Abstract
Embryonic stem cells and induced pluripotent stem cells have the ability to maintain their telomere length via expression of an enzymatic complex called telomerase. Similarly, more than 85%–90% of cancer cells are found to upregulate the expression of telomerase, conferring them with the potential to proliferate indefinitely. Telomerase Reverse Transcriptase (TERT), the catalytic subunit of telomerase holoenzyme, is the rate-limiting factor in reconstituting telomerase activity in vivo. To date, the expression and function of the human Telomerase Reverse Transcriptase (hTERT) gene are known to be regulated at various molecular levels (including genetic, mRNA, protein and subcellular localization) by a number of diverse factors. Among these means of regulation, transcription modulation is the most important, as evident in its tight regulation in cancer cell survival as well as pluripotent stem cell maintenance and differentiation. Here, we discuss how hTERT gene transcription is regulated, mainly focusing on the contribution of trans-acting factors such as transcription factors and epigenetic modifiers, as well as genetic alterations in hTERT proximal promoter.
1. Introduction
The ends of human chromosomes are capped by telomeres which protect the chromosome termini from degradation, end-to-end fusion and recombination [1,2]. Telomeres are long stretches of 5′-TTAGGG-3′ DNA repeats which end in single-stranded 3′ G-rich overhangs [3,4]. The telomeric DNA repeats are bound by shelterin protein complexes consisting of Telomeric Repeat Factor I and II (TRF1, TRF2), Repressor/Activator Protein I (RAP1), TRF1-Interacting Nuclear Protein 2 (TIN2), Tripeptidyl Peptidase I (TPP1) and Protection of Telomeres I (POT1) that distinguish naturally occurring chromosomal ends from DNA double-strand breaks [2]. Telomeric DNA repeats are synthesized by telomerase, a reverse transcriptase. Human core telomerase consists of at least two essential subunits, the protein subunit, human Telomerase Reverse Transcriptase (hTERT), and the RNA subunit, human Telomerase RNA (hTR) [5,6]. Telomerase activity is generally limited by the expression of hTERT, and is barely detected in most human adult somatic tissues, except in germ cells and some stem cells [5,6,7,8,9]. In cells lacking telomerase activity, about 50–200 bp of telomeric DNA repeats are lost during each cell division due to incomplete replication by DNA polymerase, and end processing [4,10].
Adult human somatic cells have limited telomere lengths ranging from 7 to 12 kb [11]. Therefore, progressive telomere shortening may function as an internal clock that determines the replicative capacity of normal human somatic cells [12]. When telomeres shorten to a critical limit, they become uncapped and trigger Ataxia Telangiectasia Mutated (ATM)- and/or Ataxia Telangiectasia and Rad3-Related Protein (ATR)-dependent DNA damage signaling cascades [13,14,15]. Through the downstream transducer kinases, Checkpoint kinase I and II (CHK1 and CHK2), the uncapped chromosomal ends are marked by distinct telomere dysfunction-induced foci (TIFs) [13]. As few as five dysfunctional telomeres are sufficient to trigger irreversible cell cycle arrest, termed replicative senescence, in primary human fibroblast cells [14,15,16]. As a result, normal somatic cells can only proliferate for a limited number of passages that is pre-set by their telomere length [16]. In contrast to pluripotent stem cells whose telomere length is sustained, telomerase activity in tissue-specific progenitor/stem cells is not sufficient for complete telomere maintenance [17]. Consequently, telomere length in these tissue-specific progenitor/stem cells also progressively shortens. This decrease in length limits the proliferative capacity of tissue-specific progenitor/stem cells, and contributes to normal human aging [18]. Therefore, the process of resetting telomere length during early embryogenesis is necessary to ensure sufficient telomere reserves for cell integrity during human development and aging [19].
Defects in genes that regulate telomere length homeostasis may lead to various diseases, collectively termed telomeropathies [20,21]. Patients display diverse symptoms such as premature aging and increased risk of cancer, highlighting the importance of telomere homeostasis in human health. Mutations in telomerase and telomere maintenance genes have been found in patients with telomeropathies [20,21,22], which results in decreased telomerase activity and accelerated telomere shortening. These genes control telomerase ribonucleoprotein maturation, assembly, recruitment, and engagement, revealing their importance in telomere length homeostasis. To add, telomerase knockout mouse models show remarkable similarity to the human disease phenotypes [23,24,25]. Strategies that promote the expression of hTERT and restore telomere length homeostasis could potentially delay the clinical onset of these diseases as well as normal aging in humans.
On the other hand, telomerase activity is highly elevated in 85%–90% of human cancers and over 70% of immortalized human cell lines [7,26]. This is consistent with telomerase conferring a strong selective advantage for continued growth of malignant cells [27]. These observations suggest that telomere maintenance is essential for cancer cell immortalization and that it may be possible to inhibit cancer growth by interfering with telomerase activity.
Expression and function of hTERT gene are known to be regulated at various molecular levels. However, the transcription of hTERT has been suggested to be the dominant step in the regulation of telomerase activity [7,26]. Previous studies on hTERT promoter have defined a core region encompassing 330 bp upstream of the translation start site to 228 bp downstream, extending right into the second exon of the gene [28,29,30]. A number of transcription factor binding sites have been identified in this core promoter. However, the molecular mechanism underlying hTERT gene activation during induced Pluripotent Stem (iPS) cell reprogramming [31,32] and hTERT gene silencing during cellular differentiation remains largely unclear. On the other hand, recent studies have revealed the potential role of promoter mutations and chromosomal rearrangements in the activation of telomerase in cancer cells. These results have provided potential new strategies in targeting telomerase for cancer therapy. Here, we summarize the recent advances in the understanding of the transcriptional regulation of hTERT gene, focusing our attention on trans-acting factors, namely transcription factors and epigenetic modifiers, as well as genetic alterations in hTERT proximal region.
2. Trans-Acting Regulators of hTERT Transcription
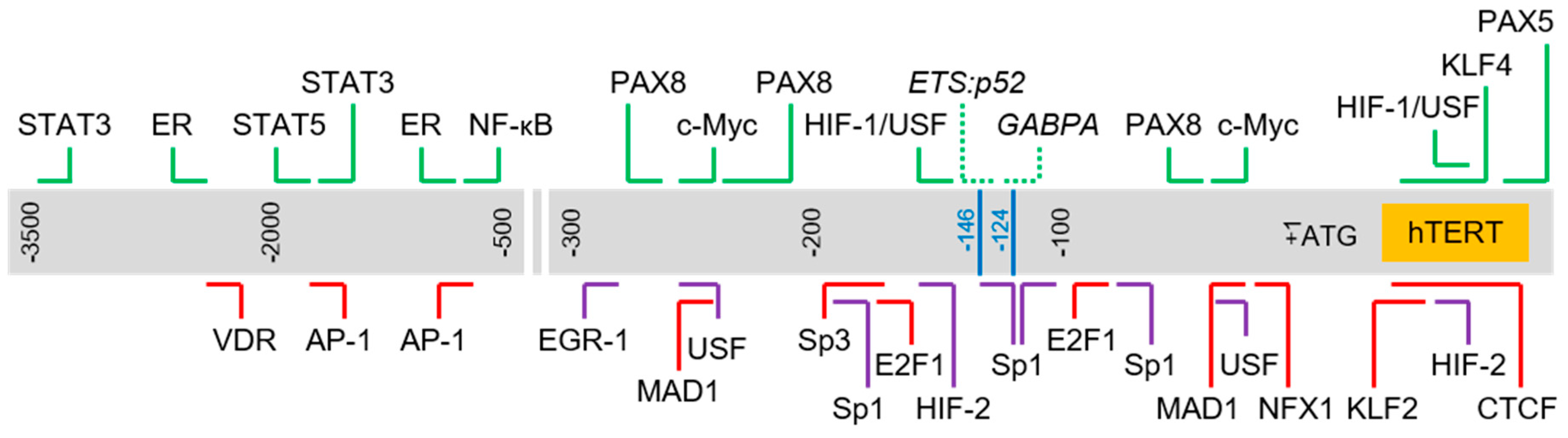
The core promoter of the hTERT gene contains several known regulatory elements including GC-motifs and E-boxes. Several other articles have elegantly reviewed the roles specific factors or protein families play in the modulation of hTERT gene expression. Here, we have chosen to focus only on factors which have been reported to bind directly to the hTERT promoter region via in vitro or in vivo DNA–protein interaction assays, such as chromatin immunoprecipitation (ChIP) and electrophoretic mobility shift assay (EMSA) (refer to Table 1 for the complete list of factors). We selected a number of well-studied factors in each category and briefly discuss its role in the regulation of the hTERT gene, specifically highlighting the complexity of the regulatory network involved in controlling the expression of hTERT. As expected of a critical gene, hTERT transcription is modulated in a context-dependent manner via a multi-tiered regulatory network involving, among other means, feedback loops, and genetic and epigenetic controls. We also highlight the complexity of the hTERT proximal promoter with regards to the numerous response elements enclosed in this region (refer to Figure 1 for a schematic of the binding sites of selected transcription factors found in this region).

Table 1.
List of factors reported to bind to human Telomerase Reverse Transcriptase (hTERT) promoter and regulate its expression.
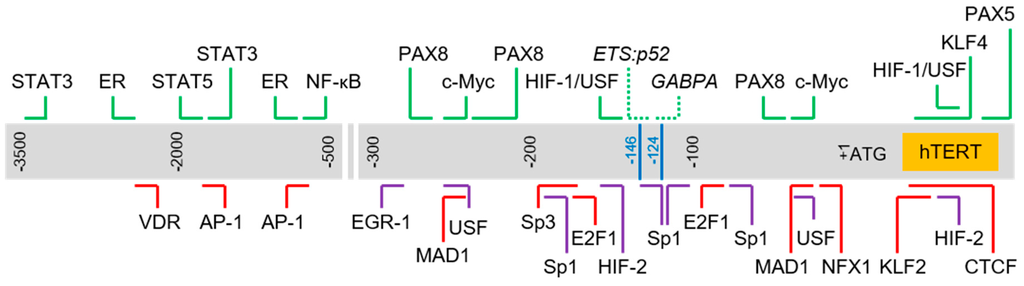
Figure 1.
Schematic of transcription factor binding sites in human Telomerase Reverse Transcriptase (hTERT) promoter. Chromosomal sequence extending from 3.5 kb upstream and 150 bp downstream of hTERT translation start site (+1) is represented by the gray box. Horizontal lines above and below the box indicate approximate binding sites of respective transcription factors. Blue lines: hotspot promoter mutations (“-124” corresponds to C228T mutation; “-146” corresponds to C250T mutation); green: activator; red: repressor; purple: regulator with dual roles; dotted line: regulator bound to sites created by hotspot mutations.
2.1. Transcription Activators of hTERT
2.1.1. c-Myc
c-Myc, together with its dimerization partner Max, binds to regulatory elements called E-boxes and recruits histone acetyltransferases (HATs) to exact an activating effect on the transcription of various genes. Human TERT gene is one of them; c-Myc binds to two E-box sequences found on the core promoter of hTERT, leading to the upregulation of the expression of the gene and telomerase activity [43,44,45]. Mutating these sites weakens the promoter activity of hTERT gene [45,46]. In addition, overexpression of c-Myc in squamous cell carcinoma cells and human foreskin keratinocyte cells resulted in the upregulation of the hTERT promoter activity [46]. The transcription activating role of c-Myc on hTERT gene is mediated by the recruitment of the histone acetyltransferase (HAT) complex called SPT3-TAF9-GCN5 acetyltransferase complex (STAGA) and the transcription co-activator Mediator complex [66].
On the other hand, c-Myc alone may not be sufficient to drive the activation of hTERT expression. E6-transduced human foreskin keratinocytes (HFKs) did not show an increase in c-Myc expression, even though the cells attained replicative immortality [205]. This suggests that additional factors may be required in order to upregulate hTERT expression in these cells. Indeed, c-Myc was found to act cooperatively with Specificity Protein 1 (Sp1) in the activation of hTERT transcription via combinatorial binding of these two factors on their respective cis elements in the hTERT promoter [53]. When the E-boxes and GC-rich motifs (response elements of Sp1) were mutated, E6-mediated activation of telomerase expression was abolished. This also explains the observation that c-Myc and Sp1 expression correlates with hTERT transcription in various cancer cell lines.
Besides Sp1, numerous other factors play a role in modulating c-Myc-mediated regulation of hTERT transcription. Estrogen has been shown to activate c-Myc expression in breast cancer cell line, MCF-7 [51]. This, on top of direct activation of hTERT by estrogen receptor (ER) (see below), enhances hTERT transcription and telomerase activity in these cells. Aurora-A activates c-Myc expression and thence hTERT promoter activity in ovarian and breast epithelial cancer cells [61]. Nuclear Transcription Factor, X Box-Binding Protein 1 variant 123 (NFX1-123) and c-Myc, together with an unknown factor which acts upstream of the hTERT core promoter, co-activate hTERT gene expression in human foreskin keratinocytes [48]. Survivin induces the phosphorylation of c-Myc (and Sp1) and enhances its transactivation of hTERT transcription [63]. p300 interacts with and stabilises c-Myc via the latter’s Topologically Associated Domain (TAD) and they both co-activate hTERT gene expression [49]. Protein Kinase C θ (PKCθ) activates Nuclear Factor κB (NF-κB) signalling which in turn enhances c-Myc binding to hTERT promoter in activated human T lymphocytes [64]. ETS Proto-Oncogene 2 (Ets2) interacts with c-Myc and together they bind to hTERT promoter sequence and mediate breast cancer proliferation [52]. Colony Stimulating Factor 1 Receptor (CSF1R), upon induction by its ligand Colony Stimulating Factor 1 (CSF1), gets internalised into activated immortalized epithelial cells which lead to elevated binding of c-Myc to hTERT promoter [68]. Protein deglycase DJ-1 regulates c-Myc expression and thus hTERT promoter activity in renal and ovarian carcinoma cells [70]. Leptin upregulates binding of c-Myc (and Signal Transducer and Activator of Transcription 3 (STAT3)) to hTERT promoter and induces hTERT expression in HepG2 cells [71]. Brain-Derived Neurotrophic Factor (BDNF) activates hTERT expression and telomerase activity in spinal cord motor neurons via Mitogen-Activated Protein Kinase (MAPK)/Phosphatidylinositol-4,5-Bisphosphate 3-Kinase (PI3K) pathway which induces, among others, c-Myc expression [72]. Matrix Metallopeptidase 9 (MMP-9) facilitates in the switch from repressive Mad/Max-bound state to activating c-Myc/Max-bound state of the hTERT promoter in glioma cells [60]. Sirtuin 1 (SIRT1) upregulates c-Myc expression through activation of Forkhead Box O3A (FOXO3a) in human umbilical cord fibroblast (HUC-F2) cells and this leads to increased hTERT expression and longer lifespan of the cells [73]. The tumor suppressor p53 and its family members, p63 and p73, are potent repressors of hTERT transcription as transient overexpression of these factors in human embryonic kidney cells results in lower c-Myc expression and consequently attenuated hTERT promoter activity [88].
Conversely, the activity of the positive regulators of c-Myc-induced hTERT activation may be counteracted by a variety of c-Myc inhibitors and suppressors. A major player is Mad, a potent antagonist of c-Myc, whose mechanism of action involves competing with c-Myc for E-box protein binding sites found in transcription regulatory regions of their target genes (see below for the discussion on the role Mad plays in repressing hTERT). In addition, the lipid ceramide was shown to destabilize c-Myc protein via ubiquitination of the transcription factor, resulting in the downregulation of hTERT expression [45]. Other factors were shown to disrupt c-Myc binding to hTERT promoter and its transcription activation: Breast cancer 1 (BRCA1) associates with c-Myc via its N-terminal domain and depletes hTERT promoter-bound c-Myc in ovarian, prostate and breast cancer cells [81,82]. Cyclin-Dependent Kinase Inhibitor P27 (p27KIP1) interferes with c-Myc binding to hTERT promoter in malignant glioma cells [206]. Hypoxia-Inducible Factor 1-alpha (HIF-1α) downregulates c-Myc-mediated activation of hTERT promoter activity in colorectal carcinoma cells [77]. Mothers Against Decapentaplegic Homolog 3 (Smad3), upon Transforming Growth Factor beta (TGFβ) induction, interacts with c-Myc which leads to the downregulation of hTERT promoter activity [79,80]. TGFβ has also been shown to inhibit hTERT expression by upregulating Snail expression in human embryonic kidney (HEK) cells and keratinocyte cells [80]. Wilms Tumor 1 (WT1) downregulates hTERT transcription in clear cell renal cell carcinoma (ccRCC) by directly binding to hTERT promoter or by repressing c-Myc expression via its promoter activity [35]. Gastrokine 1 (GKN1) was reported to inhibit hTERT expression in gastric cancer cells by binding directly to c-Myc and downregulating its expression, leading to lower hTERT promoter activity [207]. The compound arsenic trioxide (ATO) was previously shown to downregulate telomerase activity and this was recently shown to involve the downregulation of four transcription activators of hTERT, one of which is c-Myc [101]. Knockdown of c-Myc (or the other factors) via siRNA could sensitize promyelocytic leukemia cells to ATO-induced apoptosis and inhibition of cell growth.
Despite the strong evidences brought forth by the various studies above regarding the key role c-Myc plays in the modulation of hTERT expression and telomerase activity, several studies involving the use of primary tumor samples have proven the lack of correlation between c-Myc expression and hTERT mRNA levels, specifically in hepatocellular carcinoma and breast carcinoma tissue samples [208,209]. Consistent with this notion, while c-Myc is one of the four transcription factors used for iPS cell reprogramming, it is non-essential [210].
2.1.2. NF-κB
NF-κB is a transcription factor complex whose activity is induced in many cell types by various stimuli such as inflammation, cellular differentiation, tumorigenesis, and apoptosis. It is shown to play an activating role in telomerase expression and activity by regulating hTERT gene transcription via binding to the proximal promoter of the target gene, or indirectly by modulating the expression of transcription factors known to affect hTERT expression. The activation of NF-κB in primary bovine aortic endothelial cells (BAECs) and neuroepithelioma cell line via exposure to ionizing gamma radiation, and in human monocyte cells undergoing inflammation, leads to increased binding of NF-κB to hTERT promoter and consequently enhanced telomerase activity [147,148,152]. Depleting NF-κB levels by ectopically expressing NFKB Inhibitor Alpha (IκBα) or by disrupting the binding of NF-κB to hTERT promoter by eliminating its response element compromises the radiation- and lesion-induced upregulation of hTERT expression NF-κB-mediated activation of telomerase was also shown to be crucial in the recovery of intimal smooth muscle cells upon vascular injury in mediating intimal hyperplasia [57]. It was also proposed that there exists a feed-forward regulation between NF-κB and telomerase as the latter was found to bind to p65, a component of NF-κB, and modulate its transcription activity on its target genes, including factors which are important for inflammation and cancer progression [211,212]. The NF-κB response element in hTERT promoter is located more than 600 bp upstream of the translation start site, however, recently, the non-canonical NF-κB pathway was implicated in tumorigenesis specifically via a hotspot hTERT promoter mutation—C250T—which creates a binding motif for E-twenty-six (ETS) protein, a transcription activator of hTERT gene. Binding of ETS to the newly formed response element is not enough to activate hTERT transcription; it requires an activated non-canonical NF-κB signaling background to drive this transcription [213]. The NF-κB subunit, p52, is recruited to the C250T site and binds to its own half site and this facilitates the stimulation of hTERT transcription in the cancer cells.
As mentioned, NF-κB can also activate hTERT expression via an indirect way. It is known to activate strong hTERT transcription activators such as c-Myc and Sp1 in a number of human cell lines [58,64]. Besides the ones described above, other naturally occurring and synthetic chemicals were shown to modulate NF-κB-mediated activation of hTERT transcription. The plant-derived molecule, curcumin, and the drug, pelitinib, were both shown to potently inhibit NF-κB-induced hTERT activation by ionizing radiation in neurogenic cancer and tongue squamous cell carcinoma cells, respectively [148,153]. In addition, arsenic trioxide (ATO) was found to repress the expression of NF-κB (and other transcription factors namely Sp1, c-Myc and Upstream Transcription Factor 2 (USF2)) in human promyelocytic leukemia cells and reduce the transcription of hTERT [101].
2.1.3. STAT Proteins
STAT3 plays a key regulatory role in the expression of hTERT in several cancer cell lines including gastric, breast and glioblastoma, and hTERT in turn contributes to the survival of these STAT3-dependent tumors. When STAT3 expression levels were reduced in a hepatocellular carcinoma (HCC) cell line using siRNA, hTERT expression was consequently reduced [214]. Studies involving breast cancer stem cells revealed that STAT3 binds to and activates hTERT promoter in concert with Cluster of Differentiation 44 (CD44) and NF-κB, and that diminished levels of STAT3 resulted in the downregulation of hTERT expression [149]. Mechanistically, STAT3-mediated upregulation of hTERT expression may be the result of the following biological factors: leptin induction in breast cancer and HCC cells [71,188], miR-21 expression in glioblastoma cells [191], and core protein of hepatitis C virus (HCVc) in HCC cells [192]. STAT3-mediated upregulation of hTERT expression by HCVc also involves the action of DNA Methyltransferase 1 (DNMT1) which facilitates the methylation of hTERT promoter.
STAT5 has been shown to bind directly to a distal promoter region of hTERT in a chronic myelogenous leukaemia (CML) cell line and an adult T-cell leukaemia (ATL) cell line, resulting in the activation of telomerase expression [193,194]. The positive effect STAT5 has on hTERT expression was also seen in primary leukemic cells upon Interleukin 2 (IL-2) induction and has been attributed to the phosphorylation of STAT5 which permits its interaction with Janus-activated kinase (JAK)-1 and -2. Depletion of STAT5 via the introduction of specific siRNA was shown to lead to the inhibition of IL-2-induced hTERT activation [193]. However, STAT5 was also reported to indirectly activate hTERT through c-Myc, another transcription activator of hTERT. In two different leukemic cell lines, erythropoietin (EPO) was proven to be a potent activator of telomerase and this was shown to be via the induction of the JAK2/STAT5/c-Myc axis, and in turn was negatively regulated by TGFβ/Smad3 signaling [195,196].
2.1.4. Paired Box Proteins (Pax)
Pax protein family consists of paired box- and homeobox-containing transcription factors which play a crucial role in early development. Two of its members were shown to bind to regions proximal to hTERT translational start site and activate its transcription. In lymphocyte cell lines, PAX5 was found to bind to two sites, one each in exon 1 and 2 of hTERT gene [156]. Knockdown via siRNA and overexpression of PAX5 lead to increased and decreased hTERT expression, respectively. Furthermore, these effects were reported to be independent of CCCTC-binding factor (CTCF) and promoter methylation. On the other hand, PAX8 was revealed to bind to four sites upstream of the hTERT translational start site in glioma cells [157]. Interestingly, PAX8 was also shown to bind and activate hTR promoter, which fortifies the role of PAX8 in the regulation of telomerase activity.
2.1.5. Estrogen Receptor
Estrogen receptor is a nuclear hormone receptor which binds to estrogen response elements (ERE) upon stimulation by its ligand. hTERT transcription and telomerase activity are found to be activated by estradiol (E2) in ER-positive cells but not in ER-negative ones [215]. However, artificial expression of ER in ER-negative cells was found to result in hTERT transcription activation and increased telomerase activity. In vitro DNA-protein binding assays revealed that ERα specifically binds to two EREs in the hTERT promoter [51,116,118]. In addition, several biomolecules were shown to inhibit ER activation of hTERT in human cancer cells. Treatment of breast cancer cells with indole-3-carbonol (I3C) [121], colon cancer cells with 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) [84], ovarian cancer cells with miR-498 [120], and breast and endometrial cancer cells with progesterone [122], were reported to result in the suppression of ER-induced activation of hTERT expression.
2.2. Transcription Repressors of hTERT
2.2.1. Mad1
Mad1 is an antagonist of Myc protein in that it competes with the latter for E-box motif binding in promoter region of target genes and mediates the repression (as opposed to Myc’s activation) of these genes. The binding of Mad1 to hTERT proximal promoter is mediated by an N-terminal domain which is responsible for its interaction with its co-repressor, Sin3A [139]. This binding can be reversed by overexpression of Myc in mortal WI38 cells [86]. The Mad1-induced hTERT repression has clinical significance in patients suffering from myelodysplastic syndrome (MDS) as these individuals, especially those with more favorable outcomes, show higher expression levels of Mad1 as compared to healthy controls [216]. The authors of the report went on to propose that Mad1 plays an important role in reducing hTERT expression during the early stage of the disease. Thus, it is not surprising that a number of publications have focused on the effects of various molecules on Mad1-mediated repression of hTERT promoter. For example, 4-[3-(1-adamanyly)-4-hydroxyphenyl]-3-chlorocinnamic acid (3-C1-AHPC), an adamantyl-related molecule, has been shown to inhibit the expression of miR-202, which targets Mad1 gene, and hence lead to de-repression of hTERT gene and increased telomerase activity [140]. The repressive role of the 3-C1-AHPC/miR-202/Mad1 axis on hTERT expression was supported by the observation that the overexpression of pre-miR-202 and inhibition of miR-202 resulted in the decreased and increased repressive activity of Mad1 on hTERT expression, respectively. In addition, EGCG (a green tea polyphenol), sulforaphane (SFN), 15d-PGJ2 (15-deoxy-delta-12,14-prostaglandin J2), selenium, and 12-O-tetradecanoylphorbol-13-acetate have been shown to enhance Mad1’s binding to and repression of hTERT promoter [85,108,139,217,218].
2.2.2. Sp3
Sp3 is a GC-box-binding protein with an antagonistic function to Sp1. Sp3, together with Sp1, has been shown to bind to a recognition site in the proximal promoter of hTERT, resulting in the repression of the gene. This binding is important but not sufficient to govern hTERT expression; chromatin environment, such as histone modification patterns, is also essential [219]. In fact, several studies have reported that the binding of Sp3 to hTERT proximal promoter leads to the recruitment of a histone deacetylase (HDAC), HDAC1, to the promoter region and, in turn, modifies the epigenetic landscape of the region, resulting in the silencing of the hTERT gene [186]. This repression can be reversed by overexpression of dominant-negative form of Sp3. On the other hand, ceramide is known to enhance the Sp3/HDAC-mediated suppression of the hTERT gene [174]. This involves the ceramide-induced deacetylation of Sp3, an active form of the protein with higher binding capacity to hTERT promoter, and a decrease in RNA polymerase II recruitment to the promoter due to lower histone acetylation levels in the region. Ceramide has also been shown to reduce Sp1 binding to the hTERT promoter, thus abrogating its activating effect on hTERT transcription [174]. However, it is worth noting that a study has posited that Sp1, but not Sp3, plays a regulatory role in telomerase activity in Jurkat T cells, as Sp3 overexpression in these cells did not cause a significant change in hTERT mRNA levels or telomerase activity [220].
2.2.3. CTCF
CTCF is chromatin-binding factor which affects transcription of numerous genes. It mostly acts as a transcription repressor but is known to activate the transcription of several genes. The binding of CTCF to the first exon of the hTERT gene was reported to suppress its expression in telomerase-negative cells but not in cancer cells [105]. It preferentially binds to unmethylated sites of the hTERT regulatory region. The treatment of telomerase-positive cells with a strong demethylating agent (5-azadC) led to the reactivation of CTCF binding to its response element and repression of hTERT expression. This observation was supported by another study whose results showed that the introduction of sulforaphane (SFN), a potent histone deacetylase inhibitor, in human breast cancer cells resulted in CpG demethylation and hyperacetylation of the region proximal to the first exon of hTERT and this facilitated the binding of hTERT repressors, such as MAD1 and CTCF, in this region [108]. Conversely, siRNA-induced knockdown of CTCF was sufficient to partially reverse the inhibitory effect of SFN on hTERT transcription. In addition to binding to the first exon of hTERT, CTCF was also found to negatively modulate hTERT expression by binding to an enhancer element approximately 4.5 kb upstream of the hTERT transcription start site and this was confirmed by chromosome conformation capture (3C) assay [221].
2.2.4. E2F1
E2F1 is a transcription factor which binds to E2 recognition sites found in the promoter of numerous genes, especially those involved in cell cycle regulation and DNA damage response. It has also been shown to play a direct role in the transcription regulation of hTERT in human squamous cell carcinoma cells by binding to and modulating hTERT proximal promoter [110]. The overexpression of wildtype E2F1, but not its mutant, has been shown to downregulate hTERT promoter activity and telomerase activity. The effect of E2F1 on hTERT promoter has been proposed to counteract the activating effect of c-Myc on the regulatory region [100] and act downstream of the TGFβ signaling pathway as the expression of a dominant negative form of E2F1 resulted in the abrogation of the TGFβ-induced hTERT inhibition [111].
2.2.5. Hormone Nuclear Receptors
Vitamin D(3) receptor (VDR) is the nuclear receptor for 1,25-dihydroxyvitamin D(3) (VD3) and it can form a heterodimer with another nuclear receptor, retinoid X receptor (RXR), whose ligand is 9-cis-retinoic acid (9-cis-RA). In prostate cancer cells, this heterodimer was found to bind directly to a region approximately 2.5 kb upstream of hTERT translation start site and repress the expression of the gene [203]. In vivo experiments involving xenografts in nude mice recapitulated the inhibitory effects of this protein heterodimer on hTERT transcription. However, another report has argued against the direct role of VDR on hTERT transcription repression. Instead, it was proposed that the inhibitory effect of VD3 stems from its suppression of hTERT mRNA by destabilizing it [222]. This was supported by independent studies in ovarian, endometrial and breast cancer cells where VD3 was shown to repress hTERT expression through the induction of miR-498, which in turn targets the 3’ untranslated region (3’ UTR) of hTERT transcripts and thus decreases its expression [120,223].
Another nuclear receptor that has a repressive effect on hTERT transcription is androgen receptor (AR). Treatment of prostate cancer cells with AR agonist resulted in the inhibition of hTERT promoter activity and, conversely, treatment of AR antagonist failed to recapitulate this inhibition [33]. On top of that, the expression of a mutant form of AR (T877A) not only broadens ligand specificity but also precluded its binding to the hTERT promoter.
2.3. Transcription Regulators with Dual Roles
2.3.1. Specificity Protein 1 (Sp1)
Sp1 is a transcription factor that binds to GC-box motifs in the promoter of its target gene and regulates its expression, either activating or repressing the transcription process depending on cellular context. Sp1 is known to activate hTERT gene expression in telomerase-positive cells but suppresses it in telomerase-negative ones. It does this by binding to five GC-box motifs found in the proximal promoter of hTERT [46,165]. Mutation of these binding sites results in the attenuation of Sp1-mediated hTERT activation or repression in various cell lines. However, the expression and hTERT promoter-binding of Sp1 alone is insufficient to drive the desired effect. Epigenetic environment, especially the presence of suitable histone marks, is crucial in actualising these effects.
In telomerase-positive cells, particularly cancer cells, Sp1 may activate hTERT expression on its own or in conjunction with specific co-activators. Sp1 may work cooperatively with c-Myc and bind their respective response elements in hTERT proximal promoter to upregulate the transcription of the gene [46,53]. In fact, in the various cell lines examined, expression of Sp1 and c-Myc correlates positively with hTERT transcription activity. Kaposi’s sarcoma-associated herpesvirus (KSHV) latent protein, latency-associated nuclear antigen (LANA), was also found to bind directly to Sp1 in KSHV-infected body-cavity-based lymphoma (BCBL) cells and enhance the binding of Sp1 to its cognate binding sites in the hTERT proximal promoter [165,166]. In HeLa cells, the human T cell leukaemia virus type 1 protein, HTLV-1 bZIP factor (HBZ), forms heterodimers with JunD and this complex interacts with Sp1 at the hTERT promoter region and causes the activation of the target gene [36]. In addition, MBD1-Containing Chromatin-Associated Factor 1 (MCAF1) was shown to interact with Sp1 and the general transcription machinery in HeLa cells and facilitate hTERT expression [168]. Nuclear Factor Of Activated T-Cells 1 (NFAT1) can bind to hTERT proximal promoter at a site flanked by two GC-boxes and this allows for the synergistic interaction between NFAT1 and Sp1, resulting in the activation of hTERT transcription [154]. In human liver carcinoma cells, High Mobility Group AT-Hook 2 (HMGA2) binds to Sp1 and disrupts the recruitment of histone deacetylase (HDAC) to the promoter of hTERT and this leads to an increase in the expression of the gene [170]. This observation was recapitulated in cells treated with suberoylanilide hydroxamide (SAHA), a HDAC inhibitor. Numerous other factors and molecules have been shown to facilitate Sp1-mediated upregulation of hTERT gene expression in various cell lines (refer to Table 1 for a comprehensive list), and many others have been reported to play a repressive role instead. Notable inhibitors of Sp1-mediated activation of hTERT promoter activity include the tumor suppressor p53 [171,224] and its family members p63 and p73 [88,172,173], the key transcription factor E2F-1 [176 Beitzinger, 2006 #55], and the cell cycle checkpoint proteins p27Kip1 [93] and p16 [225].
As alluded to earlier, epigenetic environment is important in Sp1-mediated regulation of hTERT gene. The epigenetic mechanism behind the repression of hTERT gene expression in telomerase-negative human somatic cells is particularly well-characterized. Sp1 binds to its cognate binding motifs found in the hTERT proximal promoter and recruits HDAC proteins to this region [183,226]. The deacetylation of histone subunits leads to the silencing of the hTERT gene. This transcription repression may be reversed by treating the cells with trichostatin A (TSA), a HDAC inhibitor [226,227]. TSA-induced activation of hTERT was shown to be enhanced by the overexpression of Sp1 [227] or HDAC1 [226], or the mutation of the Sp1 binding sites [226,227]. In contrast, TGF-β-activated kinase 1 (TAK1) has the ability to facilitate the recruitment of HDAC to Sp1 on hTERT promoter and suppress hTERT activation in human lung adenocarcinoma cells [185].
2.3.2. Activator Protein 1 (AP-1)
AP-1 is a transcription factor complex which consists of components belonging to the c-Jun, c-Fos, Activating Transcription Factor (ATF) and J Domain-Containing Protein (JDP) families. Its effect on hTERT transcription has been contentious. It was initially reported to repress hTERT expression in HeLa cells, where the transient overexpression of its components (c-Fos and c-Jun or c-Fos and JunD) strongly represses hTERT promoter activity [34]. This suppression was found to be mediated by the binding of the heterodimer to two regions upstream of the hTERT translation start site. Mutation of these binding sites led to the abrogation of the repressive effect of AP-1 on hTERT. On the other hand, ectopic expression of the viral protein HBZ and JunD in HeLa cells was shown to activate hTERT promoter [36]. This involves the interaction of HBZ/JunD heterodimer with the co-activator Sp1 and their binding to GC-rich motifs in the hTERT promoter.
2.3.3. Early growth response-1 (EGR-1)
EGR-1 is a transcription factor whose activity is crucial for mitogenesis and cellular differentiation. The effect of this protein on hTERT transcription is two-fold—it can act as an activator and also a repressor depending on the tissue of origin. Ectopic expression of EGR-1 in villous cancer cells led to an increase in hTERT expression, both in the mRNA and protein levels [114]. This effect was shown to be mediated by the direct binding of the protein to a single site in the proximal promoter region of hTERT. Furthermore, expression of EGR-1 was found to correlate with that of hTERT during trophoblastic development and in patients with preeclampsia. On the contrary, the examination of mRNA levels in primary cervical cancer tissues revealed a negative correlation between the expression of EGR-1 and that of hTERT. Further studies involving epidermoid carcinoma and squamous cell carcinoma cell lines supported this observation—overexpression of EGR-1 resulted in the suppression of hTERT expression [115]. These results showcased the important role EGR-1 plays in the regulation of hTERT gene transcription.
2.3.4. Hypoxia-inducible factor 2-alpha (HIF-2α)
HIF-2α is a transcription factor which is involved in cellular oxygen regulation. Unlike another hypoxia-inducible factor, HIF-1, whose regulatory effect on hTERT gene is solely positive, HIF-2α plays dual role in modulating hTERT expression. In several renal carcinoma cell lines tested, HIF-2α overexpression led to an increase in hTERT promoter activity and its depletion resulted in lower hTERT mRNA levels [134]. This positive effect is mediated by the direct binding of HIF-2α to hTERT proximal promoter and the recruitment of p300 and histone acetyltransferase to the regulatory site. In contrast, ectopic expression of HIF-2α in three glioma cell lines led to repression of hTERT gene expression [134]. This suggests that HIF-2α may play different roles in hTERT gene regulation in different cellular context.
2.3.5. Kruppel-like family of transcription factors (KLF) Proteins
KLFs are a group of proteins which share a common structure, namely a C-terminal domain consisting of three zinc finger configurations. The human genome contains 17 KLF family genes and they may act as activators or repressors of their target genes. Two of the members of the KLF family proteins are known to play opposite roles in the regulation of hTERT expression. KLF2 was shown to bind to a putative site in the first exon of the hTERT gene in resting T cells, resulting in the repression of the latter [136]. However, upon activation of these cells, KLF2 dissociates from the regulatory element and this relieves the gene of its repression. In contrast, KLF4, a key pluripotency marker, and mediator, was found to activate hTERT expression in telomerase-negative, alternative lengthening of telomere (ALT) cells and human fibroblast cells via direct binding to a response element in the proximal promoter region of hTERT [137]. Conversely, knocking down KLF4 in telomerase-positive cancer and stem cells abrogated hTERT expression significantly. Furthermore, telomerase expression was found to be sufficient in replacing KLF4 function in the maintenance of self-renewal in human embryonic stem cells, showcasing that hTERT is one of the primary targets of KLF4 in stem cell maintenance. Interestingly, a recent study revealed that the knockdown of hTERT via shRNA in HeLa cells led to a decrease in KLF4 expression [228]. This shows that the interplay between the expression of KLF4 and hTERT is a complex and important one.
2.3.6. Nuclear Transcription Factor X Box-Binding Protein 1 (NFX1)
NFX1 is traditionally known as a transcription repressor. It plays a key role in determining the duration of inflammatory response via its interaction with and transcription regulation of the promoter of MHC class II genes. Three isoforms of NFX1 have been identified and two of these have opposite roles in the regulation of hTERT gene expression. NFX1-123, with c-Myc as a co-activator, was shown to bind to an X-box motif located adjacent to a known E-box motif, the canonical response element of c-Myc and other E-box proteins, and activate hTERT promoter activity [48]. In contrast, NFX1-91 represses hTERT expression by binding to the same X-box motif and recruiting Sin3A and histone deacetylases (HDACs) to the proximal promoter of hTERT [48]. This repressive effect can be reversed by the expression of the human papillomavirus (HPV) viral protein E6, which facilitates the ubiquitination and degradation of the NFX1-91 isoform, or by knocking down NFX1-91. Thus, alternative splicing of the NFX1 transcript plays an important role in the expression of hTERT gene in human fetal kidney cells.
2.3.7. Upstream stimulatory factors (USF) Proteins
USFs are a group of proteins belonging to the basic helix-loop-helix leucine zipper protein family. They function as transcription regulators and are known to bind to E-box motifs found in regulatory elements of their target genes. USF1 and USF2 have been shown to play both activating and repressive roles in the regulation of hTERT gene expression. USF1/2 heterodimers were found to bind directly to E-box motifs found on the proximal promoter of hTERT in both telomerase-positive and -negative cells. However, they specifically activate hTERT expression only in telomerase-positive cells [200]. This positive effect was shown to be mediated by p300, which acts as a co-activator, and p38 MAP kinase, a known activator of USF activity. In stark contrast, two other reports posited that USF1 and USF2 act as suppressors of hTERT expression in telomerase-positive oral squamous cancer cells and E6-expressing immortalized cells [54,202]. This was evident by the downregulation and upregulation of E6-mediated hTERT expression upon the forced expression and siRNA-induced knockdown of the USF genes, respectively. Moreover, binding of USF1 and USF2 to the proximal E-box motif in the hTERT promoter diminishes in cells expressing E6 and this is accompanied by an increase in c-Myc binding to the same site [54]. Interestingly, yet another group proposed that the effect USF2 has on hTERT expression is dependent on the relative abundance of the splice isoforms in the cells. They showed that both full-length and truncated forms of USF2 can bind to the hTERT proximal E-box. However, the latter has a dominant-negative effect on the former, therefore depleting the full-length isoform and abrogating its activating effect on hTERT expression [201]. This concurs with the finding that the N-terminally truncated isoform is only present in telomerase-negative resting lymphocytes but not in telomerase-positive activated lymphocytes [201].
2.4. Epigenetic Modifiers Regulating hTERT Transcription
Differentiation of pluripotent stem cells is accompanied by the downregulation of hTERT expression. hTERT promoter is eventually silenced when the cells are terminally differentiated. On the other hand, reprogramming of somatic cells to induced pluripotent stem cells results in the activation of hTERT expression. The epigenetic regulation of hTERT promoter plays an important role in these two opposite hTERT transcriptional states. The following section will focus on the known modes of epigenetic regulation of hTERT expression including methylation of hTERT promoter, histone modifications, and modulation by sirtuins and non-coding RNAs. However, it remains unclear how hTERT expression is differentially regulated during cancer development, cellular differentiation and reprogramming.
2.4.1. Histone Modifiers
In telomerase-positive cells, telomerase expression is associated with hyperacetylation of histone H3 and H4 and methylation of lysine-4 of histone H3, whereas in telomerase-negative cells (ALT cells), the silencing of the expression of telomerase is associated with hypoacetylation of H3 and H4 and methylation of lysine-9 of histone H3 [229]. Induction of the tumor suppressor, AT-Rich Interaction Domain 1A (ARID1A), increases occupancy of H3K9me3 at transcription start site (TSS) of hTERT and decreases acetylated lysine-12 of histone H4 (H4K12Ac) levels at this site. Treatment of telomerase negative cells with histone deacetylase inhibitor (HDACi), trichostatin A (TSA), could activate transcription of hTERT gene [229].TSA was shown to inhibit deacetylation of histone H3 lysine-9 or -14, leading to upregulated hTERT expression in umbilical cord mesenchymal stem cells as well [230]. Sirtuin protein family consists of seven homologs of yeast Sir2 protein which is known to be involved in a plethora of biological processes including aging [231], stress response, and tumorigenesis [232]. SIRT1, also known as nicotinamide adenine dinucleotide (NAD)-dependent deacetylase sirtuin-1, was shown to deacetylate c-Myc [233]. Deacetylated c-Myc displays enhanced association with Max, an essential partner for its activation [227], and this, in turn activates hTERT expression [233]. Knocking down SIRT1 leads to decreased expression of genes targeted by c-Myc including hTERT. These findings have been recapitulated by other groups with additional mechanism. Deletion of SIRT1 leads to significant reduction in telomerase expression, thus causing telomere dysfunction-induced foci and nuclear abnormality [234,235]. The same group found that deletion of SIRT1 is associated with substantial induction of acetylated lysine-9 of histone H3 (H3K9Ac) and reduction in trimethylated H3K9 at hTERT promoter [235]. In addition, SET and MYND Domain-Containing Protein 3 (SMYD3) knockdown in colorectal carcinoma and hepatocellular carcinoma cells was found to diminish the occupancy of c-Myc at hTERT promoter via reduction in H3K4 trimethylation and histone H3 acetylation.
2.4.2. Regulators of hTERT Promoter Methylation
DNA methylation is involved in gene silencing [236,237]. The hTERT promoter region contains clusters of CpG islands with dense GC-rich regions. This suggests that DNA methylation may play a role in the regulation of hTERT expression [238]. However, recent reports suggested that there is little or no methylation around the hTERT transcription start site (TSS) in most cancer cell lines and embryonic stem cells [239,240]. Consistent with these results, demethylation of CpG by demethylating agent 5aza-2′-deoxycytidine (5azadC) is not associated with increased hTERT expression in cancer cells [105,227,241,242]. In summary, there is an inconclusive correlation between DNA methylation and telomerase regulation. Information on the detailed results for different tissues, and regions of hTERT promoter, data interpretation, and categorization, and the DNA methylation detection methods is summarized in Table 2.

Table 2.
Detail of studies which reported methylation status of human telomerase reverse transcriptase (hTERT) promoter proximal region.
2.5. Monoallelic Expression of hTERT Gene
Several cancer cell lines contain point mutations in only one of its hTERT alleles, specifically in its promoter region (see below for a detailed discussion of hTERT promoter mutations and their effect on hTERT expression). This is attributed to the reversal of hTERT gene silencing on the one active allele while the other one remains silenced. The promoter of the active gene was found to be decorated by the permissive histone H3K4me2/3 mark, while the promoter of the silenced gene showed higher deposition of H3K27me3, a histone mark indicating transcriptionally repressed heterochromatin regions [247]. Concomitant to this epigenetic switch in the hTERT promoter region, the point mutation allows for the binding of the transcription factor GA Binding Protein Transcription Factor Alpha and -Beta 1(GABPA/B1) heterodimer to the newly created site, which in turn facilitates the recruitment of RNA Polymerase II to the promoter, resulting in the transcription of hTERT gene.
2.6. Complexity of Trans-Regulation of hTERT Gene Transcription
It is clear from the multitude of evidence presented above that hTERT transcription regulation is complex. It involves the interplay between the various components of molecular and cellular biology. This is not surprising given the crucial role telomerase plays in the maintenance of stem cell and cellular transformation. Despite the wealth of knowledge in the roles transcription factors and epigenetic modifiers play in the regulation of hTERT expression, a lot more has to be done to elucidate the mechanism underlying the switching off and on of hTERT gene during cellular differentiation and cellular reprogramming, and during cellular transformation, respectively.
3. Genetic Alterations Regulating hTERT Transcription
Recent advances in DNA sequencing technologies have enabled large-scale genome sequencing studies across various tumor types. Many alterations in protein-coding genes have been identified [248,249]. On the other hand, only a handful of the mutations in non-coding regions have been recently identified [250]. Recurrent mutations and chromosomal rearrangements in hTERT promoter have further confirmed the importance of telomerase activation in human cancers [251,252].
3.1. hTERT Promoter Mutations
Transcriptional regulation at the level of hTERT promoter mutations first came into attention with the discovery of highly recurrent mutations in this region in melanomas. In particular, two hotspot C > T point mutations were observed at the nucleotide position 124 bp and 146 bp upstream of the translation start codon (ATG) and these specific mutations were termed C228T and C250T, respectively. Other less common mutations were also detected in the hTERT promoter region such as the CC > TT mutations at −124/−125 and −138/−139 positions. The observation that the frequency of mutations detected in hTERT promoter is higher than those in the B-Raf Proto-Oncogene Serine/Threonine-Protein Kinase (BRAF) gene, and that the presence of highly recurrent point mutations in just two nucleotide positions strongly suggests that these are driver mutations which play key roles at various stages of tumorigenesis in melanoma [251,252].These important findings have provided new insights into a possible mechanism for hTERT activation in human cancers. In addition, the discovery of driver alterations in the non-coding portion of the human cancer genome was a novel advancement in understanding the role of mutated non-coding sequences in transcriptional deregulation and tumorigenesis.
3.1.1. hTERT Promoter Mutations in Different Types of Human Cancers
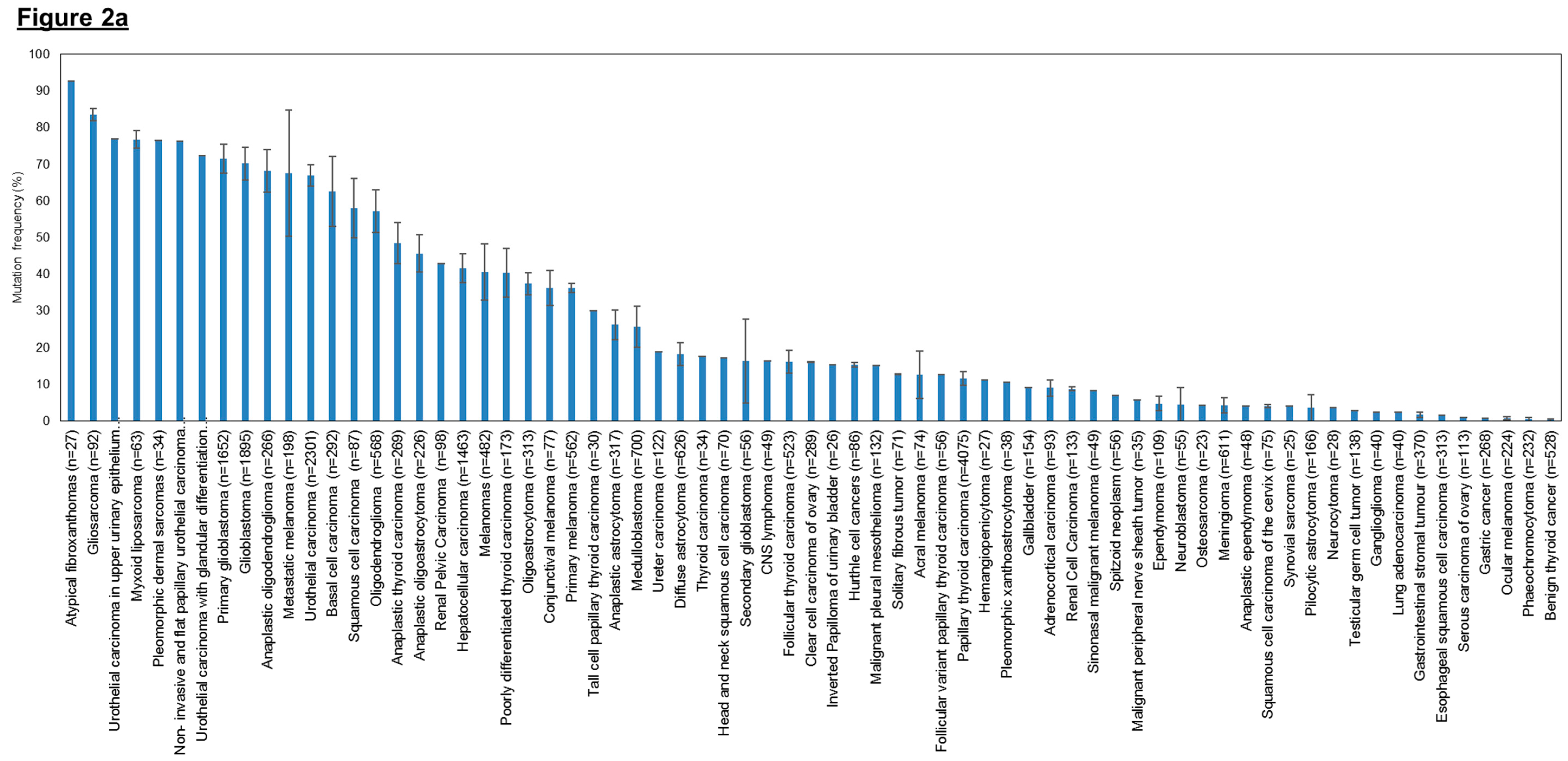
Since the publication of the studies mentioned above, there has been a surge in interest in investigating the frequency of hTERT promoter mutations in melanomas and other types of cancers, particularly at the two hotspot locations, C228T and C250T (Figure 2). To date, hTERT promoter mutations have been evaluated in more than 60 tumor types and they are found to be the most common point mutations in hepatocellular carcinoma [253,254], glioblastoma [253], bladder cancer [255] and melanoma [251,252].
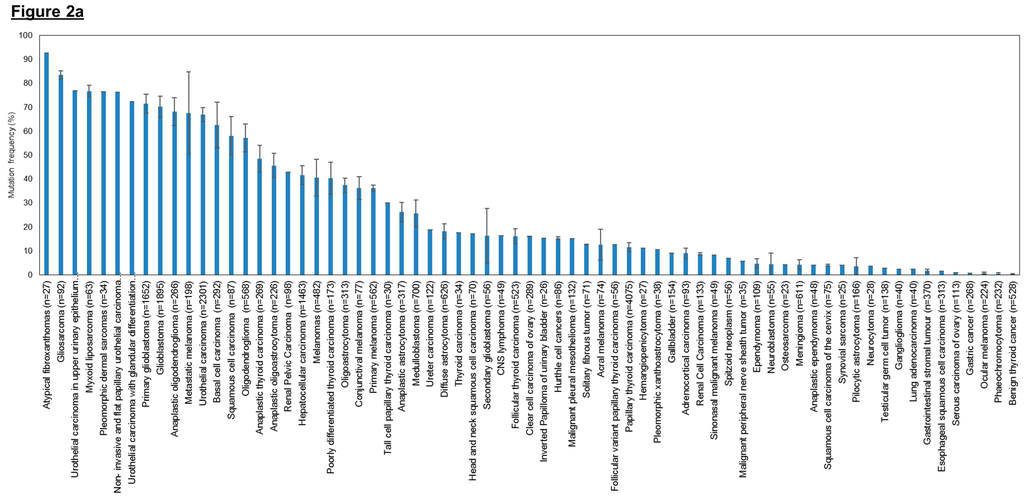
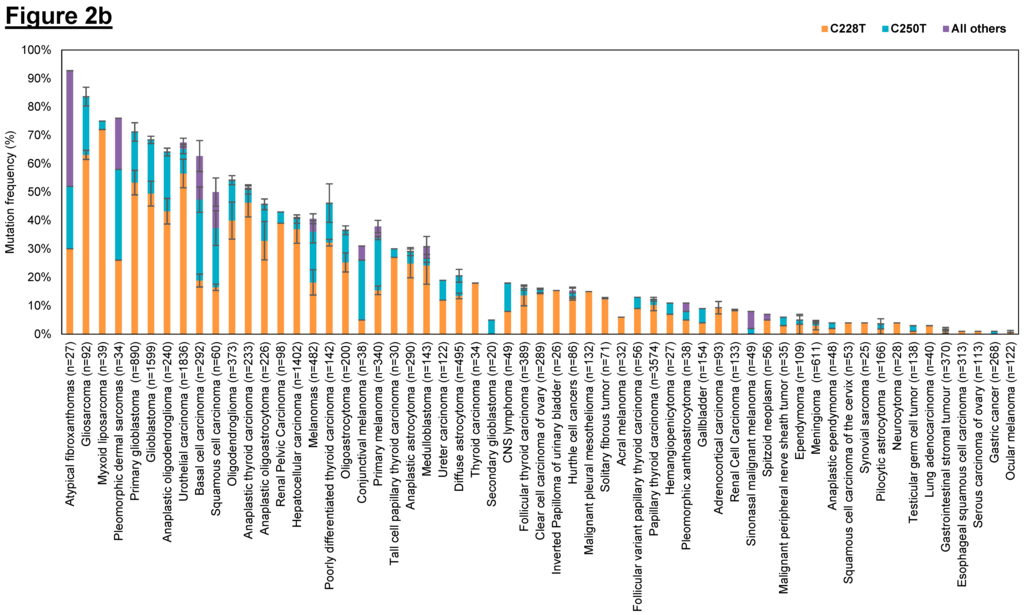
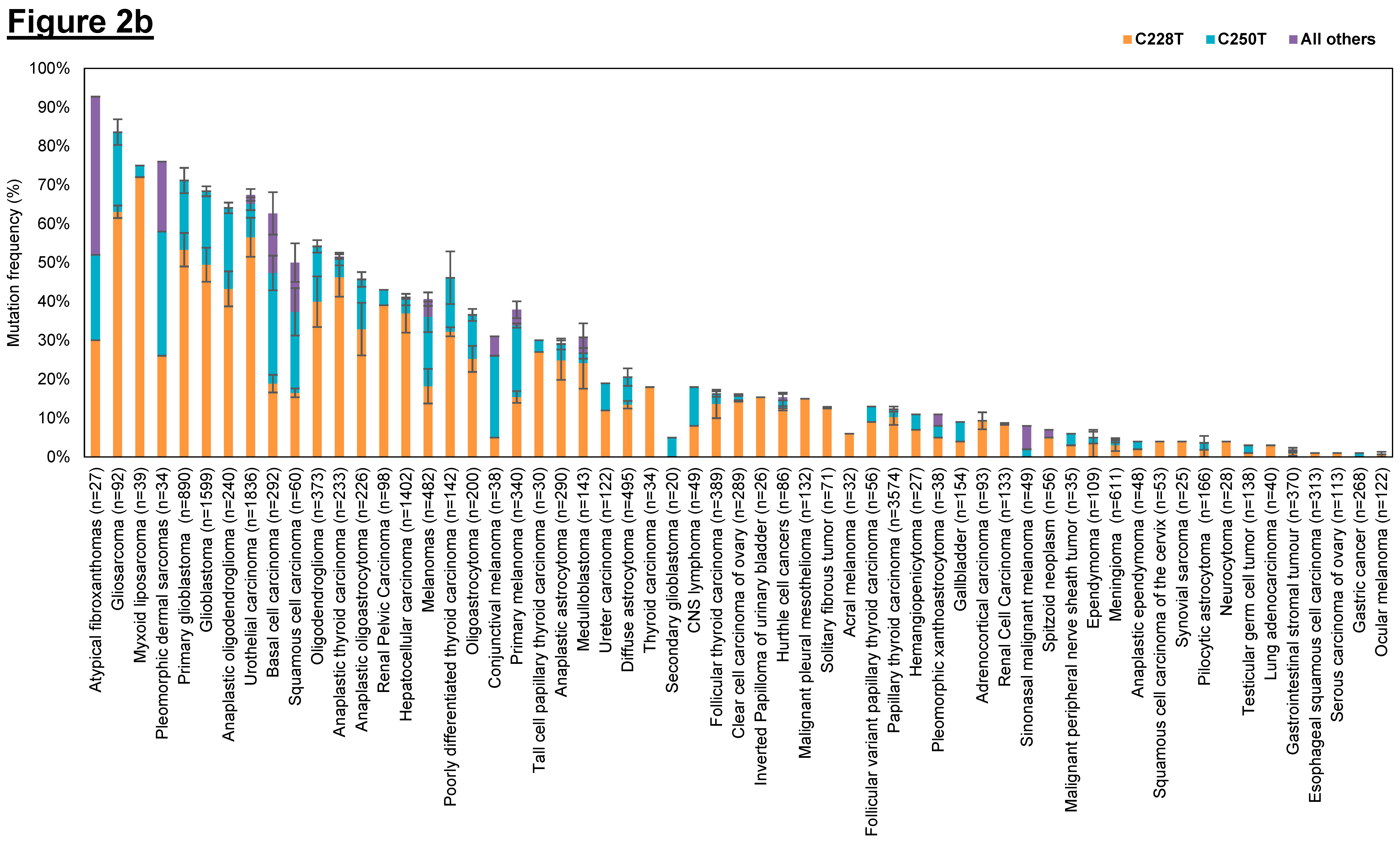
Figure 2.
Frequency of human telomerase reverse transcriptase (hTERT) promoter mutations in various cancer types. (a) Overall hTERT promoter mutation frequencies of various cancer types plotted in descending order; (b) Overall hTERT promoter mutation frequencies of various cancer types with breakdown of individual frequencies of C228T, C250T, and all other mutations. The overall mutation frequencies of hTERT promoter were compiled from all relevant publications on human cancer genome sequencing. The label “n” corresponds to the total number of tumors sequenced among different studies for the same tumor type. Error bars correspond to the standard errors in mutation frequencies calculated among different studies on the same tumor type. Only studies with at least 20 samples sequenced were included in this study. Only studies which provided the detailed breakdown of different mutation sites were included in (b). Refer to Table 3 for the list of references used to compile this figure.
Cancer types that have high overall mutation frequency generally correspond to tumors arising from cells with low turnover rate such as neurons, fibroblasts, glial cells and hepatocytes (Figure 2a). This observation concurs with previously published results that showed that hTERT promoter mutations occur most frequently in cancers that originate in tissues with low self-renewal rates [253]. Based on the hTERT promoter mutation studies conducted so far, it is tantalizing to hypothesize that cancer subtypes without hTERT promoter mutations may derive from tissues with tissue-specific stem cells as they already display high telomerase expression and therefore do not require hTERT promoter mutations to further upregulate telomerase expression to sustain telomere maintenance. In contrast, cells with lower hTERT expression levels and low turnover rates might selectively acquire hTERT promoter mutations during tumorigenesis to upregulate telomerase levels and avoid replicative senescence. We further examined the distribution of the two hotspot mutations (C228T and C250T) across the different tumor types (Figure 2b) and observed that the mutation frequency ratio of C228T:C250T is markedly lower in cancers of the cutaneous tissues such as basal cell carcinomas, squamous cell carcinoma and melanoma.
3.1.2. Mechanism by Which hTERT Promoter Mutations Leads to Enhanced Telomerase Levels
The hotspot mutations mentioned earlier create a CCGGAA/T binding motif for ETS (E-twenty six) transcription factors whose recruitment to these sites may result in increased hTERT expression. This is facilitated by the activation of non-canonical NF-κB signaling pathway which provides a co-activator, in the form of p52, to enhance the transcription activity of the hTERT promoter [213] (see above for a more comprehensive discussion of the molecular mechanism). Reporter assays carried out to assess the functional impact of these promoter mutations showed a two- to four-fold increase in hTERT promoter activity in melanomas [251,252].
3.2. Chromosomal Rearrangements and hTERT Expression
A recent study involving neuroblastoma tumors posited the significance of genomic rearrangements in telomerase activation specifically in the high-risk bracket of this tumor type [246]. They discovered from their analysis of whole-genome sequencing data that neuroblastoma tumors displayed breakpoint clusters at four genomic loci and one of them is at the 5p15.33 region where hTERT gene is located. These chromosomal rearrangements were found to consistently cluster in the region about 50 kb upstream of the hTERT transcription initiation site, but at the same time leave the coding and core promoter region intact. They showed that these chromosomal alterations result in the juxtaposition of hTERT gene to strong enhancer regions, resulting in major changes in the epigenetic landscape of the affected region. These changes, in turn, lead to the abrogation of hTERT gene silencing engendered by repressive histone modification and DNA methylation states.
4. Conclusions
Herein, we have summarized the numerous transcription activators and repressors that were found to interact with the hTERT core promoter. However, it is still unclear how hTERT is silenced during stem cell differentiation as well as reactivated during somatic cell reprogramming. These processes are likely to involve a host of these transcription regulators in a cell context-dependent manner. In addition, they are likely to be controlled by epigenetic changes accompanying these cellular events. The contribution of specific transcription modulators in these processes remains to be explored and elucidated. Given the complexity of the regulatory network, it is perhaps more meaningful to approach the issue from a wider perspective by studying the network system as a whole instead of focusing on individual players.
The identification of hTERT promoter mutations, on the other hand, may provide interesting biomarkers for diagnosis and prognosis of various cancer types. Since the publication of the abovementioned landmark papers, many studies have subsequently explored the potential application of these mutations as biomarkers. To date, the existence of hTERT promoter mutations has been associated with decreased survival in patients suffering from melanoma [279,292], bladder cancer [280,352], urogenital cancer [280,325], glioma [274], medulloblastoma [316], thyroid cancer [299,300,330], and laryngeal tumors [353].
As shown in Figure 2, urothelial carcinoma samples display up to 67% mean mutation frequency (n = 2301) in the hTERT promoter region, specifically at the two hotspots, making it the most frequently mutated gene identified so far in this region. Hence, these hTERT promoter mutations have the potential to become a good biomarker for clinicians to conduct early detection of bladder cancer and subsequent follow-up tests for cancer progression or recurrence in patients. In hepatocellular carcinomas, about 42% mean mutation frequency (n = 1463) in the hTERT promoter region was observed. Similarly, their high prevalence suggests that these hotspot mutations may be utilized as candidate biomarkers for early detection and monitoring. Lastly, the high prevalence of hTERT promoter mutations in various subtypes of gliomas also suggest that the mutations can serve as useful biomarkers to aid in classification and prognostication via extraction of samples from cerebrospinal fluid. Further studies to confirm such a causal relation in various tumor types will be necessary before genome-based clinical classifications based on hTERT promoter mutations can find their way into the clinics.
Activation of telomerase enzyme is classified as one of the classic hallmarks of cancer as it confers cells with replicative immortality [354]. Hence, blocking the activity of telomerase is an active area of research in cancer therapeutics. Previous strategies to block telomerase activity in cancer patients have focused on the use of immunotherapy, gene therapy, small molecular inhibitors and G-quadruplex ligands, of which some have entered clinical trials [355]. However, such targeting strategies can also result in non-specific inhibition of telomerase activity in tissue progenitor/stem cells, which may limit the utilization of such telomerase inhibitors in the long run. As we find out more about the mechanism of how hTERT promoter is activated or repressed, novel strategies to therapeutically target telomerase can be developed. For instance, new specific small molecular inhibitors may be developed to interfere with the binding of ETS/TCF transcription factors to the CCGGAA/T binding motif that is only present in cancer cells that carry the specific C228T or C250T mutation. In addition, as mentioned above, the presence of hTERT promoter mutation in a tumor may be used as a biomarker to predict subsequent clinical response to a telomerase inhibitor drug.
Acknowledgments
All sources of funding of the study should be disclosed. Please clearly indicate grants that you have received in support of your research work. Clearly state if you received funds for covering the costs to publish in open access.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Blackburn, E.H. Telomere states and cell fates. Nature 2000, 408, 53–56. [Google Scholar] [CrossRef] [PubMed]
- De Lange, T. How shelterin solves the telomere end-protection problem. Cold Spring Harb. Symp. Quant. Biol. 2010, 75, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Makarov, V.L.; Hirose, Y.; Langmore, J.P. Long G tails at both ends of human chromosomes suggest a C strand degradation mechanism for telomere shortening. Cell 1997, 88, 657–666. [Google Scholar] [CrossRef]
- Wright, W.E.; Tesmer, V.M.; Huffman, K.E.; Levene, S.D.; Shay, J.W. Normal human chromosomes have long G-rich telomeric overhangs at one end. Genes Dev. 1997, 11, 2801–2809. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Funk, W.D.; Wang, S.S.; Weinrich, S.L.; Avilion, A.A.; Chiu, C.P.; Adams, R.R.; Chang, E.; Allsopp, R.C.; Yu, J.; et al. The RNA component of human telomerase. Science 1995, 269, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.M.; Morin, G.B.; Chapman, K.B.; Weinrich, S.L.; Andrews, W.H.; Lingner, J.; Harley, C.B.; Cech, T.R. Telomerase catalytic subunit homologs from fission yeast and human. Science 1997, 277, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.W.; Piatyszek, M.A.; Prowse, K.R.; Harley, C.B.; West, M.D.; Ho, P.L.; Coviello, G.M.; Wright, W.E.; Weinrich, S.L.; Shay, J.W. Specific association of human telomerase activity with immortal cells and cancer. Science 1994, 266, 2011–2015. [Google Scholar] [CrossRef] [PubMed]
- Masutomi, K.; Yu, E.Y.; Khurts, S.; Ben-Porath, I.; Currier, J.L.; Metz, G.B.; Brooks, M.W.; Kaneko, S.; Murakami, S.; DeCaprio, J.A.; et al. Telomerase maintains telomere structure in normal human cells. Cell 2003, 114, 241–253. [Google Scholar] [CrossRef]
- Wright, W.E.; Piatyszek, M.A.; Rainey, W.E.; Byrd, W.; Shay, J.W. Telomerase activity in human germline and embryonic tissues and cells. Dev. Genet. 1996, 18, 173–179. [Google Scholar] [CrossRef]
- Levy, M.Z.; Allsopp, R.C.; Futcher, A.B.; Greider, C.W.; Harley, C.B. Telomere end-replication problem and cell aging. J. Mol. Biol. 1992, 225, 951–960. [Google Scholar] [CrossRef]
- De Lange, T.; Lundblad, V.; Blackburn, E.H. Telomeres, 2nd ed.; Cold Spring Harbor Laboratory Press: Plainview, NY, USA, 2005; p. 576. [Google Scholar]
- Harley, C.B.; Futcher, A.B.; Greider, C.W. Telomeres shorten during ageing of human fibroblasts. Nature 1990, 345, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Takai, H.; Smogorzewska, A.; de Lange, T. DNA damage foci at dysfunctional telomeres. Curr. Biol. 2003, 13, 1549–1556. [Google Scholar] [CrossRef]
- d’Adda di Fagagna, F.; Reaper, P.M.; Clay-Farrace, L.; Fiegler, H.; Carr, P.; Von Zglinicki, T.; Saretzki, G.; Carter, N.P.; Jackson, S.P. A DNA damage checkpoint response in telomere-initiated senescence. Nature 2003, 426, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Kaul, Z.; Cesare, A.J.; Huschtscha, L.I.; Neumann, A.A.; Reddel, R.R. Five dysfunctional telomeres predict onset of senescence in human cells. EMBO Rep. 2012, 13, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Flores, I.; Canela, A.; Vera, E.; Tejera, A.; Cotsarelis, G.; Blasco, M.A. The longest telomeres: A general signature of adult stem cell compartments. Genes Dev. 2008, 22, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Artandi, S.E.; DePinho, R.A. Telomeres and telomerase in cancer. Carcinogenesis 2010, 31, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Schaetzlein, S.; Lucas-Hahn, A.; Lemme, E.; Kues, W.A.; Dorsch, M.; Manns, M.P.; Niemann, H.; Rudolph, K.L. Telomere length is reset during early mammalian embryogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 8034–8038. [Google Scholar] [CrossRef] [PubMed]
- Holohan, B.; Wright, W.E.; Shay, J.W. Cell biology of disease: Telomeropathies: An emerging spectrum disorder. J. Cell Biol. 2014, 205, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Armanios, M.; Blackburn, E.H. The telomere syndromes. Nat. Rev. Genet. 2012, 13, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Lansdorp, P.M. Telomeres and disease. EMBO J. 2009, 28, 2532–2540. [Google Scholar] [CrossRef] [PubMed]
- Blasco, M.A.; Lee, H.W.; Hande, M.P.; Samper, E.; Lansdorp, P.M.; DePinho, R.A.; Greider, C.W. Telomere shortening and tumor formation by mouse cells lacking telomerase rna. Cell 1997, 91, 25–34. [Google Scholar] [CrossRef]
- Lee, H.W.; Blasco, M.A.; Gottlieb, G.J.; Horner, J.W., II; Greider, C.W.; DePinho, R.A. Essential role of mouse telomerase in highly proliferative organs. Nature 1998, 392, 569–574. [Google Scholar] [PubMed]
- Chou, W.C.; Chen, H.Y.; Yu, S.L.; Cheng, L.; Yang, P.C.; Dang, C.V. Arsenic suppresses gene expression in promyelocytic leukemia cells partly through Sp1 oxidation. Blood 2005, 106, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.W.; Bacchetti, S. A survey of telomerase activity in human cancer. Eur. J. Cancer 1997, 33, 787–791. [Google Scholar] [CrossRef]
- Smith, L.L.; Coller, H.A.; Roberts, J.M. Telomerase modulates expression of growth-controlling genes and enhances cell proliferation. Nat. Cell Biol. 2003, 5, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.S.; Wen, J.; Bacchetti, S. The human telomerase catalytic subunit hTERT: Organization of the gene and characterization of the promoter. Hum. Mol. Genet. 1999, 8, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, I.; Cable, P.L.; Afshari, C.; Barrett, J.C. Cloning and characterization of the promoter region of human telomerase reverse transcriptase gene. Cancer Res. 1999, 59, 826–830. [Google Scholar] [PubMed]
- Takakura, M.; Kyo, S.; Kanaya, T.; Hirano, H.; Takeda, J.; Yutsudo, M.; Inoue, M. Cloning of human telomerase catalytic subunit (hTERT) gene promoter and identification of proximal core promoter sequences essential for transcriptional activation in immortalized and cancer cells. Cancer Res. 1999, 59, 551–557. [Google Scholar] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Moehren, U.; Papaioannou, M.; Reeb, C.A.; Grasselli, A.; Nanni, S.; Asim, M.; Roell, D.; Prade, I.; Farsetti, A.; Baniahmad, A. Wild-type but not mutant androgen receptor inhibits expression of the hTERT telomerase subunit: A novel role of AR mutation for prostate cancer development. FASEB J. 2008, 22, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- Takakura, M.; Kyo, S.; Inoue, M.; Wright, W.E.; Shay, J.W. Function of AP-1 in transcription of the telomerase reverse transcriptase gene (TERT) in human and mouse cells. Mol. Cell. Biol. 2005, 25, 8037–8043. [Google Scholar] [CrossRef] [PubMed]
- Sitaram, R.T.; Degerman, S.; Ljungberg, B.; Andersson, E.; Oji, Y.; Sugiyama, H.; Roos, G.; Li, A. Wilms’ tumour 1 can suppress hTERT gene expression and telomerase activity in clear cell renal cell carcinoma via multiple pathways. Br. J. Cancer 2010, 103, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, A.S.; Villaudy, J.; Gazzolo, L.; Castellazzi, M.; Mesnard, J.M.; Duc Dodon, M. HTLV-1 HBZ cooperates with jund to enhance transcription of the human telomerase reverse transcriptase gene (hTERT). Retrovirology 2007. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.G.; Jayachandran, G.; Wu, G.; Xu, K.; Roth, J.A.; Ji, L. Tumor-specific activation of human telomerase reverses transcriptase promoter activity by activating enhancer-binding Protein-2β in human lung cancer cells. J. Biol. Chem. 2007, 282, 26460–26470. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Chen, W.; Guo, W.; Wang, J.; Tian, Y.; Shi, D.; Zhang, X.; Qiu, H.; Xiao, X.; Kang, T.; et al. Berberine targets AP-2/hTERT, NF-κB/COX-2, HIF-1α/VEGF and cytochrome-c/caspase signaling to suppress human cancer cell growth. PLoS ONE 2013, 8, e69240. [Google Scholar] [CrossRef] [PubMed]
- Suryo Rahmanto, Y.; Jung, J.G.; Wu, R.C.; Kobayashi, Y.; Heaphy, C.M.; Meeker, A.K.; Wang, T.L.; Shih Ie, M. Inactivating arid1a tumor suppressor enhances TERT transcription and maintains telomere length in cancer cells. J. Biol. Chem. 2016, 291, 9690–9699. [Google Scholar] [CrossRef] [PubMed]
- Hoffmeyer, K.; Raggioli, A.; Rudloff, S.; Anton, R.; Hierholzer, A.; Del Valle, I.; Hein, K.; Vogt, R.; Kemler, R. Wnt/β-catenin signaling regulates telomerase in stem cells and cancer cells. Science 2012, 336, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Toh, L.; Lau, P.; Wang, X. Human telomerase reverse transcriptase (hTERT) is a novel target of the Wnt/β-catenin pathway in human cancer. J. Biol. Chem. 2012, 287, 32494–32511. [Google Scholar] [CrossRef] [PubMed]
- Isenmann, S.; Cakouros, D.; Zannettino, A.; Shi, S.; Gronthos, S. hTERT transcription is repressed by Cbfa1 in human mesenchymal stem cell populations. J. Bone Miner. Res. 2007, 22, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.J.; Grandori, C.; Amacker, M.; Simon-Vermot, N.; Polack, A.; Lingner, J.; Dalla-Favera, R. Direct activation of TERT transcription by c-Myc. Nat. Genet. 1999, 21, 220–224. [Google Scholar] [PubMed]
- Xu, D.; Popov, N.; Hou, M.; Wang, Q.; Bjorkholm, M.; Gruber, A.; Menkel, A.R.; Henriksson, M. Switch from Myc/Max to Mad1/Max binding and decrease in histone acetylation at the telomerase reverse transcriptase promoter during differentiation of HL60 cells. Proc. Natl. Acad. Sci. USA 2001, 98, 3826–3831. [Google Scholar] [CrossRef] [PubMed]
- Ogretmen, B.; Kraveka, J.M.; Schady, D.; Usta, J.; Hannun, Y.A.; Obeid, L.M. Molecular mechanisms of ceramide-mediated telomerase inhibition in the a549 human lung adenocarcinoma cell line. J. Biol. Chem. 2001, 276, 32506–32514. [Google Scholar] [CrossRef] [PubMed]
- Kyo, S.; Takakura, M.; Taira, T.; Kanaya, T.; Itoh, H.; Yutsudo, M.; Ariga, H.; Inoue, M. Sp1 cooperates with c-Myc to activate transcription of the human telomerase reverse transcriptase gene (hTERT). Nucleic Acids Res. 2000, 28, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Veldman, T.; Liu, X.; Yuan, H.; Schlegel, R. Human papillomavirus E6 and Myc proteins associate in vivo and bind to and cooperatively activate the telomerase reverse transcriptase promoter. Proc. Natl. Acad. Sci. USA 2003, 100, 8211–8216. [Google Scholar] [CrossRef] [PubMed]
- Gewin, L.; Myers, H.; Kiyono, T.; Galloway, D.A. Identification of a novel telomerase repressor that interacts with the human papillomavirus type-16 E6/E6-AP complex. Genes Dev. 2004, 18, 2269–2282. [Google Scholar] [CrossRef] [PubMed]
- Faiola, F.; Liu, X.; Lo, S.; Pan, S.; Zhang, K.; Lymar, E.; Farina, A.; Martinez, E. Dual regulation of c-Myc by p300 via acetylation-dependent control of Myc protein turnover and coactivation of Myc-induced transcription. Mol. Cell. Biol. 2005, 25, 10220–10234. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.; Chang, H.S.; Lin, C.H.; Yu, W.C. HPV-18 E7 conjugates to c-Myc and mediates its transcriptional activity. Int. J. Biochem. Cell Biol. 2007, 39, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Kyo, S.; Takakura, M.; Kanaya, T.; Zhuo, W.; Fujimoto, K.; Nishio, Y.; Orimo, A.; Inoue, M. Estrogen activates telomerase. Cancer Res. 1999, 59, 5917–5921. [Google Scholar] [PubMed]
- Xu, D.; Dwyer, J.; Li, H.; Duan, W.; Liu, J.P. Ets2 maintains hTERT gene expression and breast cancer cell proliferation by interacting with c-Myc. J. Biol. Chem. 2008, 283, 23567–23580. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.T.; Kyo, S.; Laimins, L.A. Telomerase activation by human papillomavirus type 16 E6 protein: Induction of human telomerase reverse transcriptase expression through Myc and GC-rich Sp1 binding sites. J. Virol. 2001, 75, 5559–5566. [Google Scholar] [CrossRef] [PubMed]
- McMurray, H.R.; McCance, D.J. Human papillomavirus type 16 E6 activates TERT gene transcription through induction of c-Myc and release of USF-mediated repression. J. Virol. 2003, 77, 9852–9861. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dakic, A.; Zhang, Y.; Dai, Y.; Chen, R.; Schlegel, R. HPV E6 protein interacts physically and functionally with the cellular telomerase complex. Proc. Natl. Acad. Sci. USA 2009, 106, 18780–18785. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lee, T.H.; Avraham, H. A novel tricomplex of BRCA1, Nmi, and c-Myc inhibits c-Myc-induced human telomerase reverse transcriptase gene (hTERT) promoter activity in breast cancer. J. Biol. Chem. 2002, 277, 20965–20973. [Google Scholar] [CrossRef] [PubMed]
- Bu, D.X.; Johansson, M.E.; Ren, J.; Xu, D.W.; Johnson, F.B.; Edfeldt, K.; Yan, Z.Q. Nuclear factor {kappa}B-mediated transactivation of telomerase prevents intimal smooth muscle cell from replicative senescence during vascular repair. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2604–2610. [Google Scholar] [CrossRef] [PubMed]
- Sinha-Datta, U.; Horikawa, I.; Michishita, E.; Datta, A.; Sigler-Nicot, J.C.; Brown, M.; Kazanji, M.; Barrett, J.C.; Nicot, C. Transcriptional activation of hTERT through the NF-κB pathway in HTLV-I-transformed cells. Blood 2004, 104, 2523–2531. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Ogawa, K.; Ikei, T.; Fujiki, T.; Katakura, Y. FOXO3a potentiates hTERT gene expression by activating c-Myc and extends the replicative life-span of human fibroblast. PLoS ONE 2014, 9, e101864. [Google Scholar] [CrossRef] [PubMed]
- Ponnala, S.; Chetty, C.; Veeravalli, K.K.; Dinh, D.H.; Klopfenstein, J.D.; Rao, J.S. MMP-9 silencing regulates hTERT expression via β1 integrin-mediated FAK signaling and induces senescence in glioma xenograft cells. Cell Signal 2011, 23, 2065–2075. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ou, C.C.; Feldman, R.I.; Nicosia, S.V.; Kruk, P.A.; Cheng, J.Q. Aurora-a kinase regulates telomerase activity through c-Myc in human ovarian and breast epithelial cells. Cancer Res. 2004, 64, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Deng, X.; Deng, L.; Gu, H.; Fan, W.; Cao, Y. Telomerase activation by Epstein-Barr virus latent membrane protein 1 is associated with c-Myc expression in human nasopharyngeal epithelial cells. J. Exp. Clin. Cancer Res. 2004, 23, 495–506. [Google Scholar] [PubMed]
- Endoh, T.; Tsuji, N.; Asanuma, K.; Yagihashi, A.; Watanabe, N. Survivin enhances telomerase activity via up-regulation of specificity protein 1- and c-Myc-mediated human telomerase reverse transcriptase gene transcription. Exp. Cell Res. 2005, 305, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.Y.; Chen, Y.R.; Wang, T.C. A major role of PKC θ and NFκB in the regulation of hTERT in human T lymphocytes. FEBS Lett. 2006, 580, 6819–6824. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fang, X.; Ge, Z.; Jalink, M.; Kyo, S.; Bjorkholm, M.; Gruber, A.; Sjoberg, J.; Xu, D. The telomerase reverse transcriptase (hTERT) gene is a direct target of the histone methyltransferase SMYD3. Cancer Res. 2007, 67, 2626–2631. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Vorontchikhina, M.; Wang, Y.L.; Faiola, F.; Martinez, E. Staga recruits mediator to the Myc oncoprotein to stimulate transcription and cell proliferation. Mol. Cell. Biol. 2008, 28, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, Y.; Yang, H.; Cheng, J.Q.; Kruk, P.A. Pyk2/Erk 1/2 mediate Sp1- and c-Myc -dependent induction of telomerase activity by epidermal growth factor. Growth Factors 2008, 26, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, N.F.; Kocher, H.M.; Salako, M.A.; Obermueller, E.; Sandle, J.; Balkwill, F. A novel function of colony-stimulating factor 1 receptor in hTERT immortalization of human epithelial cells. Oncogene 2009, 28, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Bilsland, A.E.; Hoare, S.; Stevenson, K.; Plumb, J.; Gomez-Roman, N.; Cairney, C.; Burns, S.; Lafferty-Whyte, K.; Roffey, J.; Hammonds, T.; et al. Dynamic telomerase gene suppression via network effects of GSK3 inhibition. PLoS ONE 2009, 4, e6459. [Google Scholar] [CrossRef] [PubMed]
- Sitaram, R.T.; Cairney, C.J.; Grabowski, P.; Keith, W.N.; Hallberg, B.; Ljungberg, B.; Roos, G. The PTEN regulator DJ-1 is associated with hTERT expression in clear cell renal cell carcinoma. Int. J. Cancer 2009, 125, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Stefanou, N.; Papanikolaou, V.; Furukawa, Y.; Nakamura, Y.; Tsezou, A. Leptin as a critical regulator of hepatocellular carcinoma development through modulation of human telomerase reverse transcriptase. BMC Cancer 2010. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Yip, H.K. Neuroprotective signaling mechanisms of telomerase are regulated by brain-derived neurotrophic factor in rat spinal cord motor neurons. J. Neuropathol. Exp. Neurol. 2011, 70, 634–652. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Ogawa, K.; Ikei, T.; Udono, M.; Fujiki, T.; Katakura, Y. SIRT1 prevents replicative senescence of normal human umbilical cord fibroblast through potentiating the transcription of human telomerase reverse transcriptase gene. Biochem. Biophys. Res. Commun. 2012, 417, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Adamo, R.; Comandini, A.; Aquino, A.; Bonmassar, L.; Guglielmi, L.; Bonmassar, E.; Franzese, O. The antiretroviral agent saquinavir enhances hTERT expression and telomerase activity in human T leukaemia cells in vitro. J. Exp. Clin. Cancer Res. 2013. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, P.; Sun, X.; Zheng, J.; Wei, G.; Zhang, L.; Wang, H.; Yao, J.; Lu, S.; Jia, P. Acidified bile acids increase hTERT expression via c-Myc activation in human gastric cancer cells. Oncol. Rep. 2015, 33, 3038–3044. [Google Scholar] [CrossRef] [PubMed]
- Hrdlickova, R.; Nehyba, J.; Bose, H.R., Jr. Regulation of telomerase activity by interferon regulatory factors 4 and 8 in immune cells. Mol. Cell. Biol. 2009, 29, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Koshiji, M.; Kageyama, Y.; Pete, E.A.; Horikawa, I.; Barrett, J.C.; Huang, L.E. Hif-1alpha induces cell cycle arrest by functionally counteracting Myc. EMBO J. 2004, 23, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Cerezo, A.; Kalthoff, H.; Schuermann, M.; Schafer, B.; Boukamp, P. Dual regulation of telomerase activity through c-Myc -dependent inhibition and alternative splicing of hTERT. J. Cell Sci. 2002, 115, 1305–1312. [Google Scholar]
- Li, H.; Xu, D.; Li, J.; Berndt, M.C.; Liu, J.P. Transforming growth factor beta suppresses human telomerase reverse transcriptase (hTERT) by Smad3 interactions with c-Myc and the hTERT gene. J. Biol. Chem. 2006, 281, 25588–25600. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.S.; Park, S.; Gwak, J.; Ju, B.G.; Oh, S. Involvement of transcription repressor snail in the regulation of human telomerase reverse transcriptase (hTERT) by transforming growth factor-beta. Biochem. Biophys. Res. Commun. 2015, 465, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Fan, S.; Meng, Q.; Schramm, L.; Wang, C.; Bouzahza, B.; Zhou, J.; Zafonte, B.; Goldberg, I.D.; Haddad, B.R.; et al. Brca1 inhibition of telomerase activity in cultured cells. Mol. Cell. Biol. 2003, 23, 8668–8690. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Liu, J. Inhibition of human telomerase reverse transcriptase gene expression by BRCA1 in human ovarian cancer cells. Biochem. Biophys. Res. Commun. 2003, 303, 130–136. [Google Scholar] [CrossRef]
- Jang, K.J.; Kwon, G.S.; Jeong, J.W.; Kim, C.H.; Yoon, H.M.; Kim, G.Y.; Shim, J.H.; Moon, S.K.; Kim, W.J.; Choi, Y.H. Cordyceptin induces apoptosis through repressing hTERT expression and inducing extranuclear export of hTERT. J. Biosci. Bioeng. 2015, 119, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Moriai, M.; Tsuji, N.; Kobayashi, D.; Kuribayashi, K.; Watanabe, N. Down-regulation of hTERT expression plays an important role in 15-deoxy-delta12,14-prostaglandin J2-induced apoptosis in cancer cells. Int. J. Oncol. 2009, 34, 1363–1372. [Google Scholar] [PubMed]
- Toaldo, C.; Pizzimenti, S.; Cerbone, A.; Pettazzoni, P.; Menegatti, E.; Daniela, B.; Minelli, R.; Giglioni, B.; Dianzani, M.U.; Ferretti, C.; et al. Ppargamma ligands inhibit telomerase activity and hTERT expression through modulation of the Myc /Mad/Max network in colon cancer cells. J. Cell. Mol. Med. 2010, 14, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Song, Y.H.; Yim, J.; Kim, T.K. Identification of Mad as a repressor of the human telomerase (hTERT) gene. Oncogene 2000, 19, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Wahlstrom, T.; Belikov, S.; Arsenian Henriksson, M. Chromatin dynamics at the hTERT promoter during transcriptional activation and repression by c-Myc and Mnt in xenopus leavis oocytes. Exp. Cell Res. 2013, 319, 3160–3169. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Bellon, M.; Shelton, S.N.; Nicot, C. Tumor suppressors p53, p63TAα, p63TAy, p73α, and p73β use distinct pathways to repress telomerase expression. J. Biol. Chem. 2012, 287, 20737–20747. [Google Scholar] [CrossRef] [PubMed]
- Gabet, A.S.; Mortreux, F.; Charneau, P.; Riou, P.; Duc-Dodon, M.; Wu, Y.; Jeang, K.T.; Wattel, E. Inactivation of hTERT transcription by tax. Oncogene 2003, 22, 3734–3741. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, Y.; Kyo, S.; Takakura, M.; Kanaya, T.; Koshida, K.; Namiki, M.; Inoue, M. Demethylating reagent 5-azacytidine inhibits telomerase activity in human prostate cancer cells through transcriptional repression of hTERT. Clin. Cancer Res. 2000, 6, 2868–2875. [Google Scholar] [PubMed]
- Grandjenette, C.; Schnekenburger, M.; Karius, T.; Ghelfi, J.; Gaigneaux, A.; Henry, E.; Dicato, M.; Diederich, M. 5-aza-2’-deoxycytidine-mediated c-Myc down-regulation triggers telomere-dependent senescence by regulating human telomerase reverse transcriptase in chronic myeloid leukemia. Neoplasia 2014, 16, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Grand, C.L.; Han, H.; Munoz, R.M.; Weitman, S.; Von Hoff, D.D.; Hurley, L.H.; Bearss, D.J. The cationic porphyrin Tmpyp4 down-regulates c-Myc and human telomerase reverse transcriptase expression and inhibits tumor growth in vivo. Mol. Cancer Ther. 2002, 1, 565–573. [Google Scholar] [PubMed]
- Kanzawa, T.; Germano, I.M.; Kondo, Y.; Ito, H.; Kyo, S.; Kondo, S. Inhibition of telomerase activity in malignant glioma cells correlates with their sensitivity to temozolomide. Br. J. Cancer 2003, 89, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesh, S.; Kyo, S.; Banerjee, P.P. Genistein represses telomerase activity via both transcriptional and posttranslational mechanisms in human prostate cancer cells. Cancer Res. 2006, 66, 2107–2115. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.H.; Hsiao, Y.M.; Hsu, C.P.; Lin, M.Y.; Wang, J.C.; Huang, Y.L.; Ko, J.L. Transcriptionally mediated inhibition of telomerase of fungal immunomodulatory protein from ganoderma tsugae in A549 human lung adenocarcinoma cell line. Mol. Carcinog. 2006, 45, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Pizzimenti, S.; Briatore, F.; Laurora, S.; Toaldo, C.; Maggio, M.; De Grandi, M.; Meaglia, L.; Menegatti, E.; Giglioni, B.; Dianzani, M.U.; et al. 4-hydroxynonenal inhibits telomerase activity and hTERT expression in human leukemic cell lines. Free Radic. Biol. Med. 2006, 40, 1578–1591. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Guo, Q.L.; You, Q.D.; Lin, S.S.; Li, Z.; Gu, H.Y.; Zhang, H.W.; Tan, Z.; Wang, X. Repression of telomerase reverse transcriptase mrna and hTERT promoter by gambogic acid in human gastric carcinoma cells. Cancer Chemother. Pharmacol. 2006, 58, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.O.; Kim, M.O.; Lee, J.D.; Choi, Y.H.; Kim, G.Y. Butein suppresses c-Myc -dependent transcription and Akt-dependent phosphorylation of hTERT in human leukemia cells. Cancer Lett. 2009, 286, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.T.; Wang, C.Y.; Yang, R.C.; Chu, C.J.; Wu, H.T.; Pang, J.H. Wogonin, an active compound in Scutellaria baicalensis, induces apoptosis and reduces telomerase activity in the HL-60 leukemia cells. Phytomedicine 2010, 17, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, A.; Shen, C.; Zhang, B.; Rao, Z.; Wang, R.; Yang, S.; Ning, S.; Mao, G.; Fang, D. E2F1 acts as a negative feedback regulator of c-Myc induced hTERT transcription during tumorigenesis. Oncol. Rep. 2014, 32, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, M.; Shi, W.; Yang, Q.; Chen, C.; Wang, Z.; Zhou, X. Arsenic trioxide suppresses transcription of hTERT through down-regulation of multiple transcription factors in HL-60 leukemia cells. Toxicol. Lett. 2015, 232, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, P.; Sengupta, S.B. Role of diallyl disulfide-mediated cleavage of c-Myc and Sp-1 in the regulation of telomerase activity in human lymphoma cell line U937. Nutrition 2015, 31, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Bai, Z.; Li, X.; Hou, L.; Zhang, B. The evidences of human orphan receptor COUP-TFII inhibiting telomerase activity through decreasing hTERT transcription. Cancer Lett. 2004, 214, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Qin, L.; Wang, S.; Li, M.; Shi, D.; Tian, Y.; Wang, J.; Fu, L.; Li, Z.; Guo, W.; et al. CPSF4 activates telomerase reverse transcriptase and predicts poor prognosis in human lung adenocarcinomas. Mol. Oncol. 2014, 8, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Renaud, S.; Loukinov, D.; Abdullaev, Z.; Guilleret, I.; Bosman, F.T.; Lobanenkov, V.; Benhattar, J. Dual role of DNA methylation inside and outside of CTCF-binding regions in the transcriptional regulation of the telomerase hTERT gene. Nucleic Acids Res. 2007, 35, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Min, N.Y.; Kim, J.H.; Choi, J.H.; Liang, W.; Ko, Y.J.; Rhee, S.; Bang, H.; Ham, S.W.; Park, A.J.; Lee, K.H. Selective death of cancer cells by preferential induction of reactive oxygen species in response to (−)-epigallocatechin-3-gallate. Biochem. Biophys. Res. Commun. 2012, 421, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yao, Z.X.; Chen, J.S.; Gi, Y.J.; Munoz, N.M.; Kundra, S.; Herlong, H.F.; Jeong, Y.S.; Goltsov, A.; Ohshiro, K.; et al. TGF-β/β2-spectrin/CTCF-regulated tumor suppression in human stem cell disorder beckwith-wiedemann syndrome. J. Clin. Investig. 2016, 126, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Meeran, S.M.; Patel, S.N.; Tollefsbol, T.O. Sulforaphane causes epigenetic repression of hTERT expression in human breast cancer cell lines. PLoS ONE 2010, 5, e11457. [Google Scholar] [CrossRef] [PubMed]
- Karam, M.; Thenoz, M.; Capraro, V.; Robin, J.-P.; Pinatel, C.; Lançon, A.; Galia, P.; Sibon, D.; Thomas, X.; Ducastelle-Lepretre, S.; et al. Chromatin redistribution of the DEK oncoprotein represses hTERT transcription in leukemias. Neoplasia 2014, 16, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Crowe, D.L.; Nguyen, D.C.; Tsang, K.J.; Kyo, S. E2F-1 represses transcription of the human telomerase reverse transcriptase gene. Nucleic Acids Res. 2001, 29, 2789–2794. [Google Scholar] [CrossRef] [PubMed]
- Lacerte, A.; Korah, J.; Roy, M.; Yang, X.J.; Lemay, S.; Lebrun, J.J. Transforming growth factor-beta inhibits telomerase through SMAD3 and E2F transcription factors. Cell Signal 2008, 20, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Gizard, F.; Nomiyama, T.; Zhao, Y.; Findeisen, H.M.; Heywood, E.B.; Jones, K.L.; Staels, B.; Bruemmer, D. The PPARalpha/p16INK4a pathway inhibits vascular smooth muscle cell proliferation by repressing cell cycle-dependent telomerase activation. Circ. Res. 2008, 103, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Sekaric, P.; Cherry, J.J.; Androphy, E.J. Binding of human papillomavirus type 16 E6 to E6ap is not required for activation of hTERT. J. Virol. 2008, 82, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Akutagawa, O.; Nishi, H.; Kyo, S.; Higuma, C.; Inoue, M.; Isaka, K. Early growth response-1 mediates up-regulation of telomerase in placenta. Placenta 2007, 28, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Akutagawa, O.; Nishi, H.; Kyo, S.; Terauchi, F.; Yamazawa, K.; Higuma, C.; Inoue, M.; Isaka, K. Early growth response-1 mediates downregulation of telomerase in cervical cancer. Cancer Sci. 2008, 99, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, A.; Nanni, S.; Colussi, C.; Aiello, A.; Benvenuti, V.; Ragone, G.; Moretti, F.; Sacchi, A.; Bacchetti, S.; Gaetano, C.; et al. Estrogen receptor-alpha and endothelial nitric oxide synthase nuclear complex regulates transcription of human telomerase. Circ. Res. 2008, 103, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Goueli, B.S.; Janknecht, R. Upregulation of the catalytic telomerase subunit by the transcription factor ER81 and oncogenic HER2/Neu, Ras, or Raf. Mol. Cell. Biol. 2003, 24, 25–35. [Google Scholar] [CrossRef]
- Misiti, S.; Nanni, S.; Fontemaggi, G.; Cong, Y.S.; Wen, J.; Hirte, H.W.; Piaggio, G.; Sacchi, A.; Pontecorvi, A.; Bacchetti, S.; et al. Induction of hTERT expression and telomerase activity by estrogens in human ovary epithelium cells. Mol. Cell. Biol. 2000, 20, 3764–3771. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, K.; Tsuji, N.; Asanuma, K.; Kobayashi, D.; Watanabe, N. Inhibition of estrogen receptor beta-mediated human telomerase reverse transcriptase gene transcription via the suppression of mitogen-activated protein kinase signaling plays an important role in 15-deoxy-delta(12,14)-prostaglandin J2-induced apoptosis in cancer cells. Exp. Cell Res. 2007, 313, 3486–3496. [Google Scholar] [PubMed]
- Kasiappan, R.; Sun, Y.; Lungchukiet, P.; Quarni, W.; Zhang, X.; Bai, W. Vitamin d suppresses leptin stimulation of cancer growth through microrna. Cancer Res. 2014, 74, 6194–6204. [Google Scholar] [CrossRef] [PubMed]
- Marconett, C.N.; Sundar, S.N.; Tseng, M.; Tin, A.S.; Tran, K.Q.; Mahuron, K.M.; Bjeldanes, L.F.; Firestone, G.L. Indole-3-carbinol downregulation of telomerase gene expression requires the inhibition of estrogen receptor-alpha and Sp1 transcription factor interactions within the hTERT promoter and mediates the g1 cell cycle arrest of human breast cancer cells. Carcinogenesis 2011, 32, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Kyo, S.; Takakura, M.; Tanaka, M.; Yatabe, N.; Maida, Y.; Fujiwara, M.; Hayakawa, J.; Ohmichi, M.; Koike, K.; et al. Progesterone regulates human telomerase reverse transcriptase gene expression via activation of mitogen-activated protein kinase signaling pathway. Cancer Res. 2000, 60, 5376–5381. [Google Scholar] [PubMed]
- Hsu, C.P.; Lee, L.W.; Tang, S.C.; Hsin, I.L.; Lin, Y.W.; Ko, J.L. Epidermal growth factor activates telomerase activity by direct binding of ETS-2 to hTERT promoter in lung cancer cells. Tumour Biol. 2015, 36, 5389–5398. [Google Scholar] [CrossRef] [PubMed]
- Maida, Y.; Kyo, S.; Kanaya, T.; Wang, Z.; Yatabe, N.; Tanaka, M.; Nakamura, M.; Ohmichi, M.; Gotoh, N.; Murakami, S.; et al. Direct activation of telomerase by EGF through Ets-mediated transactivation of TERT via map kinase signaling pathway. Oncogene 2002, 21, 4071–4079. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Athanasiou, M.; Sidorov, I.A.; Horikawa, I.; Cremona, G.; Blair, D.; Barret, J.C.; Dimitrov, D.S. Role of Ets/Id proteins for telomerase regulation in human cancer cells. Exp. Mol. Pathol. 2003, 75, 238–247. [Google Scholar] [CrossRef]
- Takahashi, A.; Higashino, F.; Aoyagi, M.; Yoshida, K.; Itoh, M.; Kyo, S.; Ohno, T.; Taira, T.; Ariga, H.; Nakajima, K.; et al. EWS/EST fusions activate telomerase in ewing’s tumors. Cancer Res. 2003, 63, 8338–8344. [Google Scholar] [PubMed]
- Ogawa, D.; Nomiyama, T.; Nakamachi, T.; Heywood, E.B.; Stone, J.F.; Berger, J.P.; Law, R.E.; Bruemmer, D. Activation of peroxisome proliferator-activated receptor gamma suppresses telomerase activity in vascular smooth muscle cells. Circ. Res. 2006, 98, e50–e59. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, T.; Sandhu, R.; Qadan, M.; DeVecchio, J.; Magloire, V.; Agyeman, A.; Li, B.; Houghton, J.A. Hedgehog signaling regulates telomerase reverse transcriptase in human cancer cells. PLoS ONE 2013, 8, e75253. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Dong, Q.; Shin, K.H.; Kim, R.H.; Oh, J.E.; Park, N.H.; Kang, M.K. Grainyhead-like 2 enhances the human telomerase reverse transcriptase gene expression by inhibiting DNA methylation at the 5’-CPG island in normal human keratinocytes. J. Biol. Chem. 2010, 285, 40852–40863. [Google Scholar] [CrossRef]
- Kang, X.; Chen, W.; Kim, R.H.; Kang, M.K.; Park, N.H. Regulation of the hTERT promoter activity by MSH2, the hnRNPs K and D, and GRHL2 in human oral squamous cell carcinoma cells. Oncogene 2009, 28, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Yatabe, N.; Kyo, S.; Maida, Y.; Nishi, H.; Nakamura, M.; Kanaya, T.; Tanaka, M.; Isaka, K.; Ogawa, S.; Inoue, M. HIF-1-mediated activation of telomerase in cervical cancer cells. Oncogene 2004, 23, 3708–3715. [Google Scholar] [CrossRef] [PubMed]
- Nishi, H.; Nakada, T.; Kyo, S.; Inoue, M.; Shay, J.W.; Isaka, K. Hypoxia-inducible factor 1 mediates upregulation of telomerase (hTERT). Mol. Cell. Biol. 2004, 24, 6076–6083. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zheng, D.; Deng, X.; Bai, L.; Xu, Y.; Cong, Y.S. Lysophosphatidic acid activates telomerase in ovarian cancer cells through hypoxia-inducible factor-1α and the PI3K pathway. J. Cell. Biochem. 2008, 105, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Lou, F.; Chen, X.; Jalink, M.; Zhu, Q.; Ge, N.; Zhao, S.; Fang, X.; Fan, Y.; Bjorkholm, M.; Liu, Z.; et al. The opposing effect of hypoxia-inducible factor-2α on expression of telomerase reverse transcriptase. Mol. Cancer Res. 2007, 5, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.H.; Kim, R.; Chen, W.; Hu, S.; Shin, K.H.; Park, N.H.; Kang, M.K. Association of HSP90 to the hTERT promoter is necessary for hTERT expression in human oral cancer cells. Carcinogenesis 2008, 29, 2425–2431. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Mizuguchi, M.; Fujii, M.; Nakamura, M. Kruppel-like factor 2 represses transcription of the telomerase catalytic subunit human telomerase reverse transcriptase (hTERT) in human t cells. J. Biol. Chem. 2015, 290, 8758–8763. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.W.; Hou, P.S.; Tseng, S.F.; Chien, C.L.; Wu, K.J.; Chen, H.F.; Ho, H.N.; Kyo, S.; Teng, S.C. Kruppel-like transcription factor 4 contributes to maintenance of telomerase activity in stem cells. Stem Cells 2010, 28, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Liu, C.; Ge, Z.; Fang, X.; Zhang, X.; Straat, K.; Bjorkholm, M.; Xu, D. Lysine-specific demethylase 1 (LSD1) is required for the transcriptional repression of the telomerase reverse transcriptase (hTERT) gene. PLoS ONE 2008, 3, e1446. [Google Scholar] [CrossRef] [PubMed]
- Gunes, C.; Lichtsteiner, S.; Vasserot, A.P.; Englert, C. Expression of the hTERT gene is regulated at the level of transcriptional initiation and repressed by mad1. Cancer Res. 2000, 60, 2116–2121. [Google Scholar] [PubMed]
- Farhana, L.; Dawson, M.I.; Fontana, J.A. Down regulation of miR-202 modulates Mxd1 and Sin3A repressor complexes to induce apoptosis of pancreatic cancer cells. Cancer Biol. Ther. 2015, 16, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Katzenellenbogen, R.A.; Grandori, C.; Galloway, D.A. An unbiased in vivo screen reveals multiple transcription factors that control HPV E6-regulated hTERT in keratinocytes. Virology 2013, 446, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Su, J.M.; Lai, X.M.; Lan, K.H.; Li, C.P.; Chao, Y.; Yen, S.H.; Chang, F.Y.; Lee, S.D.; Lee, W.P. X protein of hepatitis B virus functions as a transcriptional corepressor on the human telomerase promoter. Hepatology 2007, 46, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Li, M.; Su, B. MCPH1/BRIT1 represses transcription of the human telomerase reverse transcriptase gene. Gene 2012, 495, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Kyo, S.; Hua, X.; Tahara, H.; Nakajima, M.; Takakura, M.; Sakaguchi, J.; Maida, Y.; Nakamura, M.; Ikoma, T.; et al. Role of menin in the regulation of telomerase activity in normal and cancer cells. Int. J. Oncol. 2008, 33, 333–340. [Google Scholar] [PubMed]
- Borowiak, M.; Kuhlmann, A.S.; Girard, S.; Gazzolo, L.; Mesnard, J.M.; Jalinot, P.; Dodon, M.D. HTLV-1 BZIP factor impedes the menin tumor suppressor and upregulates jund-mediated transcription of the hTERT gene. Carcinogenesis 2013, 34, 2664–2672. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, K.; Kyo, S.; Takakura, M.; Kanaya, T.; Kitagawa, Y.; Itoh, H.; Takahashi, M.; Inoue, M. Identification and characterization of negative regulatory elements of the human telomerase catalytic subunit (hTERT) gene promoter: Possible role of MZF-2 in transcriptional repression of hTERT. Nucleic Acids Res. 2000, 28, 2557–2562. [Google Scholar] [CrossRef] [PubMed]
- Gizard, F.; Heywood, E.B.; Findeisen, H.M.; Zhao, Y.; Jones, K.L.; Cudejko, C.; Post, G.R.; Staels, B.; Bruemmer, D. Telomerase activation in atherosclerosis and induction of telomerase reverse transcriptase expression by inflammatory stimuli in macrophages. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Aravindan, N.; Veeraraghavan, J.; Madhusoodhanan, R.; Herman, T.S.; Natarajan, M. Curcumin regulates low-linear energy transfer gamma-radiation-induced NFκB-dependent telomerase activity in human neuroblastoma cells. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.; Aroh, C.; Vadgama, J.V. Constitutive activation of STAT3 signaling regulates hTERT and promotes stem cell-like traits in human breast cancer cells. PLoS ONE 2013, 8, e83971. [Google Scholar] [CrossRef] [PubMed]
- Bellon, M.; Nicot, C. Central role of PI3K in transcriptional activation of hTERT in HTLV-I-Infected cells. Blood 2008, 112, 2946–2955. [Google Scholar] [CrossRef] [PubMed]
- Kawagoe, J.; Ohmichi, M.; Takahashi, T.; Ohshima, C.; Mabuchi, S.; Takahashi, K.; Igarashi, H.; Mori-Abe, A.; Saitoh, M.; Du, B.; et al. Raloxifene inhibits estrogen-induced up-regulation of telomerase activity in a human breast cancer cell line. J. Biol. Chem. 2003, 278, 43363–43372. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, M.; Mohan, S.; Konopinski, R.; Otto, R.A.; Herman, T.S. Induced telomerase activity in primary aortic endothelial cells by low-let gamma-radiation is mediated through NF-κb activation. Br. J. Radiol. 2008, 81, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Aravindan, N.; Aravindan, S.; Herman, T.S.; Natarajan, M. EGFR tyrosine kinase inhibitor pelitinib regulates radiation-induced p65-dependent telomerase activation in squamous cell carcinoma. Radiat. Res. 2013, 179, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Chebel, A.; Rouault, J.P.; Urbanowicz, I.; Baseggio, L.; Chien, W.W.; Salles, G.; Ffrench, M. Transcriptional activation of hTERT, the human telomerase reverse transcriptase, by nuclear factor of activated T cells. J. Biol. Chem. 2009, 284, 35725–35734. [Google Scholar] [CrossRef] [PubMed]
- Galloway, D.A.; Gewin, L.C.; Myers, H.; Luo, W.; Grandori, C.; Katzenellenbogen, R.A.; McDougall, J.K. Regulation of telomerase by human papillomaviruses. Cold Spring Harb. Symp. Quant. Biol. 2005, 70, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Bougel, S.; Renaud, S.; Braunschweig, R.; Loukinov, D.; Morse, H.C., III; Bosman, F.T.; Lobanenkov, V.; Benhattar, J. PAX5 activates the transcription of the human telomerase reverse transcriptase gene in B cells. J. Pathol. 2010, 220, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Campbell, H.G.; Wiles, A.K.; Eccles, M.R.; Reddel, R.R.; Braithwaite, A.W.; Royds, J.A. PAX8 regulates telomerase reverse transcriptase and telomerase RNA component in glioma. Cancer Res. 2008, 68, 5724–5732. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.L.; Ohhira, T.; Fujisaki, C.; Inoue, T.; Ohta, T.; Osaki, M.; Ohshiro, E.; Seko, T.; Aoki, S.; Oshimura, M.; et al. Identification of PITX1 as a TERT suppressor gene located on human chromosome 5. Mol. Cell. Biol. 2011, 31, 1624–1636. [Google Scholar] [CrossRef] [PubMed]
- Ohira, T.; Naohiro, S.; Nakayama, Y.; Osaki, M.; Okada, F.; Oshimura, M.; Kugoh, H. miR-19b regulates hTERT mRNA expression through targeting PITX1 mRNA in melanoma cells. Sci. Rep. 2015. [Google Scholar] [CrossRef] [PubMed]
- Luan, F.; Liu, H.; Gao, L.; Liu, J.; Sun, Z.; Ju, Y.; Hou, N.; Guo, C.; Liang, X.; Zhang, L.; et al. Hepatitis B virus protein preS2 potentially promotes HCC development via its transcriptional activation of hTERT. Gut 2009, 58, 1528–1537. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zeng, J.; Li, Q.; Zhao, L.; Liu, T.; Bjorkholm, M.; Jia, J.; Xu, D. Reptin is required for the transcription of telomerase reverse transcriptase and over-expressed in gastric cancer. Mol. Cancer 2010. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lu, J.; Qin, Y.; Wang, J.; Tian, Y.; Shi, D.; Wang, S.; Xiao, Y.; Dai, M.; Liu, L.; et al. Ret finger protein-like 3 promotes tumor cell growth by activating telomerase reverse transcriptase expression in human lung cancer cells. Oncotarget 2014, 5, 11909–11923. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Chen, W.; Xiao, Y.; Yu, W.; Cai, X.; Dai, M.; Xu, T.; Huang, W.; Guo, W.; Deng, W.; et al. RFPL3 and CBP synergistically upregulate hTERT activity and promote lung cancer growth. Oncotarget 2015, 6, 27130–27145. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Lu, J.; Dai, M.; Wu, T.; Yu, Z.; Wang, J.; Chen, W.; Shi, D.; Yu, W.; Xiao, Y.; et al. Transcriptional coactivator cbp upregulates hTERT expression and tumor growth and predicts poor prognosis in human lung cancers. Oncotarget 2014, 5, 9349–9361. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.S.; Cotter, M.A., II; Robertson, E.S. The latency-associated nuclear antigen of kaposi’s sarcoma-associated herpesvirus transactivates the telomerase reverse transcriptase promoter. J. Biol. Chem. 2001, 276, 22971–22978. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.C.; Borah, S.; Robertson, E.S. Latency-associated nuclear antigen of kaposi’s sarcoma-associated herpesvirus up-regulates transcription of human telomerase reverse transcriptase promoter through interaction with transcription factor Sp1. J. Virol. 2004, 78, 10348–10359. [Google Scholar] [CrossRef] [PubMed]
- Chau, M.N.; El Touny, L.H.; Jagadeesh, S.; Banerjee, P.P. Physiologically achievable concentrations of genistein enhance telomerase activity in prostate cancer cells via the activation of STAT3. Carcinogenesis 2007, 28, 2282–2290. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ishihara, K.; Ichimura, T.; Fujita, N.; Hino, S.; Tomita, S.; Watanabe, S.; Saitoh, N.; Ito, T.; Nakao, M. MCAF1/AM is involved in Sp1-mediated maintenance of cancer-associated telomerase activity. J. Biol. Chem. 2009, 284, 5165–5174. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shi, W.; Luan, F.; Xu, S.; Yang, F.; Sun, W.; Liu, J.; Ma, C. Hepatitis B virus X protein upregulates transcriptional activation of human telomerase reverse transcriptase. Virus Genes 2010, 40, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Li, A.Y.; Lin, H.H.; Kuo, C.Y.; Shih, H.M.; Wang, C.C.; Yen, Y.; Ann, D.K. High-mobility group a2 protein modulates hTERT transcription to promote tumorigenesis. Mol. Cell. Biol. 2011, 31, 2605–2617. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wang, Q.; Gruber, A.; Bjorkholm, M.; Chen, Z.; Zaid, A.; Selivanova, G.; Peterson, C.; Wiman, K.G.; Pisa, P. Downregulation of telomerase reverse transcriptase mrna expression by wild type p53 in human tumor cells. Oncogene 2000, 19, 5123–5133. [Google Scholar] [CrossRef] [PubMed]
- Beitzinger, M.; Oswald, C.; Beinoraviciute-Kellner, R.; Stiewe, T. Regulation of telomerase activity by the p53 family member p73. Oncogene 2006, 25, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Racek, T.; Mise, N.; Li, Z.; Stoll, A.; Putzer, B.M. C-terminal p73 isoforms repress transcriptional activity of the human telomerase reverse transcriptase (hTERT) promoter. J. Biol. Chem. 2005, 280, 40402–40405. [Google Scholar] [CrossRef] [PubMed]
- Wooten, L.G.; Ogretmen, B. Sp1/Sp3-dependent regulation of human telomerase reverse transcriptase promoter activity by the bioactive sphingolipid ceramide. J. Biol. Chem. 2005, 280, 28867–28876. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kim, H.Z.; Jeong, K.W.; Shim, Y.S.; Horikawa, I.; Barrett, J.C.; Choe, J. Human papillomavirus E2 down-regulates the human telomerase reverse transcriptase promoter. J. Biol. Chem. 2002, 277, 27748–27756. [Google Scholar] [CrossRef] [PubMed]
- Won, J.; Yim, J.; Kim, T.K. Opposing regulatory roles of E2F in human telomerase reverse transcriptase (hTERT) gene expression in human tumor and normal somatic cells. FASEB J. 2002, 16, 1943–1945. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Nakagawa, K.; Hamada, K.; Hirose, S.; Harada, H.; Kohno, S.; Nagato, S.; Ohnishi, T. Introduction of p16INK4a inhibits telomerase activity through transcriptional suppression of human telomerase reverse transcriptase expression in human gliomas. Int. J. Oncol. 2004, 24, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.C.; Lin, S.Z.; Chen, Y.L.; Chang, J.S.; Ho, L.I.; Liu, P.Y.; Chang, L.F.; Harn, Y.C.; Chen, S.P.; Sun, L.Y.; et al. Butylidenephthalide suppresses human telomerase reverse transcriptase (TERT) in human glioblastomas. Ann. Surg. Oncol. 2011, 18, 3514–3527. [Google Scholar] [CrossRef] [PubMed]
- Shapira, S.; Granot, G.; Mor-Tzuntz, R.; Raanani, P.; Uziel, O.; Lahav, M.; Shpilberg, O. Second-generation tyrosine kinase inhibitors reduce telomerase activity in K562 cells. Cancer Lett. 2012, 323, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Deeb, D.; Brigolin, C.; Gao, X.; Liu, Y.; Pindolia, K.R.; Gautam, S.C. Induction of apoptosis in pancreatic cancer cells by CDDO-Me involves repression of telomerase through epigenetic pathways. J. Carcinog. Mutagen. 2014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Sun, X.; Liu, J.; Xie, X.; Cui, W.; Zhu, Y. Homocysteine accelerates senescence of endothelial cells via DNA hypomethylation of human telomerase reverse transcriptase. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Long, C.; Wang, J.; Guo, W.; Wang, H.; Wang, C.; Liu, Y.; Sun, X. Triptolide inhibits transcription of hTERT through down-regulation of transcription factor specificity protein 1 in primary effusion lymphoma cells. Biochem. Biophys. Res. Commun. 2016, 469, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Won, J.; Yim, J.; Kim, T.K. Sp1 and Sp3 recruit histone deacetylase to repress transcription of human telomerase reverse transcriptase (hTERT) promoter in normal human somatic cells. J. Biol. Chem. 2002, 277, 38230–38238. [Google Scholar] [CrossRef] [PubMed]
- Glasspool, R.M.; Burns, S.; Hoare, S.F.; Svensson, C.; Keith, W.N. The hTERT and hTERC telomerase gene promoters are activated by the second exon of the adenoviral protein, E1 A, identifying the transcriptional corepressor CtBP as a potential rearessor of both genes. Neoplasia 2005, 7, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, T.; Miura, T.; Maura, M.; Shiraishi, H.; Nishimura, S.; Imada, Y.; Uehara, N.; Tashiro, K.; Shirahata, S.; Katakura, Y. TAK1 represses transcription of the human telomerase reverse transcriptase gene. Oncogene 2007, 26, 5258–5266. [Google Scholar] [CrossRef] [PubMed]
- Wooten-Blanks, L.G.; Song, P.; Senkal, C.E.; Ogretmen, B. Mechanisms of ceramide-mediated repression of the human telomerase reverse transcriptase promoter via deacetylation of Sp3 by histone deacetylase 1. FASEB J. 2007, 21, 3386–3397. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhu, J.; Dong, Y.; He, C.; Hu, X. Suppressor of Ty homolog-5, a novel tumor-specific human telomerase reverse transcriptase promoter-binding protein and activator in colon cancer cells. Oncotarget 2015, 6, 32841–32855. [Google Scholar] [PubMed]
- Ren, H.; Zhao, T.; Wang, X.; Gao, C.; Wang, J.; Yu, M.; Hao, J. Leptin upregulates telomerase activity and transcription of human telomerase reverse transcriptase in MCF-7 breast cancer cells. Biochem. Biophys. Res. Commun. 2010, 394, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Nakatake, M.; Kakiuchi, Y.; Sasaki, N.; Murakami-Murofushi, K.; Yamada, O. STAT3 and PKC differentially regulate telomerase activity during megakaryocytic differentiation of K562 cells. Cell Cycle 2007, 6, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Konnikova, L.; Simeone, M.C.; Kruger, M.M.; Kotecki, M.; Cochran, B.H. Signal transducer and activator of transcription 3 (STAT3) regulates human telomerase reverse transcriptase (hTERT) expression in human cancer and primary cells. Cancer Res. 2005, 65, 6516–6520. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Sun, G.; Luo, H.; Wang, X.F.; Lan, F.M.; Yue, X.; Fu, L.S.; Pu, P.Y.; Kang, C.S.; Liu, N.; et al. MiR-21 modulates hTERT through a STAT3-dependent manner on glioblastoma cell growth. CNS Neurosci. Ther. 2012, 18, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Cheng, D.; Li, Z.H.; Zhou, Q.B.; Zhou, J.J.; Lin, Q.; Zeng, B.; Liao, Q.; Chen, R.F. Transfection of HCVc improves hTERT expression through STAT3 pathway by epigenetic regulation in Huh7 cells. J. Cell. Biochem. 2012, 113, 3419–3426. [Google Scholar] [CrossRef] [PubMed]
- Yamada, O.; Ozaki, K.; Akiyama, M.; Kawauchi, K. JAK-STAT and JAK-PI3K-mTORC1 pathways regulate telomerase transcriptionally and posttranslationally in atl cells. Mol. Cancer Ther. 2012, 11, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Yamada, O.; Ozaki, K.; Furukawa, T.; Machida, M.; Wang, Y.H.; Motoji, T.; Mitsuishi, T.; Akiyama, M.; Yamada, H.; Kawauchi, K.; et al. Activation of STAT5 confers imatinib resistance on leukemic cells through the transcription of TERT and mdr1. Cell Signal 2011, 23, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Kawano, T.; Mikami-Terao, Y.; Agawa-Ohta, M.; Yamada, O.; Ida, H.; Yamada, H. Erythropoietin activates telomerase through transcriptional and posttranscriptional regulation in human erythroleukemic JAS-REN-A cells. Leuk. Res. 2011, 35, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Prade-Houdellier, N.; Frebet, E.; Demur, C.; Gautier, E.F.; Delhommeau, F.; Bennaceur-Griscelli, A.L.; Gaudin, C.; Martinel, V.; Laurent, G.; Mansat-De Mas, V.; et al. Human telomerase is regulated by erythropoietin and transforming growth factor-β in human erythroid progenitor cells. Leukemia 2007, 21, 2304–2310. [Google Scholar] [CrossRef] [PubMed]
- Terme, J.M.; Mocquet, V.; Kuhlmann, A.S.; Zane, L.; Mortreux, F.; Wattel, E.; Duc Dodon, M.; Jalinot, P. Inhibition of the hTERT promoter by the proto-oncogenic protein TAL1. Leukemia 2009, 23, 2081–2089. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Zhao, Y.; Mei, F.; Yang, S.; Li, X.; Lv, J.; Hou, L.; Zhang, B. Molecular cloning and characterization of a human gene involved in transcriptional regulation of hTERT. Biochem. Biophys. Res. Commun. 2004, 324, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Chen, C.H.; Hsu, T.I.; Hung, J.J.; Ko, J.L.; Zhang, B.; Lee, Y.C.; Chen, H.K.; Chang, W.C.; Lin, D.Y. The 58-kDa microspherule protein (MSP58) represses human telomerase reverse transcriptase (hTERT) gene expression and cell proliferation by interacting with telomerase transcriptional element-interacting factor (TEIF). Biochim. Biophys. Acta 2014, 1843, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Goueli, B.S.; Janknecht, R. Regulation of telomerase reverse transcriptase gene activity by upstream stimulatory factor. Oncogene 2003, 22, 8042–8047. [Google Scholar] [CrossRef] [PubMed]
- Yago, M.; Ohki, R.; Hatakeyama, S.; Fujita, T.; Ishikawa, F. Variant forms of upstream stimulatory factors (USFs) control the promoter activity of hTERT, the human gene encoding the catalytic subunit of telomerase. FEBS Lett. 2002, 520, 40–46. [Google Scholar] [CrossRef]
- Chang, J.T.; Yang, H.T.; Wang, T.C.; Cheng, A.J. Upstream stimulatory factor (USF) as a transcriptional suppressor of human telomerase reverse transcriptase (hTERT) in oral cancer cells. Mol. Carcinog. 2005, 44, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, N.; Uemura, H.; Ishiguro, H.; Hori, M.; Hosaka, M.; Kyo, S.; Miyamoto, K.; Takeda, E.; Kubota, Y. Combination treatment with 1α,25-dihydroxyvitamin D3 and 9-Cis-Retinoic acid directly inhibits human telomerase reverse transcriptase transcription in prostate cancer cells. Mol. Cancer Ther. 2003, 2, 739–746. [Google Scholar] [PubMed]
- Oh, S.; Song, Y.; Yim, J.; Kim, T.K. The wilms’ tumor 1 tumor suppressor gene represses transcription of the human telomerase reverse transcriptase gene. J. Biol. Chem. 1999, 274, 37473–37478. [Google Scholar] [CrossRef] [PubMed]
- Veldman, T.; Horikawa, I.; Barrett, J.C.; Schlegel, R. Transcriptional activation of the telomerase hTERT gene by human papillomavirus type 16 E6 oncoprotein. J. Virol. 2001, 75, 4467–4472. [Google Scholar] [CrossRef] [PubMed]
- Kanzawa, T.; Komata, T.; Kyo, S.; Germano, I.M.; Kondo, Y.; Kondo, S. Down-regulation of telomerase activity in malignant glioma cells by p27KIP1. Int. J. Oncol. 2003, 23, 1703–1708. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Seo, H.S.; Choi, W.S.; Kim, O.; Nam, S.W.; Lee, J.Y.; Park, W.S. Gastrokine 1 induces senescence and apoptosis through regulating telomere length in gastric cancer. Oncotarget 2014, 5, 11695–11708. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, K.L.; Newbold, R.F.; Mokbel, K. There is no correlation between c-Myc mRNA expression and telomerase activity in human breast cancer. Int. Semin. Surg. Oncol. 2004. [Google Scholar] [CrossRef] [PubMed]
- Elkak, A.E.; Meligonis, G.; Salhab, M.; Mitchell, B.; Blake, J.R.; Newbold, R.F.; Mokbel, K. hTERT protein expression is independent of clinicopathological parameters and c-Myc protein expression in human breast cancer. J. Carcinog. 2005. [Google Scholar] [CrossRef]
- Nakagawa, M.; Koyanagi, M.; Tanabe, K.; Takahashi, K.; Ichisaka, T.; Aoi, T.; Okita, K.; Mochiduki, Y.; Takizawa, N.; Yamanaka, S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotechnol. 2008, 26, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Saginc, G.; Leow, S.C.; Khattar, E.; Shin, E.M.; Yan, T.D.; Wong, M.; Zhang, Z.; Li, G.; Sung, W.K.; et al. Telomerase directly regulates NF-κB-dependent transcription. Nat. Cell Biol. 2012, 14, 1270–1281. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Xi, P.; Zhou, J.; Wang, M.; Cong, Y.S. Human telomerase reverse transcriptase regulates mmp expression independently of telomerase activity via NF-κB-dependent transcription. FASEB J. 2013, 27, 4375–4383. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, Q.L.; Sun, W.; Chandrasekharan, P.; Cheng, H.S.; Ying, Z.; Lakshmanan, M.; Raju, A.; Tenen, D.G.; Cheng, S.Y.; et al. Non-canonical NF-κB signalling and ETS1/2 cooperatively drive C250T mutant TERT promoter activation. Nat. Cell Biol. 2015, 17, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Liu, B.R.; Qu, B.; Xing, H.; Gao, S.L.; Yin, J.M.; Wang, X.F.; Cheng, Y.Q. Silencing STAT3 may inhibit cell growth through regulating signaling pathway, telomerase, cell cycle, apoptosis and angiogenesis in hepatocellular carcinoma: Potential uses for gene therapy. Neoplasma 2011, 58, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Boggess, J.F.; Zhou, C.; Bae-Jump, V.L.; Gehrig, P.A.; Whang, Y.E. Estrogen-receptor-dependent regulation of telomerase activity in human endometrial cancer cell lines. Gynecol. Oncol. 2006, 103, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Briatore, F.; Barrera, G.; Pizzimenti, S.; Toaldo, C.; Casa, C.D.; Laurora, S.; Pettazzoni, P.; Dianzani, M.U.; Ferrero, D. Increase of telomerase activity and hTERT expression in myelodysplastic syndromes. Cancer Biol. Ther. 2009, 8, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Meeran, S.M.; Patel, S.N.; Chan, T.H.; Tollefsbol, T.O. A novel prodrug of epigallocatechin-3-gallate: Differential epigenetic hTERT repression in human breast cancer cells. Cancer Prev. Res. 2011, 4, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Yu, R.A.; He, L.F.; An, S.J.; Wu, Z.G.; Yang, K.D.; Chen, X.M. Inhibitory effects of selenium on telomerase activity and hTERT expression in cadmium-transformed 16HBE cells. Biomed. Environ. Sci. 2007, 20, 307–312. [Google Scholar] [PubMed]
- Cheng, D.; Zhao, Y.; Wang, S.; Jia, W.; Kang, J.; Zhu, J. Human telomerase reverse transcriptase (hTERT) transcription requires Sp1/Sp3 binding to the promoter and a permissive chromatin environment. J. Biol. Chem. 2015, 290, 30193–30203. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.X.; Chen, X.Y.; Wu, S.G. Regulation of telomerase activity in Jurkat T cells by transcription factors Sp1 and Sp3. Di Yi Jun Yi Da Xue Xue Bao 2002, 22, 481–485. [Google Scholar] [PubMed]
- Eldholm, V.; Haugen, A.; Zienolddiny, S. Ctcf mediates the TERT enhancer-promoter interactions in lung cancer cells: Identification of a novel enhancer region involved in the regulation of TERT gene. Int. J. Cancer 2014, 134, 2305–2313. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Bao, J.; Li, P.; Nicosia, S.V.; Bai, W. Induction of ovarian cancer cell apoptosis by 1,25-dihydroxyvitamin D3 through the down-regulation of telomerase. J. Biol. Chem. 2004, 279, 53213–53221. [Google Scholar] [CrossRef] [PubMed]
- Kasiappan, R.; Shen, Z.; Tse, A.K.; Jinwal, U.; Tang, J.; Lungchukiet, P.; Sun, Y.; Kruk, P.; Nicosia, S.V.; Zhang, X.; et al. 1,25-Dihydroxyvitamin d3 suppresses telomerase expression and human cancer growth through microrna-498. J. Biol. Chem. 2012, 287, 41297–41309. [Google Scholar] [CrossRef] [PubMed]
- Kanaya, T.; Kyo, S.; Hamada, K.; Takakura, M.; Kitagawa, Y.; Harada, H.; Inoue, M. Adenoviral expression of p53 represses telomerase activity through down-regulation of human telomerase reverse transcriptase transcription. Clin. Cancer Res. 2000, 6, 1239–1247. [Google Scholar] [PubMed]
- Saito, T.; Akaike, K.; Kurisaki-Arakawa, A.; Toda-Ishii, M.; Mukaihara, K.; Suehara, Y.; Takagi, T.; Kaneko, K.; Yao, T. Promoter mutations are rare in bone and soft tissue sarcomas of japanese patients. Mol. Clin. Oncol. 2016, 4, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Wang, X.; Popov, N.; Zhang, A.; Zhao, X.; Zhou, R.; Zetterberg, A.; Bjorkholm, M.; Henriksson, M.; Gruber, A.; et al. The histone deacetylase inhibitor trichostatin a derepresses the telomerase reverse transcriptase (hTERT) gene in human cells. Exp. Cell Res. 2002, 274, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Takakura, M.; Kyo, S.; Sowa, Y.; Wang, Z.; Yatabe, N.; Maida, Y.; Tanaka, M.; Inoue, M. Telomerase activation by histone deacetylase inhibitor in normal cells. Nucleic Acids Res. 2001, 29, 3006–3011. [Google Scholar] [CrossRef] [PubMed]
- Varshney, A.; Ramakrishnan, S.K.; Sharma, A.; Santosh, B.; Bala, J.; Yadava, P.K.; Jaiswal, R.K. Global expression profile of telomerase-associated genes in HeLa cells. Gene 2014, 547, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, S.P.; Hoare, S.F.; Glasspool, R.M.; Keith, W.N. Lack of telomerase gene expression in alternative lengthening of telomere cells is associated with chromatin remodeling of the hTR and hTERT gene promoters. Cancer Res. 2005, 65, 7585–7590. [Google Scholar] [PubMed]
- Wang, Y.; Chen, T.; Yan, H.; Qi, H.; Deng, C.; Ye, T.; Zhou, S.; Li, F.R. Role of histone deacetylase inhibitors in the aging of human umbilical cord mesenchymal stem cells. J. Cell. Biochem. 2013, 114, 2231–2239. [Google Scholar] [CrossRef] [PubMed]
- Haigis, M.C.; Guarente, L.P. Mammalian sirtuins—Emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006, 20, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Jeng, Y.M.; Yuan, R.H.; Hsu, H.C.; Chen, Y.L. SIRT1 promotes tumorigenesis and resistance to chemotherapy in hepatocellular carcinoma and its expression predicts poor prognosis. Ann. Surg. Oncol. 2012, 19, 2011–2019. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.; Zhao, G.; Lv, X.; Chen, H.Z.; Xue, Z.; Yang, B.; Liu, D.P.; Liang, C.C. SIRT1 deacetylates c-Myc and promotes c-Myc /max association. Int. J. Biochem. Cell Biol. 2011, 43, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, B.; Wong, N.; Lo, A.W.; To, K.F.; Chan, A.W.; Ng, M.H.; Ho, C.Y.; Cheng, S.H.; Lai, P.B.; et al. Sirtuin 1 is upregulated in a subset of hepatocellular carcinomas where it is essential for telomere maintenance and tumor cell growth. Cancer Res. 2011, 71, 4138–4149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chen, J.; Cheng, A.S.; Ko, B.C. Depletion of sirtuin 1 (SIRT1) leads to epigenetic modifications of telomerase (TERT) gene in hepatocellular carcinoma cells. PLoS ONE 2014, 9, e84931. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Selker, E.U. Gene silencing: Repeats that count. Cell 1999, 97, 157–160. [Google Scholar] [CrossRef]
- Devereux, T.R.; Horikawa, I.; Anna, C.H.; Annab, L.A.; Afshari, C.A.; Barrett, J.C. DNA methylation analysis of the promoter region of the human telomerase reverse transcriptase (hTERT) gene. Cancer Res. 1999, 59, 6087–6090. [Google Scholar] [PubMed]
- Iliopoulos, D.; Satra, M.; Drakaki, A.; Poultsides, G.A.; Tsezou, A. Epigenetic regulation of hTERT promoter in hepatocellular carcinomas. Int. J. Oncol. 2009, 34, 391–399. [Google Scholar] [PubMed]
- Zinn, R.L.; Pruitt, K.; Eguchi, S.; Baylin, S.B.; Herman, J.G. hTERT is expressed in cancer cell lines despite promoter DNA methylation by preservation of unmethylated DNA and active chromatin around the transcription start site. Cancer Res. 2007, 67, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Guilleret, I.; Benhattar, J. Demethylation of the human telomerase catalytic subunit (hTERT) gene promoter reduced hTERT expression and telomerase activity and shortened telomeres. Exp. Cell Res. 2003, 289, 326–334. [Google Scholar] [CrossRef]
- Pettigrew, K.A.; Armstrong, R.N.; Colyer, H.A.; Zhang, S.D.; Rea, I.M.; Jones, R.E.; Baird, D.M.; Mills, K.I. Differential TERT promoter methylation and response to 5-Aza-2’-deoxycytidine in acute myeloid leukemia cell lines: TERT expression, telomerase activity, telomere length, and cell death. Genes Chromosomes Cancer 2012, 51, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Dessain, S.K.; Yu, H.; Reddel, R.R.; Beijersbergen, R.L.; Weinberg, R.A. Methylation of the human telomerase gene cpg island. Cancer Res. 2000, 60, 537–541. [Google Scholar] [PubMed]
- Guilleret, I.; Yan, P.; Grange, F.; Braunschweig, R.; Bosman, F.T.; Benhattar, J. Hypermethylation of the human telomerase catalytic subunit (hTERT) gene correlates with telomerase activity. Int. J. Cancer 2002, 101, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Castelo-Branco, P.; Choufani, S.; Mack, S.; Gallagher, D.; Zhang, C.; Lipman, T.; Zhukova, N.; Walker, E.J.; Martin, D.; Merino, D.; et al. Methylation of the TERT promoter and risk stratification of childhood brain tumours: An integrative genomic and molecular study. Lancet. Oncol. 2013, 14, 534–542. [Google Scholar] [CrossRef]
- Peifer, M.; Hertwig, F.; Roels, F.; Dreidax, D.; Gartlgruber, M.; Menon, R.; Kramer, A.; Roncaioli, J.L.; Sand, F.; Heuckmann, J.M.; et al. Telomerase activation by genomic rearrangements in high-risk neuroblastoma. Nature 2015, 526, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.L.; Theodorescu, D.; Vogelstein, B.; Papadopoulos, N.; Cech, T.R. Mutation of the TERT promoter, switch to active chromatin, and monoallelic TERT expression in multiple cancers. Genes Dev. 2015, 29, 2219–2224. [Google Scholar] [CrossRef] [PubMed]
- Ojesina, A.I.; Lichtenstein, L.; Freeman, S.S.; Pedamallu, C.S.; Imaz-Rosshandler, I.; Pugh, T.J.; Cherniack, A.D.; Ambrogio, L.; Cibulskis, K.; Bertelsen, B.; et al. Landscape of genomic alterations in cervical carcinomas. Nature 2014, 506, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Kandoth, C.; McLellan, M.D.; Vandin, F.; Ye, K.; Niu, B.; Lu, C.; Xie, M.; Zhang, Q.; McMichael, J.F.; Wyczalkowski, M.A.; et al. Mutational landscape and significance across 12 major cancer types. Nature 2013, 502, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Weinhold, N.; Jacobsen, A.; Schultz, N.; Sander, C.; Lee, W. Genome-wide analysis of noncoding regulatory mutations in cancer. Nat. Genet. 2014, 46, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.W.; Hodis, E.; Xu, M.J.; Kryukov, G.V.; Chin, L.; Garraway, L.A. Highly recurrent TERT promoter mutations in human melanoma. Science 2013, 339, 957–959. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.; Figl, A.; Rachakonda, P.S.; Fischer, C.; Sucker, A.; Gast, A.; Kadel, S.; Moll, I.; Nagore, E.; Hemminki, K.; et al. Tert promoter mutations in familial and sporadic melanoma. Science 2013, 339, 959–961. [Google Scholar] [CrossRef] [PubMed]
- Killela, P.J.; Reitman, Z.J.; Jiao, Y.; Bettegowda, C.; Agrawal, N.; Diaz, L.A., Jr.; Friedman, A.H.; Friedman, H.; Gallia, G.L.; Giovanella, B.C.; et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc. Natl. Acad. Sci. USA 2013, 110, 6021–6026. [Google Scholar] [CrossRef] [PubMed]
- Quaas, A.; Oldopp, T.; Tharun, L.; Klingenfeld, C.; Krech, T.; Sauter, G.; Grob, T.J. Frequency of TERT promoter mutations in primary tumors of the liver. Virchows Arch. 2014, 465, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Kinde, I.; Munari, E.; Faraj, S.F.; Hruban, R.H.; Schoenberg, M.; Bivalacqua, T.; Allaf, M.; Springer, S.; Wang, Y.; Diaz, L.A., Jr.; et al. TERT promoter mutations occur early in urothelial neoplasia and are biomarkers of early disease and disease recurrence in urine. Cancer Res. 2013, 73, 7162–7167. [Google Scholar] [CrossRef] [PubMed]
- Griewank, K.G.; Schilling, B.; Murali, R.; Bielefeld, N.; Schwamborn, M.; Sucker, A.; Zimmer, L.; Hillen, U.; Schaller, J.; Brenn, T.; et al. TERT promoter mutations are frequent in atypical fibroxanthomas and pleomorphic dermal sarcomas. Mod. Pathol. 2014, 27, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Adeberg, S.; Bernhardt, D.; Harrabi, S.B.; Diehl, C.; Koelsche, C.; Rieken, S.; Unterberg, A.; von Deimling, A.; Debus, J. Radiotherapy plus concomitant temozolomide in primary gliosarcoma. J. Neurooncol. 2016, 128, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.E.; Ohta, T.; Nonoguchi, N.; Satomi, K.; Capper, D.; Pierscianek, D.; Sure, U.; Vital, A.; Paulus, W.; Mittelbronn, M.; et al. Genetic alterations in gliosarcoma and giant cell glioblastoma. Brain Pathol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Koelsche, C.; Sahm, F.; Capper, D.; Reuss, D.; Sturm, D.; Jones, D.T.; Kool, M.; Northcott, P.A.; Wiestler, B.; Bohmer, K.; et al. Distribution of TERT promoter mutations in pediatric and adult tumors of the nervous system. Acta Neuropathol. 2013, 126, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.S.; Wang, Z.; He, X.J.; Diplas, B.H.; Yang, R.; Killela, P.J.; Meng, Q.; Ye, Z.Y.; Wang, W.; Jiang, X.T.; et al. Recurrent TERT promoter mutations identified in a large-scale study of multiple tumour types are associated with increased TERT expression and telomerase activation. Eur. J. Cancer 2015, 51, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Koelsche, C.; Renner, M.; Hartmann, W.; Brandt, R.; Lehner, B.; Waldburger, N.; Alldinger, I.; Schmitt, T.; Egerer, G.; Penzel, R.; et al. TERT promoter hotspot mutations are recurrent in myxoid liposarcomas but rare in other soft tissue sarcoma entities. J. Exp. Clin. Cancer Res. 2014. [Google Scholar] [CrossRef] [PubMed]
- Vail, E.; Zheng, X.; Zhou, M.; Yang, X.; Fallon, J.T.; Epstein, J.I.; Zhong, M. Telomerase reverse transcriptase promoter mutations in glandular lesions of the urinary bladder. Ann. Diagn. Pathol. 2015, 19, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Nonoguchi, N.; Ohta, T.; Oh, J.E.; Kim, Y.H.; Kleihues, P.; Ohgaki, H. TERT promoter mutations in primary and secondary glioblastomas. Acta Neuropathol. 2013, 126, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Arita, H.; Narita, Y.; Fukushima, S.; Tateishi, K.; Matsushita, Y.; Yoshida, A.; Miyakita, Y.; Ohno, M.; Collins, V.P.; Kawahara, N.; et al. Upregulating mutations in the TERT promoter commonly occur in adult malignant gliomas and are strongly associated with total 1p19q loss. Acta Neuropathol. 2013, 126, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Mosrati, M.A.; Malmstrom, A.; Lysiak, M.; Krysztofiak, A.; Hallbeck, M.; Milos, P.; Hallbeck, A.L.; Bratthall, C.; Strandeus, M.; Stenmark-Askmalm, M.; et al. TERT promoter mutations and polymorphisms as prognostic factors in primary glioblastoma. Oncotarget 2015, 6, 16663–16673. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Hosen, I.; Gousias, K.; Rachakonda, S.; Heidenreich, B.; Gessi, M.; Schramm, J.; Hemminki, K.; Waha, A.; Kumar, R. TERT promoter mutations: A novel independent prognostic factor in primary glioblastomas. Neuro-Oncology 2015, 17, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Labussiere, M.; Boisselier, B.; Mokhtari, K.; Di Stefano, A.L.; Rahimian, A.; Rossetto, M.; Ciccarino, P.; Saulnier, O.; Paterra, R.; Marie, Y.; et al. Combined analysis of TERT, EGFR, and IDH status defines distinct prognostic glioblastoma classes. Neurology 2014, 83, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, B.; Rachakonda, P.S.; Hosen, I.; Volz, F.; Hemminki, K.; Weyerbrock, A.; Kumar, R. TERT promoter mutations and telomere length in adult malignant gliomas and recurrences. Oncotarget 2015, 6, 10617–10633. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, G.; Shan, Y.; Hartmann, C.; von Deimling, A.; Xing, M. Highly prevalent TERT promoter mutations in bladder cancer and glioblastoma. Cell Cycle 2013, 12, 1637–1638. [Google Scholar] [CrossRef] [PubMed]
- Vinagre, J.; Almeida, A.; Populo, H.; Batista, R.; Lyra, J.; Pinto, V.; Coelho, R.; Celestino, R.; Prazeres, H.; Lima, L.; et al. Frequency of TERT promoter mutations in human cancers. Nat. Commun. 2013. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, N.J.; Ny, L.; Nilsson, J.A.; Larsson, E. Systematic analysis of noncoding somatic mutations and gene expression alterations across 14 tumor types. Nat. Genet. 2014, 46, 1258–1263. [Google Scholar] [CrossRef] [PubMed]
- Batista, R.; Cruvinel-Carloni, A.; Vinagre, J.; Peixoto, J.; Catarino, T.A.; Campanella, N.C.; Menezes, W.; Becker, A.P.; de Almeida, G.C.; Matsushita, M.M.; et al. The prognostic impact of TERT promoter mutations in glioblastomas is modified by the rs2853669 single nucleotide polymorphism. Int. J. Cancer 2016, 139, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Nencha, U.; Rahimian, A.; Giry, M.; Sechi, A.; Mokhtari, K.; Polivka, M.; Schmitt, Y.; Di Stefano, A.L.; Alentorn, A.; Labussiere, M.; et al. TERT promoter mutations and rs2853669 polymorphism: Prognostic impact and interactions with common alterations in glioblastomas. J. Neurooncol. 2016, 126, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Spiegl-Kreinecker, S.; Lotsch, D.; Ghanim, B.; Pirker, C.; Mohr, T.; Laaber, M.; Weis, S.; Olschowski, A.; Webersinke, G.; Pichler, J.; et al. Prognostic quality of activating TERT promoter mutations in glioblastoma: Interaction with the rs2853669 polymorphism and patient age at diagnosis. Neuro-Oncology 2015, 17, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.W.; Verhaak, R.G.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The somatic genomic landscape of glioblastoma. Cell 2013, 155, 462–477. [Google Scholar] [CrossRef] [PubMed]
- Killela, P.J.; Pirozzi, C.J.; Healy, P.; Reitman, Z.J.; Lipp, E.; Rasheed, B.A.; Yang, R.; Diplas, B.H.; Wang, Z.; Greer, P.K.; et al. Mutations in IDH1, IDH2, and in the TERT promoter define clinically distinct subgroups of adult malignant gliomas. Oncotarget 2014, 5, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Li, G.; Qu, Y.; Wang, M.; Cui, B.; Ji, M.; Shi, B.; Hou, P. TERT promoter mutations and long telomere length predict poor survival and radiotherapy resistance in gliomas. Oncotarget 2016, 7, 8712–8725. [Google Scholar] [PubMed]
- Labussiere, M.; Di Stefano, A.L.; Gleize, V.; Boisselier, B.; Giry, M.; Mangesius, S.; Bruno, A.; Paterra, R.; Marie, Y.; Rahimian, A.; et al. TERT promoter mutations in gliomas, genetic associations and clinico-pathological correlations. Br. J. Cancer 2014, 111, 2024–2032. [Google Scholar] [CrossRef] [PubMed]
- Griewank, K.G.; Murali, R.; Puig-Butille, J.A.; Schilling, B.; Livingstone, E.; Potrony, M.; Carrera, C.; Schimming, T.; Moller, I.; Schwamborn, M.; et al. TERT promoter mutation status as an independent prognostic factor in cutaneous melanoma. J. Natl. Cancer Inst. 2014. [Google Scholar] [CrossRef] [PubMed]
- Rachakonda, P.S.; Hosen, I.; de Verdier, P.J.; Fallah, M.; Heidenreich, B.; Ryk, C.; Wiklund, N.P.; Steineck, G.; Schadendorf, D.; Hemminki, K.; et al. TERT promoter mutations in bladder cancer affect patient survival and disease recurrence through modification by a common polymorphism. Proc. Natl. Acad. Sci. USA 2013, 110, 17426–17431. [Google Scholar] [CrossRef] [PubMed]
- Hurst, C.D.; Platt, F.M.; Knowles, M.A. Comprehensive mutation analysis of the TERT promoter in bladder cancer and detection of mutations in voided urine. Eur. Urol. 2014, 65, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Allory, Y.; Beukers, W.; Sagrera, A.; Flandez, M.; Marques, M.; Marquez, M.; van der Keur, K.A.; Dyrskjot, L.; Lurkin, I.; Vermeij, M.; et al. Telomerase reverse transcriptase promoter mutations in bladder cancer: High frequency across stages, detection in urine, and lack of association with outcome. Eur. Urol. 2014, 65, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, T.; Liu, C.; Meng, Y.; Yuan, X.; Liu, L.; Ge, N.; Liu, J.; Wang, C.; Ren, H.; et al. TERT promoter mutations and TERT mrna but not FGFR3 mutations are urinary biomarkers in han chinese patients with urothelial bladder cancer. Oncologist 2015, 20, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Liu, C.; Wang, K.; Liu, L.; Liu, T.; Ge, N.; Kong, F.; Yang, L.; Bjorkholm, M.; Fan, Y.; et al. The genetic difference between western and chinese urothelial cell carcinomas: Infrequent FGFR3 mutation in han chinese patients. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Huang, P.; Li, C.; Huang, Y.; Li, X.; Wang, Y.; Chen, C.; Lv, Z.; Tang, A.; Sun, X.; et al. Telomerase reverse transcriptase gene promoter mutations help discern the origin of urogenital tumors: A genomic and molecular study. Eur. Urol. 2014, 65, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Kurtis, B.; Zhuge, J.; Ojaimi, C.; Ye, F.; Cai, D.; Zhang, D.; Fallon, J.T.; Zhong, M. Recurrent TERT promoter mutations in urothelial carcinoma and potential clinical applications. Ann. Diagn. Pathol. 2016, 21, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Davidson, D.D.; Wang, M.; Lopez-Beltran, A.; Montironi, R.; Wang, L.; Tan, P.H.; MacLennan, G.T.; Williamson, S.R.; Zhang, S. Telomerase reverse transcriptase (TERT) promoter mutation analysis of benign, malignant and reactive urothelial lesions reveals a subpopulation of inverted papilloma with immortalizing genetic change. Histopathology 2015, 69, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Borah, S.; Xi, L.; Zaug, A.J.; Powell, N.M.; Dancik, G.M.; Cohen, S.B.; Costello, J.C.; Theodorescu, D.; Cech, T.R. TERT promoter mutations and telomerase reactivation in urothelial cancer. Science 2015, 347, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Tian, W.; Zhuge, J.; Zheng, X.; Huang, T.; Cai, D.; Zhang, D.; Yang, X.J.; Argani, P.; Fallon, J.T.; et al. Distinguishing nested variants of urothelial carcinoma from benign mimickers by TERT promoter mutation. Am. J. Surg. Pathol. 2015, 39, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.A.; Laughlin, T.S.; Rothberg, P.G. Mutations of the TERT promoter are common in basal cell carcinoma and squamous cell carcinoma. Mod. Pathol. 2014, 27, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Griewank, K.G.; Murali, R.; Schilling, B.; Schimming, T.; Moller, I.; Moll, I.; Schwamborn, M.; Sucker, A.; Zimmer, L.; Schadendorf, D.; et al. TERT promoter mutations are frequent in cutaneous basal cell carcinoma and squamous cell carcinoma. PLoS ONE 2013, 8, e80354. [Google Scholar] [CrossRef] [PubMed]
- Populo, H.; Boaventura, P.; Vinagre, J.; Batista, R.; Mendes, A.; Caldas, R.; Pardal, J.; Azevedo, F.; Honavar, M.; Guimaraes, I.; et al. TERT promoter mutations in skin cancer: The effects of sun exposure and x-irradiation. J. Investig. Dermatol. 2014, 134, 2251–2257. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Liao, S.L.; Hong, J.B.; Chu, C.Y.; Sheen, Y.S.; Jhuang, J.Y.; Tsai, J.H.; Liau, J.Y. TERT promoter mutations in periocular carcinomas: Implications of ultraviolet light in pathogenesis. Br. J. Ophthalmol. 2016, 100, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.A.; Kurtis, B.; Babayeva, S.; Zhuge, J.; Tantchou, I.; Cai, D.; Lafaro, R.J.; Fallon, J.T.; Zhong, M. Heterogeneity of TERT promoter mutations status in squamous cell carcinomas of different anatomical sites. Ann. Diagn. Pathol. 2015, 19, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, Z.; Sun, M.; Chen, K.; Yuan, W.; Jiang, G. The sensitive detection of telomerase reverse transcriptase promoter mutation by amplification refractory mutation system-PCR. Genet. Test. Mol. Biomark. 2016, 20, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bishop, J.; Shan, Y.; Pai, S.; Liu, D.; Murugan, A.K.; Sun, H.; El-Naggar, A.K.; Xing, M. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr. Relat. Cancer 2013, 20, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Landa, I.; Ganly, I.; Chan, T.A.; Mitsutake, N.; Matsuse, M.; Ibrahimpasic, T.; Ghossein, R.A.; Fagin, J.A. Frequent somatic TERT promoter mutations in thyroid cancer: Higher prevalence in advanced forms of the disease. J. Clin. Endocrinol. Metab. 2013, 98, E1562–E1566. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Liu, R.; Qu, S.; Zhu, G.; Bishop, J.; Liu, X.; Sun, H.; Shan, Z.; Wang, E.; Luo, Y.; et al. Association of TERT promoter mutation 1,295,228 c>t with braf v600e mutation, older patient age, and distant metastasis in anaplastic thyroid cancer. J. Clin. Endocrinol. Metab. 2015, 100, E632–E637. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.; da Rocha, A.G.; Vinagre, J.; Batista, R.; Peixoto, J.; Tavares, C.; Celestino, R.; Almeida, A.; Salgado, C.; Eloy, C.; et al. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J. Clin. Endocrinol. Metab. 2014, 99, E754–E765. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, N.; Cao, J.; Sofiadis, A.; Dinets, A.; Zedenius, J.; Larsson, C.; Xu, D. The age- and shorter telomere-dependent TERT promoter mutation in follicular thyroid cell-derived carcinomas. Oncogene 2014, 33, 4978–4984. [Google Scholar] [CrossRef] [PubMed]
- Landa, I.; Ibrahimpasic, T.; Boucai, L.; Sinha, R.; Knauf, J.A.; Shah, R.H.; Dogan, S.; Ricarte-Filho, J.C.; Krishnamoorthy, G.P.; Xu, B.; et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J. Clin. Investig. 2016, 126, 1052–1066. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, T.; Ge, N.; Liu, L.; Yuan, X.; Liu, J.; Kong, F.; Wang, C.; Ren, H.; Yan, K.; et al. TERT promoter mutations are associated with distant metastases in upper tract urothelial carcinomas and serve as urinary biomarkers detected by a sensitive castpcr. Oncotarget 2014, 5, 12428–12439. [Google Scholar] [CrossRef] [PubMed]
- Nault, J.C.; Mallet, M.; Pilati, C.; Calderaro, J.; Bioulac-Sage, P.; Laurent, C.; Laurent, A.; Cherqui, D.; Balabaud, C.; Zucman-Rossi, J. High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat. Commun. 2013. [Google Scholar] [CrossRef]
- Cevik, D.; Yildiz, G.; Ozturk, M. Common telomerase reverse transcriptase promoter mutations in hepatocellular carcinomas from different geographical locations. World J. Gastroenterol. 2015, 21, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Jeng, Y.M.; Chang, C.N.; Lee, H.J.; Hsu, H.C.; Lai, P.L.; Yuan, R.H. TERT promoter mutation in resectable hepatocellular carcinomas: A strong association with hepatitis c infection and absence of hepatitis b infection. Int. J. Surg. 2014, 12, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Guo, X.; Chen, Y.; Chen, G.; Ma, Y.; Huang, K.; Zhang, Y.; Zhao, Q.; Winkler, C.A.; An, P.; et al. Telomerase reverse transcriptase promoter mutations in hepatitis B virus-associated hepatocellular carcinoma. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Totoki, Y.; Tatsuno, K.; Covington, K.R.; Ueda, H.; Creighton, C.J.; Kato, M.; Tsuji, S.; Donehower, L.A.; Slagle, B.L.; Nakamura, H.; et al. Trans-ancestry mutational landscape of hepatocellular carcinoma genomes. Nat. Genet. 2014, 46, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- de Unamuno Bustos, B.; Murria Estal, R.; Perez Simo, G.; Oliver Martinez, V.; Llavador Ros, M.; Palanca Suela, S.; Botella Estrada, R. Lack of TERT promoter mutations in melanomas with extensive regression. J. Am. Acad. Dermatol. 2016, 74, 570–572. [Google Scholar] [CrossRef] [PubMed]
- Egberts, F.; Kruger, S.; Behrens, H.M.; Bergner, I.; Papaspyrou, G.; Werner, J.A.; Alkatout, I.; Haag, J.; Hauschild, A.; Rocken, C. Melanomas of unknown primary frequently harbor TERT-promoter mutations. Melanoma Res. 2014, 24, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.K.; Yao, Y.; Zhang, Z.; Chung, N.Y.; Liu, J.S.; Li, K.K.; Shi, Z.; Chan, D.T.; Poon, W.S.; Zhou, L.; et al. TERT promoter mutations contribute to subset prognostication of lower-grade gliomas. Mod. Pathol. 2015, 28, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, A.E.; Ober, K.; Dubbink, H.J.; Paridaens, D.; Naus, N.C.; Belunek, S.; Krist, B.; Post, E.; Zwarthoff, E.C.; de Klein, A.; et al. Prevalence and implications of TERT promoter mutation in uveal and conjunctival melanoma and in benign and premalignant conjunctival melanocytic lesions. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6024–6030. [Google Scholar] [CrossRef] [PubMed]
- Griewank, K.G.; Murali, R.; Schilling, B.; Scholz, S.; Sucker, A.; Song, M.; Susskind, D.; Grabellus, F.; Zimmer, L.; Hillen, U.; et al. TERT promoter mutations in ocular melanoma distinguish between conjunctival and uveal tumours. Br. J. Cancer 2013, 109, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, B.; Nagore, E.; Rachakonda, P.S.; Garcia-Casado, Z.; Requena, C.; Traves, V.; Becker, J.; Soufir, N.; Hemminki, K.; Kumar, R. Telomerase reverse transcriptase promoter mutations in primary cutaneous melanoma. Nat. Commun. 2014. [Google Scholar] [CrossRef] [PubMed]
- Macerola, E.; Loggini, B.; Giannini, R.; Garavello, G.; Giordano, M.; Proietti, A.; Niccoli, C.; Basolo, F.; Fontanini, G. Coexistence of TERT promoter and braf mutations in cutaneous melanoma is associated with more clinicopathological features of aggressiveness. Virchows Arch. 2015, 467, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Qasem, E.; Murugan, A.K.; Al-Hindi, H.; Xing, M.; Almohanna, M.; Alswailem, M.; Alzahrani, A.S. TERT promoter mutations in thyroid cancer: A report from a middle eastern population. Endocr. Relat. Cancer 2015, 22, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Remke, M.; Ramaswamy, V.; Peacock, J.; Shih, D.J.; Koelsche, C.; Northcott, P.A.; Hill, N.; Cavalli, F.M.; Kool, M.; Wang, X.; et al. TERT promoter mutations are highly recurrent in shh subgroup medulloblastoma. Acta Neuropathol. 2013, 126, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, J.C.; Schwalbe, E.C.; Potluri, S.; Bailey, S.; Williamson, D.; Clifford, S.C. TERT promoter mutation and aberrant hypermethylation are associated with elevated expression in medulloblastoma and characterise the majority of non-infant shh subgroup tumours. Acta Neuropathol. 2014, 127, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Kim, Y.H.; Oh, J.E.; Satomi, K.; Nonoguchi, N.; Keyvani, K.; Pierscianek, D.; Sure, U.; Mittelbronn, M.; Paulus, W.; et al. Alterations of the RRAS and ERCC1 genes at 19q13 in gemistocytic astrocytomas. J. Neuropathol. Exp. Neurol. 2014, 73, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.; Alentorn, A.; Daniau, M.; Labussiere, M.; Rahimian, A.; Tabouret, E.; Polivka, M.; Jouvet, A.; Adam, C.; Figarella-Branger, D.; et al. TERT promoter mutations in primary central nervous system lymphoma are associated with spatial distribution in the splenium. Acta Neuropathol. 2015, 130, 439–440. [Google Scholar] [CrossRef] [PubMed]
- Muzza, M.; Colombo, C.; Rossi, S.; Tosi, D.; Cirello, V.; Perrino, M.; De Leo, S.; Magnani, E.; Pignatti, E.; Vigo, B.; et al. Telomerase in differentiated thyroid cancer: Promoter mutations, expression and localization. Mol. Cell. Endocrinol. 2015, 399, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liu, T.; Sofiadis, A.; Juhlin, C.C.; Zedenius, J.; Hoog, A.; Larsson, C.; Xu, D. TERT promoter mutation as an early genetic event activating telomerase in follicular thyroid adenoma (FTA) and atypical FTA. Cancer 2014, 120, 2965–2979. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.S.; Lim, J.A.; Choi, H.; Won, J.K.; Moon, J.H.; Cho, S.W.; Lee, K.E.; Park, Y.J.; Yi, K.H.; Park do, J.; et al. Prognostic effects of TERT promoter mutations are enhanced by coexistence with BRAF or RAS mutations and strengthen the risk prediction by the ATA or TNM staging system in differentiated thyroid cancer patients. Cancer 2016, 122, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.S.; Kim, Y.; Jeon, S.; Kim, S.H.; Kim, T.J.; Lee, S.; Kim, M.H.; Lim, D.J.; Lee, Y.S.; Jung, C.K. Clinical utility of TERT promoter mutations and ALK rearrangement in thyroid cancer patients with a high prevalence of the BRAF V600E mutation. Diagn. Pathol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.N.; Chiang, Y.C.; Cheng, W.F.; Chen, C.A.; Lin, M.C.; Kuo, K.T. Molecular alterations in endometrial and ovarian clear cell carcinomas: Clinical impacts of telomerase reverse transcriptase promoter mutation. Mod. Pathol. 2015, 28, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.C.; Ayhan, A.; Maeda, D.; Kim, K.R.; Clarke, B.A.; Shaw, P.; Chui, M.H.; Rosen, B.; Shih Ie, M.; Wang, T.L. Frequent somatic mutations of the telomerase reverse transcriptase promoter in ovarian clear cell carcinoma but not in other major types of gynaecological malignancy. J. Pathol. 2014, 232, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Chindris, A.M.; Casler, J.D.; Bernet, V.J.; Rivera, M.; Thomas, C.; Kachergus, J.M.; Necela, B.M.; Hay, I.D.; Westphal, S.A.; Grant, C.S.; et al. Clinical and molecular features of hurthle cell carcinoma of the thyroid. J. Clin. Endocrinol. Metab. 2015, 100, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Tallet, A.; Nault, J.C.; Renier, A.; Hysi, I.; Galateau-Salle, F.; Cazes, A.; Copin, M.C.; Hofman, P.; Andujar, P.; Le Pimpec-Barthes, F.; et al. Overexpression and promoter mutation of the TERT gene in malignant pleural mesothelioma. Oncogene 2014, 33, 3748–3752. [Google Scholar] [CrossRef] [PubMed]
- Akaike, K.; Kurisaki-Arakawa, A.; Hara, K.; Suehara, Y.; Takagi, T.; Mitani, K.; Kaneko, K.; Yao, T.; Saito, T. Distinct clinicopathological features of NAB2-STAT6 fusion gene variants in solitary fibrous tumor with emphasis on the acquisition of highly malignant potential. Hum. Pathol. 2015, 46, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Liau, J.Y.; Tsai, J.H.; Jeng, Y.M.; Chu, C.Y.; Kuo, K.T.; Liang, C.W. TERT promoter mutation is uncommon in acral lentiginous melanoma. J. Cutan. Pathol. 2014, 41, 504–508. [Google Scholar] [CrossRef] [PubMed]
- George, J.R.; Henderson, Y.C.; Williams, M.D.; Roberts, D.B.; Hei, H.; Lai, S.Y.; Clayman, G.L. Association of TERT promoter mutation, but not BRAF mutation, with increased mortality in PTC. J. Clin. Endocrinol. Metab. 2015, 100, E1550–E1559. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Liu, R.; Liu, X.; Murugan, A.K.; Zhu, G.; Zeiger, M.A.; Pai, S.; Bishop, J. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J. Clin. Oncol. 2014, 32, 2718–2726. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Xing, M. Diagnostic and prognostic TERT promoter mutations in thyroid fine-needle aspiration biopsy. Endocr. Relat. Cancer 2014, 21, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qu, S.; Liu, R.; Sheng, C.; Shi, X.; Zhu, G.; Murugan, A.K.; Guan, H.; Yu, H.; Wang, Y.; et al. TERT promoter mutations and their association with BRAF V600E mutation and aggressive clinicopathological characteristics of thyroid cancer. J. Clin. Endocrinol. Metab. 2014, 99, E1130–E1136. [Google Scholar] [CrossRef] [PubMed]
- Dettmer, M.S.; Schmitt, A.; Steinert, H.; Capper, D.; Moch, H.; Komminoth, P.; Perren, A. Tall cell papillary thyroid carcinoma: New diagnostic criteria and mutations in braf and TERT. Endocr. Relat. Cancer 2015, 22, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Chen, E.; Dong, S.; Cai, Y.; Zhang, X.; Zhou, Y.; Zeng, R.; Yang, F.; Pan, C.; Liu, Y.; et al. BRAF and TERT promoter mutations in the aggressiveness of papillary thyroid carcinoma: A study of 653 patients. Oncotarget 2016, 7, 18346–18355. [Google Scholar] [CrossRef] [PubMed]
- Myung, J.K.; Kwak, B.K.; Lim, J.A.; Lee, M.C.; Kim, M.J. TERT Promoter mutations and tumor persistence/recurrence in papillary thyroid cancer. Cancer Res. Treat. 2016, 48, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.J.; Kim, W.G.; Sim, S.; Lim, S.; Kwon, H.; Kim, T.Y.; Shong, Y.K.; Kim, W.B. Low prevalence of somatic TERT promoter mutations in classic papillary thyroid carcinoma. Endocrinol. Metab. 2016, 31, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Bullock, M.; Ren, Y.; O’Neill, C.; Gill, A.; Aniss, A.; Sywak, M.; Sidhu, S.; Delbridge, L.; Learoyd, D.; de Vathaire, F.; et al. TERT promoter mutations are a major indicator of recurrence and death due to papillary thyroid carcinomas. Clin. Endocrinol. 2016, 85, 283–190. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Shi, L.; Wang, D.; Zhang, B.; Yang, Q.; Ji, M.; Shi, B.; Hou, P. Low frequency of TERT promoter mutations in a large cohort of gallbladder and gastric cancers. Int. J. Cancer 2014, 134, 2993–2994. [Google Scholar] [CrossRef] [PubMed]
- Papathomas, T.G.; Oudijk, L.; Zwarthoff, E.C.; Post, E.; Duijkers, F.A.; van Noesel, M.M.; Hofland, L.J.; Pollard, P.J.; Maher, E.R.; Restuccia, D.F.; et al. Telomerase reverse transcriptase promoter mutations in tumors originating from the adrenal gland and extra-adrenal paraganglia. Endocr. Relat. Cancer 2014, 21, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Brown, T.C.; Juhlin, C.C.; Andreasson, A.; Wang, N.; Backdahl, M.; Healy, J.M.; Prasad, M.L.; Korah, R.; Carling, T.; et al. The activating TERT promoter mutation C228T is recurrent in subsets of adrenal tumors. Endocr. Relat. Cancer 2014, 21, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, T.; Liu, L.; Liu, J.; Liu, C.; Wang, C.; Ge, N.; Ren, H.; Yan, K.; Hu, S.; et al. TERT promoter mutations in renal cell carcinomas and upper tract urothelial carcinomas. Oncotarget 2014, 5, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
- Jangard, M.; Zebary, A.; Ragnarsson-Olding, B.; Hansson, J. TERT promoter mutations in sinonasal malignant melanoma: A study of 49 cases. Melanoma Res. 2015, 25, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Barnhill, R.L.; Dummer, R.; Dalton, J.; Wu, J.; Pappo, A.; Bahrami, A. TERT promoter mutations are predictive of aggressive clinical behavior in patients with spitzoid melanocytic neoplasms. Sci. Rep. 2015. [Google Scholar] [CrossRef] [PubMed]
- Goutagny, S.; Nault, J.C.; Mallet, M.; Henin, D.; Rossi, J.Z.; Kalamarides, M. High incidence of activating TERT promoter mutations in meningiomas undergoing malignant progression. Brain Pathol. 2014, 24, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Sahm, F.; Schrimpf, D.; Olar, A.; Koelsche, C.; Reuss, D.; Bissel, J.; Kratz, A.; Capper, D.; Schefzyk, S.; Hielscher, T.; et al. TERT promoter mutations and risk of recurrence in meningioma. J. Natl. Cancer Inst. 2016. [Google Scholar] [CrossRef]
- Carcano, F.M.; Vidal, D.O.; van Helvoort Lengert, A.; Neto, C.S.; Queiroz, L.; Marques, H.; Baltazar, F.; da Silva Martinelli, C.M.; Soares, P.; da Silva, E.C.; et al. Hotspot TERT promoter mutations are rare events in testicular germ cell tumors. Tumour Biol. 2016, 37, 4901–4907. [Google Scholar] [CrossRef]
- Campanella, N.C.; Celestino, R.; Pestana, A.; Scapulatempo-Neto, C.; de Oliveira, A.T.; Brito, M.J.; Gouveia, A.; Lopes, J.M.; Guimaraes, D.P.; Soares, P.; et al. Low frequency of TERT promoter mutations in gastrointestinal stromal tumors (GISTs). Eur. J. Hum. Genet. 2015, 23, 877–879. [Google Scholar] [CrossRef] [PubMed]
- Akaike, K.; Toda-Ishii, M.; Suehara, Y.; Mukaihara, K.; Kubota, D.; Mitani, K.; Takagi, T.; Kaneko, K.; Yao, T.; Saito, T. TERT promoter mutations are a rare event in gastrointestinal stromal tumors. Springerplus 2015. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gao, Y.; Chen, Z.; Hu, X.; Zhou, F.; He, J. Low frequency of TERT promoter somatic mutation in 313 sporadic esophageal squamous cell carcinomas. Int. J. Cancer 2014, 134, 493–494. [Google Scholar] [CrossRef]
- Dono, M.; Angelini, G.; Cecconi, M.; Amaro, A.; Esposito, A.I.; Mirisola, V.; Maric, I.; Lanza, F.; Nasciuti, F.; Viaggi, S.; et al. Mutation frequencies of GNAQ, GNA11, BAP1, SF3B1, EIF1AX and TERT in uveal melanoma: Detection of an activating mutation in the TERT gene promoter in a single case of uveal melanoma. Br. J. Cancer 2014, 110, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Hosen, I.; Rachakonda, P.S.; Heidenreich, B.; de Verdier, P.J.; Ryk, C.; Steineck, G.; Hemminki, K.; Kumar, R. Mutations in TERT promoter and FGFR3 and telomere length in bladder cancer. Int. J. Cancer 2015, 137, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Dang, S.; Wu, K.; Shao, Y.; Yang, Q.; Ji, M.; Shi, B.; Hou, P. TERT promoter mutations predict worse survival in laryngeal cancer patients. Int. J. Cancer 2014, 135, 1008–1010. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Ruden, M.; Puri, N. Novel anticancer therapeutics targeting telomerase. Cancer Treat. Rev. 2013, 39, 444–456. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).