MTHFR Gene Polymorphisms: A Single Gene with Wide-Ranging Clinical Implications—A Review
Abstract
1. Introduction
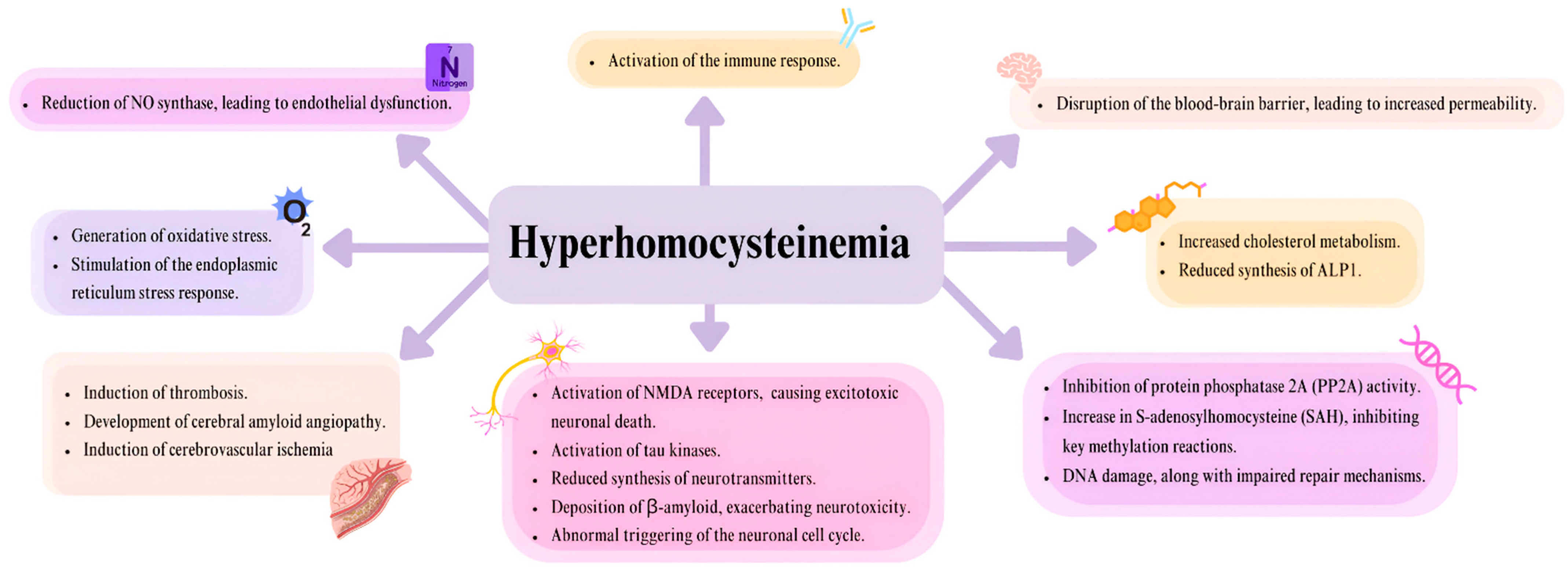
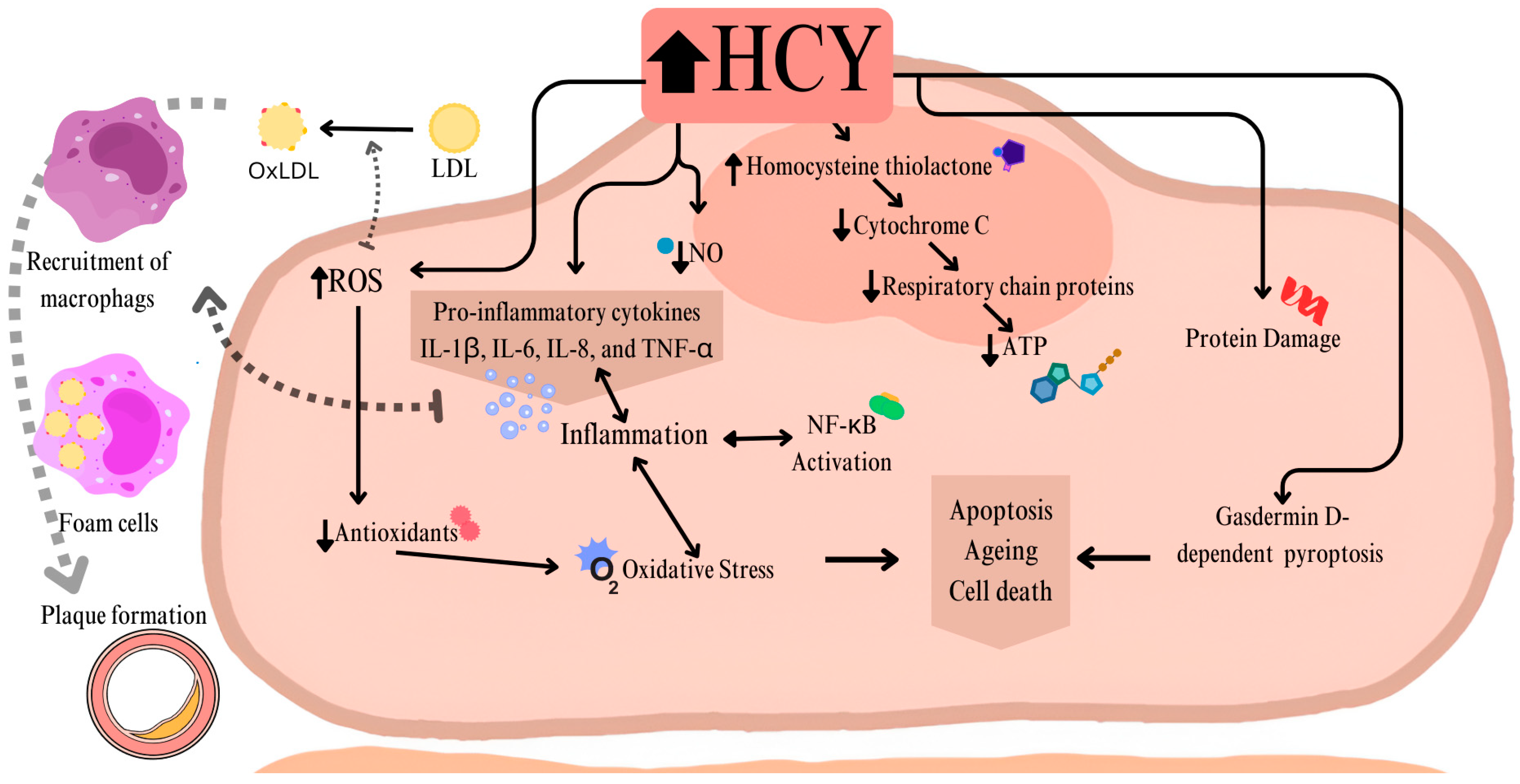
2. MTHFR in Cardiology
3. MTHFR in Oncology
3.1. Breast Cancer
3.2. Prostate Cancer
3.3. Ovarian Cancer
3.4. Leukemia
4. MTHFR in Neurology and Psychiatry
4.1. Autism Spectrum Disorder (ASD)
4.2. Alzheimer’s Disease
4.3. Schizophrenia
4.4. Major Depressive Disorder
4.5. Other Neurological and Psychiatric Disorders
5. MTHFR in Gastroenterology and Diabetology
5.1. Type 2 Diabetes Mellitus
5.2. Gestational Diabetes Mellitus
5.3. Metabolic Syndrome
5.4. Obesity
5.5. Non-Alcoholic Fatty Liver Disease
5.6. Inflammatory Bowel Disease
6. MTHFR in Pregnancy and Neonatology
6.1. Infertility
6.2. Recurrent Pregnancy Loss
6.3. Pre-Eclampsia
6.4. Preterm Birth and Low Birth Weight
6.5. Neural Tube Defects
7. MTHFR in Rheumatoid Arthritis
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| MTHFR | 5,10-methylenetetrahydrofolate reductase; Methylenetetrahydrofolate reductase |
| NTDs | Neural tube defects |
| 5,10-methylene-THF | 5,10-methylenetetrahydrofolate |
| 5-methyl-THF | 5-methyltetrahydrofolate |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| FAD | Flavin adenine dinucleotide |
| Hcy | Homocysteine |
| Met | Methionine |
| MS | Methionine synthase |
| THF | Tetrahydrofolate |
| 5,10-MTHF | 5,10-methylenetetrahydrofolate |
| 5-MTHF | 5-methyltetrahydrofolate |
| SAM | S-Adenosyl-L-methionine |
| SAH | S-Adenosyl-L-homocysteine |
| dTMP | Deoxythymidine monophosphate |
| DNA | Deoxyribonucleic acid |
| CBS | Cystathionine β-synthase |
| SNPs | Single nucleotide polymorphisms |
| Ala | Alanine |
| Val | Valine |
| Glu | Glutamine |
| HHcy | Hyperhomocysteinemia |
| A | Adenine |
| C | Cytosine |
| T | Thymine |
| G | Guanine |
| NO | Nitric Oxide |
| NMDA | N-methyl-D-aspartate |
| PP2A | Protein phosphatase 2A |
| ALP1 | Apolipoprotein 1 |
| EC | Endothelial cells |
| IL-1β | Interleukin 1 β |
| IL-6 | Interleukin 6 |
| IL-8 | Interleukin 8 |
| TNF-α | Tumor necrosis factor |
| ROS | Reactive oxygen species |
| NF-κB | Nuclear factor kappa B |
| CVD | Cardiovascular disease |
| IMH | Intramural hematoma |
| OR | Odds Ratio |
| CI | Confidence Interval |
| BC | Breast cancer |
| HER-2 | Receptor tyrosine-protein kinase erbB-2 |
| PC | Prostate cancer |
| OC | Ovarian cancer |
| CLL | Chronic lymphocytic leukemia |
| ALL | Acute lymphocytic leukemia |
| AML | Acute myeloblastic leukemia |
| CML | Chronic myeloblastic leukemia |
| ASD | Autism Spectrum Disorder |
| Fragile X | Fragile X Messenger Ribonucleoprotein 1 |
| SHANK 3 | SH3 and multiple ankyrin repeat domains 3 |
| CASPR 2 | Contactin-associated protein-like 2 |
| RFC1 | Replication factor C subunit 1 |
| FRα | Folate receptor α |
| PCFT | Proton-coupled folate transporter |
| AD | Alzheimer’s disease |
| EOAD | Early-onset Alzheimer’s disease |
| LOAD | Late-onset Alzheimer’s disease |
| APP | Amyloid precursor protein |
| PSEN1 | Presenilin 1 |
| PSEN2 | Presenilin 2 |
| APOE | Apolipoprotein E |
| aMCI | amnestic Mild Cognitive Impairment |
| SCZ | Schizophrenia |
| MDD | Major Depressive Disorder |
| TRD | Treatment-Resistant Depression |
| FDA | The United States Food and Drug Administration |
| MID | Migraine with comorbid depression |
| PD | Parkinson’s disease |
| L-DOPA | L-3,4-dihydroxyphenylalanine |
| ADHD | Attention deficit hyperactivity disorder |
| DM | Diabetes mellitus |
| T2DM | Type 2 diabetes mellitus |
| IR | Insulin resistance |
| DN | Diabetic nephropathy |
| DR | Diabetic retinopathy |
| DPN | Diabetic peripheral neuropathy |
| GDM | Gestational diabetes mellitus |
| MS | Metabolic syndrome |
| ER | Endoplasmic reticulum |
| NAFLD | Non-alcoholic fatty liver disease |
| NASH | Non-alcoholic steatohepatitis |
| IBD | Inflammatory bowel disease |
| CD | Crohn’s disease |
| UC | Ulcerative colitis |
| U-IBD | Unclassified inflammatory bowel disease |
| DNMTs | DNA methyltransferases |
| RPL | Recurrent pregnancy loss |
| PE | Pre-eclampsia |
| PTB | Preterm Birth |
| LBW | Low birth weight |
| UMFA | Unmetabolized folic acid |
| RA | Rheumatoid arthritis |
References
- Zarembska, E.; Ślusarczyk, K.; Wrzosek, M. The Implication of a Polymorphism in the Methylenetetrahydrofolate Reductase Gene in Homocysteine Metabolism and Related Civilisation Diseases. Int. J. Mol. Sci. 2023, 25, 193. [Google Scholar] [CrossRef]
- Botto, L.D.; Yang, Q. 5,10-Methylenetetrahydrofolate Reductase Gene Variants and Congenital Anomalies: A HuGE Review. Am. J. Epidemiol. 2000, 151, 862–877. [Google Scholar] [CrossRef]
- Froese, D.S.; Fowler, B.; Baumgartner, M.R. Vitamin B12, Folate, and the Methionine Remethylation Cycle—Biochemistry, Pathways, and Regulation. J. Inher Metab. Disea 2019, 42, 673–685. [Google Scholar] [CrossRef] [PubMed]
- MTHFR Methylenetetrahydrofolate Reductase [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/4524 (accessed on 12 March 2025).
- Mallhi, T.H.; Shahid, M.; Rehman, K.; Khan, Y.H.; Alanazi, A.S.; Alotaibi, N.H.; Akash, M.S.H.; Butt, M.H. Biochemical Association of MTHFR C677T Polymorphism with Myocardial Infarction in the Presence of Diabetes Mellitus as a Risk Factor. Metabolites 2023, 13, 251. [Google Scholar] [CrossRef]
- Singh, J.; Wilkins, G.; Goodman-Vincent, E.; Chishti, S.; Bonilla Guerrero, R.; McFadden, L.; Zahavi, Z.; Santosh, P. Co-Occurring Methylenetetrahydrofolate Reductase (MTHFR) Rs1801133 and Rs1801131 Genotypes as Associative Genetic Modifiers of Clinical Severity in Rett Syndrome. Brain Sci. 2024, 14, 624. [Google Scholar] [CrossRef] [PubMed]
- Barretta, F.; Uomo, F.; Fecarotta, S.; Albano, L.; Crisci, D.; Verde, A.; Fisco, M.G.; Gallo, G.; Dottore Stagna, D.; Pricolo, M.R.; et al. Contribution of Genetic Test to Early Diagnosis of Methylenetetrahydrofolate Reductase (MTHFR) Deficiency: The Experience of a Reference Center in Southern Italy. Genes 2023, 14, 980. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, D.; Sibani, S.; Rozen, R. Molecular Biology of Methylenetetrahydrofolate Reductase (MTHFR) and Overview of Mutations/Polymorphisms. In Madame Curie Bioscience Database; Landes Bioscience: Austin, TX, USA, 2005. Available online: https://www.ncbi.nlm.nih.gov/books/NBK6561/ (accessed on 12 March 2025).
- Yamada, K.; Chen, Z.; Rozen, R.; Matthews, R.G. Effects of Common Polymorphisms on the Properties of Recombinant Human Methylenetetrahydrofolate Reductase. Proc. Natl. Acad. Sci. USA 2001, 98, 14853–14858. [Google Scholar] [CrossRef]
- Bhatia, P.; Singh, N. Homocysteine Excess: Delineating the Possible Mechanism of Neurotoxicity and Depression. Fundamemntal Clin. Pharm. 2015, 29, 522–528. [Google Scholar] [CrossRef]
- Nishio, K.; Goto, Y.; Kondo, T.; Ito, S.; Ishida, Y.; Kawai, S.; Naito, M.; Wakai, K.; Hamajima, N. Serum Folate and Methylenetetrahydrofolate Reductase (MTHFR) C677T Polymorphism Adjusted for Folate Intake. J. Epidemiol. 2008, 18, 125–131. [Google Scholar] [CrossRef]
- Bjørke-Monsen, A.-L.; Ueland, P.M. Folate—A Scoping Review for Nordic Nutrition Recommendations 2023. Food Nutr. Res. 2023, 67, 10.29219/fnr.v67.10258. [Google Scholar] [CrossRef]
- Román, G.C.; Mancera-Páez, O.; Bernal, C. Epigenetic Factors in Late-Onset Alzheimer’s Disease: MTHFR and CTH Gene Polymorphisms, Metabolic Transsulfuration and Methylation Pathways, and B Vitamins. Int. J. Mol. Sci. 2019, 20, 319. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Refsum, H. Homocysteine, B Vitamins, and Cognitive Impairment. Annu. Rev. Nutr. 2016, 36, 211–239. [Google Scholar] [CrossRef]
- Austin, R.C.; Lentz, S.R.; Werstuck, G.H. Role of Hyperhomocysteinemia in Endothelial Dysfunction and Atherothrombotic Disease. Cell Death Differ. 2004, 11, S56–S64. [Google Scholar] [CrossRef]
- Yuan, D.; Chu, J.; Lin, H.; Zhu, G.; Qian, J.; Yu, Y.; Yao, T.; Ping, F.; Chen, F.; Liu, X. Mechanism of Homocysteine-Mediated Endothelial Injury and Its Consequences for Atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 1109445. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Ghosh, A.K.; Eren, M.; Miyata, T.; Vaughan, D.E. PAI-1 Contributes to Homocysteine-Induced Cellular Senescence. Cell Signal 2019, 64, 109394. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, P.; Tatarkova, Z.; Sivonova, M.K.; Racay, P.; Lehotsky, J. Homocysteine and Mitochondria in Cardiovascular and Cerebrovascular Systems. Int. J. Mol. Sci. 2020, 21, 7698. [Google Scholar] [CrossRef]
- Timkova, V.; Tatarkova, Z.; Lehotsky, J.; Racay, P.; Dobrota, D.; Kaplan, P. Effects of Mild Hyperhomocysteinemia on Electron Transport Chain Complexes, Oxidative Stress, and Protein Expression in Rat Cardiac Mitochondria. Mol. Cell. Biochem. 2016, 411, 261–270. [Google Scholar] [CrossRef]
- Perła-Kaján, J.; Marczak, Ł.; Kaján, L.; Skowronek, P.; Twardowski, T.; Jakubowski, H. Modification by Homocysteine Thiolactone Affects Redox Status of Cytochrome c. Biochemistry 2007, 46, 6225–6231. [Google Scholar] [CrossRef]
- Venditti, P.; Napolitano, G. Mitochondrial Management of ROS in Physiological and Pathological Conditions. Antioxidants 2025, 14, 43. [Google Scholar] [CrossRef]
- Chang, L.; Geng, B.; Yu, F.; Zhao, J.; Jiang, H.; Du, J.; Tang, C. Hydrogen Sulfide Inhibits Myocardial Injury Induced by Homocysteine in Rats. Amino Acids 2008, 34, 573–585. [Google Scholar] [CrossRef]
- Schwarz, K.; Siddiqi, N.; Singh, S.; Neil, C.J.; Dawson, D.K.; Frenneaux, M.P. The Breathing Heart—Mitochondrial Respiratory Chain Dysfunction in Cardiac Disease. Int. J. Cardiol. 2014, 171, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Raghubeer, S.; Matsha, T.E. Methylenetetrahydrofolate (MTHFR), the One-Carbon Cycle, and Cardiovascular Risks. Nutrients 2021, 13, 4562. [Google Scholar] [CrossRef]
- Moti Wala, S.; AlEdani, E.M.; Samuel, E.A.; Ahmad, K.; Manongi, N.J.; Rajapandian, R.; Khan, S. Exploring the Nexus: A Systematic Review on the Interplay of the Methylenetetrahydrofolate Reductase (MTHFR) Gene C677T Genotype, Hyperhomocysteinemia, and Spontaneous Cervical/Vertebral Artery Dissection in Young Adults. Cureus 2024, 16, e60878. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Guo, S.; Ma, R.; He, J.; Yan, Y.; Zhang, X.; Wang, X.; Cao, B.; Guo, H. Association between Methylenetetrahydrofolate Reductase (MTHFR) C677T Polymorphism and H-Type Hypertension: A Systematic Review and Meta-Analysis. Ann. Hum. Genet. 2022, 86, 278–289. [Google Scholar] [CrossRef]
- Bax, M.; Romanov, V.; Junday, K.; Giannoulatou, E.; Martinac, B.; Kovacic, J.C.; Liu, R.; Iismaa, S.E.; Graham, R.M. Arterial Dissections: Common Features and New Perspectives. Front. Cardiovasc. Med. 2022, 9, 1055862. [Google Scholar] [CrossRef]
- Ahmed, S.; Bogiatzi, C.; Hackam, D.G.; Rutledge, A.C.; Sposato, L.A.; Khaw, A.; Mandzia, J.; Azarpazhoo, M.R.; Hachinski, V.; Spence, J.D. Vitamin B 12 Deficiency and Hyperhomocysteinaemia in Outpatients with Stroke or Transient Ischaemic Attack: A Cohort Study at an Academic Medical Centre. BMJ Open 2019, 9, e026564. [Google Scholar] [CrossRef] [PubMed]
- Park, W.-C.; Chang, J.-H. Clinical Implications of Methylenetetrahydrofolate Reductase Mutations and Plasma Homocysteine Levels in Patients with Thromboembolic Occlusion. Vasc. Spec. Int. 2014, 30, 113–119. [Google Scholar] [CrossRef]
- Qin, X.; Spence, J.D.; Li, J.; Zhang, Y.; Li, Y.; Sun, N.; Liang, M.; Song, Y.; Zhang, Y.; Wang, B.; et al. Interaction of Serum Vitamin B12 and Folate with MTHFR Genotypes on Risk of Ischemic Stroke. Neurology 2020, 94, e1126–e1136. [Google Scholar] [CrossRef]
- Zhao, L.; Li, T.; Dang, M.; Li, Y.; Fan, H.; Hao, Q.; Song, D.; Lu, J.; Lu, Z.; Jian, Y.; et al. Association of Methylenetetrahydrofolate Reductase (MTHFR) Rs1801133 (677C>T) Gene Polymorphism with Ischemic Stroke Risk in Different Populations: An Updated Meta-Analysis. Front. Genet. 2022, 13, 1021423. [Google Scholar] [CrossRef]
- Song, Y.; Li, B.; Wang, C.; Wang, P.; Gao, X.; Liu, G. Association between 5,10-Methylenetetrahydrofolate Reductase C677T Gene Polymorphism and Risk of Ischemic Stroke: A Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2016, 25, 679–687. [Google Scholar] [CrossRef]
- Chang, G.; Kuai, Z.; Wang, J.; Wu, J.; Xu, K.; Yuan, Y.; Hu, Y. The Association of MTHFR C677T Variant with Increased Risk of Ischemic Stroke in the Elderly Population: A Meta-Analysis of Observational Studies. BMC Geriatr. 2019, 19, 331. [Google Scholar] [CrossRef] [PubMed]
- Pieroth, R.; Paver, S.; Day, S.; Lammersfeld, C. Folate and Its Impact on Cancer Risk. Curr. Nutr. Rep. 2018, 7, 70–84. [Google Scholar] [CrossRef]
- Jacques, P.F.; Bostom, A.G.; Williams, R.R.; Ellison, R.C.; Eckfeldt, J.H.; Rosenberg, I.H.; Selhub, J.; Rozen, R. Relation between Folate Status, a Common Mutation in Methylenetetrahydrofolate Reductase, and Plasma Homocysteine Concentrations. Circulation 1996, 93, 7–9. [Google Scholar] [CrossRef]
- Duthie, S.J.; Narayanan, S.; Brand, G.M.; Pirie, L.; Grant, G. Impact of Folate Deficiency on DNA Stability. J. Nutr. 2002, 132, 2444S–2449S. [Google Scholar] [CrossRef] [PubMed]
- De Cabo, S.F.; Santos, J.; Fernández-Piqueras, J. Molecular and Cytological Evidence of S-Adenosyl-L-Homocysteine as an Innocuous Undermethylating Agent in Vivo. Cytogenet. Genome Res. 1995, 71, 187–192. [Google Scholar] [CrossRef]
- Yi, P.; Melnyk, S.; Pogribna, M.; Pogribny, I.P.; Hine, R.J.; James, S.J. Increase in Plasma Homocysteine Associated with Parallel Increases in Plasma S-Adenosylhomocysteine and Lymphocyte DNA Hypomethylation. J. Biol. Chem. 2000, 275, 29318–29323. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-W.; Mason, J.B. Folate Status: Effects on Pathways of Colorectal Carcinogenesis. J. Nutr. 2002, 132, 2413S–2418S. [Google Scholar] [CrossRef]
- Baylin, S.B.; Herman, J.G. DNA Hypermethylation in Tumorigenesis: Epigenetics Joins Genetics. Trends Genet. 2000, 16, 168–174. [Google Scholar] [CrossRef]
- Ryan, B.M.; Weir, D.G. Relevance of Folate Metabolism in the Pathogenesis of Colorectal Cancer. J. Lab. Clin. Med. 2001, 138, 164–176. [Google Scholar] [CrossRef]
- Blount, B.C.; Mack, M.M.; Wehr, C.M.; MacGregor, J.T.; Hiatt, R.A.; Wang, G.; Wickramasinghe, S.N.; Everson, R.B.; Ames, B.N. Folate Deficiency Causes Uracil Misincorporation into Human DNA and Chromosome Breakage: Implications for Cancer and Neuronal Damage. Proc. Natl. Acad. Sci. USA 1997, 94, 3290–3295. [Google Scholar] [CrossRef]
- Hansen, R.S.; Ellis, N.A.; Gartler, S.M. Demethylation of Specific Sites in the 5′ Region of the Inactive X-Linked Human Phosphoglycerate Kinase Gene Correlates with the Appearance of Nuclease Sensitivity and Gene Expression. Mol. Cell. Biol. 1988, 8, 4692–4699. [Google Scholar] [CrossRef] [PubMed]
- Pogribny, I.P.; Basnakian, A.G.; Miller, B.J.; Lopatina, N.G.; Poirier, L.A.; James, S.J. Breaks in Genomic DNA and within the P53 Gene Are Associated with Hypomethylation in Livers of Folate/Methyl-Deficient Rats. Cancer Res. 1995, 55, 1894–1901. [Google Scholar] [PubMed]
- Kim, Y.; Pogribny, I.; Basnakian, A.; Miller, J.; Selhub, J.; James, S.; Mason, J. Folate Deficiency in Rats Induces DNA Strand Breaks and Hypomethylation within the P53 Tumor Suppressor Gene. Am. J. Clin. Nutr. 1997, 65, 46–52. [Google Scholar] [CrossRef]
- Kim, Y.; Shirwadkar, S.; Choi, S.; Puchyr, M.; Wang, Y.; Mason, J.B. Effects of Dietary Folate on DNA Strand Breaks within Mutation-Prone Exons of the P53 Gene in Rat Colon. Gastroenterology 2000, 119, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Aly, R.M.; Taalab, M.M.; Ghazy, H.F. MTHFR A1298C and C677T Gene Polymorphisms and Susceptibility to Chronic Myeloid Leukemia in Egypt. Int. J. Clin. Exp. Pathol. 2014, 7, 2571–2578. [Google Scholar]
- Choi, S.-W.; Kim, Y.-I.; Weitzel, J.N.; Mason, J.B. Folate Depletion Impairs DNA Excision Repair in the Colon of the Rat. Gut 1998, 43, 93–99. [Google Scholar] [CrossRef]
- Wainfan, E.; Poirier, L.A. Methyl Groups in Carcinogenesis: Effects on DNA Methylation and Gene Expression. Cancer Res. 1992, 52, 2071s–2077s. [Google Scholar]
- Sanjoaquin, M.A.; Allen, N.; Couto, E.; Roddam, A.W.; Key, T.J. Folate Intake and Colorectal Cancer Risk: A Meta-Analytical Approach. Int. J. Cancer 2005, 113, 825–828. [Google Scholar] [CrossRef]
- Zara-Lopes, T.; Silva Galbiatti-Dias, A.L.; Urbanin Castanhole-Nunes, M.M.; Padovani-Júnior, J.A.; Maniglia, J.V.; Pavarino, E.C.; Goloni-Bertollo, E.M. Polymorphisms in MTHFR, MTR, RFC1 and CßS Genes Involved in Folate Metabolism and Thyroid Cancer: A Case-Control Study. Aoms 2019, 15, 522–530. [Google Scholar] [CrossRef]
- Zhao, T.; Gu, D.; Xu, Z.; Huo, X.; Shen, L.; Wang, C.; Tang, Y.; Wu, P.; He, J.; Gong, W.; et al. Polymorphism in One-Carbon Metabolism Pathway Affects Survival of Gastric Cancer Patients: Large and Comprehensive Study. Oncotarget 2015, 6, 9564–9576. [Google Scholar] [CrossRef][Green Version]
- Yi, K.; Yang, L.; Lan, Z.; Xi, M. The Association between MTHFR Polymorphisms and Cervical Cancer Risk: A System Review and Meta Analysis. Arch. Gynecol. Obs. 2016, 294, 579–588. [Google Scholar] [CrossRef]
- Purnomo, A.F.; Daryanto, B.; Seputra, K.P.; Budaya, T.N.; Lutfiana, N.C.; Nurkolis, F.; Chung, S.; Suh, J.Y.; Park, M.N.; Seo, B.-K.; et al. Methylenetetrahydrofolate Reductase C677T (Rs1801133) Polymorphism Is Associated with Bladder Cancer in Asian Population: Epigenetic Meta-Analysis as Precision Medicine Approach. Cancers 2023, 15, 4402. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.-M.; Deng, M.-H.; Chen, W.; Zeng, X.-T.; Luo, J. MTHFR C677T Gene Polymorphism and Head and Neck Cancer Risk: A Meta-Analysis Based on 23 Publications. Dis. Markers 2015, 2015, 681313. [Google Scholar] [CrossRef]
- Jamshidi, M.; Mohammadi Pour, S.; Mahmoudian-Sani, M.-R. Single Nucleotide Variants Associated with Colorectal Cancer Among Iranian Patients: A Narrative Review. Pharmgenomics Pers. Med. 2020, 13, 167–180. [Google Scholar] [CrossRef]
- Wang, Y.; Huo, L.; Yang, C.; He, X. Methylenetetrahydrofolate Reductase C677T and A1298C Polymorphisms and Gastric Cancer Susceptibility: An Updated Meta-Analysis. Biosci. Rep. 2023, 43, BSR20222553. [Google Scholar] [CrossRef]
- Petrone, I.; Bernardo, P.S.; Dos Santos, E.C.; Abdelhay, E. MTHFR C677T and A1298C Polymorphisms in Breast Cancer, Gliomas and Gastric Cancer: A Review. Genes 2021, 12, 587. [Google Scholar] [CrossRef]
- Akilzhanova, A.; Nurkina, Z.; Momynaliev, K.; Ramanculov, E.; Zhumadilov, Z.; Rakhypbekov, T.; Hayashida, N.; Nakashima, M.; Takamura, N. Genetic Profile and Determinants of Homocysteine Levels in Kazakhstan Patients with Breast Cancer. Anticancer Res. 2013, 33, 4049–4059. [Google Scholar]
- Ajaz, S.; Zaidi, S.-Z.; Mehboob Ali, S.; Siddiqa, A.; Memon, M.A.; Abid, A.; Khaliq, S. Independent, Diplotype and Haplotype Association Analyses of the Selected MTHFR SNPs with the Risk of Breast Cancers in a South-Asian Population. medRxiv 2021. [Google Scholar] [CrossRef]
- Zara-Lopes, T.; Gimenez-Martins, A.P.A.; Nascimento-Filho, C.H.V.; Castanhole-Nunes, M.M.U.; Galbiatti-Dias, A.L.S.; Padovani-Júnior, J.A.; Maniglia, J.V.; Francisco, J.L.E.; Pavarino, E.C.; Goloni-Bertollo, E.M. Role of MTHFR C677T and MTR A2756G Polymorphisms in Thyroid and Breast Cancer Development. Genet. Mol. Res. 2016, 15, 15028222. [Google Scholar] [CrossRef]
- Castiglia, P.; Sanna, V.; Azara, A.; De Miglio, M.R.; Murgia, L.; Pira, G.; Sanges, F.; Fancellu, A.; Carru, C.; Bisail, M.; et al. Methylenetetrahydrofolate Reductase (MTHFR) C677T and A1298C Polymorphisms in Breast Cancer: A Sardinian Preliminary Case-Control Study. Int. J. Med. Sci. 2019, 16, 1089–1095. [Google Scholar] [CrossRef]
- Kumar, P.; Yadav, U.; Rai, V. Methylenetetrahydrofolate Reductase Gene C677T Polymorphism and Breast Cancer Risk: Evidence for Genetic Susceptibility. Meta Gene 2015, 6, 72–84. [Google Scholar] [CrossRef]
- You, J.; Huang, Y.; Shen, X.; Chen, Y.; Ding, X. Associations between MTHFR Gene Polymorphisms (C677T and A1298C) and Genetic Susceptibility to Prostate Cancer: A Systematic Review and Meta-Analysis. Front. Genet. 2024, 15, 1343687. [Google Scholar] [CrossRef] [PubMed]
- Cicek, M.S.; Nock, N.L.; Li, L.; Conti, D.V.; Casey, G.; Witte, J.S. Relationship between Methylenetetrahydrofolate Reductase C677T and A1298C Genotypes and Haplotypes and Prostate Cancer Risk and Aggressiveness. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1331–1336. [Google Scholar] [CrossRef]
- Singal, R.; Ferdinand, L.; Das, P.; Reis, I.; Schlesselman, J. Polymorphisms in the Methylenetetrahydrofolate Reductase Gene and Prostate Cancer Risk. Int. J. Oncol. 2004, 25, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Stevens, V.L.; Rodriguez, C.; Sun, J.; Talbot, J.T.; Thun, M.J.; Calle, E.E. No Association of Single Nucleotide Polymorphisms in One-Carbon Metabolism Genes with Prostate Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2008, 17, 3612–3614. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Bian, C.; Lin, X.; Wang, X.; Xu, K.; Zhao, X. Methylenetetrahydrofolate Reductase Gene Polymorphisms in the Risk of Polycystic Ovary Syndrome and Ovarian Cancer. Biosci. Rep. 2020, 40, BSR20200995. [Google Scholar] [CrossRef]
- Qin, Y.-T.; Zhang, Y.; Wu, F.; Su, Y.; Lu, G.-N.; Wang, R.-S. Association between MTHFR Polymorphisms and Acute Myeloid Leukemia Risk: A Meta-Analysis. PLoS ONE 2014, 9, e88823. [Google Scholar] [CrossRef]
- Sazawal, S.; Chaubey, R.; Kaur, P.; Chikkara, S.; Kumar, B.; Bakshi, S.; Arya, L.S.; Raina, V.; Das Gupta, A.; Saxena, R. MTHFR Gene Polymorphisms and the Risk of Acute Lymphoblastic Leukemia in Adults and Children: A Case Control Study in India. Indian. J. Hematol. Blood Transfus. 2014, 30, 219–225. [Google Scholar] [CrossRef]
- Kim, H.N.; Kim, Y.-K.; Lee, I.-K.; Yang, D.-H.; Lee, J.-J.; Shin, M.-H.; Park, K.-S.; Choi, J.-S.; Park, M.R.; Jo, D.Y.; et al. Association between Polymorphisms of Folate-Metabolizing Enzymes and Hematological Malignancies. Leuk. Res. 2009, 33, 82–87. [Google Scholar] [CrossRef]
- Pereira, T.V.; Rudnicki, M.; Pereira, A.C.; Pombo-de-Oliveira, M.S.; Franco, R.F. 5,10-Methylenetetrahydrofolate Reductase Polymorphisms and Acute Lymphoblastic Leukemia Risk: A Meta-Analysis. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1956–1963. [Google Scholar] [CrossRef]
- Raoufi, A.; Rahimi Kelarijani, B.; Ahadi, H.R.; Hassani Derakhshandeh, B.; Nooroollahzadeh, Z.; Hajifathali, A. Association of MTHFR C677T and A1298C Polymorphisms with Susceptibility to Chronic Lymphocytic Leukemia: A Systematic Review and Meta-Analysis. Iran. J. Public Health 2021, 50, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Lien, S.-Y.A.; Young, L.; Gau, B.-S.; K Shiao, S.P. Meta-Prediction of MTHFR Gene Polymorphism-Mutations, Air Pollution, and Risks of Leukemia among World Populations. Oncotarget 2017, 8, 4387–4398. [Google Scholar] [CrossRef] [PubMed]
- Periñán, M.T.; Macías-García, D.; Jesús, S.; Martín-Rodríguez, J.F.; Muñoz-Delgado, L.; Jimenez-Jaraba, M.V.; Buiza-Rueda, D.; Bonilla-Toribio, M.; Adarmes-Gómez, A.D.; Gómez-Garre, P.; et al. Homocysteine Levels, Genetic Background, and Cognitive Impairment in Parkinson’s Disease. J. Neurol. 2023, 270, 477–485. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Wang, W.; Fan, W.; Tang, W.; Zhang, C. Homocysteine, but Not MTHFR Gene Polymorphism, Influences Depressive Symptoms in Patients with Schizophrenia. J. Affect. Disord. 2020, 272, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Hoxha, B.; Hoxha, M.; Domi, E.; Gervasoni, J.; Persichilli, S.; Malaj, V.; Zappacosta, B. Folic Acid and Autism: A Systematic Review of the Current State of Knowledge. Cells 2021, 10, 1976. [Google Scholar] [CrossRef]
- Khan, S.; Naeem, A.; Fritts, A.; Cummins, M.; Kayes, C.; Fang, W. Discovery of Methylenetetrahydrofolate Reductase (MTHFR) Deficiency in Individuals With Common Psychiatric Comorbidities: A Retrospective Case Review. Cureus 2024, 16, e58122. [Google Scholar] [CrossRef]
- Meng, X.; Zheng, J.-L.; Sun, M.-L.; Lai, H.-Y.; Wang, B.-J.; Yao, J.; Wang, H. Association between MTHFR (677C>T and 1298A>C) Polymorphisms and Psychiatric Disorder: A Meta-Analysis. PLoS ONE 2022, 17, e0271170. [Google Scholar] [CrossRef]
- Roufael, M.; Bitar, T.; Sacre, Y.; Andres, C.; Hleihel, W. Folate-Methionine Cycle Disruptions in ASD Patients and Possible Interventions: A Systematic Review. Genes 2023, 14, 709. [Google Scholar] [CrossRef]
- Qiu, S.; Qiu, Y.; Li, Y.; Cong, X. Genetics of Autism Spectrum Disorder: An Umbrella Review of Systematic Reviews and Meta-Analyses. Transl. Psychiatry 2022, 12, 249. [Google Scholar] [CrossRef]
- Li, Y.; Qiu, S.; Shi, J.; Guo, Y.; Li, Z.; Cheng, Y.; Liu, Y. Association between MTHFR C677T/A1298C and Susceptibility to Autism Spectrum Disorders: A Meta-Analysis. BMC Pediatr. 2020, 20, 449. [Google Scholar] [CrossRef]
- Razi, B.; Imani, D.; Hassanzadeh Makoui, M.; Rezaei, R.; Aslani, S. Association between MTHFR Gene Polymorphism and Susceptibility to Autism Spectrum Disorders: Systematic Review and Meta-Analysis. Res. Autism Spectr. Disord. 2020, 70, 101473. [Google Scholar] [CrossRef]
- Tisato, V.; Silva, J.A.; Longo, G.; Gallo, I.; Singh, A.V.; Milani, D.; Gemmati, D. Genetics and Epigenetics of One-Carbon Metabolism Pathway in Autism Spectrum Disorder: A Sex-Specific Brain Epigenome? Genes 2021, 12, 782. [Google Scholar] [CrossRef] [PubMed]
- Lam, N.S.K.; Long, X.X.; Li, X.; Saad, M.; Lim, F.; Doery, J.C.; Griffin, R.C.; Galletly, C. The Potential Use of Folate and Its Derivatives in Treating Psychiatric Disorders: A Systematic Review. Biomed. Pharmacother. 2022, 146, 112541. [Google Scholar] [CrossRef]
- You, M.; Zhou, X.; Yin, W.; Wan, K.; Zhang, W.; Li, C.; Li, M.; Zhu, W.; Zhu, X.; Sun, Z. The Influence of MTHFR Polymorphism on Gray Matter Volume in Patients With Amnestic Mild Cognitive Impairment. Front. Neurosci. 2021, 15, 778123. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xiao, X.; Wen, Y.; Wan, M.; Zhou, L.; Liu, X.; Wang, X.; Guo, L.; Liu, H.; Zhou, Y.; et al. Genetic Effect of MTHFR C677T, A1298C, and A1793G Polymorphisms on the Age at Onset, Plasma Homocysteine, and White Matter Lesions in Alzheimer’s Disease in the Chinese Population. Aging 2021, 13, 11352–11362. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, X.; Zhao, M.; Ma, L.; Pei, H.; Liu, N.; Li, H. Association of Methylenetetrahydrofolate Reductase C677T Gene Polymorphisms with Mild Cognitive Impairment Susceptibility: A Systematic Review and Meta-Analysis. Behav. Neurol. 2021, 2021, 2962792. [Google Scholar] [CrossRef]
- Yoo, S.; Montazeri, A.; McNulty, H.; Kent, M.P.; Little, J. Is There a Causal Link between Folate Status and Schizophrenia? Evidence from Genetic Association Studies. Nutr. Neurosci. 2024, 2024, 2436822. [Google Scholar] [CrossRef]
- Zhang, Y.-X.; Yang, L.-P.; Gai, C.; Cheng, C.-C.; Guo, Z.-Y.; Sun, H.-M.; Hu, D. Association between Variants of MTHFR Genes and Psychiatric Disorders: A Meta-Analysis. Front. Psychiatry 2022, 13, 976428. [Google Scholar] [CrossRef]
- Liu, J.H.; Zhu, C.; Zheng, K.; Tang, W.; Gao, L.L.; Trihn, T.H.; Emily Wu, H.; Chen, D.C.; Hong Xiu, M.; Yang Zhang, X. MTHFR Ala222Val Polymorphism and Clinical Characteristics Confer Susceptibility to Suicide Attempt in Chronic Patients with Schizophrenia. Sci. Rep. 2020, 10, 5008. [Google Scholar] [CrossRef]
- Halaris, A.; Sohl, E.; Whitham, E.A. Treatment-Resistant Depression Revisited: A Glimmer of Hope. J. Pers. Med. 2021, 11, 155. [Google Scholar] [CrossRef]
- Asif, N.; Patel, A.; Vedantam, D.; Poman, D.S.; Motwani, L. Migraine With Comorbid Depression: Pathogenesis, Clinical Implications, and Treatment. Cureus 2022, 14, e25998. [Google Scholar] [CrossRef]
- Vuletić, V.; Rački, V.; Papić, E.; Peterlin, B. A Systematic Review of Parkinson’s Disease Pharmacogenomics: Is There Time for Translation into the Clinics? Int. J. Mol. Sci. 2021, 22, 7213. [Google Scholar] [CrossRef]
- Sadeghiyeh, T.; Dastgheib, S.A.; Lookzadeh, M.H.; Noori-Shadkam, M.; Akbarian-Bafghi, M.J.; Zare-Shehneh, M.; Poursharif, Z.; Neamatzadeh, H. Association of MTHFR 677C > T and 1298A > C Polymorphisms with Susceptibility to Attention Deficit and Hyperactivity Disorder. Fetal Pediatr. Pathol. 2020, 39, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Augoulea, A.; Armeni, E.; Deligeoroglou, E.; Paschou, S.A.; Papadimitriou, G.; Stergioti, E.; Karountzos, V.; Tsitsika, A.; Panoulis, K.; Economou, E.; et al. MTHFR Polymorphisms in Girls with Anorexia Nervosa: Implications on Body Weight. Endocr. Res. 2021, 46, 80–85. [Google Scholar] [CrossRef]
- Global Diabetes Data Report 2000–2045. Available online: https://diabetesatlas.org/data/en/world/ (accessed on 12 March 2025).
- Cai, Y.; Liu, B.; Zhang, Y.; Zhou, Y. MTHFR Gene Polymorphisms in Diabetes Mellitus. Clin. Chim. Acta 2024, 561, 119825. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Facts and Figures. Available online: https://idf.org/about-diabetes/diabetes-facts-figures/ (accessed on 12 March 2025).
- Bigagli, E.; Lodovici, M. Circulating Oxidative Stress Biomarkers in Clinical Studies on Type 2 Diabetes and Its Complications. Oxid. Med. Cell. Longev. 2019, 2019, 5953685. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.S.; Epstein, S.; Corkey, B.E.; Grant, S.F.A.; Gavin Iii, J.R.; Aguilar, R.B.; Herman, M.E. A Unified Pathophysiological Construct of Diabetes and Its Complications. Trends Endocrinol. Metab. 2017, 28, 645–655. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, R.; Wang, D.; Liu, X. Genetics of Diabetic Neuropathy: Systematic Review, Meta-Analysis and Trial Sequential Analysis. Ann. Clin. Transl. Neurol. 2019, 6, 1996–2013. [Google Scholar] [CrossRef]
- Meng, Y.; Liu, X.; Ma, K.; Zhang, L.; Lu, M.; Zhao, M.; Guan, M.; Qin, G. Association of MTHFR C677T Polymorphism and Type 2 Diabetes Mellitus (T2DM) Susceptibility. Mol. Gen. Gen. Med. 2019, 7, e1020. [Google Scholar] [CrossRef]
- Pathak, D.; Shrivastav, D.; Verma, A.K.; Alsayegh, A.A.; Yadav, P.; Khan, N.H.; Al-Harbi, A.I.; Khan, M.I.; Bihade, K.; Singh, D.D.; et al. Role of Metabolizing MTHFR Gene Polymorphism (Rs1801133) and Its mRNA Expression among Type 2 Diabetes. J. Diabetes Metab. Disord. 2022, 21, 511–516. [Google Scholar] [CrossRef]
- Rahimi, A.; Moridi, N.; Golestani, A.; Anani-Sarab, G.; Salmani, F.; Yaqubi, G.; Mesbahzadeh, B.; Jalalifar, M.A.; Malekaneh, M.; Sajjadi, S.M. Factor V Leiden, MTHFR, and FXIIIVal34Leu Gene Polymorphisms and Their Association with Clinical Features and Risk of Diabetic Retinopathy in Patients with Type 2 Diabetes. Casp. J. Intern. Med. 2024, 15, 101–108. [Google Scholar] [CrossRef]
- Santos, K.F.; Assunção, L.P.; Santos, R.S.; Reis, A.A.S. Machine Learning Approaches and Genetic Determinants That Influence the Development of Type 2 Diabetes Mellitus: A Genetic Association Study in Brazilian Patients. Braz. J. Med. Biol. Res. 2024, 57, e13957. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liang, H.; Yang, S.; Wang, J.; Xie, L.; Qin, X.; Li, S. Methylenetetrahydrofolate Reductase A1298C Polymorphism and Diabetes Risk: Evidence from a Meta-Analysis. Ren. Fail 2014, 36, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Huang, R.; Li, S.; Fang, A.; Chen, Y.; Chen, S.; Wang, F.; Lin, X.; Liu, Z.; Zhu, H. Associations between Serum Betaine, Methyl-Metabolizing Genetic Polymorphisms and Risk of Incident Type 2 Diabetes: A Prospective Cohort Study in Community-Dwelling Chinese Adults. Nutrients 2022, 14, 362. [Google Scholar] [CrossRef]
- Guan, H.; Xia, M.-D.; Wang, M.; Guan, Y.-J.; Lyu, X.-C. Methylenetetrahydrofolate Reductase Genetic Polymorphism and the Risk of Diabetic Nephropathy in Type 2 Diabetic Patients. Medicine 2020, 99, e21558. [Google Scholar] [CrossRef]
- Elqadi, M.; Eweidat, K.; Abu Sabha, M.; Yagmour, A.; Sabarneh, A.; Nasereddin, A.; Ereqat, S. Methylenetetrahydrofolate Reductase C677T Gene Polymorphism and the Association with Dyslipidemia in Type 2 Diabetic Palestinian Patients. J. Clin. Lab. Anal. 2021, 35, e23994. [Google Scholar] [CrossRef]
- Settin, A.; El-Baz, R.; Ismaeel, A.; Tolba, W.; Allah, W.A. Association of ACE and MTHFR Genetic Polymorphisms with Type 2 Diabetes Mellitus: Susceptibility and Complications. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 838–843. [Google Scholar] [CrossRef]
- Luo, S.; Wang, F.; Shi, C.; Wu, Z. A Meta-Analysis of Association between Methylenetetrahydrofolate Reductase Gene (MTHFR) 677C/T Polymorphism and Diabetic Retinopathy. Int. J. Environ. Res. Public Health 2016, 13, 806. [Google Scholar] [CrossRef]
- Yehya, A.; Altaany, Z.; Beni-Yonis, O. Dual Mapping of MTHFR C677T (A1298C) and BDNF G196A (Val66Met) Polymorphisms in Patients with Diabetic Peripheral Neuropathy. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 6682–6690. [Google Scholar] [CrossRef]
- Sweeting, A.; Wong, J.; Murphy, H.R.; Ross, G.P. A Clinical Update on Gestational Diabetes Mellitus. Endocr. Rev. 2022, 43, 763–793. [Google Scholar] [CrossRef]
- Dai, C.; Fei, Y.; Li, J.; Shi, Y.; Yang, X. A Novel Review of Homocysteine and Pregnancy Complications. Biomed. Res. Int. 2021, 2021, 6652231. [Google Scholar] [CrossRef]
- Tan, X.; Chen, H. Association between MTHFR Gene C677T Polymorphism and Gestational Diabetes Mellitus in Chinese Population: A Meta-Analysis. Front. Endocrinol. 2023, 14, 1273218. [Google Scholar] [CrossRef]
- Pham, N.T.N.; Huynh, C.T.N.; Nguyen, A.T.T.; Ho, C.Q.; Duong, L.M.; Bui, D.T.; Nguyen, H.H. Pre-Gestational Diabetes Mellitus, Gestational Diabetes Mellitus, and Its Association with the MTHFR C677T Polymorphism. Medicine 2024, 103, e38648. [Google Scholar] [CrossRef] [PubMed]
- Dias, S.; Adam, S.; Rheeder, P.; Pheiffer, C. No Association Between ADIPOQ or MTHFR Polymorphisms and Gestational Diabetes Mellitus in South African Women. Diabetes Metab. Syndr. Obes. 2021, 14, 791–800. [Google Scholar] [CrossRef]
- Ulloque-Badaracco, J.R.; Hernandez-Bustamante, E.A.; Alarcon-Braga, E.A.; Al-Kassab-Córdova, A.; Cabrera-Guzmán, J.C.; Herrera-Añazco, P.; Benites-Zapata, V.A. Vitamin B12, Folate, and Homocysteine in Metabolic Syndrome: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2023, 14, 1221259. [Google Scholar] [CrossRef]
- Azizi, S.; Shamshirian, A.; Alizadeh-Navaei, R.; Jafarpour, H.; Asemi, Z.; Tamtaji, O.R.; Vaziri, M.S.; Homayounfar, R.; Rezaei Shahmirzadi, A.; Alipoor, R. A Genetic Association Study of MTHFR C677T Polymorphism with Risk of Metabolic Syndrome: A Systematic Review and Meta-Analysis. Galen Med. J. 2019, 8, e1472. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, L.; Xia, H.; Li, Y.; Tang, S. Association of MTHFR C677T Gene Polymorphism with Metabolic Syndrome in a Chinese Population: A Case-Control Study. J. Int. Med. Res. 2018, 46, 2658–2669. [Google Scholar] [CrossRef]
- Wang, J.; You, D.; Wang, H.; Yang, Y.; Zhang, D.; Lv, J.; Luo, S.; Liao, R.; Ma, L. Association between Homocysteine and Obesity: A Meta-Analysis. J. Evid. Based Med. 2021, 14, 208–217. [Google Scholar] [CrossRef]
- Fu, L.; Li, Y.-N.; Luo, D.; Deng, S.; Hu, Y.-Q. Plausible Relationship between Homocysteine and Obesity Risk via MTHFR Gene: A Meta-Analysis of 38,317 Individuals Implementing Mendelian Randomization. Diabetes Metab. Syndr. Obes. 2019, 12, 1201–1212. [Google Scholar] [CrossRef]
- Leal-Ugarte, E.; Peralta-Leal, V.; Meza-Espinoza, J.P.; Durán-González, J.; Macías-Gómez, N.; Bocanegra-Alonso, A.; Lara-Ramos, J.R. Association of the MTHFR 677C>T Polymorphism with Obesity and Biochemical Variables in a Young Population of Mexico. J. Med. Biochem. 2019, 38, 461–467. [Google Scholar] [CrossRef]
- Pouwels, S.; Sakran, N.; Graham, Y.; Leal, A.; Pintar, T.; Yang, W.; Kassir, R.; Singhal, R.; Mahawar, K.; Ramnarain, D. Non-Alcoholic Fatty Liver Disease (NAFLD): A Review of Pathophysiology, Clinical Management and Effects of Weight Loss. BMC Endocr. Disord. 2022, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Nassir, F. NAFLD: Mechanisms, Treatments, and Biomarkers. Biomolecules 2022, 12, 824. [Google Scholar] [CrossRef]
- Yuan, S.; Chen, J.; Dan, L.; Xie, Y.; Sun, Y.; Li, X.; Larsson, S.C. Homocysteine, Folate, and Nonalcoholic Fatty Liver Disease: A Systematic Review with Meta-Analysis and Mendelian Randomization Investigation. Am. J. Clin. Nutr. 2022, 116, 1595–1609. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhu, J.; Meng, D.; Yu, C.; Li, Y. Association of Homocysteine Level with Biopsy-Proven Non-Alcoholic Fatty Liver Disease: A Meta-Analysis. J. Clin. Biochem. Nutr. 2016, 58, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.-Y.; Zhang, L.; Shi, S.-L.; Lin, J.-N. Associations between Methylenetetrahydrofolate Reductase (MTHFR) Polymorphisms and Non-Alcoholic Fatty Liver Disease (NAFLD) Risk: A Meta-Analysis. PLoS ONE 2016, 11, e0154337. [Google Scholar] [CrossRef]
- Hao, X.; Ma, C.; Xiang, T.; Ou, L.; Zeng, Q. Associations Among Methylene Tetrahydrofolate Reductase Rs1801133 C677T Gene Variant, Food Groups, and Non-Alcoholic Fatty Liver Disease Risk in the Chinese Population. Front. Genet. 2021, 12, 568398. [Google Scholar] [CrossRef]
- De Vincentis, A.; Mancina, R.M.; Pihlajamäki, J.; Männistö, V.; Petta, S.; Dongiovanni, P.; Fracanzani, A.L.; Valenti, L.; Tavaglione, F.; Romeo, S.; et al. Genetic Variants in the MTHFR Are Not Associated with Fatty Liver Disease. Liver Int. 2020, 40, 1934–1940. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Y.; Zhang, M.; Wang, Y.; Qin, B. The Methylenetetrahydrofolate Reductase Genotype 677CT and Non-Alcoholic Fatty Liver Disease Have a Synergistic Effect on the Increasing Homocysteine Levels in Subjects from Chongqing, China. Genes Dis. 2019, 6, 88–95. [Google Scholar] [CrossRef]
- Levine, A.; Koletzko, S.; Turner, D.; Escher, J.C.; Cucchiara, S.; de Ridder, L.; Kolho, K.-L.; Veres, G.; Russell, R.K.; Paerregaard, A.; et al. ESPGHAN Revised Porto Criteria for the Diagnosis of Inflammatory Bowel Disease in Children and Adolescents. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 795–806. [Google Scholar] [CrossRef]
- Mak, W.Y.; Zhao, M.; Ng, S.C.; Burisch, J. The Epidemiology of Inflammatory Bowel Disease: East Meets West. J. Gastroenterol. Hepatol. 2020, 35, 380–389. [Google Scholar] [CrossRef]
- Uhlig, H.H.; Charbit-Henrion, F.; Kotlarz, D.; Shouval, D.S.; Schwerd, T.; Strisciuglio, C.; de Ridder, L.; van Limbergen, J.; Macchi, M.; Snapper, S.B.; et al. Clinical Genomics for the Diagnosis of Monogenic Forms of Inflammatory Bowel Disease: A Position Paper From the Paediatric IBD Porto Group of European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 456–473. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Rodriguez-Guéant, R.-M.; Chamaillard, M.; Desreumaux, P.; Xia, B.; Bronowicki, J.-P.; Bigard, M.-A.; Guéant, J.-L. Vascular and Cellular Stress in Inflammatory Bowel Disease: Revisiting the Role of Homocysteine. Am. J. Gastroenterol. 2007, 102, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Varzari, A.; Deyneko, I.V.; Tudor, E.; Turcan, S. Polymorphisms of Glutathione S-Transferase and Methylenetetrahydrofolate Reductase Genes in Moldavian Patients with Ulcerative Colitis: Genotype-Phenotype Correlation. Meta Gene 2016, 7, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Karban, A.; Feldman, T.; Waterman, M.; Leiba, R.; Efrati, E. The Association of the MTHFR C677T Polymorphism with Inflammatory Bowel Diseases in the Israeli Jewish Population: An Example of Genetic Heterogeneity. Medicine 2016, 95, e5611. [Google Scholar] [CrossRef]
- Servy, E.J.; Jacquesson-Fournols, L.; Cohen, M.; Menezo, Y.J.R. MTHFR Isoform Carriers. 5-MTHF (5-Methyl Tetrahydrofolate) vs Folic Acid: A Key to Pregnancy Outcome: A Case Series. J. Assist. Reprod. Genet. 2018, 35, 1431–1435. [Google Scholar] [CrossRef]
- Erdoğan, K.; Sanlier, N.T.; Sanlier, N. Are Epigenetic Mechanisms and Nutrition Effective in Male and Female Infertility? J. Nutr. Sci. 2023, 12, e103. [Google Scholar] [CrossRef] [PubMed]
- More, A.; Anjankar, N.; Shrivastava, J.; Nair, N.; Jadhav, R. Correlation of MTHFR C677T Polymorphism with Male Infertility among Indian Population: Case-Control Study. J. Pharm. Bioallied Sci. 2024, 16, S2809–S2814. [Google Scholar] [CrossRef]
- Ménézo, Y.; Patrizio, P.; Alvarez, S.; Amar, E.; Brack, M.; Brami, C.; Chouteau, J.; Clement, A.; Clement, P.; Cohen, M.; et al. MTHFR (Methylenetetrahydrofolate Reductase: EC 1.5.1.20) SNPs (Single-Nucleotide Polymorphisms) and Homocysteine in Patients Referred for Investigation of Fertility. J. Assist. Reprod. Genet. 2021, 38, 2383–2389. [Google Scholar] [CrossRef]
- Liu, K.; Zhao, R.; Shen, M.; Ye, J.; Li, X.; Huang, Y.; Hua, L.; Wang, Z.; Li, J. Role of Genetic Mutations in Folate-Related Enzyme Genes on Male Infertility. Sci. Rep. 2015, 5, 15548. [Google Scholar] [CrossRef]
- Ren, F.; Fang, G.; Zhang, Z. Association between Methylenetetrahydrofolate Reductase C677T Polymorphisms and Male Oligozoospermia, Asthenozoospermia or Oligoasthenozoospermia: A Case–Control Study. Sci. Rep. 2024, 14, 25219. [Google Scholar] [CrossRef]
- Gong, M.; Dong, W.; He, T.; Shi, Z.; Huang, G.; Ren, R.; Huang, S.; Qiu, S.; Yuan, R. MTHFR 677C>T Polymorphism Increases the Male Infertility Risk: A Meta-Analysis Involving 26 Studies. PLoS ONE 2015, 10, e0121147. [Google Scholar] [CrossRef]
- Thakur, P.; Bhalerao, A. High Homocysteine Levels During Pregnancy and Its Association with Placenta-Mediated Complications: A Scoping Review. Cureus 2023, 15, e35244. [Google Scholar] [CrossRef]
- Ota, K.; Takahashi, T.; Han, A.; Damvaeba, S.; Mizunuma, H.; Kwak-Kim, J. Effects of MTHFR C677T Polymorphism on Vitamin D, Homocysteine and Natural Killer Cell Cytotoxicity in Women with Recurrent Pregnancy Losses. Hum. Reprod. 2020, 35, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
- Basha, A.; Alkhatib, Y.; Tashtoush, T.; Yousef, M.; Oweidi, L.; Alkhatib, M.; Al-Aqrabawi, S.; Jarrar, Y.; Awidi, A. Recurrent Early Pregnancy Loss and Congenital Thrombophilia: A Prospective Study. J. Clin. Med. 2024, 13, 6871. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Vishvkarma, R.; Singh, K.; Rajender, S. MTHFR 1298A>C Substitution Is a Strong Candidate for Analysis in Recurrent Pregnancy Loss: Evidence from 14,289 Subjects. Reprod. Sci. 2022, 29, 1039–1053. [Google Scholar] [CrossRef]
- Pritchard, A.M.; Hendrix, P.W.; Paidas, M.J. Hereditary Thrombophilia and Recurrent Pregnancy Loss. Clin. Obs. Gynecol. 2016, 59, 487–497. [Google Scholar] [CrossRef]
- Chen, H.; Yang, X.; Lu, M. Methylenetetrahydrofolate Reductase Gene Polymorphisms and Recurrent Pregnancy Loss in China: A Systematic Review and Meta-Analysis. Arch. Gynecol. Obs. 2016, 293, 283–290. [Google Scholar] [CrossRef]
- Memon, S.I.; Acharya, N.S. The Association Between Serum Homocysteine Levels and Placenta-Mediated Complications: A Narrative Review. Cureus 2022, 14, e31305. [Google Scholar] [CrossRef]
- Dymara-Konopka, W.; Laskowska, M. The Role of Nitric Oxide, ADMA, and Homocysteine in The Etiopathogenesis of Preeclampsia—Review. Int. J. Mol. Sci. 2019, 20, 2757. [Google Scholar] [CrossRef]
- Raijmakers, M.T.; Zusterzeel, P.L.; Steegers, E.A.; Peters, W.H. Hyperhomocysteinaemia: A Risk Factor for Preeclampsia? Eur. J. Obs. Gynecol. Reprod. Biol. 2001, 95, 226–228. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, J.; Wang, X.; Gu, H. High Level of Homocysteine Is Associated with Pre-Eclampsia Risk in Pregnant Woman: A Meta-Analysis. Gynecol. Endocrinol. 2022, 38, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.; Younas, H.; Younus, A.; Nathenial, S. A Narrative Review on the Role of Folate-Mediated One-Carbon Metabolism and Its Associated Gene Polymorphisms in Posing Risk to Preeclampsia. Clin. Exp. Hypertens. 2021, 43, 487–504. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Gao, F.; Qiu, Y.; Bao, J.; Gu, X.; Long, Y.; Liu, F.; Cai, M.; Liu, H. Association of Maternal Serum Homocysteine Concentration Levels in Late Stage of Pregnancy with Preterm Births: A Nested Case-Control Study. J. Matern. Fetal Neonatal Med. 2018, 31, 2673–2677. [Google Scholar] [CrossRef]
- Huang, L.-L.; Tong, J.-R.; Huang, Y.; Wei, Y.-N.; Chen, H.-F.; Chen, Y.; Su, J.-Y.; Deng, L. Association of MTHFR Gene C677T Polymorphism with Pregnancy Outcome. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 166–171. [Google Scholar] [CrossRef]
- Wang, B.J.; Liu, M.J.; Wang, Y.; Dai, J.R.; Tao, J.Y.; Wang, S.N.; Zhong, N.; Chen, Y. Association between SNPs in Genes Involved in Folate Metabolism and Preterm Birth Risk. Genet. Mol. Res. 2015, 14, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Dević Pavlić, S.; Šverko, R.; Barišić, A.; Mladenić, T.; Vraneković, J.; Stanković, A.; Peterlin, A.; Peterlin, B.; Ostojić, S.; Pereza, N. MTHFR Gene Polymorphisms and DNA Methylation in Idiopathic Spontaneous Preterm Birth. Medicina 2024, 60, 2028. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, P.; Geng, X.; Liu, Z.; Cui, L.; Gao, Z.; Jiang, B.; Yang, L. Genetic Polymorphism of MTHFR C677T with Preterm Birth and Low Birth Weight Susceptibility: A Meta-Analysis. Arch. Gynecol. Obstet. 2017, 295, 1105–1118. [Google Scholar] [CrossRef]
- Tiwari, D.; Bose, P.D.; Das, S.; Das, C.R.; Datta, R.; Bose, S. MTHFR (C677T) Polymorphism and PR (PROGINS) Mutation as Genetic Factors for Preterm Delivery, Fetal Death and Low Birth Weight: A Northeast Indian Population Based Study. Meta Gene 2015, 3, 31–42. [Google Scholar] [CrossRef]
- Steele, J.W.; Kim, S.-E.; Finnell, R.H. One Carbon Metabolism and Folate Transporter Genes: Do They Factor Prominently in the Genetic Etiology of Neural Tube Defects? Biochimie 2020, 173, 27–32. [Google Scholar] [CrossRef]
- Wang, X.; Yu, J.; Wang, J. Neural Tube Defects and Folate Deficiency: Is DNA Repair Defective? Int. J. Mol. Sci. 2023, 24, 2220. [Google Scholar] [CrossRef]
- Kakebeen, A.D.; Niswander, L. Micronutrient Imbalance and Common Phenotypes in Neural Tube Defects. Genesis 2021, 59, e23455. [Google Scholar] [CrossRef]
- Almekkawi, A.K.; AlJardali, M.W.; Daadaa, H.M.; Lane, A.L.; Worner, A.R.; Karim, M.A.; Scheck, A.C.; Frye, R.E. Folate Pathway Gene Single Nucleotide Polymorphisms and Neural Tube Defects: A Systematic Review and Meta-Analysis. J. Pers. Med. 2022, 12, 1609. [Google Scholar] [CrossRef]
- Li, J.; Feng, D.; He, S.; Yang, H.; Su, Z.; Ye, H. Association of MTHFR 677C > T Gene Polymorphism with Neonatal Defects: A Meta-Analysis of 81444 Subjects. J Obs. Gynaecol. 2022, 42, 1811–1822. [Google Scholar] [CrossRef]
- Soleimani-Jadidi, S.; Meibodi, B.; Javaheri, A.; Tabatabaei, R.S.; Hadadan, A.; Zanbagh, L.; Abbasi, H.; Bahrami, R.; Mirjalili, S.R.; Karimi-Zarchi, M.; et al. Association between Fetal MTHFR A1298C (Rs1801131) Polymorphism and Neural Tube Defects Risk: A Systematic Review and Meta-Analysis. Fetal Pediatr. Pathol. 2022, 41, 116–133. [Google Scholar] [CrossRef] [PubMed]
- Menezo, Y.; Elder, K.; Clement, A.; Clement, P. Folic Acid, Folinic Acid, 5 Methyl TetraHydroFolate Supplementation for Mutations That Affect Epigenesis through the Folate and One-Carbon Cycles. Biomolecules 2022, 12, 197. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzi, E.; Tiso, G.; Di Martino, D. Folic Acid versus 5- Methyl Tetrahydrofolate Supplementation in Pregnancy. Eur. J. Obs. Gynecol. Reprod. Biol. 2020, 253, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Samaniego-Vaesken, M.d.L.; Morais-Moreno, C.; Carretero-Krug, A.; Puga, A.M.; Montero-Bravo, A.M.; Partearroyo, T.; Gregorio, V.-M. Supplementation with Folic Acid or 5-Methyltetrahydrofolate and Prevention of Neural Tube Defects: An Evidence-Based Narrative Review. Nutrients 2024, 16, 3154. [Google Scholar] [CrossRef]
- Radu, A.-F.; Bungau, S.G. Management of Rheumatoid Arthritis: An Overview. Cells 2021, 10, 2857. [Google Scholar] [CrossRef]
- Yadav, U.; Kumar, P.; Gupta, S.; Rai, V. Distribution of MTHFR C677T Gene Polymorphism in Healthy North Indian Population and an Updated Meta-Analysis. Ind. J. Clin. Biochem. 2017, 32, 399–410. [Google Scholar] [CrossRef]
- Rosenberg, N.; Murata, M.; Ikeda, Y.; Opare-Sem, O.; Zivelin, A.; Geffen, E.; Seligsohn, U. The Frequent 5,10-Methylenetetrahydrofolate Reductase C677T Polymorphism Is Associated with a Common Haplotype in Whites, Japanese, and Africans. Am. J. Hum. Genet. 2002, 70, 758–762. [Google Scholar] [CrossRef]
- Cen, H.; Huang, H.; Zhang, L.-N.; Liu, L.-Y.; Zhou, L.; Xin, X.-F.; Zhuo, R.-J. Associations of Methylenetetrahydrofolate Reductase (MTHFR) C677T and A1298C Polymorphisms with Genetic Susceptibility to Rheumatoid Arthritis: A Meta-Analysis. Clin. Rheumatol. 2017, 36, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Bagheri-Hosseinabadi, Z.; Imani, D.; Yousefi, H.; Abbasifard, M. MTHFR Gene Polymorphisms and Susceptibility to Rheumatoid Arthritis: A Meta-Analysis Based on 16 Studies. Clin. Rheumatol. 2020, 39, 2267–2279. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Shao, W.; Li, Y. Associations between C677T and A1298C Polymorphisms of MTHFR and Susceptibility to Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Rheumatol. Int. 2017, 37, 557–569. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araszkiewicz, A.F.; Jańczak, K.; Wójcik, P.; Białecki, B.; Kubiak, S.; Szczechowski, M.; Januszkiewicz-Lewandowska, D. MTHFR Gene Polymorphisms: A Single Gene with Wide-Ranging Clinical Implications—A Review. Genes 2025, 16, 441. https://doi.org/10.3390/genes16040441
Araszkiewicz AF, Jańczak K, Wójcik P, Białecki B, Kubiak S, Szczechowski M, Januszkiewicz-Lewandowska D. MTHFR Gene Polymorphisms: A Single Gene with Wide-Ranging Clinical Implications—A Review. Genes. 2025; 16(4):441. https://doi.org/10.3390/genes16040441
Chicago/Turabian StyleAraszkiewicz, Antoni F., Krzysztof Jańczak, Paweł Wójcik, Bartłomiej Białecki, Szymon Kubiak, Michał Szczechowski, and Danuta Januszkiewicz-Lewandowska. 2025. "MTHFR Gene Polymorphisms: A Single Gene with Wide-Ranging Clinical Implications—A Review" Genes 16, no. 4: 441. https://doi.org/10.3390/genes16040441
APA StyleAraszkiewicz, A. F., Jańczak, K., Wójcik, P., Białecki, B., Kubiak, S., Szczechowski, M., & Januszkiewicz-Lewandowska, D. (2025). MTHFR Gene Polymorphisms: A Single Gene with Wide-Ranging Clinical Implications—A Review. Genes, 16(4), 441. https://doi.org/10.3390/genes16040441





