Physiological and Transcriptomic Analyses Unveil the Preservation Mechanism of Streptomyces albulus Ah11601 Fermentation Broth on ‘Shine Muscat’ Grapes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Grape Samples and Treatments
2.2. Determination of Hardness and Vitamin C Content
2.3. Determination of Malondialdehyde and Hydrogen Peroxide Content and the Superoxide Radical Production Rate
2.4. Enzyme Assays
2.5. Determination of the Respiration Rate and Ethylene Production
2.6. RNA Extraction, Library Construction, Sequencing, and Data Processing
2.7. Data Statistics and Analysis
3. Results
3.1. Effects of S. albulus Ah11601 Fermentation Broth (SFB) Treatment on the Hardness and Vitamin C (Vc) Content of ‘Shine Muscat’ Grapes
3.2. Effects of SFB Treatment on the Lipooxygenase Activity and Malondialdehyde Content in ‘Shine Muscat’ Grapes
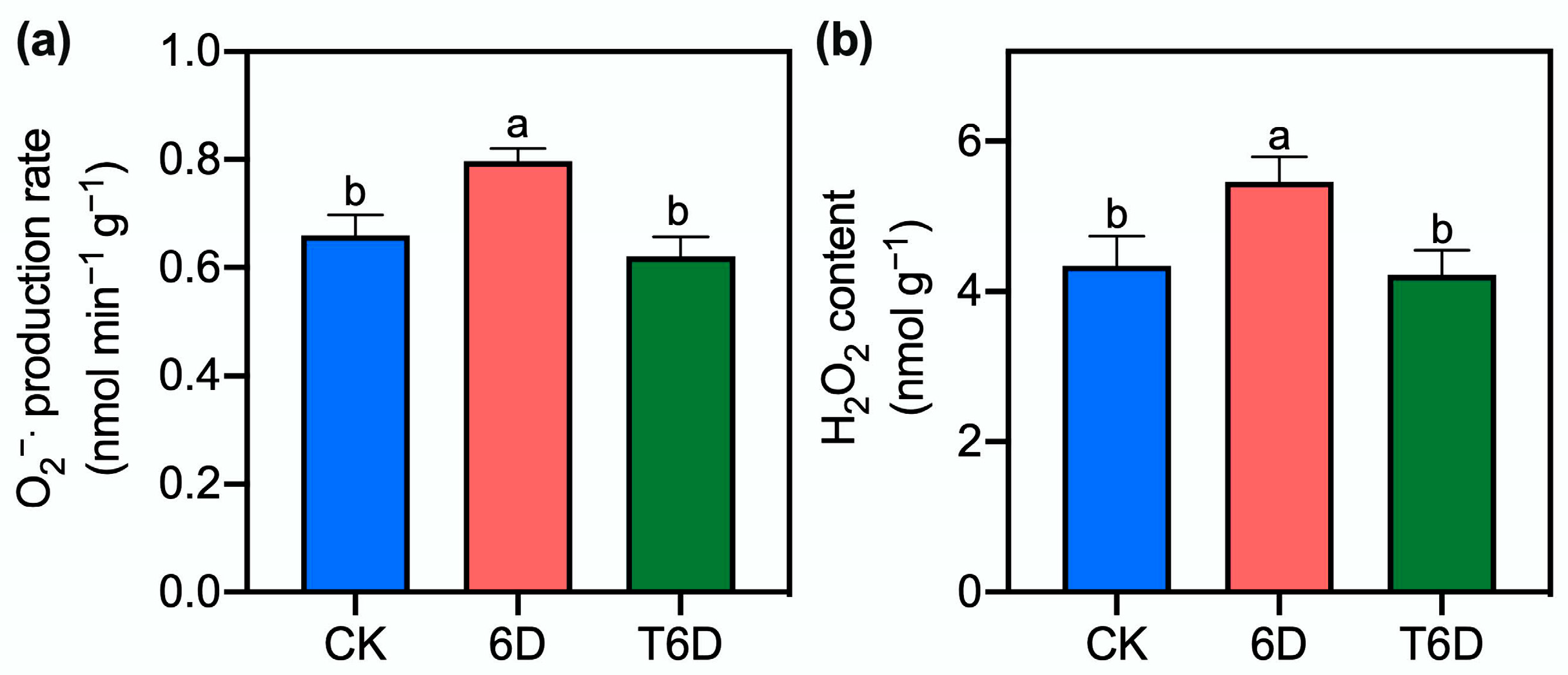
3.3. Effects of SFB Treatment on the Generation Rate of Superoxide Radicals and Content of Hydrogen Peroxide in ‘Shine Muscat’ Grapes
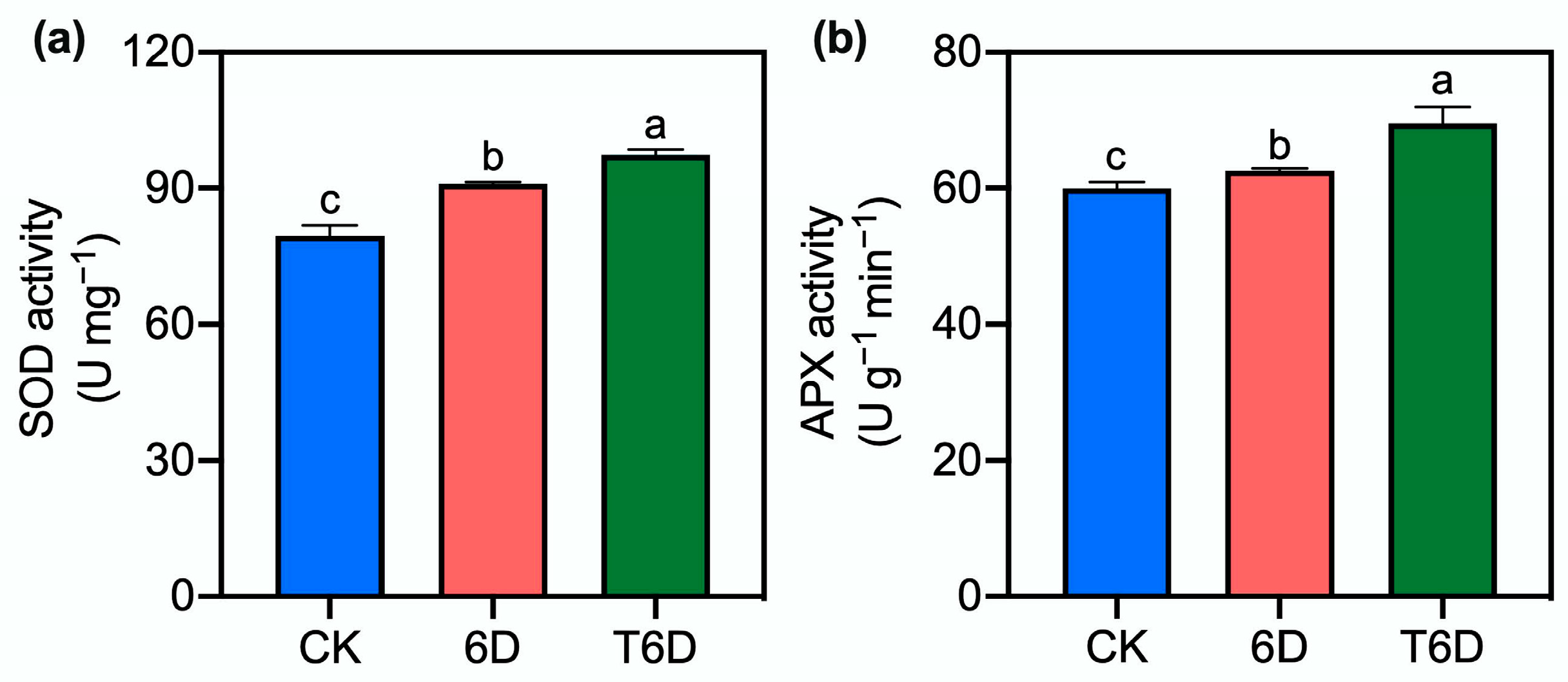
3.4. Effects of SFB Treatment on Superoxide Dismutase and Ascorbate Peroxidase Enzyme Activities in ‘Shine Muscat’ Grapes
3.5. Effects of SFB Treatment on the Respiratory Rate and Ethylene Production in ‘Shine Muscat’ Grapes
3.6. Transcriptome Data Assembly and Functional Annotation
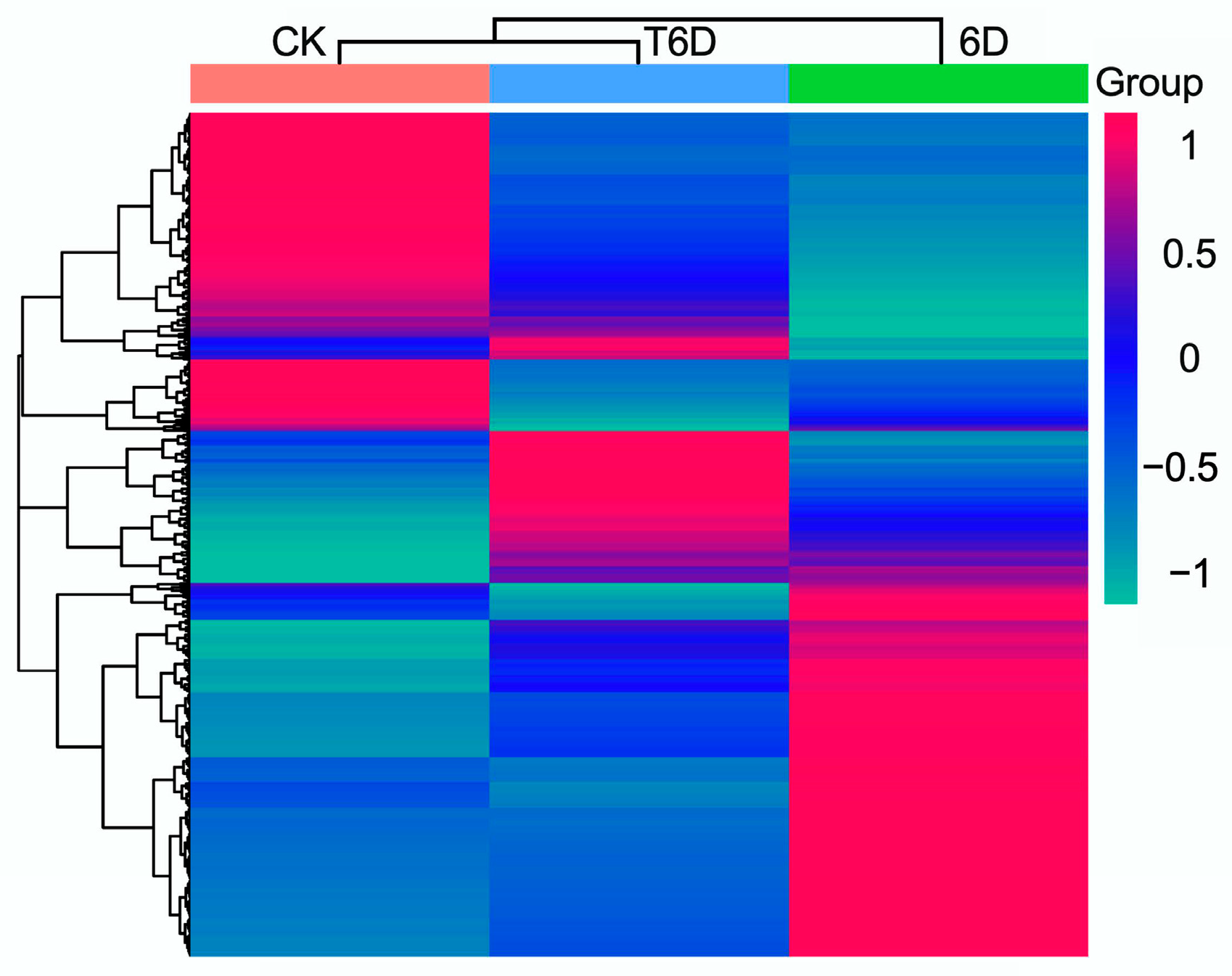
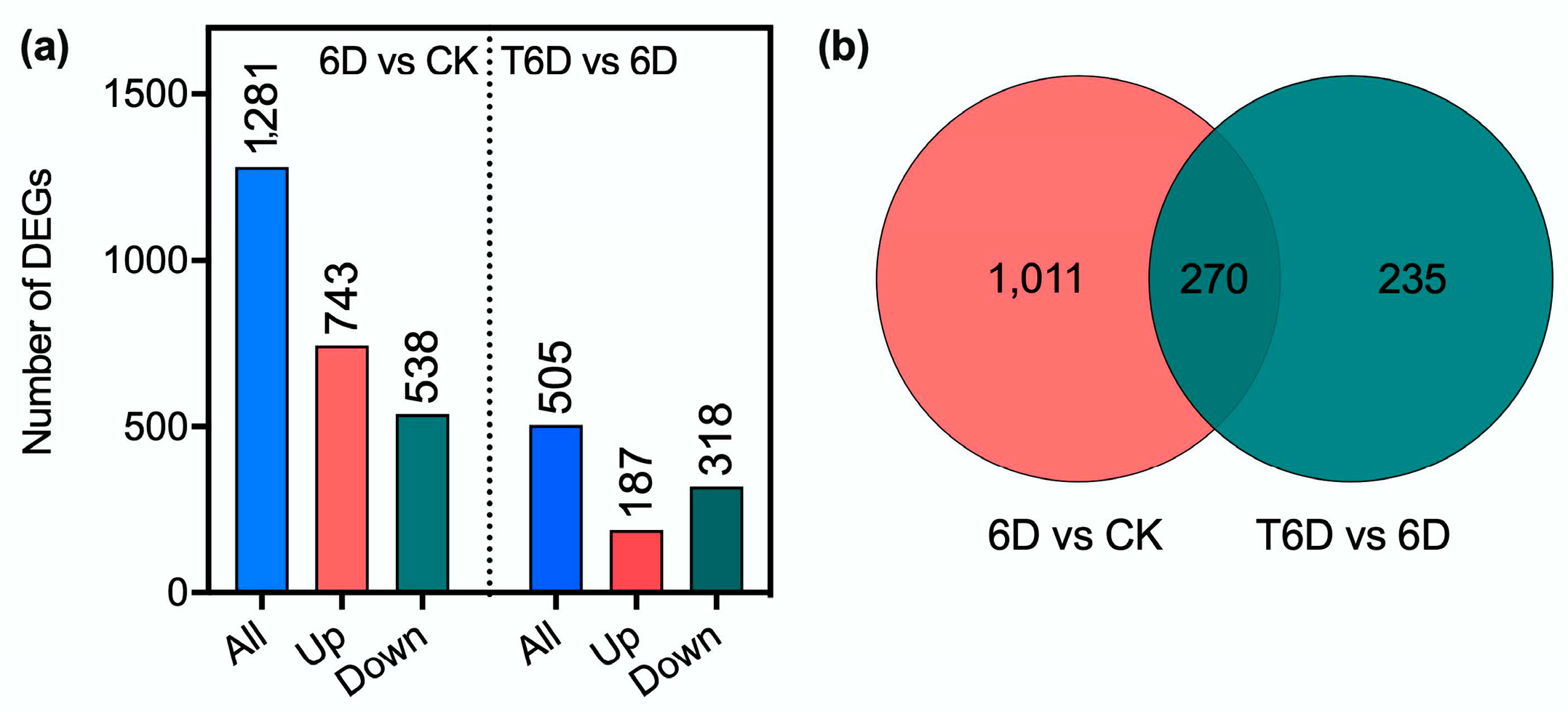
3.7. Screening of Differentially Expressed Genes
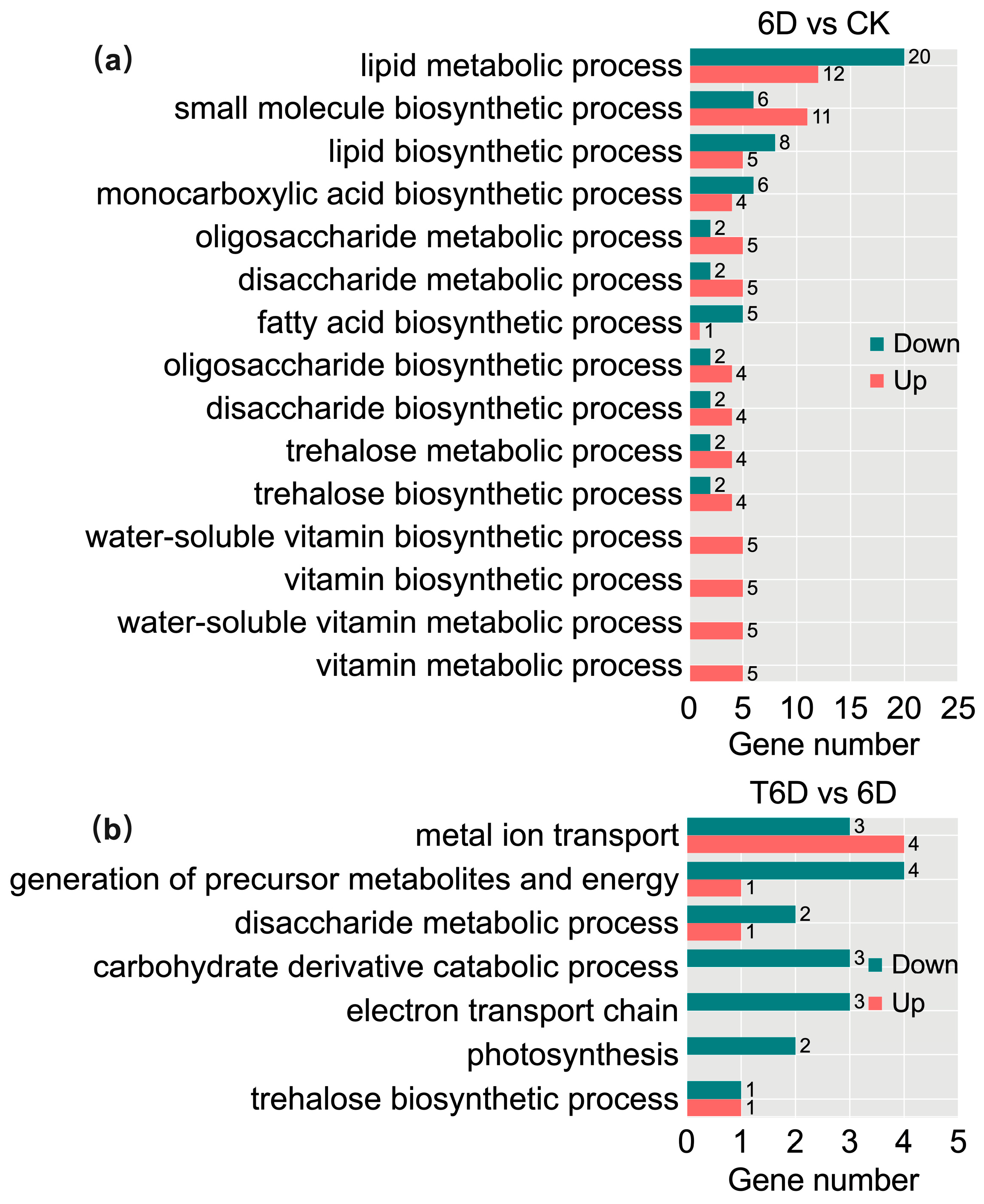
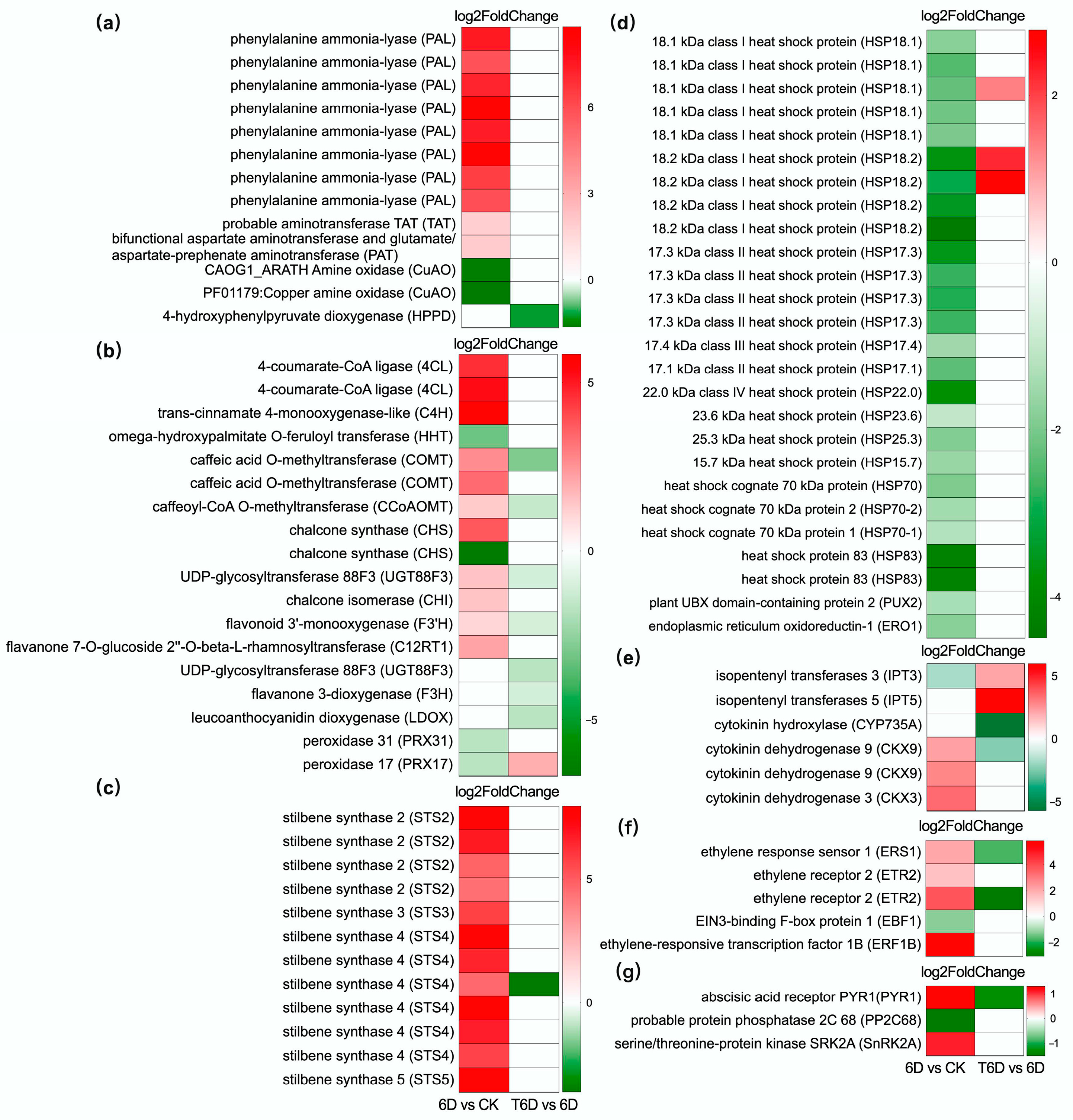
3.8. Gene Ontology and Kyoto Encyclopedia of Genes and Genomes Enrichment Analyses of DEGs
3.9. Construction and Module Analysis of the Protein–Protein Interaction Networks
4. Discussion
5. Conclusions
6. Future Perspectives
7. Limitations and Challenges
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iannone, M.; Mare, R.; Paolino, D.; Gagliardi, A.; Froiio, F.; Cosco, D.; Fresta, M. Characterization and in vitro anticancer properties of chitosan-microencapsulated flavan-3-ols-rich grape seed extracts. Int. J. Biol. Macromol. 2017, 104, 1039–1045. [Google Scholar] [CrossRef]
- Yamada, M.; Yamane, H.; Sato, A.; Hirakawa, N.; Iwanami, H.; Yoshinaga, K.; Ozawa, T.; Mitani, N.; Shiraishi, M.; Yoshioka, M. New grape cultivar ‘Shine muscat’. Bull. Natl. Inst. Fruit Tree Sci. 2008, 7, 21–38. [Google Scholar]
- Jiang, Z.H.; Li, X.J.; Peng, S.T.; Li, X.B.; Zhou, C.Y.; Zhao, X.Y. Effects of temperature on the physicochemical properties and volatile profiles of ‘Shine Muscat’ grape during postharvest storage. Food Chem. 2025, 469, 142546. [Google Scholar] [CrossRef]
- Zhao, H.D.; Liu, B.D.; Zhang, W.L.; Cao, J.K.; Jiang, W.B. Enhancement of quality and antioxidant metabolism of sweet cherry fruit by near-freezing temperature storage. Postharvest Biol. Technol. 2019, 147, 113–122. [Google Scholar] [CrossRef]
- Yan, D.M.; Yi, H.L. Transcriptome analysis provides insights into preservation mechanism of postharvest Muscat Hamburg grapes treated with SO2. Sci. Hortic. 2024, 331, 113108. [Google Scholar] [CrossRef]
- Pang, L.J.; Lu, G.Q.; Cheng, J.Y.; Lu, X.H.; Ma, D.F.; Li, Q.; Li, Z.Y.; Zheng, J.; Zhang, C.F.; Pan, S.Y. Physiological and biochemical characteristics of sweet potato (Ipomoea batatas (L.) Lam) roots treated by a high voltage alternating electric field during cold storage. Postharvest Biol. Technol. 2021, 180, 111619. [Google Scholar] [CrossRef]
- Yuan, S.; Xue, Z.H.; Zhang, S.L.; Wu, C.E.; Feng, Y.; Kou, X.H. The characterization of antimicrobial nanocomposites based on chitosan, cinnamon essential oil, and TiO2 for fruits preservation. Food Chem. 2023, 413, 135446. [Google Scholar] [CrossRef]
- Ren, W.H.; Sun, C.X.; Wang, L.; Zhu, C.X.; Ren, D.D.; Wang, T.; Wang, L.P.; Cai, Y.F.; Wang, Y.W.; Zhu, P.K.; et al. Postharvest disease, latent infection, and preharvest control of ‘Shine-Muscat’ grapes. Postharvest Biol. Technol. 2024, 214, 112989. [Google Scholar] [CrossRef]
- Wan, Y.K.; Tian, S.P.; Qin, G.Z. Enhancement of biocontrol activity of yeasts by adding sodium bicarbonate or ammonium molybdate to control postharvest disease of jujube fruits. Lett. Appl. Microbiol. 2003, 37, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Lai, C.D.; Tu, G.Q. A study on fruit preservation by using the ferment product of Streptomyces 702. Acta Agric. Univ. Jiangxiensis 2005, 27, 25–28. [Google Scholar]
- Tian, S.P.; Qin, G.Z.; Xu, Y. Survival of antagonistic yeasts under field conditions and their biocontrol ability against postharvest diseases of sweet cherry. Postharvest Biol. Technol. 2004, 33, 327–331. [Google Scholar] [CrossRef]
- Fan, Q.; Tian, S.P. Postharvest biological control of grey mold and blue mold on apple by Cryptococcus albidus (Saito) Skinner. Postharvest Biol. Technol. 2001, 21, 341–350. [Google Scholar] [CrossRef]
- Li, S.P.; Hu, J.Y.; Ning, S.Q.; Li, W.; Jiang, R.; Huang, J.G.; Li, Y. Bacillus velezensis HY19 as a sustainable preservative in post-harvest citrus (Citrus reticulata Blanco L.) fruit management. Food Control 2024, 155, 110068. [Google Scholar] [CrossRef]
- Wang, K.; Qin, Z.; Wu, S.Y.; Zhao, P.Y.; Zhen, C.Y.; Gao, H.Y. Antifungal mechanism of volatile organic compounds produced by Bacillus subtilis CF-3 on Colletotrichum gloeosporioides assessed using omics technology. J. Agric. Food Chem. 2021, 69, 5267–5278. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, L.J.; Wang, H.J.; Zhou, S.H.; Zhu, Z.Q.; Xie, T.L.; Zhou, Y.M.; Li, W.; Pang, L.T.; Sun, J.; et al. Metabolome and transcriptome analyses provide insight into the effect of 1-MCP and SO2 preservatives on the synthesis and regulation of phenols in ‘Shine Muscat ‘ storage grapes. LWT-Food Sci. Technol. 2024, 203, 116400. [Google Scholar] [CrossRef]
- Zheng, B.X.; Fang, L.C.; Li, C.L.; Gong, L.Z.; Ji, X.M. Efects of diferent preservation treatments on postharvest storage of ‘Shine Muscat’ grape. North. Hortic. 2023, 95–101. [Google Scholar] [CrossRef]
- Zhang, K.K.; Zhang, J.X.; Zheng, T.Y.; Gu, W.J.; Zhang, Y.Y.; Li, W.P.; Zhou, P.H.; Fang, Y.L.; Chen, K.Q. Preharvest application of MeJA enhancing the quality of postharvest grape berries via regulating terpenes biosynthesis and phenylpropanoid metabolisms. Food Chem. 2024, 438, 137958. [Google Scholar] [CrossRef]
- Wu, J.Y.; Zhang, F.C.; Liu, G.H.; Abudureheman, R.; Bai, S.J.; Wu, X.Y.; Zhang, C.; Ma, Y.N.; Wang, X.P.; Zha, Q.; et al. Transcriptome and coexpression network analysis reveals properties and candidate genes associated with grape (Vitis vinifera L.) heat tolerance. Front. Plant Sci. 2023, 14, 1270933. [Google Scholar] [CrossRef]
- Guan, Y.Q.; Qin, X.L.; Wei, C.Q.; Feng, Y.X.; Cheng, Y.D.; Zhang, Y.; Guan, J.F. Influence of bagging on fruit quality, incidence of peel browning spots, and lignin content of ‘Huangguan’ pears. Plants 2024, 13, 516. [Google Scholar] [CrossRef]
- Wang, X.; Li, D.Y.; Song, X.H. Effects of elevated CO2 and cadmium stress on vegetable quality and cadmium accumulation. Hortic. Sci. 2024, 51, 270–277. [Google Scholar] [CrossRef]
- Peng, Y.L.; Wang, Y.S.; Fei, J.; Sun, C.C.; Cheng, H. Ecophysiological differences between three mangrove seedlings (Kandelia obovata, Aegiceras corniculatum, and Avicennia marina exposed to chilling stress. Ecotoxicology 2015, 24, 1722–1732. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.Y.; Liu, J.Y.; Li, H.; Hu, W.J.; Shen, Z.J.; Qiao, F.; Zhu, C.Q.; Chen, J.; Liu, X.; Zheng, H.L. Proteomic analysis reveals the protective role of exogenous hydrogen sulfide against salt stress in rice seedlings. Nitric Oxide-Biol. Chem. 2021, 111, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Rao, M.V.; Paliyath, C.; Ormrod, D.P. Ultraviolet-B- and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996, 110, 125–136. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen-peroxide is scavenged by ascorbate-specific peroxidase in spinach-chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Yu, W.T.; Zhang, X.N.; Yan, W.W.; Sun, X.N.; Wang, Y.; Jia, X.H. Effects of 1-methylcyclopropene on skin greasiness and quality of ‘Yuluxiang’ pear during storage at 20 °C. J. Integr. Agric. 2024, 23, 2476–2490. [Google Scholar] [CrossRef]
- Li, H.; Lv, C.T.; Li, Y.T.; Gao, G.Y.; Meng, Y.F.; You, Y.L.; Tian, Q.; Liang, K.Q.; Chen, Y.; Chen, H.; et al. RNA-sequencing transcriptome analysis of Avicennia marina (Forsk.) Vierh. leaf epidermis defines tissue-specific transcriptional response to salinity treatment. Sci. Rep. 2023, 13, 7614. [Google Scholar] [CrossRef]
- Yan, Q.; Wu, F.; Xu, P.; Sun, Z.Y.; Li, J.; Gao, L.J.; Lu, L.Y.; Chen, D.D.; Muktar, M.; Jones, C.; et al. The elephant grass (Cenchrus purpureus) genome provides insights into anthocyanidin accumulation and fast growth. Mol. Ecol. Resour. 2021, 21, 526–542. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef]
- Mao, X.Z.; Cai, T.; Olyarchuk, J.G.; Wei, L.P. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef]
- Meng, X.H.; Li, B.Q.; Liu, J.; Tian, S.P. Physiological responses and quality attributes of table grape fruit to chitosan preharvest spray and postharvest coating during storage. Food Chem. 2008, 106, 501–508. [Google Scholar] [CrossRef]
- Li, H.; Li, Z.; Shen, Z.J.; Luo, M.R.; Liu, Y.L.; Wei, M.Y.; Wang, W.H.; Qin, Y.Y.; Gao, C.H.; Li, K.K.; et al. Physiological and proteomic responses of mangrove plant Avicennia marina seedlings to simulated periodical inundation. Plant Soil 2020, 450, 231–254. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef]
- Toral, L.; Rodriguez, M.; Bejar, V.; Sampedro, I. Antifungal activity of lipopeptides from Bacillus XT1 CECT 8661 against Botrytis cinerea. Front. Microbiol. 2018, 9, 1315. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Tewari, A.K.; Saxena, D. Activities of defensive antioxidant enzymes and biochemical compounds induced by bioagents in Indian mustard against Alternaria blight. Proc. Nat. Acad. Sci. India Sect. B-Biol. Sci. 2018, 88, 1507–1516. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiong, Q.; Yin, C.C.; Ma, B.; Chen, S.Y.; Zhang, J.S. Ethylene biosynthesis, signaling, and crosstalk with other hormones in rice. Small Methods 2020, 4, 1900278. [Google Scholar] [CrossRef]
- Hua, L.; Li, N.; Zhang, W.Y.; Ruan, C.Q.; Zeng, K.F. Photocatalytic ethylene scavenging for fresh produce preservation: A comprehensive review. Trends Food Sci. Technol. 2024, 150, 104604. [Google Scholar] [CrossRef]
- Chen, H.C.; Lai, X.H.; Wang, L.H.; Li, X.P.; Chen, W.X.; Zhu, X.Y.; Song, Z.Y. Ethylene response factor MaERF012 modulates fruit ripening by regulating chlorophyll degradation and softening in banana. Foods 2022, 11, 3882. [Google Scholar] [CrossRef]
- Wang, H.Y.; Li, Y.W.; Wassie, M.; Muhammad, M.; Bai, S.L.; Shi, H.Y. The effect of 1-methylcyclopropene on the shelf life of sand pear fruits. LWT-Food Sci. Technol. 2025, 218, 117530. [Google Scholar] [CrossRef]
- Li, B.J.; Grierson, D.; Shi, Y.N.; Chen, K.S. Roles of abscisic acid in regulating ripening and quality of strawberry, a model non-climacteric fruit. Hortic. Res. 2022, 9, uhac089. [Google Scholar] [CrossRef]
- Kou, X.H.; Yang, S.; Chai, L.P.; Wu, C.E.; Zhou, J.Q.; Liu, Y.F.; Xue, Z.H. Abscisic acid and fruit ripening: Multifaceted analysis of the effect of abscisic acid on fleshy fruit ripening. Sci. Hortic. 2021, 281, 109999. [Google Scholar] [CrossRef]
- Kai, W.B.; Wang, J.; Liang, B.; Fu, Y.; Zheng, Y.; Zhang, W.B.; Li, Q.; Leng, P. PYL9 is involved in the regulation of ABA signaling during tomato fruit ripening. J. Exp. Bot. 2019, 70, 6305–6319. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Li, Q.; Jiang, L.; Kai, W.B.; Liang, B.; Wang, J.; Du, Y.W.; Zhai, X.W.; Wang, J.L.; Zhang, Y.Q.; et al. Suppressing type 2C protein phosphatases alters fruit ripening and the stress response in tomato. Plant Cell Physiol. 2018, 59, 142–154. [Google Scholar] [CrossRef]
- Tang, N.; An, J.; Deng, W.; Gao, Y.Q.; Chen, Z.X.; Li, Z.G. Metabolic and transcriptional regulatory mechanism associated with postharvest fruit ripening and senescence in cherry tomatoes. Postharvest Biol. Technol. 2020, 168, 111274. [Google Scholar] [CrossRef]
- Zheng, R.H.; Li, S.J.; Zhang, X.; Zhao, C.Q. Biological activities of some new secondary metabolites isolated from endophytic fungi: A review study. Int. J. Mol. Sci. 2021, 22, 959. [Google Scholar] [CrossRef]
- Li, C.H.; Jiang, R.; Wang, X.X.; Lv, Z.Y.; Li, W.K.; Chen, W.S. Feedback regulation of plant secondary metabolism: Applications and challenges. Plant Sci. 2024, 340, 111983. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.X.; Feng, Y.; Yu, S.H.; Fan, Z.Q.; Li, X.L.; Li, J.Y.; Yin, H.F. The flavonoid biosynthesis network in plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef]
- Pfeiffer, P.; Hegedus, A. Review of the molecular genetics of flavonoid biosynthesis in fruits. Acta Aliment. 2011, 40, 150–163. [Google Scholar] [CrossRef]
- Pourcel, L.; Routaboul, J.M.; Cheynier, V.; Lepiniec, L.; Debeaujon, I. Flavonoid oxidation in plants: From biochemical properties to physiological functions. Trends Plant Sci. 2007, 12, 29–36. [Google Scholar] [CrossRef]
- Vannozzi, A.; Dry, I.B.; Fasoli, M.; Zenoni, S.; Lucchin, M. Genome-wide analysis of the grapevine stilbene synthase multigenic family: Genomic organization and expression profiles upon biotic and abiotic stresses. BMC Plant Biol. 2012, 12, 130. [Google Scholar] [CrossRef]
- Shigeto, J.; Kiyonaga, Y.; Fujita, K.; Kondo, R.; Tsutsumi, Y. Putative cationic cell-wall-bound peroxidase homologues in Arabidopsis, AtPrx2, AtPrx25, and AtPrx71, are involved in lignification. J. Agric. Food Chem. 2013, 61, 3781–3788. [Google Scholar] [CrossRef] [PubMed]
- Yoshikay-Benitez, D.A.; Yokoyama, Y.; Ohira, K.; Fujita, K.; Tomie, A.; Kijidani, Y.; Shigeto, J.; Tsutsumi, Y. Populus alba cationic cell-wall-bound peroxidase (CWPO-C) regulates the plant growth and affects auxin concentration in Arabidopsis thaliana. Physiol. Mol. Biol. Plants 2022, 28, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Feng, Y.H.; Xia, C.; Chen, Y.; Lu, X.Y.; Wei, Y.; Qian, L.L.; Zhu, M.Y.; Gao, G.Y.; Meng, Y.F.; et al. Physiological and transcriptomic analysis dissects the molecular mechanism governing meat quality during postmortem aging in Hu sheep (Ovis aries). Front. Nutr. 2024, 10, 1321938. [Google Scholar] [CrossRef]
- Chevet, E.; Cameron, P.H.; Pelletier, M.F.; Thomas, D.Y.; Bergeron, J.J.M. The endoplasmic reticulum: Integration of protein folding, quality control, signaling and degradation. Curr. Opin. Struct. Biol. 2001, 11, 120–124. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Xu, C.L.; Gu, R.J.; Han, R.Q.; Li, Z.Y.; Xu, X.R. Endoplasmic reticulum stress in diseases. MedComm 2024, 5, e701. [Google Scholar] [CrossRef]
- Alvim, F.C.; Carolino, S.M.B.; Cascardo, J.C.M.; Nunes, C.C.; Martinez, C.A.; Otoni, W.C.; Fontes, E.P.B. Enhanced accumulation of BiP in transgenic plants confers tolerance to water stress. Plant Physiol. 2001, 126, 1042–1054. [Google Scholar] [CrossRef]
- Valente, M.A.S.; Faria, J.; Soares-Ramos, J.R.L.; Reis, P.A.B.; Pinheiro, G.L.; Piovesan, N.D.; Morais, A.T.; Menezes, C.C.; Cano, M.A.O.; Fietto, L.G.; et al. The ER luminal binding protein (BiP) mediates an increase in drought tolerance in soybean and delays drought-induced leaf senescence in soybean and tobacco. J. Exp. Bot. 2009, 60, 533–546. [Google Scholar] [CrossRef]
- Wang, H.; Niu, H.H.; Zhai, Y.F.; Lu, M.H. Characterization of BiP genes from pepper (Capsicum annuum L.) and the role of CaBiP1 in response to endoplasmic reticulum and multiple abiotic stresses. Front. Plant Sci. 2017, 8, 1122. [Google Scholar] [CrossRef]
- Guan, C.F.; Jin, C.; Ji, J.; Wang, G.; Li, X.Z. LcBiP, a endoplasmic reticulum chaperone binding protein gene from Lycium chinense, confers cadmium tolerance in transgenic tobacco. Biotechnol. Prog. 2015, 31, 358–368. [Google Scholar] [CrossRef]
- Shafikova, T.N.; Maksimova, L.A.; Omelichkina, Y.V. Heat shock proteins in plant immunity. Russ. J. Plant Physiol. 2024, 71, 100. [Google Scholar] [CrossRef]
- Morrow, G.; Tanguay, R.M. Small heat shock protein expression and functions during development. Int. J. Biochem. Cell Biol. 2012, 44, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, H.; Khurana, N.; Nijhavan, A.; Khurana, J.P.; Khurana, P. The wheat chloroplastic small heat shock protein (sHSP26) is involved in seed maturation and germination and imparts tolerance to heat stress. Plant Cell Environ. 2012, 35, 1912–1931. [Google Scholar] [CrossRef]
- Prieto-Dapena, P.; Castaño, R.; Almoguera, C.; Jordano, J. Improved resistance to controlled deterioration in transgenic seeds. Plant Physiol. 2006, 142, 1102–1112. [Google Scholar] [CrossRef] [PubMed]
- Frand, A.R.; Kaiser, C.A. The ERO1 gene of yeast is required for oxidation of protein dithiols in the endoplasmic reticulum. Mol. Cell 1998, 1, 161–170. [Google Scholar] [CrossRef]
- Tu, B.P.; Weissman, J.S. The FAD- and O2-dependent reaction cycle of Ero1-mediated oxidative protein folding in the endoplasmic reticulum. Mol. Cell 2002, 10, 983–994. [Google Scholar] [CrossRef]
- Ruggiano, A.; Foresti, O.; Carvalho, P. ER-associated degradation: Protein quality control and beyond. J. Cell Biol. 2014, 204, 868–878. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, C.-T.; Li, H.; Hua, R.-M. Physiological and Transcriptomic Analyses Unveil the Preservation Mechanism of Streptomyces albulus Ah11601 Fermentation Broth on ‘Shine Muscat’ Grapes. Genes 2025, 16, 468. https://doi.org/10.3390/genes16040468
Lv C-T, Li H, Hua R-M. Physiological and Transcriptomic Analyses Unveil the Preservation Mechanism of Streptomyces albulus Ah11601 Fermentation Broth on ‘Shine Muscat’ Grapes. Genes. 2025; 16(4):468. https://doi.org/10.3390/genes16040468
Chicago/Turabian StyleLv, Chao-Tian, Huan Li, and Ri-Mao Hua. 2025. "Physiological and Transcriptomic Analyses Unveil the Preservation Mechanism of Streptomyces albulus Ah11601 Fermentation Broth on ‘Shine Muscat’ Grapes" Genes 16, no. 4: 468. https://doi.org/10.3390/genes16040468
APA StyleLv, C.-T., Li, H., & Hua, R.-M. (2025). Physiological and Transcriptomic Analyses Unveil the Preservation Mechanism of Streptomyces albulus Ah11601 Fermentation Broth on ‘Shine Muscat’ Grapes. Genes, 16(4), 468. https://doi.org/10.3390/genes16040468




