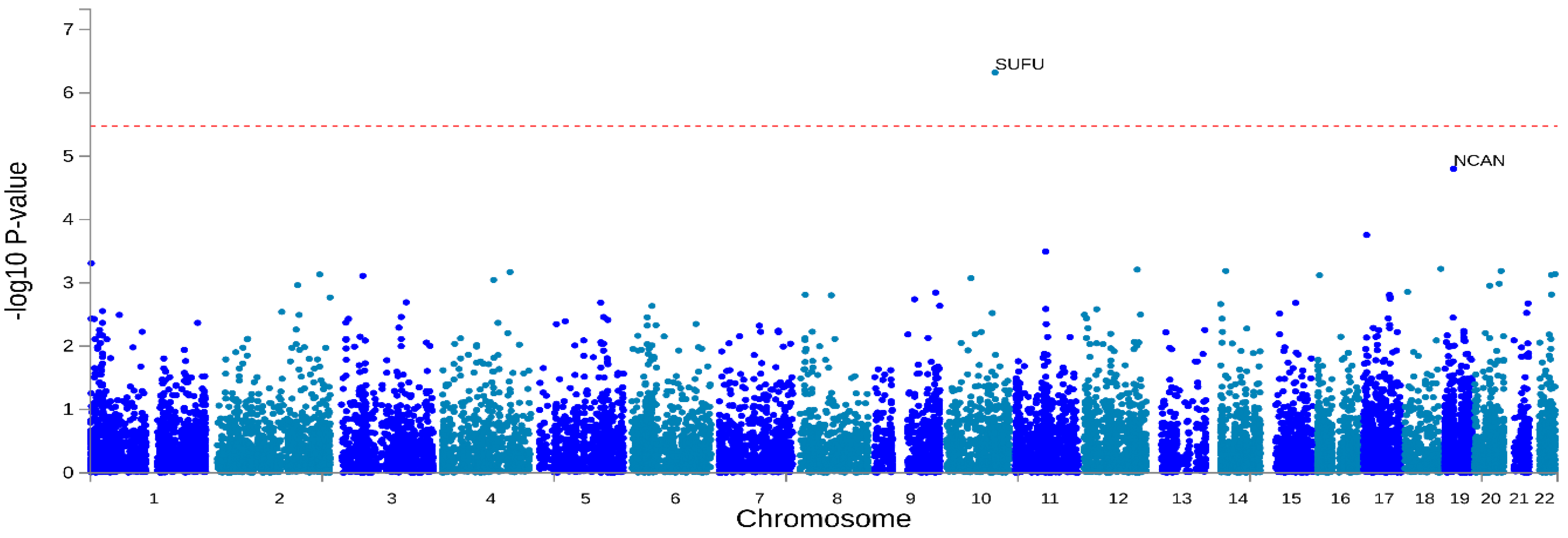
3.2. Genome-Wide Association Analysis of Non-Affective Psychosis
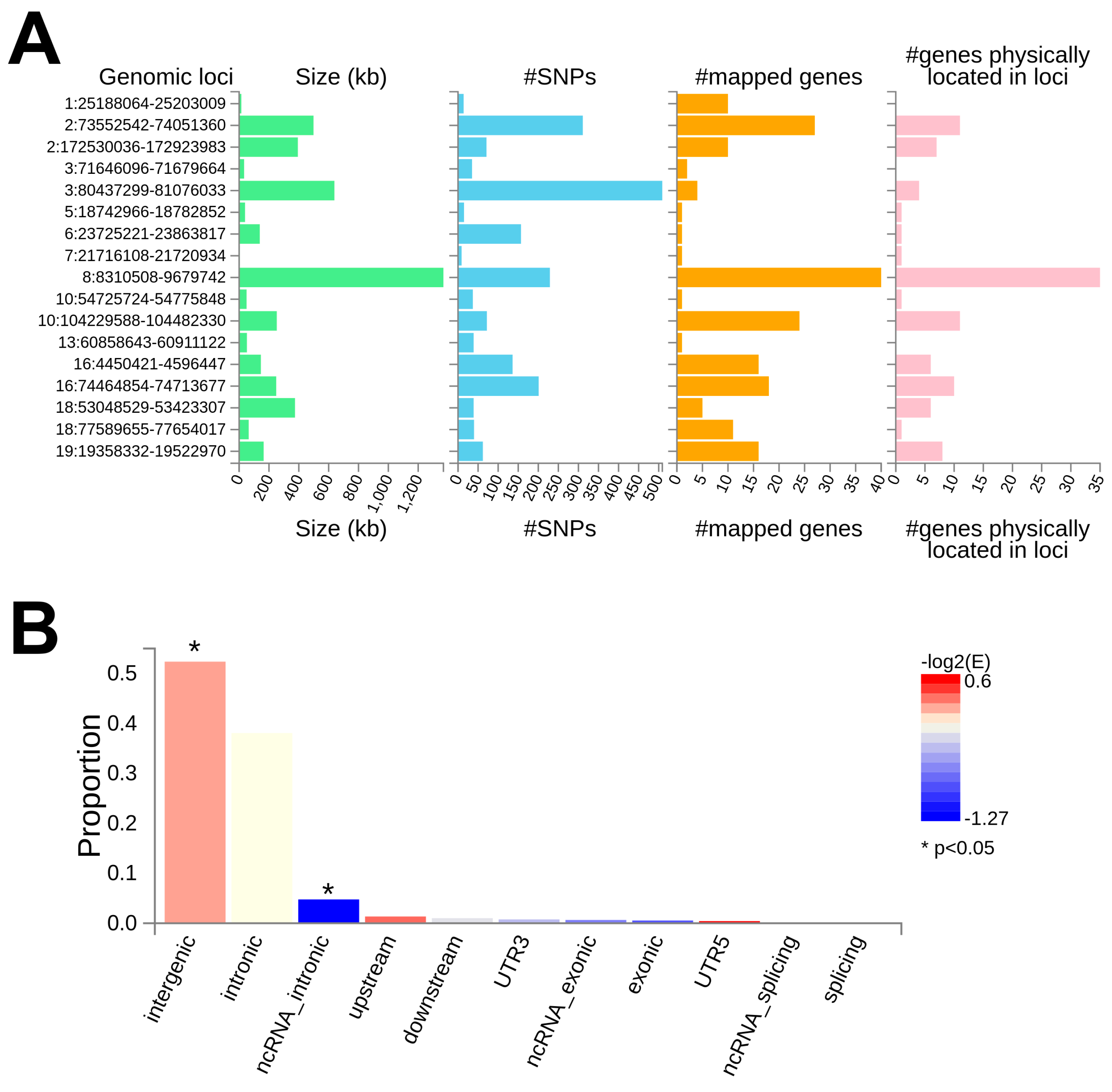
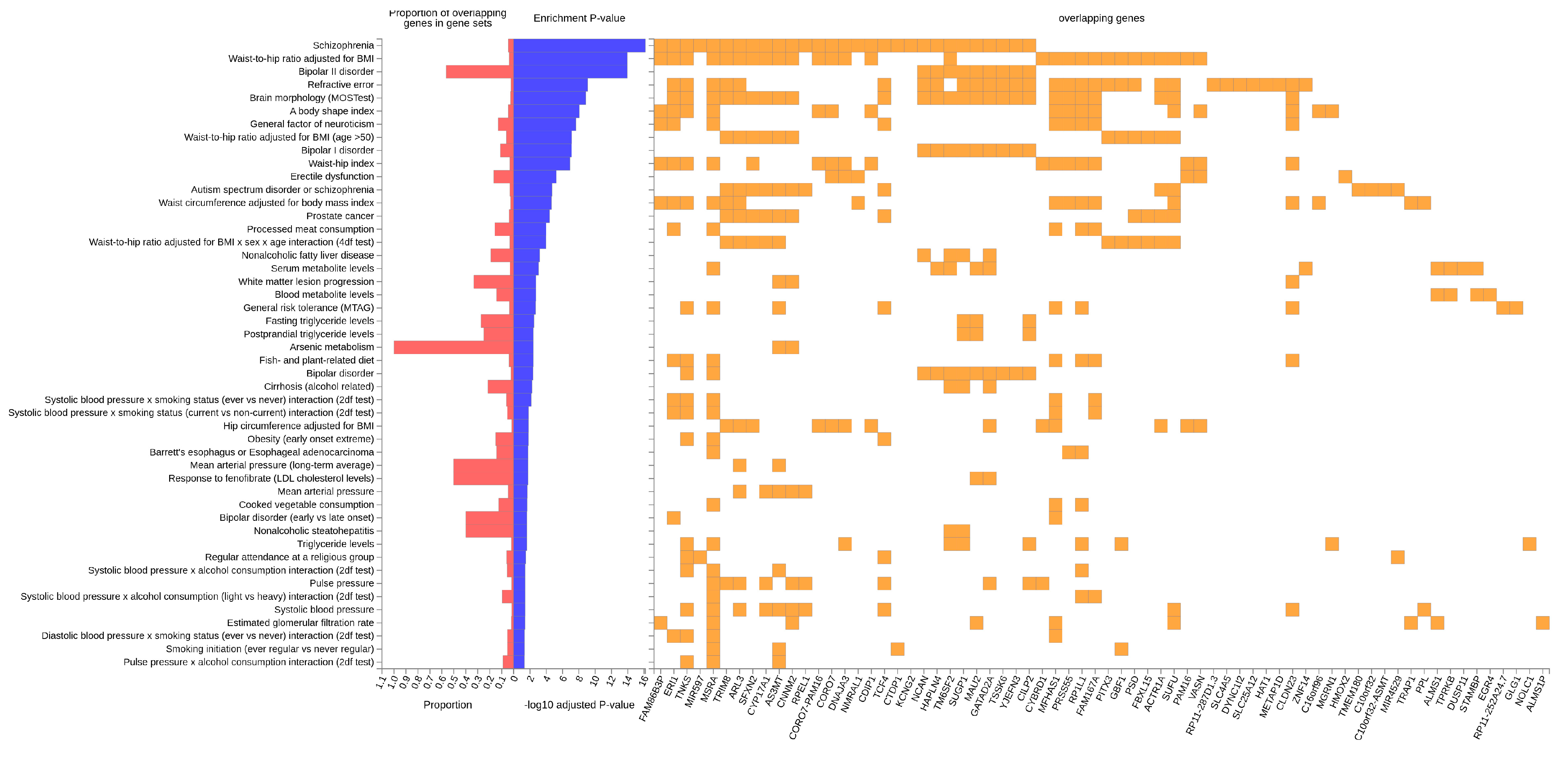
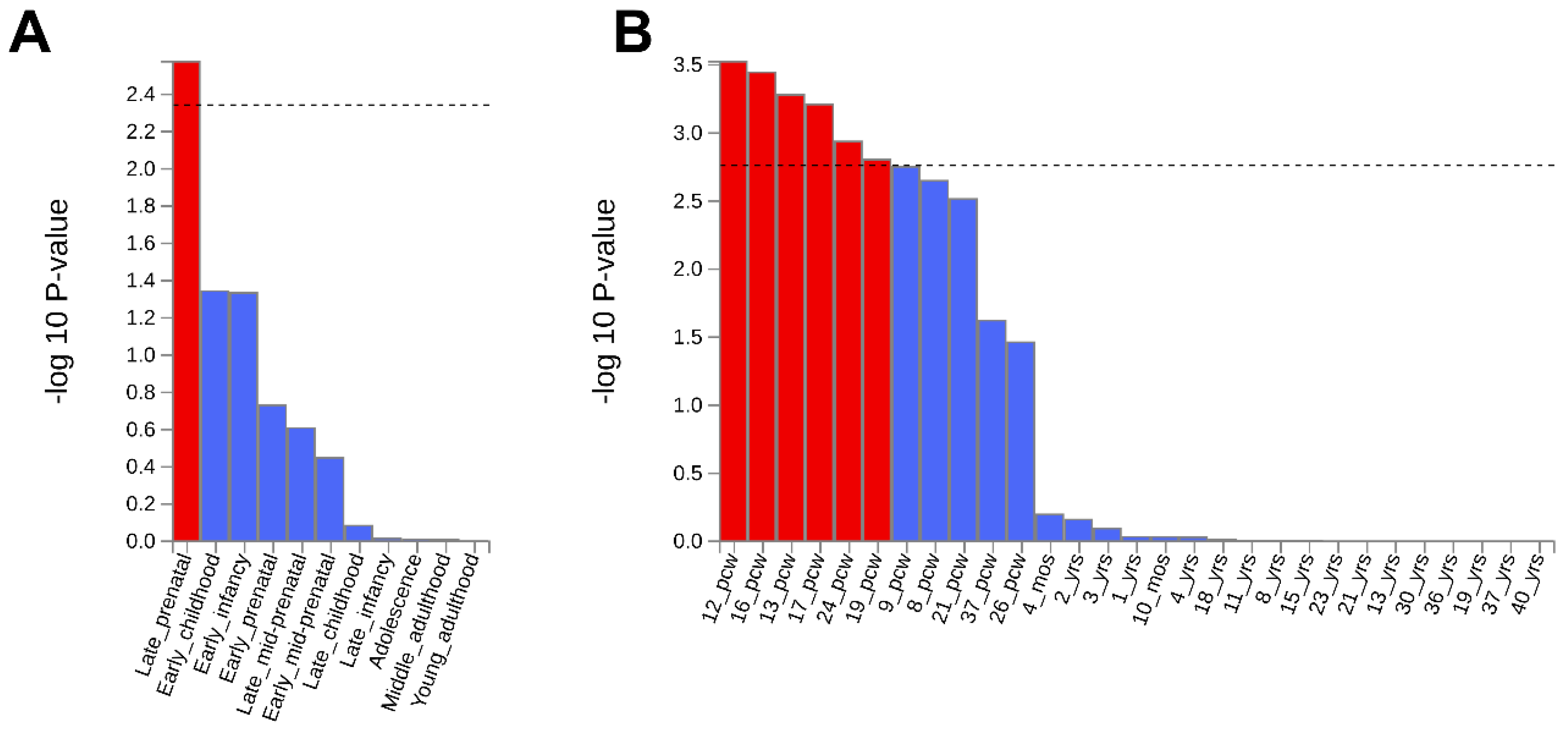
3.3. In Silico Functional Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013; ISBN 0-89042-554-X. [Google Scholar]
- Lasalvia, A.; Bonetto, C.; Tosato, S.; Zanatta, G.; Cristofalo, D.; Salazzari, D.; Lazzarotto, L.; Bertani, M.; Bissoli, S.; Santi, K.D.; et al. First-Contact Incidence of Psychosis in North-Eastern Italy: Influence of Age, Gender, Immigration and Socioeconomic Deprivation. Br. J. Psychiatry 2014, 205, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.J.; Sawa, A.; Mortensen, P.B. Schizophrenia. Lancet 2016, 388, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, D.R. The Pathogenesis of Schizophrenia: A Neurodevelopmental Theory. In The Neurology of Schizophrenia; Weinberger, D.R., Nasrallah, H., Eds.; Elsevier: Amsterdam, The Netherlands, 1986; pp. 397–406. [Google Scholar]
- Murray, R.M.; Bhavsar, V.; Tripoli, G.; Howes, O. 30 Years on: How the Neurodevelopmental Hypothesis of Schizophrenia Morphed Into the Developmental Risk Factor Model of Psychosis. Schizophr. Bull. 2017, 43, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Tosato, S.; Bonetto, C.; Vassos, E.; Lasalvia, A.; De Santi, K.; Gelmetti, M.; Cristofalo, D.; Richards, A.; Ruggeri, M.; on behalf of the PICOS-Veneto Group. Obstetric Complications and Polygenic Risk Score: Which Role in Predicting a Severe Short-Term Outcome in Psychosis? Genes 2021, 12, 1895. [Google Scholar] [CrossRef]
- Ursini, G.; Punzi, G.; Chen, Q.; Marenco, S.; Robinson, J.F.; Porcelli, A.; Hamilton, E.G.; Mitjans, M.; Maddalena, G.; Begemann, M.; et al. Convergence of Placenta Biology and Genetic Risk for Schizophrenia. Nat. Med. 2018, 24, 792–801. [Google Scholar] [CrossRef]
- Vassos, E.; Kou, J.; Tosato, S.; Maxwell, J.; Dennison, C.A.; Legge, S.E.; Walters, J.T.R.; Owen, M.J.; O’Donovan, M.C.; Breen, G.; et al. Lack of Support for the Genes by Early Environment Interaction Hypothesis in the Pathogenesis of Schizophrenia. Schizophr. Bull. 2022, 48, 20–26. [Google Scholar] [CrossRef]
- Birnbaum, R.; Jaffe, A.E.; Hyde, T.M.; Kleinman, J.E.; Weinberger, D.R. Prenatal Expression Patterns of Genes Associated with Neuropsychiatric Disorders. Am. J. Psychiatry 2014, 171, 758–767. [Google Scholar] [CrossRef]
- Jaffe, A.E.; Shin, J.; Collado-Torres, L.; Leek, J.T.; Tao, R.; Li, C.; Gao, Y.; Jia, Y.; Maher, B.J.; Hyde, T.M.; et al. Developmental Regulation of Human Cortex Transcription and Its Clinical Relevance at Single Base Resolution. Nat. Neurosci. 2015, 18, 154–161. [Google Scholar] [CrossRef]
- Hannon, E.; Spiers, H.; Viana, J.; Pidsley, R.; Burrage, J.; Murphy, T.M.; Troakes, C.; Turecki, G.; O’Donovan, M.C.; Schalkwyk, L.C.; et al. Methylation QTLs in the Developing Brain and Their Enrichment in Schizophrenia Risk Loci. Nat. Neurosci. 2016, 19, 48–54. [Google Scholar] [CrossRef]
- Jaffe, A.E.; Gao, Y.; Deep-Soboslay, A.; Tao, R.; Hyde, T.M.; Weinberger, D.R.; Kleinman, J.E. Mapping DNA Methylation across Development, Genotype and Schizophrenia in the Human Frontal Cortex. Nat. Neurosci. 2016, 19, 40–47. [Google Scholar] [CrossRef]
- Weinberger, D.R. Future of Days Past: Neurodevelopment and Schizophrenia. Schizophr. Bull. 2017, 43, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Trubetskoy, V.; Pardiñas, A.F.; Qi, T.; Panagiotaropoulou, G.; Awasthi, S.; Bigdeli, T.B.; Bryois, J.; Chen, C.-Y.; Dennison, C.A.; Hall, L.S.; et al. Mapping Genomic Loci Implicates Genes and Synaptic Biology in Schizophrenia. Nature 2022, 604, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.J. Recent Genetic Findings in Schizophrenia and Their Therapeutic Relevance. J. Psychopharmacol. 2015, 29, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.; Mandal, A.; Jambhale, A.; Narnur, P.; Chen, G.; Akula, N.; Kramer, R.; Kolachana, B.; Xu, Q.; McMahon, F.J.; et al. Schizophrenia Risk-Associated SNPs Affect Expression of microRNA 137 Host Gene: A Postmortem Study. Hum. Mol. Genet. 2024, 33, 1939–1947. [Google Scholar] [CrossRef]
- Owen, M.J.; Legge, S.E.; Rees, E.; Walters, J.T.R.; O’Donovan, M.C. Genomic Findings in Schizophrenia and Their Implications. Mol. Psychiatry 2023, 28, 3638–3647. [Google Scholar] [CrossRef]
- Legge, S.E.; Jones, H.J.; Kendall, K.M.; Pardiñas, A.F.; Menzies, G.; Bracher-Smith, M.; Escott-Price, V.; Rees, E.; Davis, K.A.S.; Hotopf, M.; et al. Association of Genetic Liability to Psychotic Experiences With Neuropsychotic Disorders and Traits. JAMA Psychiatry 2019, 76, 1256–1265. [Google Scholar] [CrossRef]
- Mallard, T.T.; Karlsson Linnér, R.; Grotzinger, A.D.; Sanchez-Roige, S.; Seidlitz, J.; Okbay, A.; de Vlaming, R.; Meddens, S.F.W.; Palmer, A.A.; Davis, L.K.; et al. Multivariate GWAS of Psychiatric Disorders and Their Cardinal Symptoms Reveal Two Dimensions of Cross-Cutting Genetic Liabilities. Cell Genom. 2022, 2, 100140. [Google Scholar] [CrossRef]
- Lasalvia, A.; Tosato, S.; Brambilla, P.; Bertani, M.; Bonetto, C.; Cristofalo, D.; Bissoli, S.; De Santi, K.; Lazzarotto, L.; Zanatta, G.; et al. Psychosis Incident Cohort Outcome Study (PICOS). A Multisite Study of Clinical, Social and Biological Characteristics, Patterns of Care and Predictors of Outcome in First-Episode Psychosis. Background, Methodology and Overview of the Patient Sample. Epidemiol. Psychiatr. Sci. 2012, 21, 281–303. [Google Scholar] [CrossRef]
- Tosato, S.; Lasalvia, A.; Bonetto, C.; Mazzoncini, R.; Cristofalo, D.; De Santi, K.; Bertani, M.; Bissoli, S.; Lazzarotto, L.; Marrella, G.; et al. The Impact of Cannabis Use on Age of Onset and Clinical Characteristics in First-Episode Psychotic Patients. Data from the Psychosis Incident Cohort Outcome Study (PICOS). J. Psychiatr. Res. 2013, 47, 438–444. [Google Scholar] [CrossRef]
- World Health Organization. ICD-10: International Statistical Classification of Diseases and Related Health Problems: 10th Revision, 2nd ed.; World Health Organization: Geneva, Switzerland, 2004; ISBN 92-4-154649-2. [Google Scholar]
- Tosato, S.; Bonetto, C.; Lopizzo, N.; Cattane, N.; Barcella, M.; Turco, G.; Ruggeri, M.; Provasi, S.; Tomassi, S.; Dazzan, P.; et al. Childhood and Adulthood Severe Stressful Experiences and Biomarkers Related to Glucose Metabolism: A Possible Association? Front. Psychiatry 2021, 12, 629137. [Google Scholar] [CrossRef]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The Development and Validation of a Structured Diagnostic Psychiatric Interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59 (Suppl. S20), 22–33, quiz 34–57. [Google Scholar]
- Spitzer, R.L.; Williams, J.B.W.; Gibbon, M.; First, M.B. The Structured Clinical Interview for DSM-III-R (SCID): I: History, Rationale, and Description. Arch. Gen. Psychiatry 1992, 49, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-Generation PLINK: Rising to the Challenge of Larger and Richer Datasets. GigaScience 2015, 4, s13742-015-0047-8. [Google Scholar] [CrossRef]
- Graffelman, J.; Moreno, V. The Mid P-Value in Exact Tests for Hardy-Weinberg Equilibrium. Stat. Appl. Genet. Mol. Biol. 2013, 12, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Treccani, M.; Locatelli, E.; Patuzzo, C.; Malerba, G. A Broad Overview of Genotype Imputation: Standard Guidelines, Approaches, and Future Investigations in Genomic Association Studies. Biocell 2023, 47, 1225–1241. [Google Scholar] [CrossRef]
- Das, S.; Forer, L.; Schönherr, S.; Sidore, C.; Locke, A.E.; Kwong, A.; Vrieze, S.I.; Chew, E.Y.; Levy, S.; McGue, M.; et al. Next-Generation Genotype Imputation Service and Methods. Nat. Genet. 2016, 48, 1284–1287. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional Annotation of Genetic Variants from High-Throughput Sequencing Data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef]
- Watanabe, K.; Taskesen, E.; van Bochoven, A.; Posthuma, D. Functional Mapping and Annotation of Genetic Associations with FUMA. Nat. Commun. 2017, 8, 1826. [Google Scholar] [CrossRef]
- De Leeuw, C.A.; Mooij, J.M.; Heskes, T.; Posthuma, D. MAGMA: Generalized Gene-Set Analysis of GWAS Data. PLoS Comput. Biol. 2015, 11, e1004219. [Google Scholar] [CrossRef]
- The GTEx Consortium; Ardlie, K.G.; Deluca, D.S.; Segrè, A.V.; Sullivan, T.J.; Young, T.R.; Gelfand, E.T.; Trowbridge, C.A.; Maller, J.B.; Tukiainen, T.; et al. The Genotype-Tissue Expression (GTEx) Pilot Analysis: Multitissue Gene Regulation in Humans. Science 2015, 348, 648–660. [Google Scholar] [CrossRef]
- Kang, H.J.; Kawasawa, Y.I.; Cheng, F.; Zhu, Y.; Xu, X.; Li, M.; Sousa, A.M.M.; Pletikos, M.; Meyer, K.A.; Sedmak, G.; et al. Spatio-Temporal Transcriptome of the Human Brain. Nature 2011, 478, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, S.; Warrell, J.; Won, H.; Shi, X.; Navarro, F.C.P.; Clarke, D.; Gu, M.; Emani, P.; Yang, Y.T.; et al. Comprehensive Functional Genomic Resource and Integrative Model for the Human Brain. Science 2018, 362, eaat8464. [Google Scholar] [CrossRef]
- Ramasamy, A.; Trabzuni, D.; Guelfi, S.; Varghese, V.; Smith, C.; Walker, R.; De, T.; Coin, L.; de Silva, R.; Cookson, M.R.; et al. Genetic Variability in the Regulation of Gene Expression in Ten Regions of the Human Brain. Nat. Neurosci. 2014, 17, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.; Falkai, P.; Papiol, S. Neurodevelopmental Disturbances in Schizophrenia: Evidence from Genetic and Environmental Factors. J. Neural Transm. 2023, 130, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Roussos, P.; Mitchell, A.C.; Voloudakis, G.; Fullard, J.F.; Pothula, V.M.; Tsang, J.; Stahl, E.A.; Georgakopoulos, A.; Ruderfer, D.M.; Charney, A.; et al. A Role for Noncoding Variation in Schizophrenia. Cell Rep. 2014, 9, 1417–1429. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Li, S.; Li, X.; Yang, J.; Dang, X.; Mu, C.; Li, Y.; Li, K.; Li, J.; et al. Genetic Regulatory and Biological Implications of the 10q24.32 Schizophrenia Risk Locus. Brain 2023, 146, 1403–1419. [Google Scholar] [CrossRef]
- Del Casale, A.; Modesti, M.N.; Gentile, G.; Guariglia, C.; Ferracuti, S.; Simmaco, M.; Borro, M. Is the Hedgehog Pathway Involved in the Pathophysiology of Schizophrenia? A Systematic Review of Current Evidence of Neural Molecular Correlates and Perspectives on Drug Development. Curr. Issues Mol. Biol. 2024, 46, 5322–5336. [Google Scholar] [CrossRef]
- Zhou, X.-H.; Brakebusch, C.; Matthies, H.; Oohashi, T.; Hirsch, E.; Moser, M.; Krug, M.; Seidenbecher, C.I.; Boeckers, T.M.; Rauch, U.; et al. Neurocan Is Dispensable for Brain Development. Mol. Cell. Biol. 2001, 21, 5970. [Google Scholar] [CrossRef]
- Wang, P.; Cai, J.; Ni, J.; Zhang, J.; Tang, W.; Zhang, C. The NCAN Gene: Schizophrenia Susceptibility and Cognitive Dysfunction. Neuropsychiatr. Dis. Treat. 2016, 12, 2875–2883. [Google Scholar] [CrossRef]
- Mühleisen, T.W.; Mattheisen, M.; Strohmaier, J.; Degenhardt, F.; Priebe, L.; Schultz, C.C.; Breuer, R.; Meier, S.; Hoffmann, P.; Rivandeneira, F.; et al. Association between Schizophrenia and Common Variation in Neurocan (NCAN), a Genetic Risk Factor for Bipolar Disorder. Schizophr. Res. 2012, 138, 69–73. [Google Scholar] [CrossRef]
- Anderson, M.S.; Su, M.A. Aire and T Cell Development. Curr. Opin. Immunol. 2011, 23, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.; Bray, N.J. Schizophrenia Genomics: Convergence on Synaptic Development, Adult Synaptic Plasticity, or Both? Biol. Psychiatry 2022, 91, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Fromer, M.; Pocklington, A.J.; Kavanagh, D.H.; Williams, H.J.; Dwyer, S.; Gormley, P.; Georgieva, L.; Rees, E.; Palta, P.; Ruderfer, D.M.; et al. De Novo Mutations in Schizophrenia Implicate Synaptic Networks. Nature 2014, 506, 179–184. [Google Scholar] [CrossRef]
- Weinberger, D.R. The Neurodevelopmental Origins of Schizophrenia in the Penumbra of Genomic Medicine. World Psychiatry 2017, 16, 225–226. [Google Scholar] [CrossRef]
- Davies, C.; Segre, G.; Estradé, A.; Radua, J.; Micheli, A.D.; Provenzani, U.; Oliver, D.; de Pablo, G.S.; Ramella-Cravaro, V.; Besozzi, M.; et al. Prenatal and Perinatal Risk and Protective Factors for Psychosis: A Systematic Review and Meta-Analysis. Lancet Psychiatry 2020, 7, 399–410. [Google Scholar] [CrossRef]
- Gropman, A.L.; Summar, M.; Leonard, J.V. Neurological Implications of Urea Cycle Disorders. J. Inherit. Metab. Dis. 2007, 30, 865–879. [Google Scholar] [CrossRef]
- Bonnot, O.; Herrera, P.M.; Tordjman, S.; Walterfang, M. Secondary Psychosis Induced by Metabolic Disorders. Front. Neurosci. 2015, 9, 177. [Google Scholar] [CrossRef][Green Version]
- Fassier, T.; Guffon, N.; Acquaviva, C.; D’Amato, T.; Durand, D.V.; Domenech, P. Misdiagnosed Postpartum Psychosis Revealing a Late-Onset Urea Cycle Disorder. AJP 2011, 168, 576–580. [Google Scholar] [CrossRef]
- He, Y.; Yu, Z.; Giegling, I.; Xie, L.; Hartmann, A.M.; Prehn, C.; Adamski, J.; Kahn, R.; Li, Y.; Illig, T.; et al. Schizophrenia Shows a Unique Metabolomics Signature in Plasma. Transl. Psychiatry 2012, 2, e149. [Google Scholar] [CrossRef]
- Bernstein, H.-G.; Bogerts, B.; Keilhoff, G. The Many Faces of Nitric Oxide in Schizophrenia. A Review. Schizophr. Res. 2005, 78, 69–86. [Google Scholar] [CrossRef]
- Avigdor, B.E.; Yang, K.; Shinder, I.; Orsburn, B.C.; Rais, R.; Kano, S.; Sawa, A.; Pevsner, J. Characterization of Antipsychotic Medications, Amino Acid Signatures, and Platelet-Activating Factor in First-Episode Psychosis. Biomark. Neuropsychiatry 2021, 5, 100045. [Google Scholar] [CrossRef]
- Cuesta, M.J.; Moreno-Izco, L.; Ribeiro, M.; López-Ilundain, J.M.; Lecumberri, P.; Cabada, T.; Lorente-Omeñaca, R.; Sánchez-Torres, A.M.; Gómez, M.S.; Peralta, V. Motor Abnormalities and Cognitive Impairment in First-Episode Psychosis Patients, Their Unaffected Siblings and Healthy Controls. Schizophr. Res. 2018, 200, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Howes, O.D.; Onwordi, E.C. The Synaptic Hypothesis of Schizophrenia Version III: A Master Mechanism. Mol. Psychiatry 2023, 28, 1843–1856. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.R.; Szeto, R.A.; Carvalho, V.M.A.; Muotri, A.R.; Papes, F. Transcription Factor 4 and Its Association with Psychiatric Disorders. Transl. Psychiatry 2021, 11, 19. [Google Scholar] [CrossRef]
- Ahn, J.; Febbraio, M.; Silverstein, R.L. A Novel Isoform of Human Golgi Complex-Localized Glycoprotein-1 (Also Known as E-Selectin Ligand-1, MG-160 and Cysteine-Rich Fibroblast Growth Factor Receptor) Targets Differential Subcellular Localization. J. Cell Sci. 2005, 118, 1725–1731. [Google Scholar] [CrossRef]
- Yavarna, T.; Al-Dewik, N.; Al-Mureikhi, M.; Ali, R.; Al-Mesaifri, F.; Mahmoud, L.; Shahbeck, N.; Lakhani, S.; AlMulla, M.; Nawaz, Z.; et al. High Diagnostic Yield of Clinical Exome Sequencing in Middle Eastern Patients with Mendelian Disorders. Hum. Genet. 2015, 134, 967–980. [Google Scholar] [CrossRef]
- Bansal, V.; Mitjans, M.; Burik, C.a.P.; Linnér, R.K.; Okbay, A.; Rietveld, C.A.; Begemann, M.; Bonn, S.; Ripke, S.; de Vlaming, R.; et al. Genome-Wide Association Study Results for Educational Attainment Aid in Identifying Genetic Heterogeneity of Schizophrenia. Nat. Commun. 2018, 9, 3078. [Google Scholar] [CrossRef]
- Liu, J.; Yang, A.; Zhang, Q.; Yang, G.; Yang, W.; Lei, H.; Quan, J.; Qu, F.; Wang, M.; Zhang, Z.; et al. Association between Genetic Variants in SLC25A12 and Risk of Autism Spectrum Disorders: An Integrated Meta-Analysis. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2015, 168, 236–246. [Google Scholar] [CrossRef]
- Taheri, M.; Pourtavakoli, A.; Eslami, S.; Ghafouri-Fard, S.; Sayad, A. Assessment of Expression of Calcium Signaling Related lncRNAs in Epilepsy. Sci. Rep. 2023, 13, 17993. [Google Scholar] [CrossRef]
- Aoki, Y.; Cortese, S. Mitochondrial Aspartate/Glutamate Carrier SLC25A12 and Autism Spectrum Disorder: A Meta-Analysis. Mol. Neurobiol. 2016, 53, 1579–1588. [Google Scholar] [CrossRef]
- Rees, E.; Creeth, H.D.J.; Hwu, H.-G.; Chen, W.J.; Tsuang, M.; Glatt, S.J.; Rey, R.; Kirov, G.; Walters, J.T.R.; Holmans, P.; et al. Schizophrenia, Autism Spectrum Disorders and Developmental Disorders Share Specific Disruptive Coding Mutations. Nat. Commun. 2021, 12, 5353. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.J.; Escott-Price, V.; Davies, G.; Bailey, M.E.S.; Colodro-Conde, L.; Ward, J.; Vedernikov, A.; Marioni, R.; Cullen, B.; Lyall, D.; et al. Genome-Wide Analysis of over 106 000 Individuals Identifies 9 Neuroticism-Associated Loci. Mol. Psychiatry 2016, 21, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Smeland, O.B.; Wang, Y.; Lo, M.-T.; Li, W.; Frei, O.; Witoelar, A.; Tesli, M.; Hinds, D.A.; Tung, J.Y.; Djurovic, S.; et al. Identification of Genetic Loci Shared between Schizophrenia and the Big Five Personality Traits. Sci. Rep. 2017, 7, 2222. [Google Scholar] [CrossRef]
- Johnson, E.C.; Kapoor, M.; Hatoum, A.S.; Zhou, H.; Polimanti, R.; Wendt, F.R.; Walters, R.K.; Lai, D.; Kember, R.L.; Hartz, S.; et al. Investigation of Convergent and Divergent Genetic Influences Underlying Schizophrenia and Alcohol Use Disorder. Psychol. Med. 2023, 53, 1196–1204. [Google Scholar] [CrossRef]
- Wiström, E.D.; O’Connell, K.S.; Karadag, N.; Bahrami, S.; Hindley, G.F.L.; Lin, A.; Cheng, W.; Steen, N.E.; Shadrin, A.; Frei, O.; et al. Genome-Wide Analysis Reveals Genetic Overlap between Alcohol Use Behaviours, Schizophrenia and Bipolar Disorder and Identifies Novel Shared Risk Loci. Addiction 2022, 117, 600–610. [Google Scholar] [CrossRef]
- Vonberg, F.W.; Bigdeli, T.B. Genetic Correlation Between Schizophrenia and Epilepsy. JAMA Neurol. 2016, 73, 125–126. [Google Scholar] [CrossRef]
- Cascella, N.G.; Schretlen, D.J.; Sawa, A. Schizophrenia and Epilepsy: Is There a Shared Susceptibility? Neurosci. Res. 2009, 63, 227–235. [Google Scholar] [CrossRef]
- Hiraide, T.; Hayashi, T.; Ito, Y.; Urushibata, R.; Uchida, H.; Kitagata, R.; Ishigaki, H.; Ogata, T.; Saitsu, H.; Fukuda, T. Case Report: Novel Compound Heterozygous TPRKB Variants Cause Galloway-Mowat Syndrome. Front. Pediatr. 2024, 12, 1360867. [Google Scholar] [CrossRef]
- Owen, M.J.; O’Donovan, M.C. Schizophrenia and the Neurodevelopmental Continuum:Evidence from Genomics. World Psychiatry 2017, 16, 227–235. [Google Scholar] [CrossRef]

| Non-Affective Psychosis N = 147, N (%) | Healthy Controls N = 102, N (%) | |
|---|---|---|
| Sex | ||
| Female | 62 (42.2%) | 57 (55.9%) |
| Male | 85 (57.8%) | 45 (44.1%) |
| Age, years (SD) | - | 33.89 ± 10.99 |
| At onset, years (SD) | 29.68 ± 9.04 | - |
| Educational level | 10 missing | |
| Primary school | 5 (3.6%) | 13 (12.7%) |
| Higher school | 132 (96.4%) | 89 (87.3%) |
| Living condition | 10 missing | 1 missing |
| Alone | 13 (9.5%) | 22 (21.8%) |
| With partner and/or children | 27 (19.7%) | 38 (37.6%) |
| With parents | - | 33 (32.7%) |
| With other relatives | 92 (67.2%) | 1 (1%) |
| With other people | 5 (3.6%) | 7 (6.9%) |
| Working status | 12 missing | 2 missing |
| Employed | 55 (40.7%) | 71 (71%) |
| Unemployed | 46 (34.1%) | 10 (10%) |
| Student/Retired/Other condition | 30 (25.2%) | 19 (19%) |
| Marital status | 16 missing | |
| Single | 101 (77.1%) | 54 (52.9%) |
| Married | 21 (16.0%) | 40 (39.2%) |
| Separated/Divorced | 9 (6.9%) | 8 (7.9%) |
| Diagnosis | ||
| Schizophrenia | 42 (28.6%) | - |
| Other non-affective psychosis | 105 (71.4%) | - |
| Chrom | Position (bp) | RSID | Effect Allele | p-Value | Gene | Distance (bp) |
|---|---|---|---|---|---|---|
| 2 | 73,735,674 | rs7606483 | A | 5.9 × 10−14 | ALMS1 | 0 |
| 2 | 172,897,761 | rs7591150 | G | 7.7 × 10−14 | METAP1D | 0 |
| 3 | 63,842,629 | rs832190 | A | 8.9 × 10−14 | THOC7 | 0 |
| 3 | 71,661,862 | rs830650 | A | 8.9 × 10−14 | RP11-154H23.3 | 25,302 |
| 3 | 80,670,963 | rs72897474 | A | 2.0 × 10−13 | RP11-47P18.1 | 139,230 |
| 4 | 48,471,170 | rs7670045 | G | 9.4 × 10−14 | ||
| 5 | 45,882,593 | rs9763350 | A | 2.0 × 10−13 | ||
| 5 | 46,121,882 | rs8185209 | G | 1.2 × 10−13 | ||
| 5 | 49,478,029 | rs8188217 | A | 3.5 × 10−13 | ||
| 5 | 49,674,996 | rs74865196 | A | 2.8 × 10−13 | ||
| 6 | 23,798,867 | rs9393484 | A | 7.3 × 10−14 | SPTLC1P2 | 58,059 |
| 6 | 26,211,146 | rs9358912 | A | 1.1 × 10−13 | ||
| 6 | 26,958,528 | rs9467936 | G | 2.5 × 10−10 | ||
| 6 | 157,099,430 | A | 2.6 × 10−13 | |||
| 8 | 8691,622 | rs13270070 | T | 1.5 × 10−13 | MFHAS1 | 0 |
| 8 | 9620,359 | rs9657509 | A | 9.4 × 10−14 | TNKS | 0 |
| 8 | 89,588,626 | rs7819570 | A | 8.9 × 10−14 | ||
| 10 | 104,300,638 | rs9665626 | G | 1.2 × 10−13 | SUFU | 0 |
| 11 | 133,959,472 | rs12802468 | G | 1.2 × 10−13 | JAM3 | 0 |
| 14 | 81,735,565 | rs8007595 | A | 2.8 × 10−13 | STON2 | 0 |
| 16 | 4,515,145 | rs7206782 | A | 8.9 × 10−14 | CDIP1 | 0 |
| 16 | 74,649,232 | rs8047523 | A | 7.2 × 10−14 | HSPE1P7 | 0 |
| 18 | 53,301,359 | rs78294462 | A | 2.6 × 10−13 | TCF4 | 0 |
| 19 | 9,764,421 | rs200076265 | A | 5.9 × 10−12 | ZNF562 | 0 |
| 19 | 19,358,332 | rs7253952 | A | 4.6 × 10−13 | NCAN | 0 |
| 19 | 31,045,360 | rs8102611 | A | 1.1 × 10−13 | ZNF536 | 0 |
| 21 | 33,175,364 | rs62221589 | A | 4.6 × 10−13 |
| Trait | RSID | Genes | p-Value | PMID |
|---|---|---|---|---|
| Schizophrenia | rs7591150 | METAP1D | 7 × 10−7 | 35396580 |
| rs72897474 | Intergenic | 8 × 10−8 | 26198764 | |
| rs9665626 | SUFU | 5 × 10−9 | 26198764 | |
| rs9665626 | ARL3 | 3 × 10−9 | 27922604 | |
| rs7206782 | CORO7-PAM16 | 6 × 10−10 | 31740837 | |
| rs7206782 | DNAJA3 | 3 × 10−7 | 26198764 | |
| rs7206782 | DNAJA3 | 9 × 10−10 | 35396580 | |
| rs7253952 | MAU2 | 4 × 10−8 | 30285260 | |
| rs9665626 | TRIM8, ARL3 | 4 × 10−10 | 33479212 | |
| Waist circumference adjusted for body mass index | rs13270070 | PRAG1, RN7SL178P | 9 × 10−18 | 34021172, 30239722 |
| rs9657509 | CLDN23, MFHAS1 | 3 × 10−8 | 34021172 | |
| rs7206782 | TNKS | 1 × 10−11 | 34021172 | |
| Alcohol dependence | rs3131513 | CLIC4, RUNX3 | 2 × 10−6 | 23942779 |
| Autism spectrum disorder | rs9393484 | SPTLC1P2 | 1 × 10−6 | 28540026 |
| Bipolar disorder | rs9665626 | SUFU, TRIM8 | 2 × 10−9 | 33479212 |
| Depression | rs13270070 | CLDN23, MFHAS1 | 8 × 10−16 | 33859377 |
| Educational attainment | rs7606483 | ALMS1P1, RNU6-111P, RPSAP28 | 3 × 10−11 | 35361970 |
| General cognitive ability | rs7606483 | RNU6-111P RPSAP28 | 2 × 10−8 | 29844566 |
| Neuroticism | rs13270070 | MFHAS1 | 6 × 10−30 | 27067015, 29255261, 29255261 |
| Gene Set | N. Genes | β | SE | p-Value | Pbon |
|---|---|---|---|---|---|
| REACTOME_UREA_CYCLE | 6 | 1.9558 | 0.30306 | 5.6363 × 10−11 | 9.5625 × 10−7 |
| ROZANOV_MMP14_TARGETS_DN | 26 | 0.8407 | 0.1762 | 9.2779 × 10−7 | 1.5739 × 10−2 |
| ZHAN_MULTIPLE_MYELOMA_MS_DN | 37 | 0.6046 | 0.1401 | 8.0401 × 10−6 | 1.1363 × 10−1 |
| GOLDRATH_HOMEOSTATIC_PROLIFERATION | 101 | 0.3758 | 0.0899 | 1.4548 × 10−5 | 2.4678 × 10−1 |
| GOBP_REGULATION_OF_DNA_DEMETHYLATION | 10 | 1.0509 | 0.2629 | 3.2099 × 10−5 | 5.4446 × 10−1 |
| GOBP_POSITIVE_REGULATION_OF_DNA_DEMETHYLATION | 8 | 1.1283 | 0.2841 | 3.5860 × 10−5 | 6.0822 × 10−1 |
| GOBP_POSITIVE_REGULATION_OF_INTRINSIC_APOPTOTIC_SIGNALING_PATHWAY | 39 | 0.5700 | 0.1469 | 5.2322 × 10−5 | 8.8738 × 10−1 |
| GOBP_POSITIVE_REGULATION_OF_RELEASE_OF_SEQUESTERED_CALCIUM_ION_INTO_CYTOSOL | 32 | 0.5695 | 0.1482 | 6.9526 × 10−5 | 1 |
| GOBP_NEGATIVE_REGULATION_OF_NUCLEOCYTOPLASMIC_TRANSPORT | 23 | 0.6782 | 0.1775 | 6.6904 × 10−5 | 1 |
| GOBP_POSITIVE_REGULATION_OF_POST_TRANSCRIPTIONAL_GENE_SILENCING | 13 | 1.0190 | 0.2674 | 6.0945 × 10−5 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Treccani, M.; Maggioni, L.; Di Giovanni, C.; Veschetti, L.; Cristofalo, D.; Patuzzo, C.; Lasalvia, A.; Ristic, B.; Kumar, R.; The PICOS-Veneto Group; et al. A Genome-Wide Association Study of First-Episode Psychosis: A Genetic Exploration in an Italian Cohort. Genes 2025, 16, 439. https://doi.org/10.3390/genes16040439
Treccani M, Maggioni L, Di Giovanni C, Veschetti L, Cristofalo D, Patuzzo C, Lasalvia A, Ristic B, Kumar R, The PICOS-Veneto Group, et al. A Genome-Wide Association Study of First-Episode Psychosis: A Genetic Exploration in an Italian Cohort. Genes. 2025; 16(4):439. https://doi.org/10.3390/genes16040439
Chicago/Turabian StyleTreccani, Mirko, Lucia Maggioni, Claudia Di Giovanni, Laura Veschetti, Doriana Cristofalo, Cristina Patuzzo, Antonio Lasalvia, Branko Ristic, Roushan Kumar, The PICOS-Veneto Group, and et al. 2025. "A Genome-Wide Association Study of First-Episode Psychosis: A Genetic Exploration in an Italian Cohort" Genes 16, no. 4: 439. https://doi.org/10.3390/genes16040439
APA StyleTreccani, M., Maggioni, L., Di Giovanni, C., Veschetti, L., Cristofalo, D., Patuzzo, C., Lasalvia, A., Ristic, B., Kumar, R., The PICOS-Veneto Group, Ruggeri, M., Bonetto, C., Malerba, G., & Tosato, S. (2025). A Genome-Wide Association Study of First-Episode Psychosis: A Genetic Exploration in an Italian Cohort. Genes, 16(4), 439. https://doi.org/10.3390/genes16040439








