The Silencing of a 14-3-3ɛ Homolog in Tenebrio molitor Leads to Increased Antimicrobial Activity in Hemocyte and Reduces Larval Survivability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insect Rearing, Microorganism Culture, and Challenge Experiments
2.2. Full-Length cDNA Cloning and in Silico Analysis of the Putative Protein
2.3. Expression Analysis of Tm14-3-3ɛ Transcripts and RNAi
2.4. Larval Mortality Assay
2.5. Antibacterial Activity Assay
2.6. Statistical Analysis
3. Results and Discussion
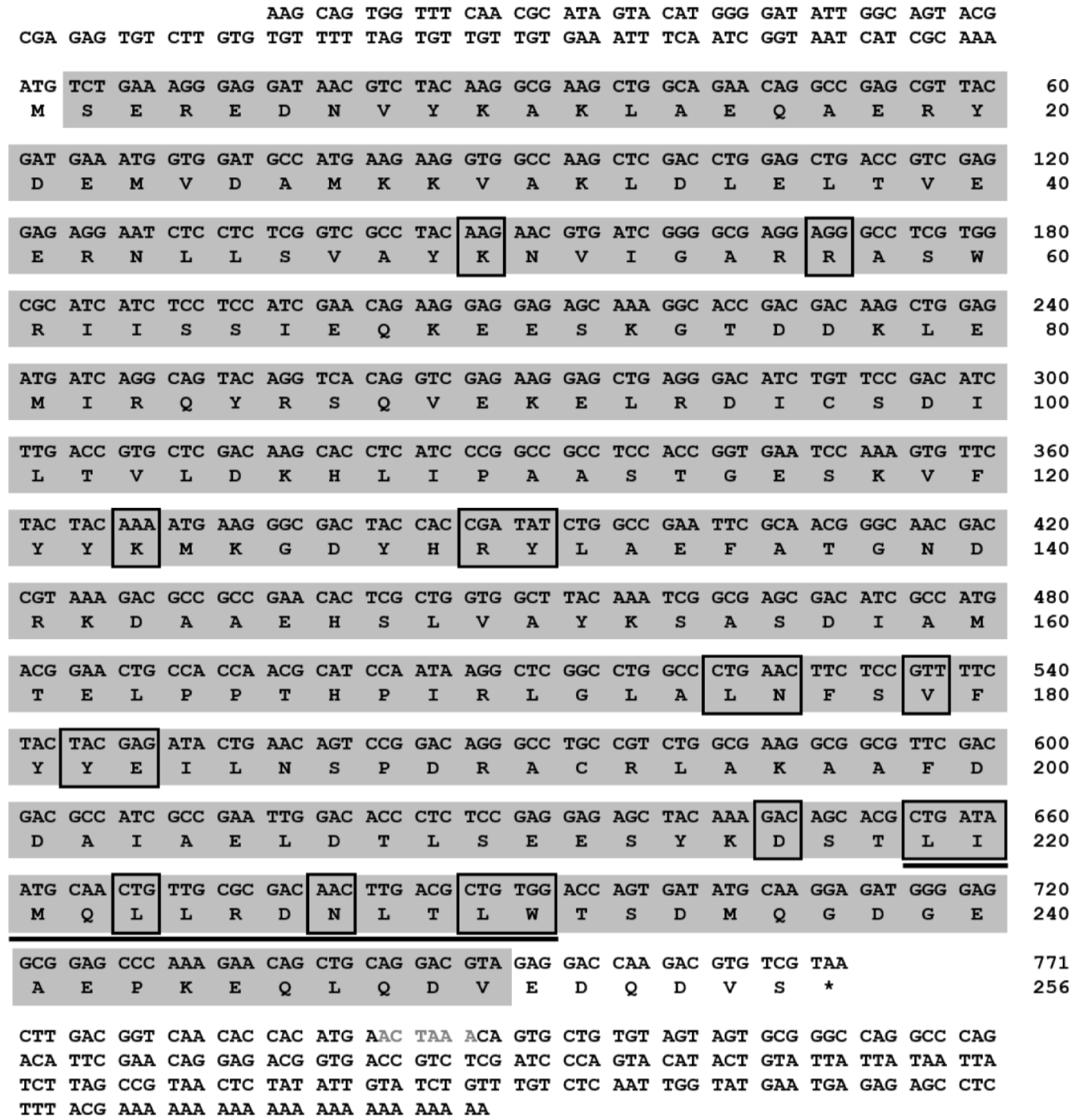
3.1. Molecular Cloning and Sequence Analysis of Tm14-3-3ɛ
3.2. Expression of Tm14-3-3ɛ Transcripts and Innate Immune Function in Hosts
3.3. Tm14-3-3ɛ and Hemocyte Antimicrobial Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aitken, A. 14-3-3 proteins: A historic overview. Semin. Cancer Biol. 2006, 16, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lee, W.H.; Sobott, F.; Papagrigoriou, E.; Robinson, C.V.; Grossmann, J.G.; Sundstrom, M.; Doyle, D.A.; Elkins, J.M. Structural basis for protein-protein interactions in the 14-3-3 protein family. Proc. Natl. Acad. Sci. USA 2006, 103, 17237–17242. [Google Scholar] [CrossRef] [PubMed]
- De Vetten, N.C.; Lu, G.; Feri, R.J. A maize protein associated with the G-box binding complex has homology to brain regulatory proteins. Plant Cell 1992, 4, 1295–1307. [Google Scholar] [CrossRef] [PubMed]
- Van Heusden, G.P.; Griffiths, D.J.; Ford, J.C.; Chin, A.W.T.F.; Schrader, P.A.; Carr, A.M.; Steensma, H.Y. The 14-3-3 proteins encoded by the BMH1 and BMH2 genes are essential in the yeast Saccharomyces cerevisiae and can be replaced by a plant homolog. Eur. J. Biochem. 1995, 229, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Toker, A.; Sellers, L.A.; Amess, B.; Patel, Y.; Harris, A.; Aitken, A. Multiple isoforms of a protein kinase C inhibitor (KCIP-1/14-3-3) from sheep brain. Amino acid sequence of phosphorylated forms. Eur. J. Biochem. 1992, 206, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Benton, R.; Palacios, I.M.; Johnston, D. Drosophila 14-3-3/PAR-5 is an essential mediator of PAR-1 function in axis formation. Dev. Cell 2002, 3, 659–671. [Google Scholar] [CrossRef]
- Shandala, T.; Woodcock, J.M.; Ng, Y.; Biggs, L.; Skoulakis, E.M.; Brooks, D.A.; Lopez, A.F. Drosophila 14-3-3ɛ has a crucial role in anti-microbial peptide secretion and innate immunity. J. Cell Sci. 2011, 124, 2165–2174. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Hwang, S.H.; Han, Y.S.; Cho, S. Isolation and expression analysis of a homolog of the 14-3-3ɛ gene in the diamondback moth, Plutella xylostella. Arch. Insect Biochem. Physiol. 2011, 76, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Feng, E.; Chen, H.; Li, Y.; Jiang, W.; Wang, Z.; Yin, Y. Gene cloning, expression, and function analysis of SpL14-3-3ζ in Spodoptera litura and its response to the entomopathogenic fungus Nomuraea rileyi. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Lv, Z.; Chen, J.; Nie, Z.; Wang, D.; Shen, H.; Wang, X.; Wu, X.; Zhang, Y. Expression analysis and tissue distribution of two 14-3-3 proteins in silkworm. Biochim. Biophys. Acta 2007, 1770, 1598–1604. [Google Scholar]
- Veisova, D.; Rezabkova, L.; Stepanek, M.; Novotna, P.; Herman, P.; Vecer, J.; Obsil, T.; Obsilova, V. The C-terminal segment of yeast BMH proteins exhibits different structure compared to other 14-3-3 protein isoforms. Biochemistry 2010, 49, 3853–3861. [Google Scholar] [CrossRef] [PubMed]
- Obsilova, V.; Kopecka, M.; Kosek, D.; Kacirova, M.; Kylarova, S.; Rezabkova, L.; Obsil, T. Mechanisms of the 14-3-3 protein function: Regulation of protein function through conformational modulation. Physiol. Res. 2014, 1, S155–S164. [Google Scholar]
- Van Hemert, M.J.; Steensma, H.Y.; van Heusden, G.P. 14-3-3 proteins: Key regulators of cell division, signalling and apoptosis. BioEssays 2001, 23, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Niemantsverdriet, M.; Wagner, K.; Visser, M.; Backendorf, C. Cellular functions of 14-3-3ζ in apoptosis and cell adhesion emphasize its oncogenic character. Oncogene 2008, 27, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Rubin, G.M. 14-3-3ɛ positively regulates ras-mediated signaling in Drosophila. Genes Dev. 1997, 11, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.D.; Luo, X.; Biteau, B.; Syverson, K.; Jasper, H. 14-3-3ɛ antagonizes FoxO to control growth, apoptosis and longevity in Drosophila. Aging Cell 2008, 7, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Shandala, T.; Brooks, D.A. Innate immunity and exocytosis of antimicrobial peptides. Commu. Integr. Biol. 2012, 5, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Ulvila, J.; Vanha-aho, L.M.; Kleino, A.; Vaha-Makila, M.; Vuoksio, M.; Eskelinen, S.; Hultmark, D.; Kocks, C.; Hallman, M.; Parikka, M.; et al. Cofilin regulator 14-3-3ζ is an evolutionarily conserved protein required for phagocytosis and microbial resistance. J. Leuk. Biol. 2011, 89, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Tabunoki, H.; Shimada, T.; Banno, Y.; Sato, R.; Kajiwara, H.; Mita, K.; Satoh, J. Identification of Bombyx mori 14-3-3 orthologs and the interactor Hsp60. Neurosci. Res. 2008, 61, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Kim, C.H.; Kim, J.H.; Je, B.R.; Roh, K.B.; Kim, S.J.; Lee, H.H.; Ryu, J.H.; Lim, J.H.; Oh, B.H.; et al. Clustering of Peptidoglycan recognition protein-SA is required for sensing lysine-type peptidoglycan in insects. Proc. Natl. Acad. Sci. USA 2007, 104, 6602–6607. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Kim, S.J.; Kan, H.; Kwon, H.M.; Roh, K.B.; Jiang, R.; Yang, Y.; Park, J.W.; Lee, H.H.; Ha, N.C.; et al. A three-step proteolytic cascade mediates the activation of the peptidoglycan-induced toll pathway in an insect. J. Biol. Chem. 2008, 283, 7599–7607. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Kim, E.H.; Gong, J.H.; Kwon, H.M.; Kim, C.H.; Ryu, K.H.; Park, J.W.; Kurokawa, K.; Zhang, J.; Gubb, D.; et al. Three pairs of protease-serpin complexes cooperatively regulate the insect innate immune responses. J. Biol. Chem. 2009, 284, 35652–35658. [Google Scholar] [CrossRef] [PubMed]
- Roh, K.B.; Kim, C.H.; Lee, H.; Kwon, H.M.; Park, J.W.; Ryu, J.H.; Kurokawa, K.; Ha, N.C.; Lee, W.J.; Lemaitre, B.; et al. Proteolytic cascade for the activation of the insect toll pathway induced by the fungal cell wall component. J. Biol. Chem. 2009, 284, 19474–19481. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kwon, H.M.; Park, J.W.; Kurokawa, K.; Lee, B.L. N-terminal GNBP homology domain of Gram-negative binding protein 3 functions as a beta-1,3-glucan binding motif in Tenebrio molitor. BMB Rep. 2009, 42, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Park, J.W.; Kwon, H.M.; Hwang, H.O.; Jang, I.H.; Masuda, A.; Kurokawa, K.; Nakayama, H.; Lee, W.J.; Dohmae, N.; et al. Diversity of innate immune recognition mechanism for bacterial polymeric meso-diaminopimelic acid-type peptidoglycan in insects. J. Biol. Chem. 2010, 285, 32937–32945. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.H.; Park, J.W.; Kurokawa, K.; Matsushita, M.; Lee, B.L. The molecular activation and regulation mechanisms of proteolytic Toll signaling cascade in insect innate immunity. Invert. Surviv. J. 2010, 7, 181–191. [Google Scholar]
- Park, S.H.; Jiang, R.; Piao, S.; Zhang, B.; Kim, E.H.; Kwon, H.M.; Jin, X.L.; Lee, B.L.; Ha, N.C. Structural and functional characterization of a highly specific serpin in the insect innate immunity. J. Biol. Chem. 2011, 286, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, B.B.; Patnaik, H.H.; Seo, G.W.; Jo, Y.H.; Lee, Y.S.; Lee, B.L.; Han, Y.S. Gene structure, cDNA characterization and RNAi-based functional analysis of a myeloid differentiation factor 88 homolog in Tenebrio molitor larvae exposed to Staphylococcus aureus infection. Dev. Comp. Immunol. 2014, 46, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.H.; Kim, M.S.; Choi, H.W.; Joo, C.H.; Cho, M.Y.; Lee, B.L. A masquerade-like serine proteinase homolog is necessary for phenoloxidase activity in the coleopteran insect, Holotrichia diomphalia larvae. Eur. J. Biochem. 2000, 267, 6188–6196. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Zhang, R.; Kim, M.S.; Park, J.W.; Park, H.Y.; Kawabata, S.; Lee, B.L. A zymogen form of masquerade-like serine proteinase homolog is cleaved during pro-phenoloxidase activation by Ca2+ in coleopteran and Tenebrio molitor larvae. Eur. J. Biochem. 2002, 269, 4375–4383. [Google Scholar] [CrossRef] [PubMed]
- Tindwa, H.; Patnaik, B.B.; Kim, D.H.; Mun, S.; Jo, Y.H.; Lee, B.L.; Lee, Y.S.; Kim, N.J.; Han, Y.S. Cloning, characterization and effect of TmPGRP-LE gene silencing on survival of Tenebrio molitor against Listeria monocytogenes infection. Int. J. Mol. Sci. 2013, 14, 22462–22482. [Google Scholar] [CrossRef] [PubMed]
- Tindwa, H.; Jo, Y.H.; Patnaik, B.B.; Lee, Y.S.; Kang, S.S.; Han, Y.S. Molecular cloning and characterization of autophagy-related gene TmAtg8 in Listeria-invaded hemocytes of Tenebrio molitor. Dev. Comp. Immunol. 2015, 51, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Tindwa, H.; Jo, Y.H.; Patnaik, B.B.; Noh, M.Y.; Kim, D.H.; Kim, I.; Han, Y.S.; Lee, Y.S.; Lee, B.L.; Kim, N.J. Depletion of autophagy-related genes Atg3 and Atg5 in Tenebrio molitor leads to decreased survivability against an intracellular pathogen, Listeria monocytogenes. Arch. Insect Biochem. Physiol. 2015, 88, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.Y.; Patnaik, B.B.; Tindwa, H.; Seo, G.W.; Kim, D.H.; Patnaik, H.H.; Jo, Y.H.; Lee, Y.S.; Lee, B.L.; Kim, N.J.; et al. Genomic organization, sequence characterization and expression analysis of Tenebrio molitor apolipophorin-III in response to an intracellular pathogen, Listeria monocytogenes. Gene 2014, 534, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, B.B.; Patnaik, H.H.; Park, K.B.; Jo, Y.H.; Lee, Y.S.; Han, Y.S. Silencing of Apolipophorin III causes abnormal adult morphological phenotype and susceptibility to Listeria monocytogenes infection in Tenebrio molitor. Entomol. Res. 2015, 45, 116–121. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Lee, S.Y.; Kurata, S.; Natori, S.; Lee, B.L. Purification and molecular cloning of cDNA for an inducible antibacterial protein from larvae of the coleopteran, Tenebrio molitor. J. Biochem. 1994, 116, 53–58. [Google Scholar] [PubMed]
- Lopez-Girona, A.; Furnari, B.; Mondesert, O.; Russell, P. Nuclear localization of CDC25 is regulated by DNA damage and a 14-3-3 protein. Nature 1999, 397, 172–175. [Google Scholar] [PubMed]
- Wang, W.; Shakes, D.C. Molecular evolution of the 14-3-3 protein family. J. Mol. Evol. 1996, 43, 384–398. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Guthridge, M.; Woodcock, J.; Lopez, A. 14-3-3 protein signaling in development and growth factor responses. Curr. Top. Dev. Biol. 2005, 67, 285–303. [Google Scholar] [PubMed]




| Name | Primer sequences |
|---|---|
| Tm14-3-3ε5’-RACE GSP1 Tm14-3-3ε 5’-RACE nGSP2 | 5’-GCTCCTTCTCGACCTGTGAC-3’ 5’-ACCTTCTTCATGGCATCCAC-3’ |
| Tm14-3-3ε 3’-RACE GSP1 Tm14-3-3ε 3’-RACE nGSP2 | 5’-CTCGCTGGTGGCTTACAAAT-3’ 5’-GCAGGACGTAGAGGACCAAG-3’ |
| dsTm14-3-3ε-Fw dsTm14-3-3ε-Rv | 5’-TAATACGACTCACTATAGGGAGAACAGGTCGAGAAGGAGCTGA-3’ 5’-TAATACGACTCACTATAGGGAGAACGTCCTGCAGCTGTTCTTT-3’ |
| dsTm14-3-3ζ-Fw dsTm14-3-3ζ-Rv | 5’-TAATACGACTCACTATAGGGTAGAAACGGGCGTAGAACTCA-3’ 5’-TAATACGACTCACTATAGGGTGCATCATCGAAAGCCTGTTT-3’ |
| dsEGFP-Fw dsEGFP-Rv | 5’-TAATACGACTCACTATAGGGTACGTAAACGGCCACAAGTTC-3’ 5’-TAATACGACTCACTATAGGGTTGCTCAGGTAGTGGTTGTCG-3’ |
| Tm14-3-3ε-qPCR-Fw Tm14-3-3ε-qPCR-Rv | 5’-TGGTGGATGCCATGAAGAAG-3’ 5’-CTGTTCGATGGAGGAGATGATG-3’ |
| Tm14-3-3ζ-qPCR-Fw Tm14-3-3ζ-qPCR-Rv | 5’-TTTGGCGGAAGTAGCCACAGGAGA-3’ 5’-TAATCTGATGGGATGTGTGGGCGT-3’ |
| TmL27a-qPCR-Fw TmL27a-qPCR-Rv | 5’-TCATCCTGAAGGCAAAGCTCCAGT-3’ 5’-AGGTTGGTTAGGCAGGCACCTTTA-3’ |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, G.W.; Jo, Y.H.; Seong, J.H.; Park, K.B.; Patnaik, B.B.; Tindwa, H.; Kim, S.-A.; Lee, Y.S.; Kim, Y.J.; Han, Y.S. The Silencing of a 14-3-3ɛ Homolog in Tenebrio molitor Leads to Increased Antimicrobial Activity in Hemocyte and Reduces Larval Survivability. Genes 2016, 7, 53. https://doi.org/10.3390/genes7080053
Seo GW, Jo YH, Seong JH, Park KB, Patnaik BB, Tindwa H, Kim S-A, Lee YS, Kim YJ, Han YS. The Silencing of a 14-3-3ɛ Homolog in Tenebrio molitor Leads to Increased Antimicrobial Activity in Hemocyte and Reduces Larval Survivability. Genes. 2016; 7(8):53. https://doi.org/10.3390/genes7080053
Chicago/Turabian StyleSeo, Gi Won, Yong Hun Jo, Jeong Hwan Seong, Ki Beom Park, Bharat Bhusan Patnaik, Hamisi Tindwa, Sun-Am Kim, Yong Seok Lee, Yu Jung Kim, and Yeon Soo Han. 2016. "The Silencing of a 14-3-3ɛ Homolog in Tenebrio molitor Leads to Increased Antimicrobial Activity in Hemocyte and Reduces Larval Survivability" Genes 7, no. 8: 53. https://doi.org/10.3390/genes7080053
APA StyleSeo, G. W., Jo, Y. H., Seong, J. H., Park, K. B., Patnaik, B. B., Tindwa, H., Kim, S. -A., Lee, Y. S., Kim, Y. J., & Han, Y. S. (2016). The Silencing of a 14-3-3ɛ Homolog in Tenebrio molitor Leads to Increased Antimicrobial Activity in Hemocyte and Reduces Larval Survivability. Genes, 7(8), 53. https://doi.org/10.3390/genes7080053







